Summary
Hypercapnia has been utilized as a stimulus to elicit changes in cerebral blood flow (CBF). However, in many instances it has been delivered in a non-controlled method that is often difficult to reproduce. The purpose of this study was to examine the within- and between-visit reproducibility of blood oxygen level-dependent (BOLD) signal changes to an iso-oxic square wave alteration in end-tidal carbon dioxide partial pressure (PetCO2). Two 3-Tesla (3T) MRI scans were performed on the same visit, with two square wave alterations administered per scan. The protocol was repeated on a separate visit with minimum of 3 days between scanning sessions. PetCO2 was altered to stimulate changes in cerebral vascular reactivity (CVR), while PetO2 was held constant. Eleven subjects (six females; mean age 26·5 ± 5·7 years) completed the full testing protocol. Excellent within-visit square wave reproducibility (ICC > 0·75) was observed. Similarly, square waves were reproducible between scanning sessions (ICC > 0·7). This study demonstrates BOLD signal changes in response to alterations in PetCO2 are reproducible both within- and between-visit MRI scans.
Keywords: cerebral vascular reactivity, hypercapnia, magnetic resonance imaging, vascular function
Introduction
Cerebrovascular reactivity (CVR) is defined as a change in cerebral blood flow due to a vasodilatory stimulus (Schwertfeger et al., 2006). Impairment in CVR has been observed in a number of cerebral disease processes, such as traumatic brain injury (Len & Neary, 2010), moyamoya vasculopathy (Han et al., 2011) and seizures (Fierstra et al., 2011). While a number of different vasodilatory stimuli such as acetazolamide (Asghar et al., 2011), inhalation of fixed concentrations of carbon dioxide (CO2) (Prisman et al., 2008) and breath holding (Murphy et al., 2011) have been used to induce cerebral vasodilation, these vasodilatory stimuli are not without issues that ultimately limit their use.
The development of a computer-controlled sequential rebreathing device (RespirAct™; Thornhill Research, Inc. Toronto, Canada) has enabled researchers to deliver a controlled CO2 stimulus while concurrently maintaining end-tidal oxygen volumes (PetO2) (Slessarev et al., 2007; Prisman et al., 2008; Mark et al., 2010; Mutch et al., 2012). Coupling this vasodilatory stimulus with blood oxygen level-dependent (BOLD) magnetic resonance imaging (MRI) is being used more frequently to measure CVR (Spano et al., 2013) due to its availability in clinical settings, larger signal-to-noise ratio as well as its less dependence on mean arterial pressure (MAP) (Halani et al., 2015) compared to arterial spin labelling (ASL). However, to date no studies have specifically explored the within- and between-day reproducibility of this methodology to measure grey matter CVR.
Mark et al. (2010) compared computer-controlled prospective end-tidal targeting to traditional manual techniques; however, the authors failed to report neither the quantified values nor the between-day reproducibility. Kassner et al. (2010) described the reproducibility of BOLD CVR using PetCO2; however, the method used by the authors did not clamp PetO2 when creating hypercapnia. Thus, it is not possible to decouple the effects of PCO2 from the effects of PO2 changes on BOLD CVR. Therefore, there is an important gap in the literature relating to using BOLD CVR as a reliable method in research as well as a future clinical tool. The purpose of this study was to examine the reproducibility of grey matter CVR under hypercapnic, iso-oxic conditions.
Subjects and methods
Eleven subjects (five males, six females) between the ages of 18 and 40 years were recruited and submitted written informed consent in compliance with all guidelines set by the University of Minnesota Institutional Review Board and the Health Insurance Portability and Accountability Act (HIPAA). All subjects were free of neurologic disorders and fasted and abstain from caffeine consumption for 12 h, as well as refrain from vigorous exercise for 24 h prior to each scan.
Prior to the MRI studies, subjects were fitted with a specialized rebreathing circuit connected to a custom gas blender (RespirAct™), which was utilized to manipulate and analyse PetCO2 and PetO2 values. Subjects were instructed to synchronize their breathing rate to 12 breaths/min. PetO2 values were targeted to 100 mmHg throughout the protocol. Baseline flow and PetCO2 values were established, and two square wave alterations (+10 mmHg above baseline) were performed during the 30-min familiarization period. Once the initial testing session was successfully completed, subjects were scheduled for full MRI protocol visits. The study protocol consisted of two MRI scans performed on the same visit, with two square wave alterations, similar to the initial session, administered per scan (four squares per visit). The protocol was then repeated on a separate visit with minimum of 3 days between visits.
All imaging were performed at the CMRR at the University of Minnesota with the Siemens (Erlangen, Germany) 3-Tesla Connectome Skyra, a MRI scanner specially modified for the Human Connectome Project (http://humanconnectome.org/). A 32-channel receive-only matrix head coil was used during acquisition. The MRI protocol included short localizer scans to allow for scan prescription, a high-resolution T1-weighted scan used for tissue segmentation (TR = 2530 ms, TE = 3·52 ms, TI = 1100 ms, flip angle = 7°, voxel = 1 mm isotropic, grappa = 2 4 min), a field map scan used to correct the BOLD data for susceptibility-induced geometric distortions (TR = 300 ms, TE = 2·79 and 5·25 ms, flip angle = 50°, voxel = 3·4 × 3·4 × 4 mm, 39 s) and two long BOLD EPI scans (TR = 2000 ms, TE = 30 ms, flip angle = 77°, voxel = 3·4 × 3·4 × 4 mm, 840 volumes, 28 min/scan) used to compute CVR.
Image processing was performed using tools from the FSL toolkit (BET, FLIRT, FEAT, FAST, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Processing of the high-resolution T1-weighted imaging consisted of brain extraction using BET and a 3-compartment tissue segmentation, using FAST. Preprocessing of the BOLD imaging was conducted within FEAT and included brain extraction, correction for magnetic field induced geometric distortion (using the separately acquired field map) and slice timing correction. No temporal or spatial smoothing was applied to the data. The extracted brain from the T1 scan was aligned to the distortion corrected mean BOLD image, using FLIRT. The grey matter partial volume estimate (PVE) map from the T1 segmentation was aligned to the BOLD data using the same transform, and a grey matter mask was created in BOLD space by thresholding the aligned PVE map at a 50% partial volume estimate. The grey matter BOLD signal, which is the mean voxel intensity within the grey matter mask per time point, was then computed.
Mean CVR in grey matter was computed from the measured PetCO2 and grey matter BOLD time courses. First, a linear detrend was applied to both the BOLD and PetCO2 waveforms to remove the effects of scanner drift from the BOLD time course and any linear drift in baseline PetCO2. Next, the PetCO2 waveform was recalculated from the measured values at each point of exhale to represent a sample every 2 seconds using spline interpolation within MATLAB (version 7·9·0, Natick, MA). Timing differences between the measured BOLD and PetCO2 wave forms were corrected, and the CVR (% BOLD signal change/mmHg CO2) was computed in MATLAB using the robust linear least squares fit to the correlation between the two time courses.
Statistical analysis
SAS Software Package (Version 9·4; SAS Inc., Cary, NC) was used for statistical analysis. Demographic characteristics are expressed as mean ± standard deviation (SD). A paired t-test was used to compare baseline PCO2 levels between visit one and visit two. An α value of 0·05 was used to signify statistical significance for t-test measures. Square-specific characteristics (PetO2, BOLD CVR, CVR standard deviation (dCVR) and R2) are expressed as mean ± standard error of the mean (SEM). An independent sample t-test was used to determine whether average PetO2 values were iso-oxic at 100 mmHg.
Test–retest reproducibility of CVR was performed both within visits and between visits using a two-way agreement intraclass correlation coefficient (ICC, or coefficient of reliability), as well as coefficient of within-subject variation (CV), for squares one and two at each visit. Bland–Altman analyses were also performed. GraphPad Prism Software (Prism version 6·0, GraphPad Software, Inc., La Jolla, CA) was used to create Bland–Altman analysis plots.
Within-visit ICC and CV comparisons were made between MRI scans one and two for each square separately, and between-visit ICC and CV comparisons were made between similar square measurements for each scan separately. Individual ICC and CV comparisons were also made between square one and square two for each visit and scan (i.e. within-scan), separately.
Results
Subject mean age was 26·5 ± 5·7 years (males: 30·4 ± 5·8 years, females: 23·2 ± 3·1 years). Subject-specific baseline PetCO2 levels were not statistically different between visit one and visit two (31·2 ± 0·8 versus 31·8 ± 1·1 mmHg, P = 0·504), respectively. Additionally, subject-specific average PetO2 levels were not statistically different between visit one and visit two (102·9 ± 2·9 versus 101·1 ± 3·3 mmHg, P = 0·07), respectively.
Average BOLD CVR was 0·43 and .42% BOLD signal change/mmHg CO2 on visits one and two, respectively. Independent t-tests showed CVR values were not significantly different between squares, scans or days (P>0·50 for all tests). Test–retest reproducibility (ICC) and CV of CVR for within scan were excellent and between visits were good. These results as well as visual representation of the study protocol format are displayed in Fig. 1. The Bland–Altman plots (Fig. 2) display good agreement between- and within-day CVR in our study population.
Figure 1.
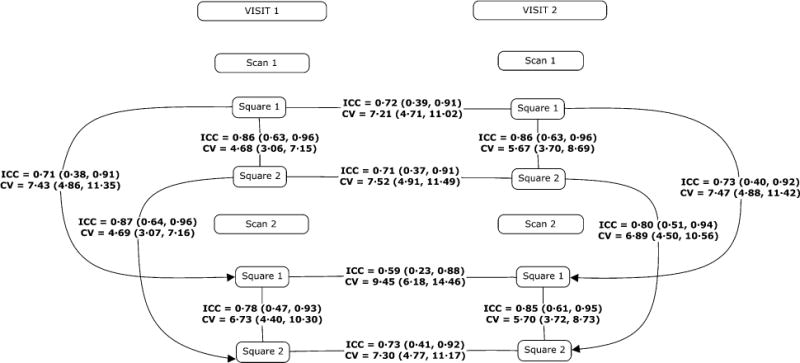
Study protocol and corresponding test–retest reliability comparisons. Estimates are presented with their confidence intervals. ICC: intra-class correlation coefficient; CV: coefficient of variation (%).
Figure 2.
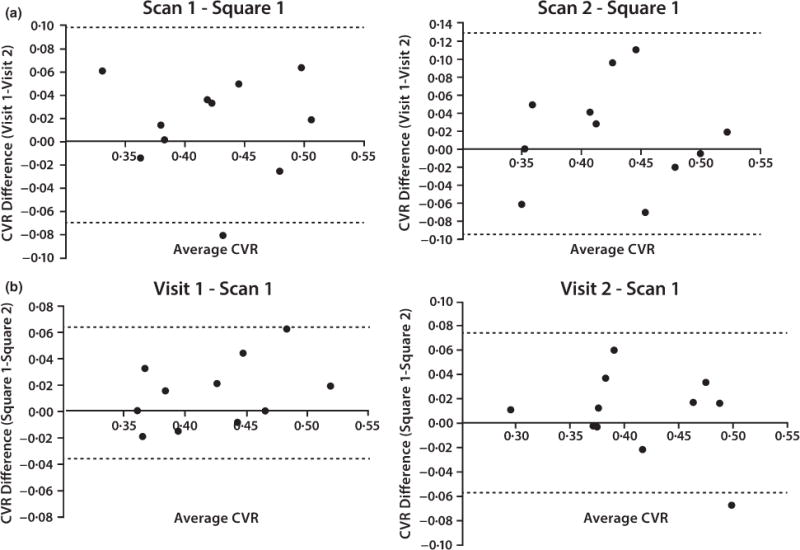
Bland–Altman plots of within-day (Panel a) and between-day (Panel b) reproducibility. Cerebrovascular reactivity (CVR) reflects the change in bold signal (%BOLD) relative to the change in end-tidal partial pressure of carbon dioxide (CVR = %BOLD/mmHg PETCO2).
Discussion
The objective of this study was to examine the reproducibility of grey matter CVR using a 10-mmHg square wave change in PetCO2. In addition, we examined the repeatability of this 10 mmHg square wave change in PetCO2. This study demonstrated that BOLD signal changes in response to alterations in PetCO2 are reproducible both within- and between-visit MRI scans.
Square waves similar to our protocol are commonly used in CVR studies (Mark et al., 2010; Mutch et al., 2012). In the present study, we observed good CVR reproducibility using back-to-back square waves that increase PetCO2 by 10 mmHg which indicates using the proposed breathing technology and BOLD imaging is a reliable method in CVR assessment and may be useful not only in intervention-based studies but also in case–control comparisons and clinical applications.
In the present study, CVR within-scan reproducibility was very good (average ICC = 0·84). The differences in CVR measurements were less than 6·75% in all within-scan trials (average CV = 5·70%). The between-scan reproducibility (e.g. square 1/scan 1 versus square 1/scan 2) was also very good to excellent (average ICC = 0·81, CV = 6·83%). Based on these data, we conclude within-visit CVR using this method is reproducible. CVR reproducibility between visit one and visit two (separated by at least 3 days) was good (average ICC = 0·69, CV = 7·87). The slightly lower between-visit reproducibility may reflect normal variation in day-to-day physiologic difference within subjects; however, these values are still very reproducible. These results suggest that high degree of reproducibility in measuring CVR using the techniques employed in the current study.
To our knowledge, only one other study specifically investigated the within- and between-day reproducibility of BOLD CVR measures using rapid changes in PetCO2 (Kassner et al., 2010). It should be noted that Kassner et al. (2010) used different techniques from the current study (8- to 45-s. duration, ~15 mmHg increases in CO2); however, the reproducibility within grey matter was similar to our results. It should be noted that the mean CVR value (0·162 ± 0·007%) reported in the study by Kassner et al. (2010) was lower than what we observed in the present study (0·421 ± 0·006%). The differences in CVR values from our study and those of Kassner et al. (2010) may be due in part to the differences in MRI field strength (1·5T versus 3·0T). In addition, it should be pointed that Kassner et al. (2010) did not clamp the PetO2 levels during the study, which may have also contributed to the lower measured CVR (Mark et al., 2010). In the present study, we eliminated the possible effect of alterations in PetO2 levels on CVR values by clamping PetO2.
There are some limitations to the present study. It has been suggested that over the entire physiologic range, BOLD CVR follows a sigmoidal relationship (Sobczyk et al., 2014). However, in this experiment, the range increase from each subject’s baseline PetCO2 may be assumed to be within the linear portion of this relationship although this was not shown empirically in this experiment.
In conclusion, CVR measured using two consecutive square waves (+10 mmHg PetCO2 from baseline) using controlled iso-oxic hypercapnic stimuli is very reproducible both within and between visits. This establishes an accurate method for measuring CVR in individuals that may be used not only in the comparison between various groups of individuals but also in longitudinal studies interested in treatment or examination of CVR over time (i.e., ageing studies, traumatic brain injury evaluation).
Acknowledgments
We thank Joseph Fisher MD for technical collaboration. Grants: This study was partially funded by an Equipment grant from the University of Minnesota Medical Foundation and a University of Minnesota Grant-in-Aid (D.R.D.)
Footnotes
Conflict of interest
The authors have no conflict of interests.
References
- Asghar MS, Hansen AE, Pedersen S, et al. Pharmacological modulation of the BOLD response: a study of acetazolamide and glyceryl trinitrate in humans. J Magn Reson Imaging. 2011;34:921–927. doi: 10.1002/jmri.22659. [DOI] [PubMed] [Google Scholar]
- Fierstra J, Conklin J, Krings T, et al. Impaired peri-nidal cerebrovascular reserve in seizure patients with brain arteriovenous malformations. Brain. 2011;134:100–109. doi: 10.1093/brain/awq286. [DOI] [PubMed] [Google Scholar]
- Halani S, Kwinta JB, Golestani AM, et al. Comparing cerebrovascular reactivity measured using BOLD and cerebral blood flow MRI: the effect of basal vascular tension on vasodilatory and vasoconstrictive reactivity. NeuroImage. 2015;110:110–123. doi: 10.1016/j.neuroimage.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Abou-Hamden A, Mandell DM, et al. Impact of extracranial-intracranial bypass on cerebrovascular reactivity and clinical outcome in patients with symptomatic moyamoya vasculopathy. Stroke. 2011;42:3047–3054. doi: 10.1161/STROKEAHA.111.615955. [DOI] [PubMed] [Google Scholar]
- Kassner A, Winter JD, Poublanc J, et al. Blood-oxygen level dependent MRI measures of cerebrovascular reactivity using a controlled respiratory challenge: reproducibility and gender differences. J Magn Reson Imaging. 2010;31:298–304. doi: 10.1002/jmri.22044. [DOI] [PubMed] [Google Scholar]
- Len TK, Neary JP. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin Physiol Funct Imaging. 2010;31:85–93. doi: 10.1111/j.1475-097X.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- Mark CI, Slessarev M, Ito S, et al. Precise control of end-tidal carbon dioxide and oxygen improves BOLD and ASL cerebrovascular reactivity measures. Magn Reson Med. 2010;64:749–756. doi: 10.1002/mrm.22405. [DOI] [PubMed] [Google Scholar]
- Murphy K, Harris AD, Wise RG. Robustly measuring vascular reactivity differences with breath-hold: normalising stimulus-evoked and resting state BOLD fMRI data. NeuroImage. 2011;54:369–379. doi: 10.1016/j.neuroimage.2010.07.059. [DOI] [PubMed] [Google Scholar]
- Mutch WAC, Mandell DM, Fisher JA, et al. Approaches to brain stress testing: BOLD magnetic resonance imaging with computer-controlled delivery of carbon dioxide. PLoS ONE. 2012;7:e47443. doi: 10.1371/journal.pone.0047443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisman E, Slessarev M, Han J, et al. Comparison of the effects of independently-controlled end-tidal PCO2 and PO2 on blood oxygen level-dependent (BOLD) MRI. J Magn Reson Imaging. 2008;27:185–191. doi: 10.1002/jmri.21102. [DOI] [PubMed] [Google Scholar]
- Schwertfeger N, Neu P, Schlattmann P, et al. Cerebrovascular reactivity over time course in healthy subjects. J Neurol Sci. 2006;249:135–139. doi: 10.1016/j.jns.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Slessarev M, Han J, Mardimae A, et al. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J Physiol. 2007;581:1207–1219. doi: 10.1113/jphysiol.2007.129395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczyk O, Battisti-Charbonney A, Fierstra J, et al. A conceptual model for CO2-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. NeuroImage. 2014;92:56–68. doi: 10.1016/j.neuroimage.2014.01.051. [DOI] [PubMed] [Google Scholar]
- Spano VR, Mandell DM, Poublanc J, et al. CO2 blood oxygen level–dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology. 2013;266:592–598. doi: 10.1148/radiol.12112795. [DOI] [PubMed] [Google Scholar]


