SUMMARY
A cancer mass consists of a complex composition of cancer cells, stromal cells, endothelial cells and also immune cells, which can represent more than half of the cellularity of a solid cancer. These immune cells become activated when they sense cancer antigens and stress ligands. Innate immune cells also detect various aspects of cellular stress that characterize a growing tumor mass. These key hallmarks of cellular stress are also detected by the cancer cell itself. In this review, we highlight studies that show that the cancer cell itself could be considered an “innate cell” that senses and reacts to non-immunologic hallmarks of cancer, including displaced nucleic acids, proteotoxic stress, oxidative stress, and metabolic alterations.
Introduction
Although cancer cells can display unique neoantigens that drive personalized approaches to cancer therapy, they can also exhibit shared “hallmarks” which accompany uncontrolled cellular proliferation. These hallmarks include not only cellular processes such as resistance to apoptosis and autonomous replication [1] but also subcellular characteristics such as oxidative stress, DNA damage, and aberrant metabolism [2, 3]. Notably, evading immunity and inflammation-driven cancer progression also are hallmarks of cancer [4], but how these hallmarks relate to the non-immunologic hallmarks is not clear. In this review, we highlight recent studies that demonstrate that the non-immunologic hallmarks of cancer can be sensed in the tumor microenvironment, leading to immunologic effector activities of innate cells.
Previous reviews have summarized how innate immune cells sense antigens, stress, or changes in the microenvironment during cancer immune surveillance. We will briefly cover the newest insights into this well-known concept at the end of every section below. However, we begin each section by focusing on the idea that cancer cells themselves can function as “innate cells” by sensing intrinsic or extrinsic changes in their microenvironment, leading to direct immune effector functions. We will focus on sensing of various hallmarks of cancer, including ectopic nucleic acids (Fig. 1), aneuploidy and proteotoxic stress (Fig. 2), oxidative stress (Fig. 3), and metabolic aberrations (Fig. 4), with specific regard to the anti-tumor effector activities downstream of innate sensing.
Fig. 1. Sensing of nucleic acids by cancer cells and innate immune cells.
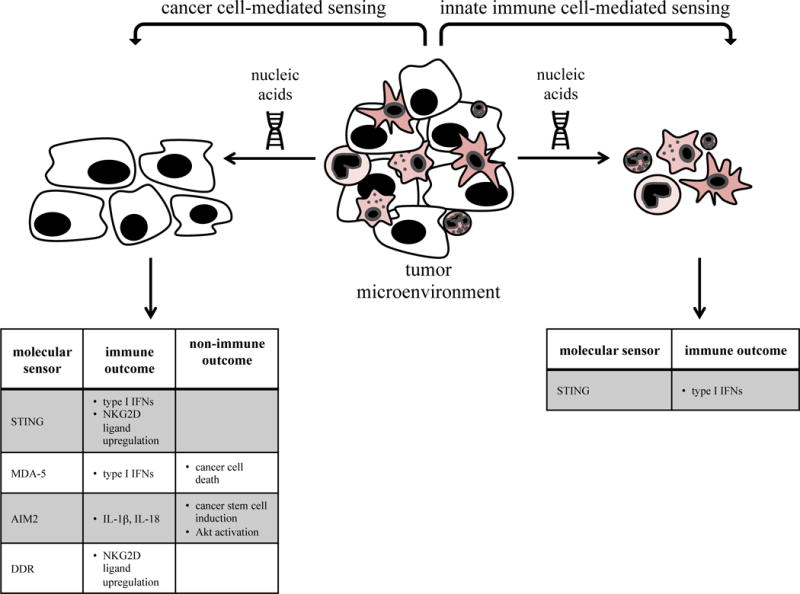
Distinct molecular sensors mediate nucleic acid sensing by either cancer cells (left) or innate immune cells (right).
Fig. 2. Sensing of proteotoxic stress by cancer cells and innate immune cells.
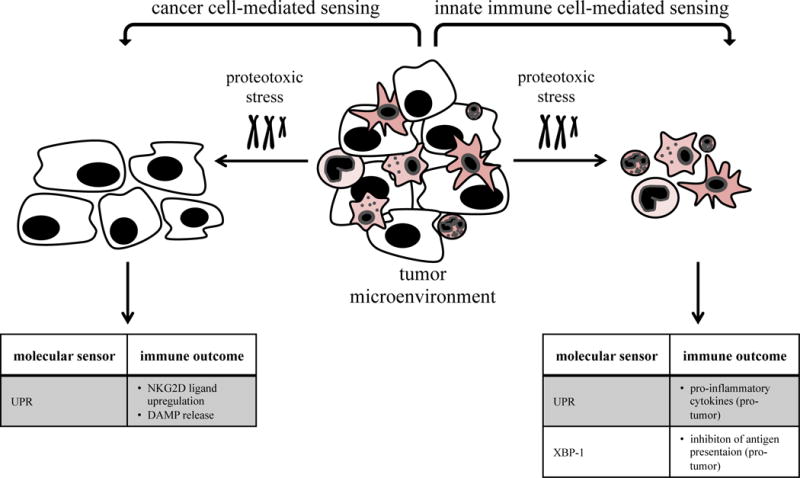
Cancer cells and innate immune cells can experience and sense proteotoxic stress induced by hyperploidy, proteotoxic stress-inducing agents or cell-to-cell transmission. Proteotoxic stress is sensed by the UPR, resulting in distinct outcomes on innate immunity.
Fig. 3. Sensing of oxidative stress by cancer cells and innate immune cells.
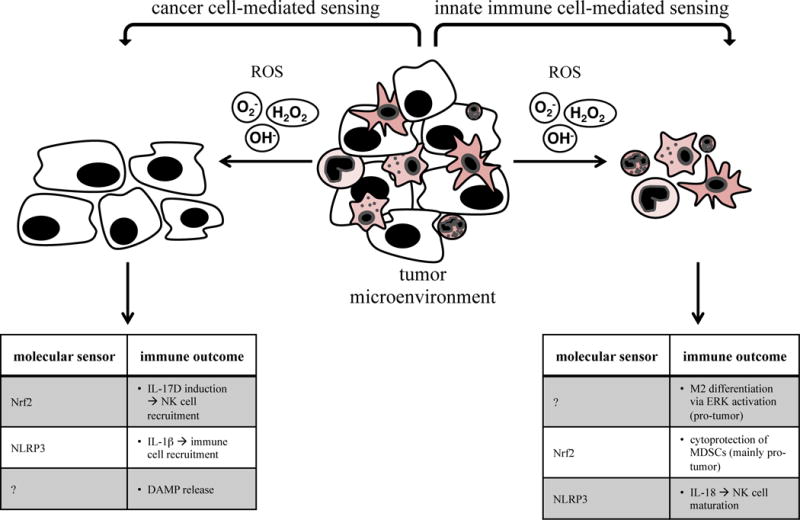
Reactive oxygen species in the tumor environment are produced and sensed by both cancer cells and innate immune cells. They are mostly detrimental, but can also induce anti-cancer immunity.
Fig. 4. Sensing of metabolic aberrations by cancer cells and innate immune cells.
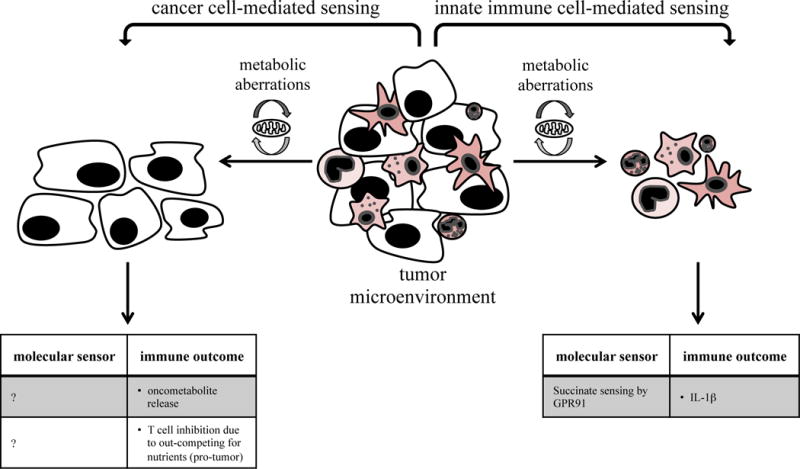
A progressively growing tumor features several metabolic aberrations such as hypoxia, abundant ROS and oncometabolites or nutrient deprivation. Those can be sensed by both cancer cells and innate immune cells with different outcomes on immunity.
Sensing of nucleic acids by cancer cells and other non-immune cells
Hallmarks of cancer such as extensive replication, genomic instability and mutation, oxidative stress, or a high metabolic rate can cause damage in the cell’s nuclear and/or mitochondrial (mt) DNA. Nuclear DNA damage is detected by the DNA damage response (DDR) pathway leading to activation of the kinases ATM/ATR [5]. In some instances, DNA damage can lead to displacement of endogenous nucleic acids into ectopic locations (i.e. extranuclear or extracellular). Ectopic nucleic acids are sensed by a panoply of receptors (reviewed in [6]), including toll-like receptors (TLRs), NOD-like receptors (NLRs), absent in melanoma (AIM) 2, and RIG-I-like receptors (RLRs). These receptors have independent signaling pathways that converge on activation of transcription factors such as interferon (IFN) regulatory factors (IRFs) or nuclear factor (NF)- κB, leading to the upregulation of type I IFNs (which are IFNs α, β, and the less well described ε, κ and ω) and other pro-inflammatory cytokines. Activation of the signaling module composed of cyclic GMP-AMP (cGAMP) synthase (cGAS) and stimulator of IFN genes (STING) also results in type I IFN induction (reviewed in [7]). In the case of certain NLRs and AIM2, activation leads to inflammasome activity and production of interleukin (IL)-1β and IL-18. This section will review the role of some of these nucleic acid sensors in cancer progression, with focus on their activity in non-immune cells and the outcome on innate immunity (Fig. 1).
Classic studies showed that DNA damage sensed by ATM/ATR could induce NKG2D ligands, leading to NK cell activation [8]. Recent studies demonstrated that induction of the NKG2D ligand RAE required sensing of cytosolic DNA by STING [9, 10]. In these studies, this sensing led to autonomous, i.e., tumor derived, production of type I IFNs in lymphoma cells. Interestingly, the “basal” level of DNA damage resulted in both constitutive expression of NKG2D ligands and autonomous IFN secretion.
Other studies found that forced activation of STING after intratumoral injection of cyclic dinucleotides could induce anti-tumor immune responses. This study did not define which cells, tumor or dendritic cells (DCs), were the targets of STING agonists in vivo [11]. Another group found that activation of STING and subsequent IFN production after intratumoral injection of cGAMP controlled the growth of melanoma and colon cancers by enhancing CD8+ T cell immunity [12]. In this study, the STING-induced secretion of type I IFNs into the tumor microenvironment was mediated mostly by tumor endothelial cells rather than DCs, in contrast to another study proposing cGAMP as a cancer immune therapy agent through its activation of DCs [13].
Cancer cell expression and activity of the RLR melanoma differentiation associated protein (MDA)-5 could also mediate anti-tumor activities. Yu et al., showed that transducing either full-length or truncated MDA-5 led to direct cell death in cancer cells but not normal cells. Interestingly, IFNβ was induced only by full-length, but not truncated, MDA-5. Intratumoral delivery of full-length MDA-5 led to cancer regression, which was mediated by type I IFN-induced antitumor immunity. These studies suggest that ectopic dsRNA (the ligand for MDA-5) could potentially induce cancer cell death, if present in high enough levels, while also stimulating production of IFNβ by cancer cells to activate immunity [14]. In contrast, the RLR laboratory of genetics and physiology (LGP) 2 is a negative regulator of MDA-5 and was found to be beneficial for tumor growth after radiotherapy by shutting off IFN synthesis [15, 16]. This suggests that cancer cells might develop defense mechanisms targeting type I IFN production to protect themselves from immune-mediated destruction.
AIM2 is a cytosolic DNA sensor that induces inflammasome activity. In two recent similar studies, Aim2−/− mice developed more colitis-associated cancer compared to control animals [17, 18]. The effects were attributed to uncontrolled proliferation of intestinal tumor-initiating stem cells and a dysbiotic gut microbiota [17] and/or hyperactivation of Akt [17, 18], rather than by immune activity. Nevertheless, these studies suggest that AIM2 could normally prevent cancer, a result that is supported by previous observations that it is downregulated in a variety of cancers and cancer cell lines [19, 20]. Moreover, AIM2 expression positively correlated with patient survival in Epstein-Barr virus (EBV)-associated nasopharyngeal carcinoma [21]. Altogether, these studies on MDA-5 and AIM2 document a different role for innate immune sensing molecules: they may exert activity in non-immune cells and may even influence cancer progression via non-immunologic effector pathways.
Sensing of nucleic acids by innate immune cells
The importance of type I IFN expression by antigen-presenting cells (APCs) in response to nucleic acid sensing during cancer immune surveillance has been reviewed elsewhere [22], so we will briefly cover only the newest insights into this topic (Fig. 1). It is now clear that DNA sensing pathways can be activated in innate cells during both conditions in which cancer cell death is induced (i.e., irradiation (IR) and chemotherapy) as well as during natural tumor progression. For example, the cytosolic DNA sensor pathway cGAS/STING was required for the antitumor effect of IR and this was mediated by type I IFN production in DCs [23]. In other studies, STING activation in APCs could be detected spontaneously during cancer progression or after stimulation with ectopic tumor-derived DNA, leading to increased IFNβ induction [24]. This was also seen in de novo gliomas, in which newly infiltrating CD11b+ innate leukocytes were a major source of type I IFNs [25]. Interestingly, the two cytosolic DNA sensor pathways mediated by STING or AIM2, respectively, might be antagonistic because activating AIM2 in macrophages or DCs reduced activation of STING in these cells [26].
Sensing of aneuploidy and proteotoxic stress in cancer cells
Cancer cells can feature and sense an abnormal content of DNA and chromosomes, termed aneuploidy. In consequence to an increased number of chromosomes, the expression of mRNA and cellular proteins increases, which can overload the endoplasmic reticulum (ER) folding capacity and lead to proteotoxic stress. Therefore, the sensing of aneuploidy is indirect, via the unfolded protein response (UPR) pathway that is activated by proteotoxic stress (Fig. 2).
Prototoxic stress has known effects on immunity, (also reviewed elsewhere [27]). For example, hyperploidy has been shown to induce immunogenicity, mediated by sensing of proteotoxic stress inside the cancer cell, which leads to the upregulation of cell surface calreticulin (ecto-CRT), an “eat-me” signal for macrophages [28]. Moreover, hyperploidy induces the expression of ligands for the activating receptors NKG2D and DNAM-1 in a number of cancer cell lines, which directly alerts the immune system [29]. Ecto-CRT and ATP are highly immunogenic damage-associated molecular patterns (DAMPs) released after cell death but recently also have been shown to be induced after proteotoxic stress sensing before apoptosis induction [30].
Sensing of proteotoxic stress by innate immune cells
Cancer associated innate immune cells are not known to be aneuploid but can experience and sense proteotoxic stress either induced by proteotoxic stress-inducing agents [31] or by transmission of proteotoxic stress from tumor cells [32], with mostly pro-tumor outcomes due to the release of pro-inflammatory cytokines (Fig. 2). One recent study showed that DCs in the tumor environment featured proteotoxic stress, and resulting hyperactivation of the sensor XBP-1 inhibited antigen presentation by dysregulating lipid metabolism [33]. This suggests that cancers might transmit proteotoxic stress to immune cells to escape immune-mediated destruction.
Sensing of oxidative stress by cancer cells
Cancer cells can sense intracellular or extracellular reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), and superoxide (O2−) or hydroxyl radicals (OH) as stress signals (Fig. 3). Oxidative stress is defined as an imbalance between ROS and detoxifying ROS scavengers. It is well-accepted that the cancer microenvironment generally features an abnormal oxidative status, mainly caused by extensive proliferation of cancer cells, metabolic aberrations as well as accumulation of ROS-secreting myeloid cells [34]. ROS are generally detrimental to cells, underlining that cancer cells must have developed distinct sensing mechanisms to be able to cope with an aberrant oxidative environment.
One key player in oxidant sensing and anti-oxidant responses is the transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (reviewed in [35]). Nrf2 is activated by electrophilic compounds, including ROS, leading to induction of anti-oxidant genes. These target genes can suppress or promote cancer depending on how far a cancer is along the progression process. In somatic and premalignant cells, Nrf2 suppresses transformation by detoxifying carcinogens, while in transformed cells, it protects from ROS or chemotherapeutics, thus explaining why many cancer cells have constitutively active Nrf2.
Although the role of Nrf2 during tumorigenesis and cancer progression has been widely analyzed, little is known about how this stress-sensing pathway can influence the immune system. A recent study revealed a direct connection between Nrf2 activation and induction of anti-tumor immunity [36]. This study showed that Nrf2 directly induced the cytokine IL-17D, which was previously found to recruit NK cells into the tumor bed by inducing the chemokine CCL2 in tumor endothelial cells [37]. Of note, the Nrf2/IL-17D pathway was activated during basal conditions but could also be therapeutically co-opted using an Nrf2 agonist, leading to induction of IL-17D and anti-tumor immunity mediated by NK cells. It is interesting to speculate that the Nrf2/IL-17D- induced immunity pathway evolved as the host’s response to the cancer’s increased defense mechanisms against oxidative stress.
An interesting study showed that ROS can exert immune-inducing functions by activating the NLRP3 inflammasome in cancer cells, which led to the IL-1β-mediated recruitment of anti-cancer immune cells [21]. In another publication, ROS sensing by DCs led to IL-6 production. Interestingly, this was tied to sensing of mutations in mitochondrial DNA (mtDNA) by lung carcinoma cells, which resulted in ROS overproduction and tumor suppression in vivo [38]. In a study using photodynamic therapy to induce ROS, ROS regulated proteotoxic stress-mediated secretion of ecto-CRT, leading to increased immunogenicity of colon carcinoma cells [30].
Sensing of oxidative stress by innate immune cells
Myeloid cells not only produce ROS in the tumor microenvironment but also respond to them (Fig. 3). One study showed that ROS production activates the ERK pathway and can promote M2 macrophage polarization [39].
APCs can also use the cytoplasmic sensor NLRP3, one of the inflammasome receptors that is known to be activated by multiple signals, including ROS [40], as a tumor suppressor. A recent study found that NLRP3-deficient mice featured exacerbated liver colorectal cancer metastatic growth mediated by impaired IL-18 signaling. This study suggested that the NLRP3 inflammasome was activated in Kupffer cells after sensing of an unknown signal (presumably ROS, nucleic acids, or DAMPs), leading to IL-18 mediated NK cell maturation and subsequent tumor killing [41].
Concerning the master antioxidant transcription factor Nrf2, most studies point towards a pro-cancer role in innate immune cells because of its protective effect from high ROS in pro-tumor myeloid derived suppressor cells (MDSCs) [42]. In some instances, however, MDSCs can also have anti-tumor functions by inhibiting TReg cell differentiation dependent on ROS [43].
Sensing of metabolic aberrations in cancer cells
Another form of stress tumors can sense is metabolic stress, caused by increased metabolism, leading to hypoxia, mtDNA damage, release of oncometabolites and autophagy.
For example, sensing of altered metabolism in cancer cells may result in the release of so-called oncometabolites, metabolic byproducts that are different from those of healthy cells (Fig. 4). Most of these, such as lactate or 2-hydroxyglutarate (2-HG), are pro-tumor because they negatively affect immune surveillance or stimulate immune suppressive MDSCs and M2 macrophages [44, 45]. In contrast, the oncometabolite succinate, an intermediate in the citric acid cycle, could possibly have anti-tumor effects by inducing IL-1β secretion from macrophages in the context of LPS-induced activation [46]. In this study, macrophages switched their metabolism from oxidative phosphorylation to glycolysis, resulting in increased succinate production. It is not clear whether cancer cells can sense their own oncometabolites although switching their metabolism to glycolysis is a common feature of cancer cells.
Sensing of metabolic aberrations by immune cells
Immune cells are influenced by factors resulting from metabolic stress in the tumor environment, for example hypoxia, oncometabolites or ROS. Progressively growing tumors have been shown to compete with T cells for nutrients, thereby leading to glycolysis and defective IFNγ production in T cells [47]. Likewise, innate immune cells might also be influenced by metabolic alterations in the tumor environment.
Conclusions
In conclusion, tumor cells have evolved to sense distinct signals and stresses intrinsically or in their immediate environment. As such, they may be viewed as innate cells themselves because their perception of abnormalities can lead to the induction of immunity that might result in their eventual elimination.
Highlights.
The cancer cell as a sensing innate cell
Cancer cells sense intrinsic and extrinsic non-immunologic hallmarks
Cancer cells sense nucleic acids, proteotoxic stress, oxidative stress, metabolic aberrations
Innate immune cells sense non-immunologic and immunologic hallmarks
Innate sensing by PRRs can mediate both immunologic and non-immunologic effector outcomes
Acknowledgments
J.D.B is funded by NIH grant CA157885 and a grant from The Hartwell Foundation. R.S. is additionally funded by the DFG (SE 2418/1-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflicts of interest regarding this manuscript.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136(5):823–37. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seelige R, Searles S, Bui JD. Mechanisms regulating immune surveillance of cellular stress in cancer. Cell Mol Life Sci. 2017 doi: 10.1007/s00018-017-2597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Brzostek-Racine S, Gordon C, Van Scoy S, Reich NC. The DNA damage response induces IFN. J Immunol. 2011;187(10):5336–45. doi: 10.4049/jimmunol.1100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38(5):870–80. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142–9. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 8.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam AR, Le Bert N, Ho SS, Shen YJ, Tang ML, Xiong GM, Croxford JL, Koo CX, Ishii KJ, Akira S, Raulet DH, Gasser S. RAE1 ligands for the NKG2D receptor are regulated by STING-dependent DNA sensor pathways in lymphoma. Cancer Res. 2014;74(8):2193–203. doi: 10.1158/0008-5472.CAN-13-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen YJ, Le Bert N, Chitre AA, Koo CX, Nga XH, Ho SS, Khatoo M, Tan NY, Ishii KJ, Gasser S. Genome-derived cytosolic DNA mediates type I interferon-dependent rejection of B cell lymphoma cells. Cell Rep. 2015;11(3):460–73. doi: 10.1016/j.celrep.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW, Jr, Gajewski TF. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015;11(7):1018–30. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, Gaide O, Michielin O, Hwu P, Petrova TV, Martinon F, Modlin RL, Speiser DE, Gilliet M. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci U S A. 2015;112(50):15408–13. doi: 10.1073/pnas.1512832112. Elegant study showing that artificially activating STING by intratumoral injection of agonist or tumor DNA in vitro induces the expression of IFNβ in tumor endothelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li T, Cheng H, Yuan H, Xu Q, Shu C, Zhang Y, Xu P, Tan J, Rui Y, Li P, Tan X. Antitumor Activity of cGAMP via Stimulation of cGAS-cGAMP-STING-IRF3 Mediated Innate Immune Response. Sci Rep. 2016;6:19049. doi: 10.1038/srep19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Yu X, Wang H, Li X, Guo C, Yuan F, Fisher PB, Wang XY. Activation of the MDA-5-IPS-1 Viral Sensing Pathway Induces Cancer Cell Death and Type I IFN-Dependent Antitumor Immunity. Cancer Res. 2016;76(8):2166–76. doi: 10.1158/0008-5472.CAN-15-2142. This study shows that the cytosolic dsRNA sensor MDA-5 might have anticancer functions in two ways; first by directly inducing cancer cell death, second, by activating immunity via upregulation of type I IFN expression in tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widau RC, Parekh AD, Ranck MC, Golden DW, Kumar KA, Sood RF, Pitroda SP, Liao Z, Huang X, Darga TE, Xu D, Huang L, Andrade J, Roizman B, Weichselbaum RR, Khodarev NN. RIG-I-like receptor LGP2 protects tumor cells from ionizing radiation. Proc Natl Acad Sci U S A. 2014;111(4):E484–91. doi: 10.1073/pnas.1323253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranoa DR, Parekh AD, Pitroda SP, Huang X, Darga T, Wong AC, Huang L, Andrade J, Staley JP, Satoh T, Akira S, Weichselbaum RR, Khodarev NN. Cancer therapies activate RIG-I-like receptor pathway through endogenous non-coding RNAs. Oncotarget. 2016;7(18):26496–515. doi: 10.18632/oncotarget.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RK, Gurung P, Neale G, Olsen SR, Carter RA, McGoldrick DJ, Wu G, Finkelstein D, Vogel P, Gilbertson RJ, Kanneganti TD. Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell. 2015;162(1):45–58. doi: 10.1016/j.cell.2015.06.001. In this study similar to Ref. 18, Man et al. show that mice deficient for the DNA sensor Aim2 are more susceptible to colorectal cancer, largely mediated by an expansion of cancer-initiating stem cells and a dysbiotic gut microbiota, but seemingly independent of inflammasome-mediated immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, Muhlbauer M, Chou WC, Barker BR, Jobin C, Allbritton NL, Ramsden DA, Davis BK, Ting JP. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med. 2015;21(8):906–13. doi: 10.1038/nm.3908. In this study similar to Ref. 17, Wilson et al. show that Aim2−/− mice are more susceptible to colorectal cancer, mainly attributed to hyperactivation of the tumor promoter Akt, but not to inflammasome activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponomareva L, Liu H, Duan X, Dickerson E, Shen H, Panchanathan R, Choubey D. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res. 2013;11(10):1193–202. doi: 10.1158/1541-7786.MCR-13-0145. [DOI] [PubMed] [Google Scholar]
- 20.Dihlmann S, Tao S, Echterdiek F, Herpel E, Jansen L, Chang-Claude J, Brenner H, Hoffmeister M, Kloor M. Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int J Cancer. 2014;135(10):2387–96. doi: 10.1002/ijc.28891. [DOI] [PubMed] [Google Scholar]
- 21.Chen LC, Wang LJ, Tsang NM, Ojcius DM, Chen CC, Ouyang CN, Hsueh C, Liang Y, Chang KP, Chen CC, Chang YS. Tumour inflammasome-derived IL-1beta recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol Med. 2012;4(12):1276–93. doi: 10.1002/emmm.201201569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–14. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 23.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, Watkins SC, Sarkar SN, Okada H. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res. 2014;2(12):1199–208. doi: 10.1158/2326-6066.CIR-14-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Corrales L, Woo SR, Williams JB, McWhirter SM, Dubensky TW, Jr, Gajewski TF. Antagonism of the STING Pathway via Activation of the AIM2 Inflammasome by Intracellular DNA. J Immunol. 2016;196(7):3191–8. doi: 10.4049/jimmunol.1502538. Study suggesting that the two intracellular DNA sensing pathways mediated by STING and AIM2 are antagonized because activating AIM2 in macrophages and DCs led to reduced activation of STING. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. 2016;16(8):469–84. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M, Shen S, Marino G, Criollo A, Boileve A, Job B, Ladoire S, Ghiringhelli F, Sistigu A, Yamazaki T, Rello-Varona S, Locher C, Poirier-Colame V, Talbot M, Valent A, Berardinelli F, Antoccia A, Ciccosanti F, Fimia GM, Piacentini M, Fueyo A, Messina NL, Li M, Chan CJ, Sigl V, Pourcher G, Ruckenstuhl C, Carmona-Gutierrez D, Lazar V, Penninger JM, Madeo F, Lopez-Otin C, Smyth MJ, Zitvogel L, Castedo M, Kroemer G. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337(6102):1678–84. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 29.Acebes-Huerta A, Lorenzo-Herrero S, Folgueras AR, Huergo-Zapico L, Lopez-Larrea C, Lopez-Soto A, Gonzalez S. Drug-induced hyperploidy stimulates an antitumor NK cell response mediated by NKG2D and DNAM-1 receptors. Oncoimmunology. 2016;5(2):e1074378. doi: 10.1080/2162402X.2015.1074378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, Annaert W, Golab J, de Witte P, Vandenabeele P, Agostinis P. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31(5):1062–79. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheeler MC, Rizzi M, Sasik R, Almanza G, Hardiman G, Zanetti M. KDEL-retained antigen in B lymphocytes induces a proinflammatory response: a possible role for endoplasmic reticulum stress in adaptive T cell immunity. J Immunol. 2008;181(1):256–64. doi: 10.4049/jimmunol.181.1.256. [DOI] [PubMed] [Google Scholar]
- 32.Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A. 2011;108(16):6561–6. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, Ellenson LH, Caputo T, Lee AH, Conejo-Garcia JR, Glimcher LH. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161(7):1527–38. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell. 2015;27(2):156–7. doi: 10.1016/j.ccell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Taguchi K, Yamamoto M. The KEAP1-NRF2 System in Cancer. Front Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Saddawi-Konefka R, Seelige R, Gross ET, Levy E, Searles SC, Washington A, Jr, Santosa EK, Liu B, O’Sullivan TE, Harismendy O, Bui JD. Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance. Cell Rep. 2016;16(9):2348–58. doi: 10.1016/j.celrep.2016.07.075. First study to make a connection between the oxidative stress responder Nrf2 in cancer cells and NK cell-mediated antitumor immunity. Saddawi-Konefka & Seelige et al. show that Nrf2 activation in tumors leads to the upregulation of the cytokine IL-17D, which recruits NK cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Sullivan T, Saddawi-Konefka R, Gross E, Tran M, Mayfield SP, Ikeda H, Bui JD. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell Rep. 2014;7(4):989–98. doi: 10.1016/j.celrep.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imanishi H, Takibuchi G, Kobayashi T, Ishikawa K, Nakada K, Mori M, Kikkawa Y, Takenaga K, Toyama-Sorimachi N, Hayashi J. Specific mtDNA mutations in mouse carcinoma cells suppress their tumor formation via activation of the host innate immune system. PLoS One. 2013;8(9):e75981. doi: 10.1371/journal.pone.0075981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23(7):898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Y, Hara H, Nunez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 2016;41(12):1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton V, Colpitts SL, Beauchemin N, Saleh M. The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity. Immunity. 2015;43(4):751–63. doi: 10.1016/j.immuni.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Beury DW, Carter KA, Nelson C, Sinha P, Hanson E, Nyandjo M, Fitzgerald PJ, Majeed A, Wali N, Ostrand-Rosenberg S. Myeloid-Derived Suppressor Cell Survival and Function Are Regulated by the Transcription Factor Nrf2. J Immunol. 2016;196(8):3470–8. doi: 10.4049/jimmunol.1501785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centuori SM, Trad M, LaCasse CJ, Alizadeh D, Larmonier CB, Hanke NT, Kartchner J, Janikashvili N, Bonnotte B, Larmonier N, Katsanis E. Myeloid-derived suppressor cells from tumor-bearing mice impair TGF-beta-induced differentiation of CD4+CD25+FoxP3+ Tregs from CD4+CD25-FoxP3- T cells. J Leukoc Biol. 2012;92(5):987–97. doi: 10.1189/jlb.0911465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–63. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191(3):1486–95. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 46.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–42. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, Tonc E, Schreiber RD, Pearce EJ, Pearce EL. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162(6):1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


