Abstract
Exposure to stressors primes the neuroinflammatory and microglial proinflammatory response to subsequent immune challenges, suggesting that stress might attenuate immunoregulatory mechanisms in the CNS microenvironment. CD200:CD200R is a key immunoregulatory signaling dyad that constrains microglial activation, and disruption of CD200:CD200R signaling primes microglia to subsequent immune challenges. Therefore, the present study examined the mediating role of CD200:CD200R signaling in stress-induced microglial priming. Here, we found that exposure to an acute stressor reduced CD200R expression across sub-regions of the hippocampus, amygdala as well as in isolated hippocampal microglia. A transcriptional suppressor of CD200R, CAAT/Enhancer Binding Proteinβ, was induced by stress and inversely associated with CD200R expression. To examine whether disrupted CD200:CD200R signaling plays a mediating role in stress-induced microglial priming, a soluble fragment of CD200 (mCD200Fc) was administered intra-cisterna magna prior to stressor exposure and stress-induced microglia priming assessed ex vivo 24h later. Treatment with mCD200Fc blocked the stress-induced priming of the microglial pro-inflammatory response. Further, treatment with mCD200R1Fc recapitulated the effects of stress on microglial priming. We previously found that stress increases the alarmin high mobility group box-1 (HMGB1) in hippocampus, and that HMGB1 mediates stress-induced priming of microglia. Thus, we examined whether stress-induced increases in hippocampal HMGB1 are a consequence of disrupted CD200:CD200R signaling. Indeed, treatment with mCD200Fc prior to stress exposure blocked the stress-induced increase in hippocampal HMGB1. The present study suggests that stress exposure disrupts immunoregulatory mechanisms in the brain, which typically constrain the immune response of CNS innate immune cells. This attenuation of immunoregulatory mechanisms may thus permit a primed activation state of microglia to manifest.
Keywords: immunoregulation, CD200R, stress, microglia, priming, neuroinflammation, alarmin
1. Introduction
Prior exposure to acute and chronic stressors potentiates the neuroinflammatory and microglial pro-inflammatory response to subsequent immunologic challenges (de Pablos et al., 2006; Espinosa-Oliva et al., 2011; Frank et al., 2007; Johnson et al., 2003; Johnson et al., 2004; Munhoz et al., 2006; Wohleb et al., 2011), suggesting that stressors sensitize or prime microglia, which are considered a key substrate of this stress-induced phenomenon (Frank et al., 2015b). Microglia are highly labile and can fluidly shift across a spectrum of activation states (Colonna and Butovsky, 2017). Of these states, the immunophenotype and function of a primed microglial state have been characterized in a number of normal and pathological conditions (Perry et al., 2007). In general, a hallmark of primed microglia is the lack of an overt pro-inflammatory profile despite a shift in immunophenotype. However, upon exposure to a pro-inflammatory stimulus, primed microglia exhibit an exaggerated pro-inflammatory response (Perry et al., 2007) suggesting that inhibitory control over microglia has been attenuated.
In the CNS microenvironment, microglia are held in a surveillant or quiescent state of activation through several inhibitory signaling dyads (Hoarau et al., 2011; Ransohoff and Cardona, 2010). Of these, the CD200:CD200R dyad is particularly noteworthy because disruption of CD200:CD200R signaling potentiates the pro-inflammatory response of microglia to immune stimuli (Costello et al., 2011; Denieffe et al., 2013) as well as exacerbates disease severity and progression in neuroinflammatory disease models (Hoek et al., 2000; Meuth et al., 2008; Wright et al., 2000). Further, disruption of CD200:CD200R signaling has been implicated in neuroinflammatory processes observed in aging (Lyons et al., 2007), neuropathic pain (Hernangomez et al., 2016) and Alzheimers disease (Walker et al., 2009). These findings prompted us to explore the notion that disruption of CD200:CD200R signaling may play a role in stress-induced neuroinflammatory and microglial priming.
In the innate immune system, CD200 is thought to constitutively inhibit myeloid cell function via engagement of CD200R (Gorczynski, 2005). CD200 is a membrane glycoprotein that is expressed ubiquitously in the CNS on neurons, endothelial cells and oligodendrocytes, while its cognate receptor CD200R is expressed almost exclusively on microglia as well as other CNS macrophages (Koning et al., 2009; Wright et al., 2000). Upon binding CD200R, CD200 initiates an intra-cellular signaling cascade that results in general inhibition of myeloid cell function including pro-inflammatory cytokine responses to immune stimuli (Gorczynski et al., 2008; Jenmalm et al., 2006; Zhang et al., 2004).
Here, we explored the possibility that stress-induced disruption of CD200:C200R signaling might serve as a trigger to disinhibit microglia, thereby sensitizing the neuroinflammatory and microglial response to subsequent immune challenges.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (60–90 d old; Envigo) were pair-housed with food and water available ad libitum. The colony was maintained at 22 °C on a 12h light/dark cycle (lights on at 07:00h). All experimental procedures were conducted in accord with the University of Colorado Institutional Animal Care and Use Committee.
2.2. Drug Treatments
mCD200Fc
Soluble recombinant mouse CD200 Fc (mCD200Fc) chimera (R & D Systems; cat. no. 3555-CD) and human IgG1Fc (R & D Systems; cat. no. 110-HG) were dissolved in sterile 1x PBS. Human IgG1Fc (hIgG1Fc) served as a control protein. Briefly, mCD200Fc is a fusion protein consisting of the extracellular domain of mCD200 and the Fc domain of human IgG1. CD200Fc fusion proteins were originally developed by Cherwinski and colleagues (Cherwinski et al., 2005) and found to be highly effective at inhibiting the pro-inflammatory response of myeloid cells in vitro (Cherwinski et al., 2005; Gorczynski et al., 2008; Gorczynski et al., 2004; Jenmalm et al., 2006). Further, mCD200Fc was found to specifically bind CD200R (Hatherley et al., 2005), which mediates the inhibitory effects of mCD200Fc (Gorczynski et al., 2008). Hernangomez and colleagues found that intrathecal injection of mCD200Fc in rats blocked spinal pro-inflammatory responses to chronic constriction injury of the sciatic nerve (Hernangomez et al., 2016). As such, their findings served as the basis for our dose selection of mCD200Fc, which was utilized here to block stress-induced priming of microglia. Here, mCD200Fc was used as a surrogate to increase endogenous CD200 levels in hippocampus and thus increase CD200-mediated inhibitory drive on microglia. mCD200R1Fc: Soluble recombinant mouse CD200R1Fc chimera (R & D Systems; cat. no. 2554-CD) and human IgG1Fc (R & D Systems; cat. no. 110-HG) were dissolved in sterile 1x PBS. Human IgG1Fc (hIgG1Fc) served as a control protein. mCD200R1Fc was used here as a decoy receptor to bind endogenous CD200 and thus inhibit endogenous CD200:CD200R signaling. The purpose of this experimental approach was to test whether disruption of CD200:CD200R signaling outside the context of stress is sufficient to prime the microglial response to LPS. LPS. Lipopolysaccharide (LPS; E. coli serotype 0111:B4; Sigma; cat. no. L3012) was dissolved in pyrogen free, sterile 0.9% saline and was used to challenge microglia ex vivo.
2.3. Intra-cisterna magna (ICM) injections
mCD200Fc
mCD200Fc (5 ug total at 1 ug/ul) or hIgG1Fc (equimolar concentration; 5 ul total) was injected ICM immediately prior to stress exposure. Hippocampal microglial priming and HMGB1 protein levels were measured 24h after stress exposure. mCD200R1Fc: mCD200R1Fc (5 ug total at 1 ug/ul) or hIgG1Fc (equimolar concentration; 5 ul total) was injected ICM. 24h post-injection, hippocampal microglial priming was measured. We have demonstrated that ICM injected substances reach distal target regions in the CNS (i.e. hippocampus) consistent with more typical ICV procedures, and this procedure produces no detectable inflammatory responses (Frank et al., 2012a). Rats were anesthetized with 5% isoflurane in oxygen and then maintained on 3% isoflurane during the brief procedure (~3 min). The dorsal aspect of the skull was shaved and swabbed with 70% EtOH. A sterile 27-gauge needle attached via sterile PE50 tubing to a 25ul Hamilton syringe was inserted into the cisterna magna (verified by withdrawing 2 ul of clear CSF) and drug injected over a 30s period. After injection, the needle was left in place for 30s to allow for diffusion of drug.
2.4. Inescapable tail-shock (IS)
Details of the stressor protocol have been published previously and this protocol reliably potentiates pro-inflammatory cytokine responses in the hippocampus after peripheral immune challenge (Johnson et al., 2003) as well as in isolated hippocampal microglia to LPS ex vivo (Frank et al., 2007). Briefly, animals were placed in Plexiglas tubes (23.4 cm in length x 7 cm in diameter) and exposed to 100-1.6 mA, 5 s tail-shocks with a variable inter-trial interval (ITI) ranging from 30 – 90 s (average ITI = 60 s). All IS treatments occurred between 09:00 and 11:00 h. IS animals were returned to their home cages immediately after termination of shock. Home cage control (HCC) animals remained undisturbed in their home cages.
2.5. Tissue collection
Animals were given a lethal dose of sodium pentobarbital. Animals were fully anesthetized and transcardially perfused with ice-cold saline (0.9%) for 3 min to remove peripheral immune leukocytes from the CNS vasculature. Brain was rapidly extracted, placed on ice and hippocampus dissected. Hippocampus was a focus given our prior findings of robust stress-induced priming effects in this region (Frank et al., 2015b). For in vivo experiments, hippocampus was flash frozen in liquid nitrogen for whole tissue analysis. For micropunching of hippocampal and amygdalar sub-regions, whole brain was flash frozen in isopentane. All tissue samples were stored at −80°C. For ex vivo experiments, hippocampal microglia were immediately isolated.
2.6. Hippocampal and amygdala micropunching
Brains were sectioned at 50 μm increments on a Leica cryostat at −20 °C until the region of interest was reached. Tissue punches were then excised from discrete regions of dorsal hippocampus (CA1, CA3, and dentate gyrus) and basolateral (BLA) and central nucleus (CEA) amygdala using a brain punch tool (1 mm diameter × 1 mm depth). Tissue punches (2 per region per hemisphere) were stored at −80 °C until assayed. One hemisphere was used for assay of gene expression and one for protein.
2.7. Ex vivo immune stimulation of hippocampal microglia with LPS
Hippocampal microglia were isolated using a Percoll density gradient as previously described (Frank et al., 2006). This procedure of isolating cells takes ~1.5h. We have previously shown (Frank et al., 2006) that this microglia isolation procedure yields highly pure microglia (Iba-1+/MHCII+/CD163-/GFAP-). In the present experiments, immunophenotype and purity of microglia was assessed using real time RT-PCR. Microglia were suspended in DMEM+10% FBS and microglia concentration determined by trypan blue exclusion. Microglia concentration was adjusted to a density of 1 × 104 cells/100 μl and 100 μl added to individual wells of a 96-well v-bottom plate. LPS was utilized to challenge microglia ex vivo as we have previously determined the optimal in vitro conditions under which LPS stimulates a microglia pro-inflammatory cytokine response (Frank et al., 2006). Cells were incubated with LPS (1, 10, and 100 ng/ml) or media alone for 2h at 37° C, 5% CO2. The plate was centrifuged at 1000 × g for 10 min at 4 °C to pellet cells and cells washed 1x in ice cold PBS and centrifuged at 1000 × g for 10 min at 4 °C. Cell lysis/homogenization and cDNA synthesis was performed according to the manufacturer’s protocol using the SuperScript III CellsDirect cDNA Synthesis System (Invitrogen).
2.8. Real time RT-PCR measurement of gene expression
Total RNA was isolated from hippocampus and amygdala utilizing a standard method of phenol:chloroform extraction (Chomczynski and Sacchi, 1987). For detailed descriptions of RNA isolation, cDNA synthesis and PCR amplification protocols refer to prior publication (Frank et al., 2007). A detailed description of the PCR amplification protocol has been published previously (Frank et al., 2006). cDNA sequences were obtained from Genbank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). Primer sequences were designed using the Operon Oligo Analysis Tool (http://www.operon.com/technical/toolkit.aspx) and tested for sequence specificity using the Basic Local Alignment Search Tool at NCBI (Altschul et al., 1997). Primers were obtained from Invitrogen. Primer specificity was verified by melt curve analyses. All primers were designed to span exon/exon boundaries and thus exclude amplification of genomic DNA (See Table 1 for primer description and sequences).
Table 1.
Primer Sequences.
| Gene | Primer Sequence 5′ → 3′ | Function |
|---|---|---|
| β-Actin | F: TTCCTTCCTGGGTATGGAAT R: GAGGAGCAATGATCTTGATC |
Cytoskeletal protein (Housekeeping gene) |
| CD68 | F: CAAGCAGCACAGTGGACATTC R: CAAGAGAAGCATGGCCCGAA |
Macrophage antigen that binds LDL |
| CD163 | F: GTAGTAGTCATTCAACCCTCAC R: CGGCTTACAGTTTCCTCAAG |
Hemoglobin receptor expressed by macrophages, but not microglia |
| CD200 | F: CTCTCTATGTACAGCCCATAG R: GGGAGTGACTCTCAGTACTAT |
Neuronal antigen that binds CD200R to inhibit macrophage function |
| CD200R | F: TAGAGGGGGTGACCAATTAT R: TACATTTTCTGCAGCCACTG |
Cognate receptor for CD200 |
| CEBPβ | F: GGGGTTGTTGCTGTTGATGT R: GCTCGAAACGGAAAAGGTTC |
Transcription factor that regulates CD200R transcription and other neuroinflammatory genes |
| CX3CL1 | F:3ATCATCCTGGAGACGAGACAGC R: CCACACGCTTCTCAAACTTGCC |
Neuronal antigen that binds CX3CR1 to inhibit macrophage function |
| CX3CR1 | F:3TCAGGACCTCACCATGCCTA R: CGAACGTGAAGACAAGGGAG |
Cognate receptor for CX3CL1 |
| GFAP | F: AGATCCGAGAAACCAGCCTG R: CCTTAATGACCTCGCCATCC |
Astrocyte antigen |
| IL-1β | F: CCTTGTGCAAGTGTCTGAAG R: GGGCTTGGAAGCAATCCTTA |
Pro-inflammatory cytokine |
| IL-4 | F:3GAACTCACTGAGAAGCTGCA R: GAAGTGCAGGACTGCAAGTA |
Anti-inflammatory cytokine |
| IL-6 | F: AGAAAAGAGTTGTGCAATGGCA R: GGCAAATTTCCTGGTTATATCC |
Pro-inflammatory cytokine |
| Iba-1 | F: GGCAATGGAGATATCGATAT R: AGAATCATTCTCAAGATGGC |
Microglia/Macrophage antigen |
| MHCII | F: AGCACTGGGAGTTTGAAGAG R: AAGCCATCACCTCCTGGTAT |
Microglia/Macrophage antigen |
| NF-κBIα | F: CACCAACTACAACGGCCACA R: GCTCCTGAGCGTTGACATCA |
Induced by NFκB to inhibit NFκB function |
| NLRP3 | F: AGAAGCTGGGGTTGGTGAATT R: GTTGTCTAACTCCAGCATCTG |
Inflammasome component mediating caspase-1/IL-1β activation |
| RAGE | F: AGGACAAGCTGCAGGCTCTG R: TTCTGGGGCCTTCCTCTCCT |
Receptor for HMGB1 that mediates chemotaxis and inflammatory responses. |
| TLR4 | F: TCCCTGCATAGAGGTACTTC R: CACACCTGGATAAATCCAGC |
Receptor for LPS and DAMPs |
| TNFα | F: CAAGGAGGAGAAGTTCCCA R: TTGGTGGTTTGCTACGACG |
Pro-inflammatory cytokine |
Abbreviations: CD, cluster of differentiation; CEBPβ, CAAT/enhancer binding protein β; CX3CL1, chemokine (C-X3-C motif) ligand 1; CX3CR1, CX3C chemokine receptor 1; GFAP, glial fibrillary acidic protein; IL, interleukin; Iba-1, ionized calcium-binding adaptor molecule-1; MHCII, Major histocompatibility complex II; NF-κBIα, nuclear factor kappa light chain enhancer of activated B cells inhibitor alpha; NLRP3, NACHT Domain-, Leucine-Rich Repeat-, And PYD-Containing Protein 3; RAGE, receptor for advanced glycation end-products; TLR4, toll-like receptor-4; TNFα, tumor necrosis factor-α.
PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen). Formation of PCR product was monitored in real time using the MyiQ Single-Color Real-Time PCR Detection System (BioRad). Relative gene expression was determined using β-Actin as the housekeeping gene and the 2−ΔΔCT method (Livak and Schmittgen, 2001).
2.9. Western Blot
Hippocampal tissue was sonicated in a mixture containing extraction buffer (Invitrogen) and protease inhibitors (Sigma). Ice-cold tissue samples were centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was removed and the protein concentration for each sample was quantified using the Bradford method. Samples were heated to 100 °C for 10 min and 40 μg total protein loaded into a standard polyacrylamide 4–12% Bis-Tris gel (Invitrogen). SDS-PAGE was performed in 3-(N-morpholino)-propanesulfonic acid running buffer (Invitrogen) at 160 V for 1h. Protein was transferred onto a nitrocellulose membrane using an iBlot dry transfer system (Invitrogen). The membrane was then blocked with Odyssey blocking buffer (LI-COR Biosciences) for 1h and incubated overnight at 4°C in blocking buffer (LI-COR Biosciences) with the following primary antibodies: rabbit polyclonal to high mobility group box (HMGB)1 (1:1000, Abcam; cat. no. ab18256), mouse monoclonal to CD200R (1:1000, ThermoFisher Scientific; cat. no. MA5-16894), mouse monoclonal to CD200 (1:500, ThermoFisher Scientific; cat. no. MA1-90805) rabbit monoclonal to CAAT/Enhancer Binding Protein (C/EBP)β (1:1000, Abcam; cat. no. ab32358) and mouse monoclonal to β-actin (1:100,000, Sigma-Aldrich; cat. no. A5316). The membrane was washed 4x in 1x PBS + 0.1% Tween and then incubated in blocking buffer containing either goat anti-rabbit (LI-COR; cat. no. 925-32211) or goat anti-mouse (LI-COR; cat. no. 925-32210) IRDye 800CW secondary antibody at a concentration of 1:10,000 for 1 h at room temperature and the membrane was washed 4x in 1x PBS + 0.1% Tween. Protein expression was quantified using an Odyssey Infrared Imager (LI-COR Biosciences) and expressed relative to the housekeeping protein β-actin.
2.10. HMGB1 ELISA
Hippocampus was sonicated in a mixture containing extraction buffer (Invitrogen) and protease inhibitors (Sigma). Ice-cold tissue samples were centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was removed and the protein concentration for each sample was quantified using the Bradford method. HMGB1 protein was measured using a standard colorimetric sandwich ELISA (LifeSpan Biosciences, Inc.; cat. no. LS-F4039). HMGB1 protein was quantified as pg/mg total protein.
2.11. Statistical analysis and data presentation
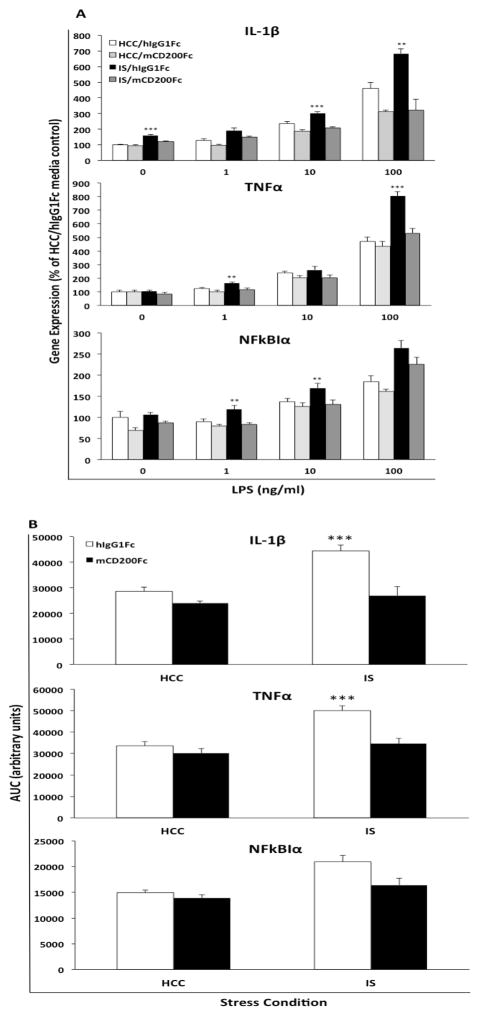
All data are presented as mean ± SEM. Statistical analyses consisted of ANOVA followed by post-hoc tests (Tukey’s HSD) using Prism 5 (Graphpad Software, Inc.). In several instances, multiple t-tests were carried out on the same samples, which increases the risk of Type I error. A Bonferroni correction of p-values (corr p) was conducted to mitigate this risk. Threshold for statistical significance was set at α = 0.05. Sample sizes are provided in figure captions. In several instances, data is scaled to the mean of the HCC animals and presented as a percent of the HCC mean. Here, the mean of the HCC group was computed and all individual data points for a particular analyte were divided by the HCC mean * 100, which sets the HCC mean at 100% with a specific standard error. Data presented in Fig. 5 is derived from a 2 (HCC vs IS) × 2 (hIgG1Fc vs mCD200Fc) × 4 (0, 1, 10, 100 ng/ml LPS) factorial design with stress and mCD200Fc as a between subjects factors and LPS as a within subjects factor. A 2 (HCC vs IS) × 2 (hIgG1Fc vs mCD200Fc) factorial analysis was conducted at each concentration of LPS (Fig. 5A). Area under the LPS concentration curve (AUC) was computed to capture the cumulative effect of stress and mCD200Fc treatment on the cytokine response to LPS ex vivo (Fig. 5B). This transformation of the raw data presented in Fig. 5A results in a cumulative cytokine response measure for each animal as a function of stress and mCD200Fc treatment.
Fig. 5. Effect of mCD200Fc on IS-induced microglial priming.
Animals were injected ICM with control protein (hIgG1Fc) or mCD200Fc. Immediately after injection, animals were exposed to IS or served as HCC. 24h after stress exposure, hippocampal microglia were isolated and treated with LPS for 2h. A) the cytokine response (IL-1β, TNFα and NFkBIα) at each concentration of LPS was captured for the following treatment groups: HCC/hIgG1Fc (N = 8), IS/hIgG1Fc (N = 8), HCC/mCD200Fc (N = 8), and IS/mCD200Fc (N = 7). B) The cumulative cytokine response across concentrations of LPS (area under the curve; AUC) was computed. Data are presented as mean ± sem. In panel A, the effects of stress and mCD200Fc treatment on the cytokine response were analyzed at each concentration of LPS. The cytokine response for the IS/hIgG1Fc group significantly differed from all other treatment groups, ** p < 0.01, *** p < 0.001. In panel B, the cytokine response of the IS/hIgG1Fc group was significantly different from all other experimental groups, *** p < 0.001.
3. Results
3.1. Effect of inescapable tailshock (IS) on CD200 and CD200R mRNA in hippocampus
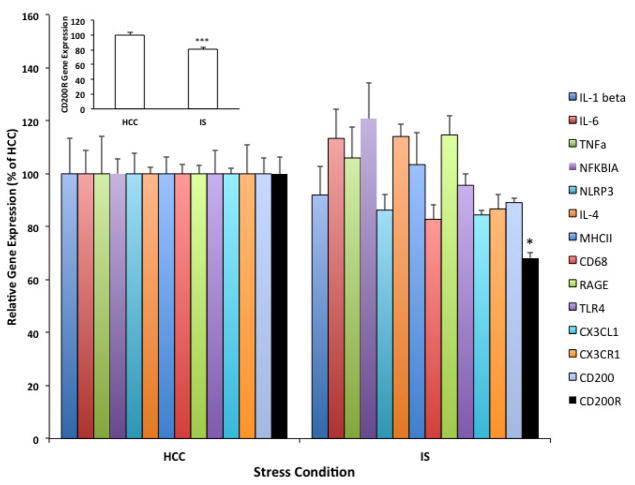
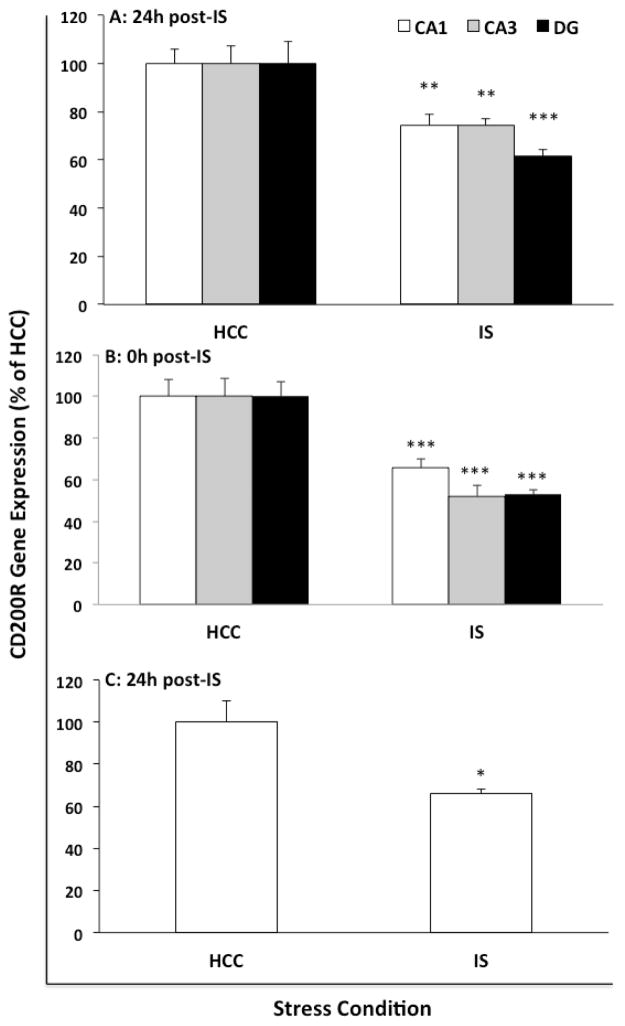
As an initial experiment, CD200 and CD200R mRNA were characterized in the context of an array of immune-related genes in whole hippocampus 24h after exposure to IS or in HCC (Fig. 1). This time-point post-IS was selected given our prior findings that robust priming of microglia and the neuroinflammatory response occurs at this time-point after stressor exposure (Frank et al., 2007; Johnson et al., 2003). We found that IS selectively down-regulated CD200R compared to HCC (t = 4.67, df = 10, corr p = 0.013), an effect replicated in a separate cohort of animals (see Fig. 1 inset). Not surprisingly since sacrifice was 24h after IS, IS failed to modulate the expression of all other genes examined including the immunoregulatory signaling dyad CX3CL1:CX3CR1, which plays a similar role to CD200:CD200R (Ransohoff and Cardona, 2010). To confirm this result and assess regional differences in CD200R expression in the hippocampus, gene expression of CD200R and CD200 was measured in micropunches of CA1, CA3 and dentate gyrus (DG) 24h after IS or in HCC (Fig. 2A). Consistent with our initial findings, IS induced a significant down-regulation of CD200R compared to HCC in CA1 (t = 3.41, df = 26, corr p = 0.006), CA3 (t = 3.39, df = 26, corr p = 0.006) and DG (t = 4.02, corr p = 0.0012). IS failed to significantly change CD200 expression in all hippocampal sub-regions (data not shown). To examine whether the effects of IS on CD200R extended to other limbic structures, we examined CD200R and CD200 expression in amygdalar sub-regions (BLA and CEA) 24h post-stress exposure. Consistent with the effect of stress in hippocampus, we found that IS induced a significant reduction of CD200R in BLA (df = 14, t = 3.87, p = 0.002) and CEA (df = 14, t = 3.86, p = 0.002), however CD200 expression was not signifcantly modulated by stress (Suppl. Fig. 1). In light of these findings, we examined whether IS-induced reductions in CD200R were present immediately after stress-exposure (Fig. 2B). Indeed, IS down-regulated CD200R expression in CA1 (t =3.69, df = 10, corr p = 0.012), CA3 (t = 4.84, corr p = 0.002) and DG (t = 6.25, corr p = 0.0003) immediately after termination of the stressor. Because CD200R is expressed by CNS macrophages (Koning et al., 2009; Wright et al., 2000), we examined the effect of IS on microglial expression of CD200R. Hippocampal microglia were isolated from HCC animals and animals exposed to IS 24h previously. Consistent with the results observed in whole tissue, IS down-regulated microglial CD200R expression compared to HCC (Fig. 2C; t = 3.31, df = 6, p = 0.02).
Fig. 1. Effect of IS on whole hippocampal gene expression of CD200R.
Animals were exposed to IS (N = 6) or served as HCCs (N = 6). 24h after stress exposure, expression of genes related to innate immune function were measured in whole hippocampus. Inset shows a replication of this effect of IS on CD200R in a second cohort of animals (HCC, N = 12; IS, N = 12). Data are presented as the mean ± sem. Significant mean differences between HCC vs IS, * corr p < 0.05, *** p < 0.001.
Fig. 2. Effect of IS on CD200R gene expression in hippocampal sub-regions and microglia.
Gene expression of CD200R was measured in (A) hippocampal sub-regions (CA1, CA3 and dentate gyrus; DG) of IS (N = 8) or HCCs (N = 8) 24h after stress exposure, (B) hippocampal sub-regions of IS (N = 6) or HCCs (N = 6) immediately after stress exposure and (C) hippocampal microglia isolated from IS (N = 4) or HCC animals (N = 4) 24h after stress exposure. Data are presented as mean ± sem. For sub-regions, data are scaled as a percent of the HCC mean for each sub-region. Significant mean differences between HCC vs IS, * p < 0.05, ** corr p < 0.01, *** corr p < 0.001.
3.2. Effect of IS on CAAT/Enhancer Binding Protein (C/EBP)β
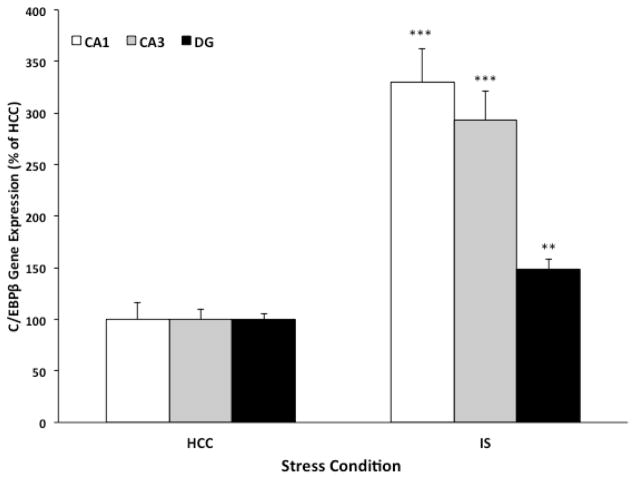
C/EBPβ is a transcription factor that plays a pivotal role in the regulation of genes involved in neuroinflammatory processes (Pulido-Salgado et al., 2015). Dentesano and colleagues found that C/EBPβ binds the promoter region of CD200R and induction of C/EBPβ is necessary for down-regulation of CD200R expression (Dentesano et al., 2012), suggesting that C/EBPβ is a suppressor of CD200R transcription. Given these findings, we explored the possibility that IS may induce C/EBPβ expression. To test this possibility, C/EBPβ expression was assessed in hippocampal sub-regions immediately after stress exposure and in HCC animals. Of note, the duration of a single session of IS is ~2h. Therefore, this immediate time-point post-IS was selected in light of evidence demonstrating that the mRNA half-life of C/EBPβ ranges from 40 min to 2h (Pulido-Salgado et al., 2015), thereby increasing the likelihood of observing stress effects on C/EBPβ expression. As depicted in Fig. 3, we found that IS induced a robust increase in C/EBPβ expression compared to HCC in CA1 (df = 10, t = 6.22, corr p < 0.001), CA3 (df = 10, t = 6.44, corr p < 0.001) and DG (df = 10, t = 4.06, corr p < 0.01). In addition, C/EBPβ expression was inversely correlated with CD200R expression (r = −0.52, p = 0.0009).
Fig. 3. Effect of IS on the CD200R transcriptional regulator C/EBPβ.
Animals were exposed to IS (N = 6) or served as HCCs (N = 6). Immediately after stress exposure, C/EBPβ gene expression was measured in sub-regions (CA1, CA3 and dentate gyrus; DG) of the hippocampus. Data are presented as mean ± sem and scaled as a percent of the HCC mean for each sub-region. Significant mean differences between HCC vs IS, ** corr p < 0.01, *** corr p < 0.001.
3.3. Effect of IS on CD200R and C/EBPβ total protein
To examine whether the effects of IS on CD200R and C/EBPβ mRNA extended to the protein level, we examined the immediate time-point post-IS exposure given the robust effects of IS on gene expression of both targets. Given that the effects of IS on gene expression were found in all hippocampal sub-regions, only DG was analyzed. Western blot analysis of total protein from DG (Fig. 4) demonstrated that IS induced a significant reduction in CD200R protein (df =12, t = 3.87, corr p < 0.01) concomitant with an increase in C/EBPβ protein (df = 12, t = 12.01, corr p < 0.001) compared to protein levels observed in HCC animals. The magnitude of these IS-induced effects were largely comparable to the effect sizes of IS on gene expression.
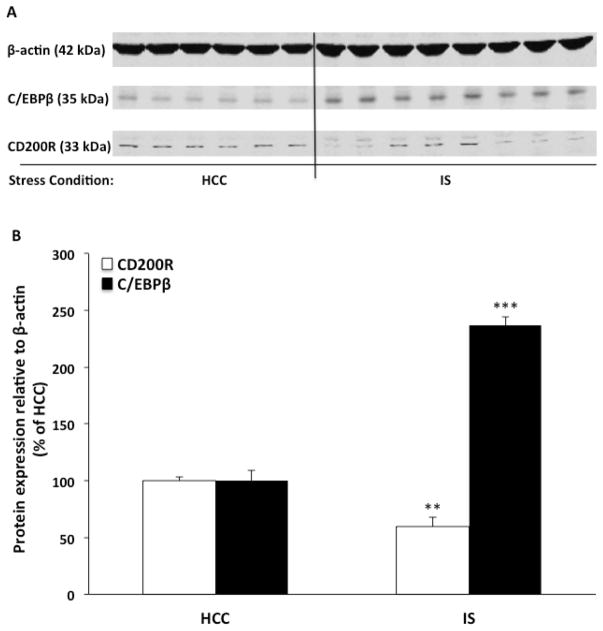
Fig. 4. Effect of IS on CD200R and C/EBPβ total protein.
Animals were exposed to IS (N = 8) or served as HCCs (N = 6). Immediately after stress exposure, CD200R and C/EBPβ total protein levels was measured in dentate gyrus. Data are presented as mean ± sem. Significant mean differences between HCC vs IS, ** corr p < 0.01, *** corr p < 0.001.
3.4. Effect of mCD200Fc on IS-induced microglial priming
The findings presented thus far prompted us to explore the possibility that IS-induced down-regulation of CD200R may have resulted in disrupted CD200:CD200R signaling, thereby disinhibiting or priming microglia pro-inflammatory processes to a subsequent immune challenge. To test this notion, mCD200Fc was utilized to increase microglial inhibitory drive, which may have been reduced as a result of IS-induced down-regulation of CD200R. mCD200Fc or control (hIgG1Fc) was injected ICM immediately prior to IS exposure or in HCC animals. 24h post-IS, hippocampal microglia were isolated from HCC and IS-exposed animals. Microglia were then treated with LPS in vitro to elicit a pro-inflammatory response and ascertain the effects of mCD200Fc on IS-induced potentiation (priming) of the microglial pro-inflammatory response. Depicted in Fig. 5A is data showing the pro-inflammatory response of microglia to several concentrations of LPS in vitro and the differential effects of stress and drug treatment. For IL-1β, the interaction between stress and mCD200Fc treatment was significant at 0 ng/ml (df = 1, 27, F = 7.2, p = 0.012), 10 ng/ml (df = 1, 27, F = 4.36, p = 0.046) and 100 ng/ml (df = 1, 27, F = 7.12, p = 0.013) LPS. Post-hoc comparisons demonstrate that in control-treated (hIgG1Fc) animals, IS potentiated the IL-1β response compared to HCC at 0 ng/ml (p < 0.001), 10 ng/ml (p < 0.001) and 100 ng/ml (p < 0.01) LPS. Treatment with mCD200Fc in stress-exposed animals blocked this stress-induced potentiation of the cytokine response at 0 ng/ml (p < 0.001), 10 ng/ml (p < 0.001) and 100 ng/ml (p < 0.001) LPS. For TNFα, the interaction between stress and mCD200Fc treatment was significant at 1 ng/ml (df = 1, 27, F = 4.38, p = 0.046) and 100 ng/ml (df = 1, 27, F = 12.27, p = 0.002) LPS. Post-hoc comparisons demonstrate that in control-treated (hIgG1Fc) animals, IS potentiated the TNFα response compared to HCC at 0 ng/ml (p < 0.01) and 100 ng/ml (p < 0.001) LPS. Treatment with mCD200Fc in stress-exposed animals blocked this stress-induced potentiation of the cytokine response at 0 ng/ml (p < 0.01) and 100 ng/ml (p < 0.001) LPS. For NFκBIα, the interaction between stress and mCD200Fc treatment was significant at 1 ng/ml (df = 1, 27, F = 7.09, p = 0.013) and 10 ng/ml (df = 1, 27, F = 4.99, p = 0.034) LPS. Post-hoc comparisons demonstrate that in control-treated (hIgG1Fc) animals, IS potentiated the NFκBIα response compared to HCC at 1 ng/ml (p < 0.01) and 10 ng/ml (p < 0.05) LPS. Treatment with mCD200Fc in stress-exposed animals blocked this stress-induced potentiation of the cytokine response at 1 ng/ml (p < 0.01) and 10 ng/ml (p < 0.01) LPS. For NLRP3, the interaction between stress and mCD200Fc treatment did not attain statistical significance at each concentration of LPS (data not shown).
Area under the curve (AUC)(Fig. 5B) was used to represent the overall magnitude of the pro-inflammatory response across LPS concentrations and to examine the interaction between stress and drug treatment on this response. Consistent with the effects shown in Fig. 5A, treatment with mCD200Fc blocked the stress-induced potentiation of the microglial response to LPS for IL-1β (stress x drug interaction, df = 1, 25, F = 7.76, p = 0.01) and TNFα (stress x drug interaction, df = 1, 25, F = 7.49, p = 0.01). The effect on NFκBIα showed a similar trend (stress x drug interaction, df = 1, 25, F = 3.3, p = 0.08), whereas NLRP3 expression was unaffected (stress x drug interaction, df = 1, 27, F = 1.23, p = 0.28). In animals treated with hIgG1Fc, post-hoc comparisons demonstrate that prior exposure to IS potentiated the IL-1β (p < 0.001) and TNFα (p < 0.001) response to LPS compared to the cytokine response in microglia from HCC animals. In IS-exposed animals, treatment with mCD200Fc completely blocked this potentiation of the IL-1β (p < 0.001) and TNFα (p < 0.001) response to LPS compared to hIgG1Fc treatment.
CD200Fc fusion proteins have been found to inhibit the pro-inflammatory response of myeloid cells in vitro to a variety of immune stimuli (Cherwinski et al., 2005; Gorczynski et al., 2008; Gorczynski, 2005; Jenmalm et al., 2006). Thus, a concern regarding the present experiment was that mCD200Fc treatment could result in a general inhibition of microglial pro-inflammatory function, thereby precluding a clear determination of the effect of mCD200Fc on stress-induced priming of the LPS-induced cytokine response. However, post-hoc comparisons demonstrate that the dose of mCD200Fc used here failed to significantly modulate the IL-1β (p > 0.05) and TNFα (p > 0.05) cytokine response to LPS in HCC animals suggesting that treatment with this dose of mCD200Fc did not affect microglial responses to LPS per se. Similarly, mCD200Fc failed to affect the NFκBIα response to LPS in HCC animals (p > 0.05) again suggesting that the microglial response to LPS remained intact in mCD200Fc-treated animals.
3.5. Effect of mCD200R1Fc on hippocampal microglial priming
In light of the effects of mCD200Fc on stress-induced microglial priming, we examined whether disruption of CD200:CD200R signaling in naive animals might be sufficient to recapitulate the effects of stress on microglial priming. Here, soluble mCD200R1 (mCD200R1Fc) or control protein (hIgG1Fc) was injected ICM with the aim of disrupting endogenous CD200:CD200R signaling, thereby disinhibiting microglia and promoting a primed immunophenotype. 24h post-injection, hippocampal microglia were isolated and treated ex vivo with several concentrations of LPS (0, 1, 10 and 100 ng/ml) for 2h. mCD200R1Fc treatment resulted in robust priming of the microglial response to LPS (Suppl. Fig. 3). Area under the LPS concentration curve (AUC) was computed to capture the cumulative effect of mCD200R1Fc on the cytokine response across concentrations of LPS (Fig. 6). We found that mCD200R1Fc potentiated the IL-1β (p < 0.01), TNFα (p < 0.01) and IL-6 (p < 0.0001) response to LPS compared to control treatment.
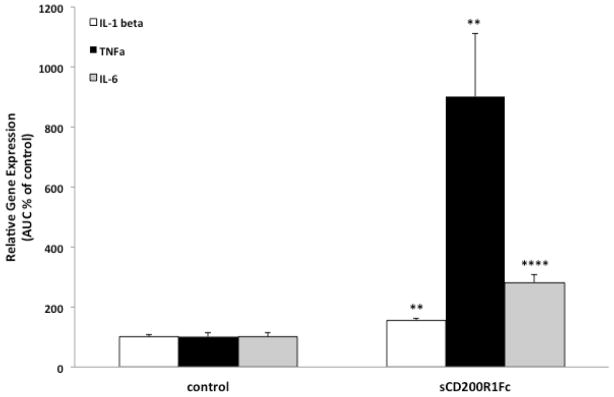
Fig. 6. Effect of mCD200R1Fc on hippocampal microglia priming.
Animals were injected ICM with control (hIgG1) or mCD200R1Fc (5 ug). 24h post-injection, hippocampal microglia were isolated and challenged with LPS for 2h ex vivo. The overall magnitude of the cytokine response (area under the curve; AUC) was computed. N = 5/experimental group. Data are presented as mean ± SEM. mCD200R1Fc treatment significantly different from control treatment, ** p < 0.01, **** p < 0.0001.
3.6. Effect of mCD200Fc on IS-induced HMGB1
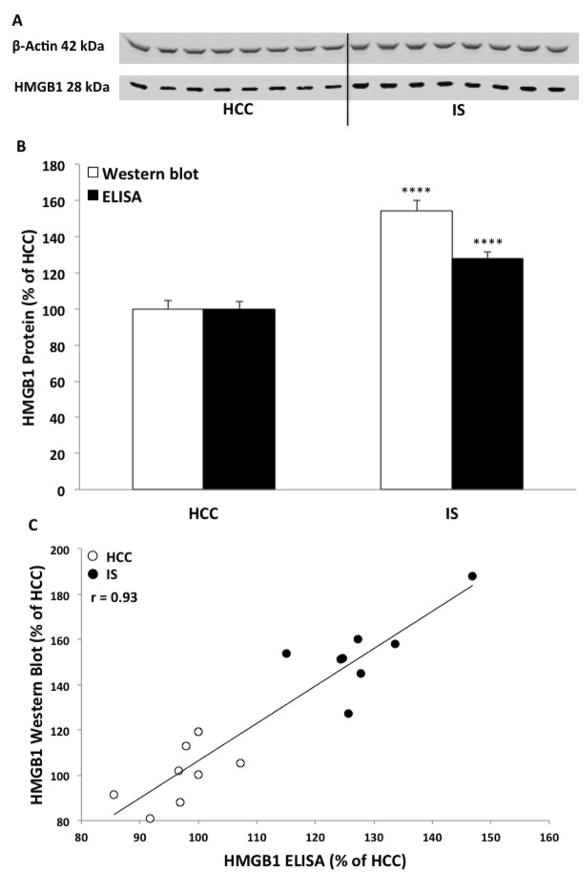
We have previously demonstrated that stress increases the alarmin HMGB1 in hippocampus and that HMGB1, at least in part, mediates stress-induced priming of hippocampal microglia (Weber et al., 2015). The effects of mCD200Fc on stress-induced microglial priming prompted us to explore whether the effects of stress on HMGB1 were a consequence of disrupted CD200:CD200R signaling. Therefore, we examined the effects of mCD200Fc on stress-induced increases in hippocampal HMGB1 protein. Given the results presented in Fig. 2 demonstrating that the effects of stress on CD200R were largely uniform across sub-regions of the hippocampus, the present experiment focused on whole hippocampus. In our prior study, we had examined the effect of IS on whole hippocampal HMGB1 using Western blot and found that IS increased hippocampal HMGB1 protein levels immediately after and 24h post-IS (Weber et al., 2015). Here, we conducted an initial experiment to replicate the effects of stress on HMGB1 using an untested, commercially available HMGB1 ELISA, which we validated using Western blot. As shown in Fig. 7, we found that IS increased hippocampal HMGB1 protein levels immediately after stress compared to levels observed in HCC animals using both Western blot (t = 7.24, df = 14, corr p < 0.0001) and ELISA (t = 7.9, df = 14, corr p < 0.0001). The results of each method showed a high degree of linearity (r = 0.93). Further, we found that IS increased HMGB1 protein levels 24h post-IS compared to HCC (Fig. 8A) using ELISA (t = 5.54, df = 14, p < 0.0001).
Fig. 7. Effect of IS on hippocampal HMGB1 protein.
Animals were exposed to IS (N = 8) or served as HCCs (N = 8). Immediately after IS, hippocampal HMGB1 protein was measured using (A) Western blot, (B) quantified comparing Western blot and ELISA and (C) the correlation of results from both methodological approaches. Data are presented as mean ± SEM. HCC significantly different from IS, **** p < 0.0001.
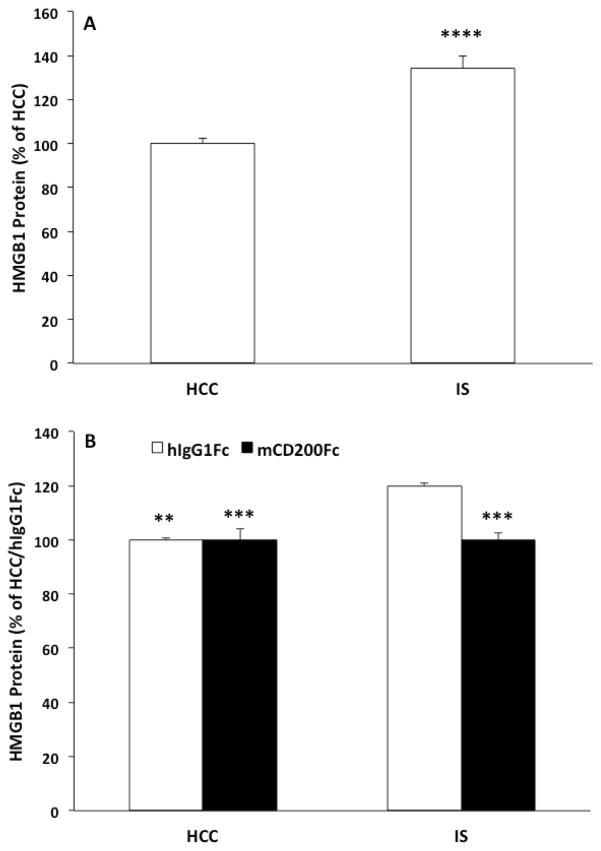
Fig. 8. Effect of mCD200Fc on IS-induced HMGB1 protein.
(A) Animals were exposed to IS (N = 8) or served as HCCs (N = 8). 24h after IS, hippocampal HMGB1 protein was measured. (B) Animals were injected ICM with control protein (hIgG1Fc) or mCD200Fc. Animals were then exposed to IS or served as HCCs. 24h after stress exposure, hippocampal HMGB1 protein was measured. Data are presented as mean ± sem. For data presented in A, IS significantly differed from HCC, **** p < 0.0001. For data presented in B, the hIgG1Fc/IS treatment group significantly differed from all other treatment groups, ** p < 0.01, *** p < 0.001.
To determine whether stress-induced increases in HMGB1 are down-stream of disrupted CD200:CD200R signaling, animals were injected ICM with mCD200Fc or control (hIgG1Fc) prior to stress exposure. 24h after termination of the stressor, hippocampal HMGB1 was measured in IS and HCC animals using ELISA. As depicted in Fig. 8B, the IS-induced increase in HMGB1 was blocked by mCD200Fc treatment (stress x drug interaction, F = 13.76, df = 1, 12, p = 0.003). Post-hoc tests show that IS significantly increased HMGB1 compared to control treated (p < 0.01) and mCD200Fc treated HCCs (p < 0.001). In IS-exposed animals, mCD200Fc significantly reduced this effect of IS compared to control treatment (p < 0.001).
An important consideration regarding the effects of mCD200Fc is whether mCD200Fc treatment altered expression of endogenous CD200. To test this possibility, we measured CD200 protein levels in these same hippocampal samples, in which HMGB1 was measured. We found that mCD200Fc treatment failed to significantly alter endogenous hippocampal CD200 protein compared to control treatment (Suppl. Fig. 2). The interaction between stress and mCD200Fc (F = 2.17, df = 1, 11, p = 0.17) as well as the main effect of stress (F = 0.001, df = 1, 11, p = 0.97) and CD200Fc (F = 2.74, df = 1, 11, p = 0.13) were not significant.
4. Discussion
Exposure to stressors can shift the activation state of microglia and render them sensitized to subsequent immune stimuli, without producing a classical pro-inflammatory state of activation (Frank et al., 2016). Thus, there is a potentiated neuroinflammatory response to these stimuli after prior stressor exposure. This suggests that stress might alter the CNS microenvironment and attenuate the immunoregulatory mechanisms that constrain microglial and neuroinflammatory processes. Immunoregulation in the CNS is mediated, in part, by a set of inhibitory signaling dyads including CD200:CD200R (Ransohoff and Cardona, 2010). Here, we explored the possibility that stress may weaken inhibitory controls over microglia, thereby permitting these cells to shift from a surveillant state to a primed state of activation. Interestingly, prior studies have found that disruption of CD200:CD200R signaling potentiates the microglial pro-inflammatory response to subsequent immune challenges (Costello et al., 2011; Denieffe et al., 2013), which served as the basis for focusing on CD200:CD200R signaling here.
Our initial experiment examined the effects of stress on hippocampal CD200 and CD200R gene expression in the context of a small array of innate immune-related genes, which included pro- and anti-inflammatory cytokines, pattern recognition receptors, inflammasome components, myeloid antigens and alternate inhibitory signaling dyads. Consistent with prior findings (Frank et al., 2007), this transcriptional profile demonstrated a distinct absence of a classical pro-inflammatory immunophenotype, which is a hallmark of a primed activation state. Although clearly not an exhaustive profile of innate-immune related gene expression, this experiment demonstrated that prior stress only reduced expression of CD200R, but not that of its ligand CD200 as well as other innate-immune genes. We have previously found that exposure to stress reduces gene expression of CD200 (Frank et al., 2007), but in the present study we failed to replicate this finding. In addition, expression of CX3CL1 and CX3CR1, which also constitutes a microglial inhibitory signaling dyad (Ransohoff and Cardona, 2010), were not altered by stress exposure. To further explore the expression pattern of CD200R in hippocampus, the effects of stress on CD200R in sub-regions of the hippocampus were examined. As with whole hippocampal expression, stress exposure produced a robust decrease in CD200R uniformly across sub-regions of the hippocampus at the 24h time-point. Furthermore, we found that the effects of stress on CD200R were not limited to hippocampus as we observed that stress down-regulated expression of CD200R in the BLA and CEA sub-regions of the amygdala. A question that arose from these finding was whether the effects of stress on CD200R were already present immediately after stressor exposure, or developed during the interval between stress termination and the 24h time-point. We found that stress-induced reductions in CD200R protein and gene expression were already present immediately after offset of the stressor, suggesting that the effect of stress on CD200R occurred during stress exposure and persisted for at least 24h post-stress.
CD200R in the CNS is primarily expressed on microglia and other cells of the myeloid lineage (Koning et al., 2009; Wright et al., 2000). Therefore, to further characterize the effects of stress on CD200R, we isolated hippocampal microglia 24h after the stressor session. Consistent with the effects of stress on expression in whole hippocampal tissue, we found that stress reduced microglial expression of this inhibitory receptor. Interestingly, Blandino and colleagues found that inescapable footshock reduced CD200R gene expression in hypothalamus, but not hippocampus (Blandino et al., 2009). The stressor protocol used by Blandino and colleagues differed from the protocol used here, which might account for these discrepant findings in hippocampus.
CD200R gene expression is regulated, in part, by the transcription factor C/EBPβ, which has been found to bind the CD200R promoter and inhibit transcription of CD200R (Dentesano et al., 2012). This finding led us to test the prediction that stress exposure would induce C/EBPβ expression. Indeed, we found that stress increased C/EBPβ protein and gene expression immediately after stress exposure and C/EBPβ gene expression was inversely correlated with CD200R expression. Of note, C/EBPβ expression is positively regulated by glucocorticoids (Hazra et al., 2007), and C/EBPβ and the glucocorticoid receptor interact to modulate the transcription of target genes (Pulido-Salgado et al., 2015). These findings are of relevance here as we have recently reported that glucocorticoids down-regulate microglial CD200R expression in vitro (Fonken et al., 2016) and have demonstrated that glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses (Frank et al., 2012b). Glucocorticoids have also been shown to mediate priming of neuroinflammatory processes in chronic stress paradigms (de Pablos et al., 2006; Espinosa-Oliva et al., 2011; Munhoz et al., 2006). In addition, exogenous glucocorticoids are sufficient to prime neuroinflammatory and microglia responses to immune challenges (Frank et al., 2014; Frank et al., 2010; Loram et al., 2011; Munhoz et al., 2010). Indeed, a considerable number of reports have challenged the dogma that glucocorticoids are universally anti-inflammatory (Sorrells et al., 2009). Taken together, these findings raise the intriguing possibility that stress-induced glucocorticoids may prime microglia via induction of CEBP/β, which down-regulates CD200R, thereby effectively disinhibiting (priming) microglia.
Our initial findings demonstrated that stress down-regulates CD200R in the hippocampus, which suggested the possibility that decreases in CD200R might result in a disruption in CD200:CD200R signaling. The central notion examined here was that disruption in CD200:CD200R signaling may then result in disinhibition of microglia as a consequence of reduced CD200R-mediated inhibitory drive. To test this notion, we took an experimental approach to increase inhibitory drive through central administration of a soluble CD200 fragment (mCD200Fc), which has been found to inhibit myeloid cell activation via CD200R1 (Cherwinski et al., 2005; Gorczynski et al., 2008; Gorczynski et al., 2004; Jenmalm et al., 2006). The approach here was to increase levels of a ligand for the CD200R, thereby compensating for stress-induced reductions in CD200R and revert CD200R-mediated inhibitory drive to a normative state. We found that central administration of mCD200Fc blocked the stress-induced potentiation of the microglial pro-inflammatory response suggesting that mCD200Fc treatment had increased CD200R-mediated inhibitory drive, thus blocking stress-induced priming of microglia. Furthermore, this result suggests that stress disrupts CD200:CD200R signaling, which leads to microglial disinhibition and a primed activation state. It is important to note that mCD200Fc treatment largely failed to modulate the main pro-inflammatory effect of LPS on microglia, but only blocked the stress-induced potentiation of this effect. These findings suggest that microglial sensitivity to LPS per se was not altered by mCD200Fc treatment, which obviates a potential confound of this experimental approach.
To further characterize the role of disrupted CD200:CD200R signaling in microglial priming, we examined whether disruption of CD200:CD200R signaling in naive animals was sufficient to induce microglial priming and thus recapitulate the effects of stress on microglia. Here, a soluble fragment of CD200R1 (mCD200R1Fc) was administered ICM, which should function like a decoy receptor through binding endogenous CD200 and effectively inhibit endogenous CD200R from binding CD200. If mCD200R1Fc disrupts endogenous CD200:CD200R signaling, microglia should become disinhibited and thus primed. Indeed, we found that treatment with mCD200R1Fc potentiated the microglial pro-inflammatory response to LPS suggesting that disruption of CD200:CD200R signaling is sufficient to prime microglia.
A number of studies have examined potential mediators of stress-induced neuroinflammatory and microglial priming (Frank et al., 2012b; Johnson et al., 2005; Johnson et al., 2004; Weber et al., 2015; Wohleb et al., 2011). We have recently found that stressor exposure increases levels of the danger-associated molecular pattern HMGB1 in hippocampus (Weber et al., 2015), a finding replicated in alternate stress paradigms (Cheng et al., 2016; Lian et al., 2017). Indeed, HMGB1 functions as one of these mediators of priming, in that blockade of HMGB1 signaling blocked priming (Weber et al., 2015). In light of the effects of mCD200Fc on stress-induced priming of microglia, we examined the possibility that stress-induced increases in hippocampal HMGB1 may be a consequence of disrupted CD200:CD200R signaling. To test this possibility, the effect of mCD200Fc on stress-induced HMGB1 was examined. Consistent with our previous findings (Weber et al., 2015), stress exposure increased hippocampal HMGB1 levels immediately after and 24h post-IS. However, prior treatment with mCD200Fc blocked this stress-induced increase in HMGB1. A concern regarding the use of mCD200Fc is that this fragment of CD200 might upregulate endogenous CD200, which would present an experimental confound. However, we found that mCD200Fc treatment failed to alter protein levels of hippocampal CD200, which suggests that the effects of mCD200Fc are not due to up-regulation of endogenous CD200. Thus, the present results suggest that the stress-induced increase in hippocampal HMGB1 is downstream of disrupted CD200:CD200R signaling. Further, the larger implication is that stressor exposure disinhibits microglia, which leads to increased HMGB1 levels because the immunoregulatory constraints on microglia have been attenuated via stress exposure. Our prior findings (Weber et al., 2015) suggest that this stress-induced increase in HMGB1 then functions to prime microglia via receptor signaling at TLR4 and/or RAGE. A further consideration regards the relationship between microglial disinhibition and priming. The present findings suggest that stress-induced disinhibition of microglia must occur initially. Once the immunoregulatory constraints on microglia are loosened, microglia can then release factors such as HMGB1, which then acts in an autocrine/paracrine fashion to prime microglia. It is important to note that we previously found that stress increases the extra-cellular release of HMGB1 from hippocampal microglia (Weber et al., 2015), however in the present study the cellular source of HMGB1 was not characterized. Nonetheless, the present findings suggest that disinhibited microglia are likely a source of the stress-induced increase in hippocampal HMGB1. It is important to clarify that the antibody-based methods used here to measure HMGB1 do not differentiate between the various redox states of HMGB1 (Venereau et al., 2012), which is a critical factor in microglial priming. We have found that the disulfide form, but not the reduced form of HMGB1 primes the neuroinflammatory and microglial response to subsequent immune challenges (Frank et al., 2015a).
A hallmark of the stress response is a disruption of homeostatic mechanisms that regulate key physiological systems, including the immune system. The present findings suggest that stress exposure disrupts immunoregulatory mechanisms in the brain, which typically constrain the immune response of innate immune cells. This attenuation of immunoregulatory mechanisms may thus permit a primed activation state of microglia to manifest, which may then allow for an enhanced innate immune response to ensue upon encounter with a subsequent immunological threat.
Supplementary Material
Gene expression of (A) CD200R and (B) CD200 was measured in amygdalar sub-regions (BLA and CEA) of IS (N = 8) or HCCs (N = 8) 24h after stress exposure. Data are presented as mean ± sem. For sub-regions, data are scaled as a percent of the HCC mean for each sub-region. Significant mean differences between HCC vs IS, ** p < 0.01.
Animals were injected ICM with control protein (hIgG1Fc) or mCD200Fc. Animals were then exposed to IS or served as HCCs. 24h after stress exposure, hippocampal CD200 protein was measured. Data are presented as mean ± sem.
Animals were injected ICM with control (hIgG1) or mCD200R1Fc (5 ug). 24h post-injection, hippocampal microglia were isolated and challenged with LPS for 2h ex vivo and gene expression of pro-inflammatory cytokines measured. N = 5/experimental group. Data are presented as mean ± sem.
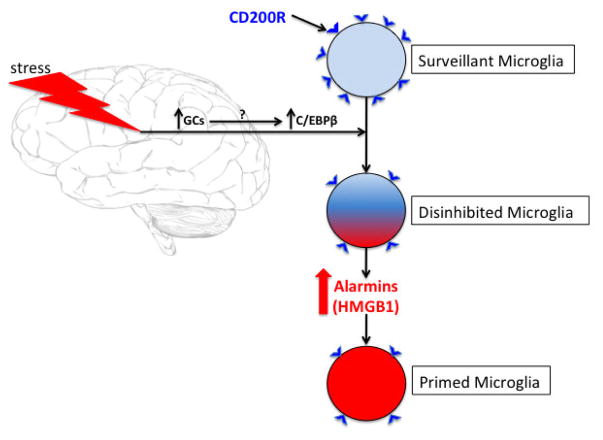
Fig. 9. Schematic of the proposed mechanism of stress-induced disinhibition/priming of microglia.
We propose that exposure to a stressor increases brain levels of glucocorticoids (GCs), which then lead to the induction of the transcription factor CAAT/enhancer binding protein (C/EBP)β. This transcription factor has been found to bind the promoter region of CD200R and reduce its expression. In the CNS, CD200R is expressed predominately on microglia as well as other brain macrophages, while CD200 is expressed ubiquitously on neurons, endothelial cells and oligodendrocytes. In light of the role that the CD200:CD200R signaling dyad plays in microglial immunoregulation, this stress-induced reduction in CD200R might then lead to disinhibition of microglia. We propose that this disinhibited state of microglia results in the increased synthesis and release of the alarmin high mobility group box (HMGB1) from microglia, which then signals microglia in an autocrine or paracrine manner to prime microglia, presumably through Toll-like receptor 4 or the receptor of advanced glycation end-products.
Acute stress down-regulates the microglial inhibitory receptor CD200R in hippocampus.
Treatment with mCD200Fc blocks stress-induced microglia priming and the alarmin HMGB1.
Stress-induced reductions in CD200R results in disinhibition of microglia.
Acknowledgments
This work was supported by grants from the National Institutes of Health to M.G.F and S.F.M (R01MH108523) and to L.K.F. (F32AG048672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Pardo M, Armini RS, Martinez A, Mouhsine H, Zagury JF, Jope RS, Beurel E. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwinski HM, Murphy CA, Joyce BL, Bigler ME, Song YS, Zurawski SM, Moshrefi MM, Gorman DM, Miller KL, Zhang S, Sedgwick JD, Phillips JH. The CD200 receptor is a novel and potent regulator of murine and human mast cell function. J Immunol. 2005;174:1348–1356. doi: 10.4049/jimmunol.174.3.1348. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colonna M, Butovsky O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello DA, Lyons A, Denieffe S, Browne TC, Cox FF, Lynch MA. Long term potentiation is impaired in membrane glycoprotein CD200-deficient mice: a role for Toll-like receptor activation. J Biol Chem. 2011;286:34722–34732. doi: 10.1074/jbc.M111.280826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denieffe S, Kelly RJ, McDonald C, Lyons A, Lynch MA. Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav Immun. 2013;34:86–97. doi: 10.1016/j.bbi.2013.07.174. [DOI] [PubMed] [Google Scholar]
- Dentesano G, Straccia M, Ejarque-Ortiz A, Tusell JM, Serratosa J, Saura J, Sola C. Inhibition of CD200R1 expression by C/EBP beta in reactive microglial cells. J Neuroinflammation. 2012;9:165. doi: 10.1186/1742-2094-9-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Oliva AM, de Pablos RM, Villaran RF, Arguelles S, Venero JL, Machado A, Cano J. Stress is critical for LPS-induced activation of microglia and damage in the rat hippocampus. Neurobiol Aging. 2011;32:85–102. doi: 10.1016/j.neurobiolaging.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Weber MD, Daut RA, Kitt MM, Frank MG, Watkins LR, Maier SF. Stress-induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology. 2016;66:82–90. doi: 10.1016/j.psyneuen.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Thompson BM, Weber MD, Watkins LR, Maier SF. IL-1RA injected intra-cisterna magna confers extended prophylaxis against lipopolysaccharide-induced neuroinflammatory and sickness responses. Journal of neuroimmunology. 2012a;252:33–39. doi: 10.1016/j.jneuroim.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain, behavior, and immunity. 2012b;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Fonken LK, Hershman SA, Watkins LR, Maier SF. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome. Brain Behav Immun. 2015a doi: 10.1016/j.bbi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF. Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun. 2015b doi: 10.1016/j.bbi.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF. Stress-induced neuroinflammatory priming: A liability factor in the etiology of psychiatric disorders. Neurobiology of stress. 2016;4:62–70. doi: 10.1016/j.ynstr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Gorczynski R, Boudakov I, Khatri I. Peptides of CD200 modulate LPS-induced TNF-alpha induction and mortality in vivo. J Surg Res. 2008;145:87–96. doi: 10.1016/j.jss.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Gorczynski R, Chen Z, Kai Y, Lee L, Wong S, Marsden PA. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J Immunol. 2004;172:7744–7749. doi: 10.4049/jimmunol.172.12.7744. [DOI] [PubMed] [Google Scholar]
- Gorczynski RM. CD200 and its receptors as targets for immunoregulation. Current opinion in investigational drugs. 2005;6:483–488. [PubMed] [Google Scholar]
- Hatherley D, Cherwinski HM, Moshref M, Barclay AN. Recombinant CD200 protein does not bind activating proteins closely related to CD200 receptor. J Immunol. 2005;175:2469–2474. doi: 10.4049/jimmunol.175.4.2469. [DOI] [PubMed] [Google Scholar]
- Hazra A, DuBois DC, Almon RR, Jusko WJ. Assessing the dynamics of nuclear glucocorticoid-receptor complex: adding flexibility to gene expression modeling. Journal of pharmacokinetics and pharmacodynamics. 2007;34:333–354. doi: 10.1007/s10928-007-9049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernangomez M, Klusakova I, Joukal M, Hradilova-Svizenska I, Guaza C, Dubovy P. CD200R1 agonist attenuates glial activation, inflammatory reactions, and hypersensitivity immediately after its intrathecal application in a rat neuropathic pain model. J Neuroinflammation. 2016;13:43. doi: 10.1186/s12974-016-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoarau JJ, Krejbich-Trotot P, Jaffar-Bandjee MC, Das T, Thon-Hon GV, Kumar S, Neal JW, Gasque P. Activation and control of CNS innate immune responses in health and diseases: a balancing act finely tuned by neuroimmune regulators (NIReg) CNS & neurological disorders drug targets. 2011;10:25–43. doi: 10.2174/187152711794488601. [DOI] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. Regulation of myeloid cell function through the CD200 receptor. J Immunol. 2006;176:191–199. doi: 10.4049/jimmunol.176.1.191. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:R422–432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Koning N, Swaab DF, Hoek RM, Huitinga I. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol. 2009;68:159–167. doi: 10.1097/NEN.0b013e3181964113. [DOI] [PubMed] [Google Scholar]
- Lian YJ, Gong H, Wu TY, Su WJ, Zhang Y, Yang YY, Peng W, Zhang T, Zhou JR, Jiang CL, Wang YX. Ds-HMGB1 and fr-HMGB induce depressive behavior through neuroinflammation in contrast to nonoxid-HMGB1. Brain Behav Immun. 2017;59:322–332. doi: 10.1016/j.bbi.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loram LC, Taylor FR, Strand KA, Frank MG, Sholar P, Harrison JA, Maier SF, Watkins LR. Prior exposure to glucocorticoids potentiates lipopolysaccharide induced mechanical allodynia and spinal neuroinflammation. Brain Behav Immun. 2011;25:1408–1415. doi: 10.1016/j.bbi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth SG, Simon OJ, Grimm A, Melzer N, Herrmann AM, Spitzer P, Landgraf P, Wiendl H. CNS inflammation and neuronal degeneration is aggravated by impaired CD200-CD200R-mediated macrophage silencing. J Neuroimmunol. 2008;194:62–69. doi: 10.1016/j.jneuroim.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, de Lima LS, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Sorrells SF, Caso JR, Scavone C, Sapolsky RM. Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci. 2010;30:13690–13698. doi: 10.1523/JNEUROSCI.0303-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nature reviews. Immunology. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Pulido-Salgado M, Vidal-Taboada JM, Saura J. C/EBPbeta and C/EBPdelta transcription factors: Basic biology and roles in the CNS. Prog Neurobiol. 2015;132:1–33. doi: 10.1016/j.pneurobio.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, Bianchi ME. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. The Journal of experimental medicine. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp Neurol. 2009;215:5–19. doi: 10.1016/j.expneurol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress Induces the Danger-Associated Molecular Pattern HMGB-1 in the Hippocampus of Male Sprague Dawley Rats: A Priming Stimulus of Microglia and the NLRP3 Inflammasome. J Neurosci. 2015;35:316–324. doi: 10.1523/JNEUROSCI.3561-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, Barclay AN. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–242. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cherwinski H, Sedgwick JD, Phillips JH. Molecular mechanisms of CD200 inhibition of mast cell activation. J Immunol. 2004;173:6786–6793. doi: 10.4049/jimmunol.173.11.6786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene expression of (A) CD200R and (B) CD200 was measured in amygdalar sub-regions (BLA and CEA) of IS (N = 8) or HCCs (N = 8) 24h after stress exposure. Data are presented as mean ± sem. For sub-regions, data are scaled as a percent of the HCC mean for each sub-region. Significant mean differences between HCC vs IS, ** p < 0.01.
Animals were injected ICM with control protein (hIgG1Fc) or mCD200Fc. Animals were then exposed to IS or served as HCCs. 24h after stress exposure, hippocampal CD200 protein was measured. Data are presented as mean ± sem.
Animals were injected ICM with control (hIgG1) or mCD200R1Fc (5 ug). 24h post-injection, hippocampal microglia were isolated and challenged with LPS for 2h ex vivo and gene expression of pro-inflammatory cytokines measured. N = 5/experimental group. Data are presented as mean ± sem.