Abstract
Infective endocarditis (IE) is a condition mainly associated with valvular disease or prosthetic valve and intravenous drug use as a risk factor. Here, we describe a rare case of a previously healthy patient with endocarditis due to Lactococcus lactis associated with cattle contact, where antibiotic treatment resulted in full recovery.
1. Introduction
The Lactococcus genus has two main representatives, L. lactis and L. cremoris. Lactococcus lactis is an anaerobic, catalase-negative, and Gram-positive microorganism widely used in cheese production and dairy milk products like yogurt and sour cream. L. lactis is considered to have low virulence and pathogenic potential although it has been associated with some diseases in healthy, immunocompetent, or immunocompromised patients. A few cases including infective endocarditis (IE) in adults and in children have been documented. Herein, we describe a case of infective endocarditis in a young adult due to Lactococcus lactis.
2. Case Presentation
A 35-year-old blacksmith presented at the emergency department with a 3-week history of fever to 38.8°C, chills, weakness, and night sweats. The patient denied the existence of shortness of breath, chest pain, vomiting, diarrhea, abdominal pain, dysuria, and any other symptoms. His previous medical history was clear, and he mentioned no use of cigarettes, alcohol, unprocessed dairy products, or intravenous drugs. In the past, he had dental implants, and his last visit at the dentist was 6 months ago. He lives in a village, where he takes care of some chickens, rabbits, and lambs. Also, one week prior to his hospital admission, he visited his family doctor who suggested the administration of levofloxacin (500 mg/d per os) for ten days.
On admission, he was in a good level of consciousness with blood pressure of 150/76 mmHg, atmospheric air oxygen saturation of 99%, and heart rate at 93 beats/min. On clinical examination, a diastolic murmur was audible at the second right intercostal space (aortic valve area). No pathologic findings were found on lung and abdominal examination. No other mucocutaneous signs of endocarditis were observed.
Blood tests showed CRP of 2.5 mg/dl, erythrocyte sedimentation rate (ESR) of 20 mm/h, and white blood cell (WBC) count of 12.000/μL. Urine analysis and urine culture were negative. The chest X-ray, the electrocardiography, and the ultrasound of the abdomen were normal. Two sets (aerobic and anaerobic) of blood cultures were obtained with a difference of 20 minutes between them, and a total of 4 sets of blood cultures were finally received. The patient was initially treated with intravenous levofloxacin 500 mg and ceftriaxone 2 g per day. The serum immunological investigation for HIV, HAV, HBV, HCV, CMV, Toxoplasma, EBV, HSV, Coxsackie, RF, C3, C4, ANA, ANCA, Coxiella burnetii, Leishmania, Leptospira, and Brucella was found to be negative. The ophthalmologic examination with fundoscopy had no findings. Transthoracic and transesophageal echocardiography showed left ventricle dilation (ejection fraction 65%) and a degenerated bicuspid aortic valve with a small vegetation (0.5 × 0.6 cm) at the left cusp and a moderate-to-severe regurgitation. These findings are well depicted in Figures 1 and 2. Two blood cultures defined the underlying pathogen, which was Lactococcus lactis, all in anaerobic bottles. The isolate was sensitive to ampicillin, ceftriaxone, clindamycin, chloramphenicol, erythromycin, oxacillin, teicoplanin, and vancomycin. An alternative antimicrobial therapy with ceftriaxone 2 g q.d. and gentamicin 80 mg t.i.d. was initiated. During the following days, the inflammation markers dropped (CRP: 0.55 and WBC 7.53 × 103/μL), and from the fourth day of antibiotic treatment, the patient was afebrile. Two sets of blood cultures were obtained whose results were negative. On hospital day 10, administration of gentamicin was ended, and a 6-week course of intravenous ceftriaxone was given. A new transthoracic echocardiography revealed a further decrease in vegetation size. In 3 months of follow-up, the patient was symptom-free.
Figure 1.
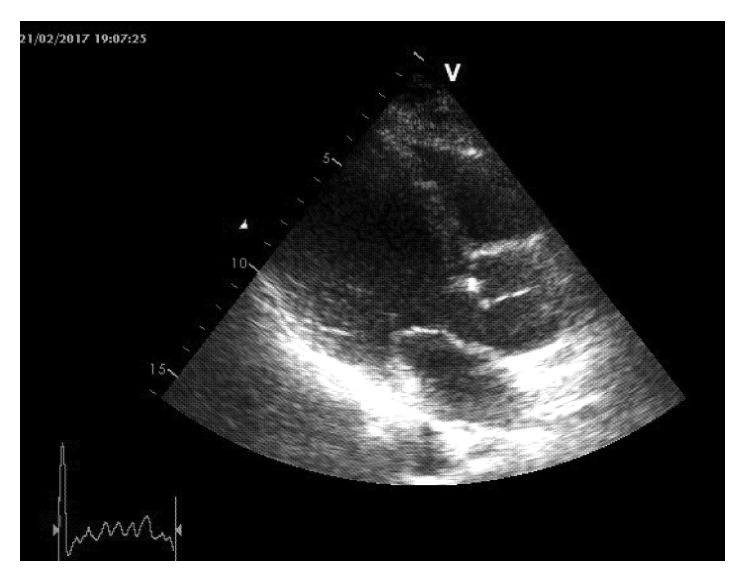
Transthoracic ultrasound showing a degenerated bicuspid aortic valve, combined with left ventricle dilation. A small mass on the left aortic cusp is distinguished as septic vegetation, due to L. lactis.
Figure 2.

Doppler echocardiography and colour flow mapping revealed moderate-to-severe (3/4) aortic valve failure.
3. Discussion
Taking into consideration the clinical presentation and the further investigation (persistent fever, endocardial involvement documented by transthoracic and transesophageal ultrasound, bicuspid aortic valve, and sustained bacteremia), the patient was diagnosed with IE, according to the modified Duke criteria [1]. There are only few cases with lactococcal IE; thus, the therapeutic protocol was based on the susceptibility of the lactococcal isolate.
Lactococcus lactis (former Streptococcus lactis) is a spherical-shaped mesophilic, microaerophilic fermenting bacterium. Among the subspecies of Lactococcus species, the main bacteria are L. lactis and L. cremoris, which seem to be skin commensals in the cattle. They are really popular in dairy industry because of their use for cheese and fermented milk products. Lately, their reputation is also growing in the vaccine industry, and there is research in animal models supporting vaccination for infectious diseases such as avian flu and pneumococcal infections [2, 3]. Lactococcus lactis has low virulence, and in general, it is nonpathogenic, but recently, it is considered as the opportunistic pathogen microbe. A possible condition affecting the virulence and the infectious potential of Lactococcus lactis in infective endocarditis is its ability of heterologous expression of surface glycoprotein Cnm which promotes adherence to cardiac tissue [4]. There are some reports that indicate the involvement of L. lactis in emergency situations such as septic arthritis [5], liver and cerebral abscess [6, 7], peritonitis [8], and osteomyelitis [9]. These reports include immunocompetent and immunocompromised patients.
Lactococcal IE is very rare, and only few cases in adults and fewer cases in children/infants can be found in the literature [10–19]. These cases are summarized in Table 1. In 2 cases [15], including ours, the affected valve was the aortic valve, which was bicuspid. However, in other cases, endocarditis seemed to attack the atrioventricular valves [10–14, 16–19].
Table 1.
Reported cases of Lactococcus lactis–associated infective endocarditis.
| References | Heart disease | Complications | Clinical outcome |
|---|---|---|---|
| Wood et al. [10] | No history of heart disease | — | Recovered |
| Mannion and Rothburn [11] | Rheumatic valve disease | Infarction/dysphasia | Recovered |
| Pellizzer et al. [12] | No history of heart disease | — | Recovered |
| Halldorsdottir et al. [13] | No history of heart disease | — | Recovered |
| Zechini et al. [14] | Atrial myxoma and mitral regurgitation | — | Recovered after surgery |
| Resch et al. [15] | Bicuspid aortic valve | Multiple mycotic aneurysms | Recovered after surgery |
| Lin et al. [16] | No history of heart disease | Intracerebral hemorrhage/infraction | Deceased |
| Rostagno et al. [17] | Bileaflet mitral valve prolapse (prosthetic valve repair) | Embolic infraction | Recovered after surgery |
| Taniguchi et al. [18] | No history of heart disease | Arrhythmia (severe inflammation of the conductive system) | Deceased |
| Mansour et al. [19] | No history of heart disease | Pulmonary septic emboli | Recovered |
Rheumatic valvular disease or other valvular impairment, prosthetic valve, intravenous drug use, and congenital heart disease are well-documented lifetime-predisposing factors for IE. However, only five patients with lactococcal IE including ours [11, 14, 15, 17] had a predisposing condition associated with high risk of IE. In the rest of them, there was not a history of valvular heart disease [10, 12, 13, 16, 18, 19].
L. lactis is not considered a human pathogen, and human infections in people with immunosuppresion or impaired defence mechanisms are opportunistic infections. Due to the rarity of the Lactococcus infection, the route of this infection is not well demonstrated. The hypotheses about the source of infection include exposure by ingestion or contact with unpasteurized dairy products or raw milk [10, 13, 15]. Another proposed mechanism which is also suggested for our patient, who was occupied with sheep, chickens, and rabbits, is direct intraluminal spreading from contaminated hands. However, many patients declared that they did not consume any dairy products or raw milk [7, 11, 12, 14, 16, 17, 19].
From the literature, we can easily assume that this condition might be associated with severe complications. Most of the patients developed cerebral embolism, and in one of them, this caused hemorrhage [16] and death. Another patient developed multiple mycotic aneurysms [15], and there is also a case of young child presenting with pulmonary embolization [19]. Another reported complication was cardiac arrhythmia due to excessive inflammation of the conduction system of the heart in an infant who did not survive the infection [18].Our patient recovered and was free of complications in 3 months of follow-up.
4. Conclusion
Lactococcal endocarditis is an extremely rare condition that can be presented at any age. Despite its low virulence and pathogenic potential, Lactococcus should be treated as serious infection because of the 2 referred deaths and its complications. It is essential for the clinician to suspect lactococcal endocarditis, when he notices (1) long-lasting fever with (2) newly audible murmur or (3) cerebrovascular event in a patient with a (4) history of unpasteurized dairy product consumption. As long as there is no specific guidance, antimicrobial therapy should be in compliance with the susceptibility of the pathogen isolated from the cultures. Further cases need to be reported and analyzed to develop a therapeutic and preventive strategy for this disease.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.Habib G., Lancellotti P., Antunes M. J., et al. 2015 ESC Guidelines for the management of infective endocarditis. European Heart Journal. 2015;36(44):3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 2.Medina M. S., Vintiñi E. O., Villena J., Raya R. R., Alvarez S. G. Lactococcus lactis as an adjuvant and delivery vehicle of antigens against pneumococcal respiratory infections. Bioengineered Bugs. 2010;1(5):313–325. doi: 10.4161/bbug.1.5.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei H., Peng X., Ouyang J., et al. Protective immunity against influenza H5N1 virus challenge in chickens by oral administration of recombinant Lactococcus lactis expressing neuraminidase. BMC Veterinary Research. 2015;11(1):p. 85. doi: 10.1186/s12917-015-0399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freires I. A., Avilés-Reyes A., Kitten T., et al. Heterologous expression of Streptococcus mutans Cnm in Lactococcus lactis promotes intracellular invasion, adhesion to human cardiac tissues and virulence. Virulence. 2017;8(1):18–29. doi: 10.1080/21505594.2016.1195538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell P., Dealler S., Lawton J. O. Septic arthritis and unpasteurised milk. Journal of Clinical Pathology. 1993;46(11):1057–1058. doi: 10.1136/jcp.46.11.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H. S., Park D. W., Youn Y. K., et al. Liver abscess and empyema due to Lactococcus lactis cremoris. Journal of Korean Medical Science. 2010;25(11):1669–1671. doi: 10.3346/jkms.2010.25.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhaddar A., El Mostarchid B., Gazzaz M., et al. Cerebellar abscess due to Lactococcus lactis: a new pathogen. Acta Neurochirurgica. 2002;144(3):305–306. doi: 10.1007/s007010200041. [DOI] [PubMed] [Google Scholar]
- 8.Guz G., Colak B., Hizel K., Suyani E., Sindel S. Peritonitis due to Lactococcus lactis in a CAPD patient. Scandinavian Journal of Infectious Diseases. 2006;38(8):698–699. doi: 10.1080/00365540500438407. [DOI] [PubMed] [Google Scholar]
- 9.Kiss J., Zahár A., Nyíri P., Prinz G. A case of femoral osteomyelitis caused by Lactococcus. Orvosi Hetilap. 2005;146:613–618. [PubMed] [Google Scholar]
- 10.Wood H. F., Jacobs K., McCarty M. Streptococcus lactis isolated from a patient with subacute bacterial endocarditis. American Journal of Medicine. 1955;18(2):345–347. doi: 10.1016/0002-9343(55)90247-3. [DOI] [PubMed] [Google Scholar]
- 11.Mannion P. T., Rothburn M. M. Diagnosis of bacterial endocarditis caused by Streptococcus lactis and assisted by immunoblotting of serum antibodies. Journal of Infection. 1990;21(3):317–318. doi: 10.1016/0163-4453(90)94149-t. [DOI] [PubMed] [Google Scholar]
- 12.Pellizzer G., Benedetti P., Biavasco F., et al. Bacterial endocarditis due to Lactococcus lactis subsp. cremoris: case report. Clinical Microbiology and Infection. 1996;2(3):230–232. doi: 10.1016/s1198-743x(14)65148-x. [DOI] [PubMed] [Google Scholar]
- 13.Halldorsdottir H. D., Haraldsdottir V., Bodvarsson A., Þorgeirsson G., Kristjánsson M. Endocarditis caused by Lactococcus cremoris. Scandinavian Journal of Infectious Diseases. 2002;34(3):205–206. doi: 10.1080/00365540110080377. [DOI] [PubMed] [Google Scholar]
- 14.Zechini B., Cipriani P., Papadopoulou S., Nucci G. D., Petrucca A., Teggi A. Endocarditis caused by Lactococcus lactis subsp. lactis in a patient with atrial myxoma: a case report. Diagnostic Microbiology and Infectious Disease. 2006;56(3):325–328. doi: 10.1016/j.diagmicrobio.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Resch M., Schichtl T., Endemann D. H., et al. General aneurysmatosis due to cheese consumption: complications of an endocarditis caused by Lactococcus cremoris. International Journal of Cardiology. 2008;126(1):e8–e9. doi: 10.1016/j.ijcard.2006.12.068. [DOI] [PubMed] [Google Scholar]
- 16.Lin K. H., Sy C. L., Chen C. S., Lee C. H., Lin Y. T., Li J. Y. Infective endocarditis complicated by intracerebral hemorrhage due to Lactococcus lactis subsp. cremoris. Infection. 2010;38(2):147–149. doi: 10.1007/s15010-010-9219-3. [DOI] [PubMed] [Google Scholar]
- 17.Rostagno C., Pecile P., Stefàno P. L. Early Lactococcus lactis endocarditis after mitral valve repair: a case report and literature review. Infection. 2013;41(4):897–899. doi: 10.1007/s15010-012-0377-8. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi K., Nakayama M., Nakahira K., et al. Sudden infant death due to Lactococcal infective endocarditis. Legal Medicine. 2016;19:107–111. doi: 10.1016/j.legalmed.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Mansour B., Habib A., Asli N., et al. A case of infective endocarditis and pulmonary septic emboli caused by Lactococcus lactis. Case reports in Pediatrics. 2016;2016:4. doi: 10.1155/2016/1024054.1024054 [DOI] [PMC free article] [PubMed] [Google Scholar]


