Summary
Drug development in HCC has been characterized in the past by many failures. Despite good rationales and promising phase II data, many phase III trials failed. Immunotherapy represents an alternate treatment approach and has been successful in many different types of cancer. Being an inflammation induced cancer HCC represents a very interesting target for immune based approaches and indeed early results from clinical trials testing immune checkpoint inhibitors are not only promising but have already led to evaluation of such in a phase III setting. Here we summarize our current knowledge on the rationale, mechanism of action and clinical data for immune checkpoint blockade in HCC. In addition, we provide an overview about other novel immune based approaches currently under development for the treatment of HCC such as adoptive cell based and antibody-based approaches.
The story of drug development for hepatocellular carcinoma (HCC) has been disappointing in the past eight years with many drugs failing in phase III trials [1]. Only the RESORCE trial testing regorafenib in patients progressing on sorafenib resulted in increased survival [2]. Immune based approaches focused on vaccination strategies, cytokines or non-specific T cell activation have been tested for many years in HCC with mostly disappointing results [3,4]. However, the era of immune-oncology has dramatically changed with the FDA approval immune checkpoint inhibitors for the treatment of different types of cancer (table 1). In 2013, the journal Science declared cancer immunotherapy as the breakthrough of the year [5] and in the two last years, the American Society of Clinical Oncology considered immunotherapy back to back the Advance of the Year. As of today, the FDA has approved six different immune checkpoint inhibitors. Great interest has sparked for immune based treatment approaches to treat patients with hepatocellular carcinoma (HCC). First results from three published clinical trials using immune checkpoint inhibitors (tremelimumab (anti-CTLA-4) and nivolumab (anti-PD1)) as well as preliminary results from other ongoing trials published in form of abstracts suggest a promising role for immunotherapy in the treatment of HCC (table 2). One immune checkpoint inhibitor (nivolumab) is currently being tested in a phase III trial in the first line setting against sorafenib (NCT02576509).
Table 1.
Immunotherapy agents approved by FDA for the treatment of cancer.
| Disease | Class of agent(s) |
|---|---|
| AIDS-related Kaposi. | interferon alpha-2b |
| Hairy cell leukemia. | interferon alpha-2b |
| Lymphoma (Hodgkin and non-Hodgkin) | anti-PD-1 mAb & interferon alpha-2b |
| Merckle cell carcinoma | anti-PD-L1 mAb |
| Urothelial carcinoma | anti-PD-1 & anti-PD-L1 mAb |
| Melanoma | anti-CTLA4 mAb & anti-PD-1 mAb |
| interferon alpha-2b & interleukin 2 | |
| oncolytic HSV-1 encoding GM-CSF | |
| Non small cell lung cancer | anti-PD-1 & anti-PD-L1 mAb |
| Prostate carcinoma | autologous DC vaccine against PAP |
| Renal cell carcinoma | anti-PD-1 mAb & interleukin 2 |
| Squamous cell carcinoma of the head and neck | anti-PD-1 mAb |
mAb: monoclonal antibody. HSV-1: herpes simplex type-1 virus. GM-CSF: granulocyte-macrophage colony stimulating factor. DC: dendritic cells. PAP: prostatic-acid phosphatase.
Table 2.
Efficacy data from clinical trials of immune checkpoint inhibitors in advanced hepatocellular carcinoma.
| Agent, dose | n | BCLC stage | Sorafenib exposure | ORR/DCR | TTP | OS | Ref. |
|---|---|---|---|---|---|---|---|
| Tremelimumab 30 mg q 3 months |
21 | 3/6/12 | Naive, intolerant or progressed to Sorafenib | 3/17 (17.6%) PR 13/17 (76.4%) DCR |
6.48 months | 8.2 months | [16] |
| Tremelimumab 10 mg q 28 days + ablation |
32 | -/7/21 | Progressed to Sorafenib | 5/19 (26.3%) PR | 7.4 months | 12.3 months | [17] |
| Nivolumab 3 mg/kg q 15 days * |
80 | Naive to Sorafenib | 1/80 (1.2%) CR 17/80 (21.2%) PR 50/80 (62.5%) DCR |
Not reported | 28.6 months | [23] | |
| Nivolumab 3 mg/kg q 15 days * |
182 | Intolerant or progressed to Sorafenib | 7/182 (3.8%) CR 27/182 (14.8%) PR 114/182 (62.6%) DCR |
Not reported | 15.6 months | [23] |
BCLC: Barcelona Clinic Liver Cancer. ORR: overall response rate. DCR: disease control rate. TTP: time to progression. OS: overall survival.
Dose used in the expansion cohort
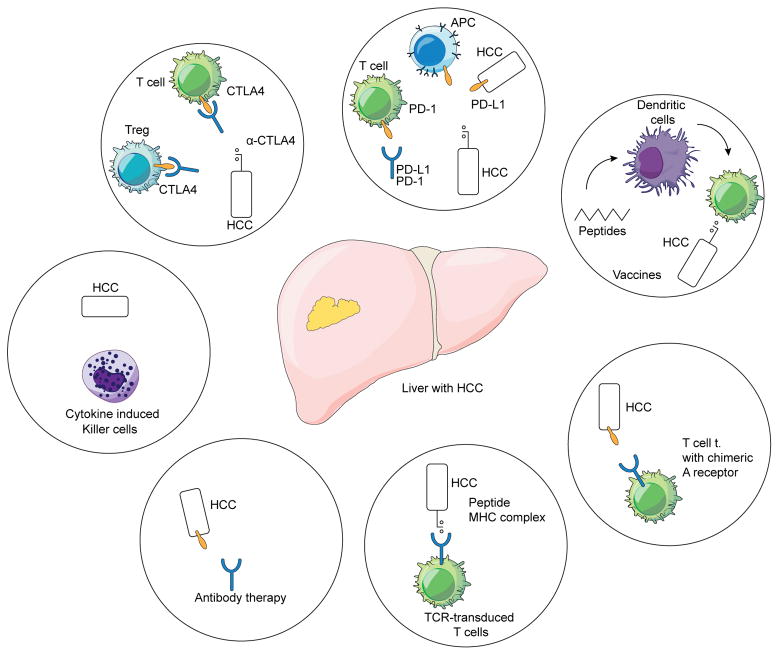
Here we describe the rationale and mechanism of action of immune interventions for the treatment of patients with HCC, with particular emphasis on immune checkpoint inhibitors (figure 1). We summarize currently available data and ongoing clinical trials. We discuss future developments and provide an overview over alternate immune based treatment options for HCC.
Figure 1.
Immune based approaches in HCC
Checkpoint inhibitors: development and mechanisms of action
Immune checkpoints are a specific subtype of membrane-bound molecules that provide fine-tuning of the immune response. Different cell types involved in the immune response express immune checkpoints, including B and T cells, natural killer (NK) cells, dendritic cells (DC), tumor associated macrophages (TAM), monocytes, and myeloid-derived suppressor cells (MDSC). The physiological function of these complexes is to prevent continuous T cell effector function upon initial stimulation and engagement of antigen-specific T cells. Thus, most of these molecules display an immunosuppressive activity that prevents uncontrolled T cell responses against infection and limit collateral tissue damage. The immune checkpoints most studied in human cancer are cytotoxic T-lymphocyte protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), lymphocyte activation gene 3 protein (LAG-3), B and T lymphocyte attenuator (BTLA), and T-cell immunoglobulin and mucin-domain containing (TIM-3). A comprehensive review of their variety and functions can be obtained in [6,7].
CTLA-4 is essential for the activation of CD4+ T cells and the priming phase of the immune response. Expressed on activated T cells, CTLA-4 has great affinity for CD80 and CD86 and may thus antagonize the interaction of CD28 with these receptors, with resulting decreased T cell activation upon antigen presentation. Regulatory T cells (Treg) also express CTLA-4 constitutively. Treg are CD4+ T cells that can be characterized by the presence of CD25, CTLA-4, CD62L and FoxP3 molecules in their membrane. Activated by TCR engagement concurrent with IL-10 and TGF-β signaling, Treg inhibit the immune response through various mechanisms including depletion of IL-2 and secretion of immunosuppressive factors such as TGF-β, IL-10 or adenosine, as well as competition with co-stimulatory CD28 via CTLA-4. Hence, CTLA-4 is also required for Treg to exert its suppressive activity on activated T cells [8]. But the role of CTLA-4 is not restricted to the priming phase. Inside the tumor, CTLA-4 also promotes immunosuppression by inducing Treg activity and differentiation and up-regulating IDO and IL-10 in DC [9].
PD-1 is a key factor in the effector phase of the immune response. It is expressed by activated CD8+ and CD4+ T cells, B cells, NK, Treg, MDSC, monocytes and DC. PD-L1 and PD-L2 are the ligands of PD-1. PD-L1 is expressed in hematopoietic cells, including APC and MDSC, and in different types of parenchymal cells too, while PD-L2 expression is limited to the hematopoietic compartment. Various cytokines up-regulate PD-L1, particularly IFN-γ. Upon binding to its ligands, PD-1 inhibits CD8+ T cell activation by blocking the TCR signaling, and inhibits CD4+ activation and proliferation through increased secretion of IL-10. Cancer cells may also express PD-L1 and PD-L2 and use this mechanism to escape from immunosurveillance. Indeed, in a situation of chronic antigen exposure such as the tumor microenvironment, IFN-γ produced by TAA-specific T cells induces PD-1 expression on reactive T lymphocytes and up-regulates PD-L1 in APC and tumor cells. PD-1–PD-L1 engagement then blocks TCR signaling and inhibits T cell proliferation and secretion of cytotoxic mediators, in a process called T cell exhaustion [10]. IFN-γ release enhances the expression of PD-L1 under the hypoxic conditions present in most tumors.
TIM-3 is a transmembrane protein expressed on cells of the innate and adaptive immune system that interacts with several ligands including phosphatidylserine on the membrane of apoptotic cells, galectin-9 and others. Galectin-9 is a soluble protein produced by cells from many different tissue types (including the liver) that regulates cell differentiation, adhesion and cell death. Evidence indicates that galectin-9 suppresses T-cell responses, which supports the concept that TIM-3 acts as an inhibitory receptor for T cells. Furthermore, CD8+ Tim-3+ T cells in animal models co-express PD-1, and these dual-expressing cells exhibit greater defects in both cell-cycle progression and effector cytokine production IL-2, TNF, and IFN-γ than cells that express PD-1 alone. The TIM-3 pathway may thus cooperate with the PD-1 pathway to promote the development of a severe dysfunctional phenotype in CD8+ T cells in cancer [11].
LAG-3 is a membrane protein that binds MHC class II molecules with high affinity, thus reducing the co-stimulatory functions of DC. LAG-3 is not expressed on resting T cells but is up-regulated upon activation. It is a marker of exhausted T cells and acts synergistically with PD-1 to promote cancer evasion from immunity [12] [13]. Finally, BTLA is an immunoglobulin-like molecule expressed by several immune cells including B and T lymphocytes, NK and antigen presenting cells. BTLA is able to inhibit T cell proliferation and cytokine production upon binding to its ligand, herpesvirus entry mediator (HVEM), which can be expressed in HCC [14,15].
Clinical experience with the use of checkpoint inhibitors in hepatocellular carcinoma
In the field of HCC, clinical development has focused so far on CTLA-4 and PD-1/PD-L1 pathways. Among CTLA-4 targeted therapies, tremelimumab (a fully human IgG2 monoclonal antibody) was the first molecule clinically evaluated in HCC. A phase II, non-controlled, multicenter trial targeted the population of patients with HCC and chronic HCV infection who were not eligible for surgery or locoregional therapy [16]. The trial had the dual intention to test the antitumor and antiviral activity of tremelimumab in a single study. The study was 80% powered to reject the null hypothesis that objective response rate did not exceed 5% at a 0.05 level of significance if true objective response rate was >25%. Based on a Simon’s optimal 2-stage design 3 tumor responses among 17 evaluable patients were needed to reject the null hypothesis. Twenty-one patients with fairly advanced disease (57% were at BCLC C stage) were enrolled, most of them (57%) having progressed to previous therapies. Importantly, a significant proportion of patients (42.9%) were in Child-Pugh stage B, indicating some degree of liver dysfunction. Patients received what we now know is a suboptimal dose of 15 mg/kg tremelimumab every 90 days to a maximum of four doses unless tumor progression or unacceptable toxicities occurred. Despite this suboptimal dosing, three partial responses were observed among 17 evaluable patients and the trial was found to be positive based on the initial assumptions. Stable disease was the best response in 10 additional patients, accounting for a remarkable disease control rate of 76.4%. Quite importantly, almost half (45%) of these stabilizations lasted longer than 6 months. Among 11 patients that had alpha-fetoprotein levels higher than 100 ng/ml at baseline, 36% showed a >50% drop following treatment, providing further evidence of antitumor activity. Median time to progression was 6.48 months (95%CI 3.95–9.14 months). Although potentially biased by a long tumor assessment interval, this prolonged time to progression compares favorably with several targeted agents as shown in table 3. The observed overall survival of 8.2 months (95%CI 4.64–21.34 months) was not much different from what could be observed in patients receiving placebo in second-line trials but the high proportion of Child B patients in this cohort likely had a significant impact in this outcome.
Table 3.
Combination therapies based on PD1/PD-L1 blockade under study for the treatment of hepatocellular carcinoma.
| Anti-PD-1/PD-L1 agent | Combining agent | Mechanism of action | Patients | Population | NCT number |
|---|---|---|---|---|---|
| Combinations with other immunotherapies | |||||
| Nivolumab | Ipilimumab | anti-CTLA4 | 620 * | HCC | 01658878 |
| Durvalumab | Tremelimumab | anti-CTLA4 | 144 | HCC | 02519348 |
| Nivolumab | Pexavec | GMCSF-armed oncolytic virus | 30 | HCC | 03071094 |
| Combinations with antiangiogenics | |||||
| Durvalumab | Ramucirumab | anti-VEGFR2 mAb | 114 | HCC and other histologies | 02572687 |
| Pembrolizumab | Lenvatinib | TKI | 30 | HCC | 03006926 |
| Pembrolizumab | Nintedanib | TKI | 18 | HCC and other histologies | 02856425 |
| SHR-1210 | Apatinib | TKI | 30 | HCC and other histologies | 02942329 |
| PDR001 | Sorafenib | TKI | 50 | HCC | 02988440 |
| Combinations with other targeted agents | |||||
| Nivolumab | Galunisertib | TGF-beta inhibitor | 75 | HCC | 02423343 |
| Nivolumab | CC-122 | Pleiotropic pathway modifier | 50 | HCC | 02859324 |
| Pembrolizumab | XL888 | Hsp90 inhibitor | 50 | HCC and other histologies | 03095781 |
| PDR001 | INC280 | c-met inhibitor | 108 | HCC | 02474537 |
| PDR001 | FGF401 | FGFR4 inhibitor | 238 | HCC | 02325739 |
| Combinations with locoregional therapies | |||||
| Nivolumab | TACE | Ischemia | 14 | HCC | 03143270 |
| Nivolumab | Y90 | Beta radiation | 40 | HCC | 03033446 |
| Nivolumab | Y90 | Beta radiation | 35 | HCC | 02837029 |
| Pembrolizumab | Y90 | Beta radiation | 30 | HCC | 03099564 |
includes nivolumab monotherapy.
Regarding safety, tremelimumab was well tolerated, with few patients experiencing grade 3 disabling adverse events, even in the presence of liver dysfunction among patients in the Child-Pugh B class. No patient received systemic steroids and there were no treatment-related deaths. An itching skin rash was the most frequent adverse event (65%), which was successfully managed with topic agents and oral antihistamine drugs. Diarrhea occurred in 30% of patients but reached grade 3 in only one patient. A remarkable rise in serum transaminases was observed after the first dose in more than half of the patients, being grade 3 or higher in 45% of cases but with no other signs of liver dysfunction. This effect on transaminases was transient, did not recur in the following cycles, and was not related to the antitumor or antiviral responses, or with changes in circulating cytokines.
Following the same path, a second trial tested a very appealing hypothesis i.e. whether an antigenic stimulation provided by means of incomplete tumor ablation using percutaneous radiofrequency (RFA) or transarterial chemoembolization (TACE) could safely enhance the effects of tremelimumab [17]. The rationale for this combination is based on the fact that RFA or TACE could induce immunogenic tumor cell death and this in turn could stimulate a peripheral systemic immune response that may be further amplified by immune checkpoint blockade. In a phase I/II trial, increasing doses of tremelimumab were given followed by subtotal tumor ablation and tumor response was evaluated in those lesions not targeted by RFA, cryoablation or TACE procedures. This was a pilot study with no specific sample size assumptions. It enrolled 32 patients with mostly advanced HCC (75% at BCLC C stage), 78% having progressed to previous therapies. Patient characteristics were therefore quite similar to the previous study except that liver function was preserved in the vast majority of patients, with only 14% of patients in Child-Pugh class B. Most patients (75%) had viral hepatitis as cause of liver cirrhosis.
Enrolled patients were treated this time with an optimal dose of tremelimumab at two dose levels (3.5 and 10 mg/kg IV) given every 4 weeks for a total of 6 doses, followed by 3-monthly infusions until off-treatment criteria were met. The interventional radiologic procedure (TACE for BCLC B and thermal ablation for BCLC C patients) was performed 5 weeks after first dose of tremelimumab. Nineteen patients were evaluable for response because they had measurable lesions that were not targeted by RFA or TACE. Of these patients, partial response was recorded in 5 patients (26%), and stable disease in 12 patients (63%), accounting for a disease control rate of 89%. Again, almost half (45%) of the stabilizations lasted longer than 6 months and median time to progression was 7.4 months (95%CI 4.7–9.4 months). Given the small number of patients in both tremelimumab trials, the small differences in response rates and time to progression seem of little relevance but provide a signal of the consistency of the antitumor effect. The better overall survival of 12.3 months (95%CI 9.3–15.4 months) in the combination trial could be explained on the basis of the good liver function but a true enhancing effect of prior ablation may not be ruled out. Regarding safety, one relevant observation was that there was no clear trend in adverse events across the different dose cohorts. The most common clinical toxicity was pruritus, although less frequent than in the previous trial (9%), and was predominantly grade 1. Less frequent side effects were diarrhea (6%), autoimmune pneumonitis (3%) and angioedema (3%). Again, the most frequent laboratory alteration was hypertransaminasemia, which occurred in 34% of patients and was grade 3 or 4 in 21% of them.
The encouraging signs of antitumor activity of tremelimumab in advanced HCC and its good safety profile in cirrhotic patients of viral etiology, provided a strong reason to test other checkpoints inhibitors [18]. The PD-L1/PD-1 pathway provides another mechanism of tumor-induced immune tolerance. PD-1 expression on effector phase CD8+T cells is increased in HCC patients compared to cirrhotic patients or healthy controls [19]. Indeed, HCC patients with higher numbers of tumor infiltrating and circulating PD-1+CD8+ T cells showed earlier and more frequent disease progression after hepatic resection. PD-L1 is also highly expressed on peritumoral stromal cells (Kupffer cells, LSEC, and monocytes) as well as cancer cells, promoting a PD-L1/PD-1 pathway-driven inhibition of antitumor T cell responses [20,21]. Thus, a strong rationale supports the use of PD-1 and PD-L1 blocking antibodies against HCC. Building on the experience with tremelimumab, a clinical trial has assessed the safety and clinical benefit of nivolumab (a fully human IgG4 monoclonal antibody targeting PD-1) as a first or second-line treatment in patients with advanced HCC across different etiologies (HCV infection, HBV infection, non-viral cirrhosis)[22].
The target population of the CheckMate 040 trial included patients with intermediate or advanced HCC and preserved liver function (Child-Pugh A) that were candidates to systemic therapy and had progressed or were intolerant to sorafenib or had refused this drug. First, a dose-escalation cohort of 48 patients received doses that ranged from 0.3mg/kg to 10 mg/kg every 2 weeks with the primary endpoint of establishing the safety and tolerability of nivolumab in HCC patients. Afterwards, the 3 mg/kg dose level was chosen for an expansion cohort of 214 patients in whom the primary endpoint was efficacy evaluated as objective response rate using RECIST 1.1 criteria. Patients in this expansion cohort were divided in four specific groups of uninfected patients progressing to sorafenib, uninfected patients naive or intolerant to sorafenib, patients with HCV infection and patients with HBV infection. In both cohorts, HBV-infected patients had to be on effective antiviral therapy (circulating v iral DNA < 100 UI/ml).
Contrary to the tremelimumab trials, this study recruited patients from Europe, Asia and America. Most were at the advanced BCLC stage C (88%), had extrahepatic metastases (68%), and had received prior systemic therapy (76%), mainly Sorafenib. Treatment was by and large well tolerated. Adverse events were observed at similar rates across dose levels and a maximal tolerated dose was not reached. The most frequent symptomatic adverse events in the large expansion cohort treated with 3 mg/kg were rash (23%), pruritus (21%) and diarrhea (13%), that were usually mild. Grade 3 or higher treatment-related symptomatic adverse events occurred in less than 2% of patients. Hypertransaminasemia was the most frequent laboratory alteration (20%) reached grade 3 or higher in only 5% of patients. Regarding etiologies, the rates of symptomatic treatment-related AEs were comparable in the uninfected and HCV- or HBV-infected cohorts. Overall, frequencies of grade 3/4 treatment-related AEs and treatment-related serious AEs overall were 20% and 7%, respectively, while no treatment-related deaths occurred. Immune related hepatitis needing steroid therapy occurred very rarely. Only 3% of patients discontinued nivolumab due to treatmentrelated adverse events and no treatment-related deaths were reported.
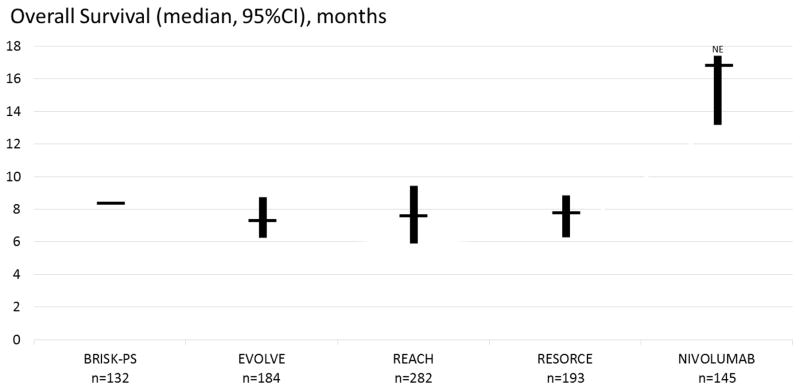
Convincing signs of efficacy were reported. In the escalation and expansion cohorts, objective tumor responses occurred in 15% and 20% of patients, respectively. They were meaningful, durable responses that lasted for a median of 17 months. An additional 45% of patients had stable disease that was frequently durable too, lasting more than 6 months in most cases. The majority of objective responses occurred during the first 3 months of treatment. It has to be stressed that response rates were similar across different etiologies, and both in sorafenib-naive and sorafenib-exposed patients. These signs of efficacy were consistent with the more recently reported median overall survival of 15.6 months (95%CI 13.2–18.9) in the population naive to sorafenib, and 28.6 months (95%CI 16.6–NE) in the population exposed to sorafenib (90% progressors) [23]. This median survival was observed irrespective of prior Sorafenib treatment, and compares well with any other phase 2 or 3 clinical trial of targeted agents including regorafenib, the first agent shown to prolong survival following sorafenib in a selected group of sorafenib-tolerant patients. Indeed, these results support nivolumab as a viable second-line therapy following Sorafenib (figure 2).
Figure 2.
Survival following Nivolumab among patients that progressed or are intolerant to Sorafenib. The survival reported in placebo-treated arms in large clinical trials addressing the second-line advanced HCC population is presented for comparison [2,22,50–52].
Results from correlative studies
The identification of prognostic markers, which will help identify patients, who benefit from a treatment with immune checkpoint inhibitors is an important point. Different experimental studies have already been conducted to better understand what and how HCC patients respond to treatment with checkpoint inhibitors. Here one aspect needs to be taken under consideration, which makes HCC patients distinct from other patients with cancer, namely the fact that the majority of HCC patients worldwide also suffer from chronic viral hepatitis.
Tremelimumab also has a significant antiviral effect. In the tremelimumab alone trial, a decrease in median HCV viral load from 3.78 × 105 IU/ml at day 0 to 3.02 × 104 IU/ml at day 120 (n = 11, p = 0.011), and 1.69 × 103 IU/ml at day 210 (n = 6, p = 0.017) was observed [16]. The progressive course of this decline in viral load was observed in most patients followed for at least 3 months, and three patients had a transient complete viral response during follow-up. The antiviral activity was confirmed in the tremelimumab plus ablation trial in which the HCV viral load of 14 quantifiable patients decreased after 3 months in 12 patients, with a median HCV viral load decrease from 1,275 × 103 UI/ml to 351 × 103 UI/ml [17]. In the first trial, the immunological origin of this viral response was supported by the fact that it was observed in 75% of patients with an immune response (defined as a >5-fold increase at any time in the sum of IFN-g-producing cells against viral antigen) versus 20% of patients with no immune response. Patients with an early decrease in IL-6 had a higher chance of having a viral response (100%) than those with increased values at that time (43%). The anti-tumoral effect was not associated to this antiviral effect or to patient characteristics including systemic inflammatory signals such as C reactive protein. The lack of repeated tumor biopsies precludes any interpretation of the mechanism behind the antitumor activity while the expansion in circulating Treg following tremelimumab therapy was in line with observations in other tumor types [24].
The second tremelimumab trial was enriched with important correlative studies. Peripheral blood CD3, CD4, CD8, CD38 and HLA-DR positive cells were counted after every cycle by multicolor flow cytometry. Tumor biopsies were obtained from some patients immediately before ablation (after 2 doses of tremelimumab). The number of cytotoxic T cells (CD3 and CD8 positive) was measured by immunohistochemistry in these samples and compared to archival samples obtained prior to enrollment. Interestingly, the number of peripheral activated CD4+ and CD8+ T cells increased after tremelimumab. Such increase was especially intense and sustained for CD8+T cells. Immune cell tumor infiltration was observed in all 12 patients in whom post-tremelimumab tumor samples could be evaluated. Among those 6 patients with paired tumor samples, an increase in both CD3+ and CD8+ cells was observed although the differences were not statistically significant, likely because of the small number of cases. Patients with objective remissions in non-ablated lesions had a higher posttremelimumab CD3+ and CD8+ infiltration compared to non-responders. Unfortunately, the effect of ablation on T-cell infiltration could not be evaluated and in the absence of a remarkable difference in patient outcomes, the synergy between TACE/RFA and CTLA-4 blockade remains an appealing hypothesis to be confirmed.
A comprehensive biomarker analysis has not yet been reported for the CheckMate 040 trial. Expression of PD-L1 prior to nivolumab was studied in fresh or archival tumor specimens. The rate was remarkably low. Even with a cut-off for positivity of 1% of tumor cells exhibiting membrane PD-L1 staining of any intensity, only 20% of 174 evaluable patients had PD-L1 positive tumors. Objective remissions occurred in 26% of PD-L1 positive patients and 19% of PD-L1 negative patients, making this marker unsuitable for patient selection. The more relevant rate of PD-L1 expression in tumor stromal cells and its association with response to nivolumab have not been reported yet.
Combination therapies
While clinical trials evaluating the safety and efficacy of anti CTLA-4 and anti-PD1 / PD-L1 are ongoing, different investigators have already initiated trials evaluating the combination of immune checkpoint inhibitors with other drugs or the combination of anti CTLA-4 with anti PD1 or PD-L1. Based on promising data in melanoma, these combinations are currently being tested mostly in the absence of any preclinical data. Combination therapies include combination of different checkpoint inhibitors, combination with oncolytic viruses, small molecules and ablative therapies. A summary of ongoing combination studies is presented in Table 3. These combinations may be based on the potential additive effect of a therapy with a treatment benefit that has been proven (TACE or sorafenib) or is being investigated (ramucirumab, cabozantinib). However, they should preferably be based on exploiting synergistic effects or avoiding primary resistance.
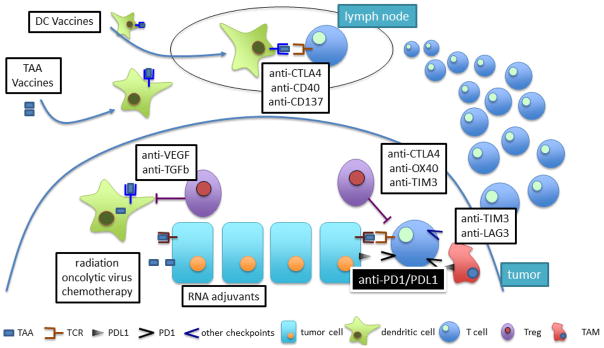
Hence, the understanding the mechanisms of resistance to anti-PD1/PDL1 therapies is important for the development of combination therapies (figure 3). It has been proposed that mechanisms promoting primary or acquired resistance are largely conserved, and must affect either tumor immunogenicity, antigen presentation and generation of effector T cells, contact of antigen and PDL1 by tumor-specific T cells, efficacy tumor cell killing, or the induction of immunological memory [25]. Although it may appear early to discuss HCC-specific resistance mechanisms to immune checkpoint blockade in the treatment of HCC one should not that primary and adaptive resistance mechanisms have been observed and described in melanoma patients [26]). One may expect to see similar mechanisms occurring in patients with HCC than the ones described in melanoma, where tumors have been found to mutate and become invisible to tumor-specific CTL responses by MHC downregulation or modulating the immediate tumor microenvironment. Interestingly, we observed in our study using anti-CTLA4 that viral load increased in patients at the time when tumors started to progress suggesting that not only tumor-specific T cells responses become weak over time leading to potential treatment failure.
Figure 3.
Strategies to increase the efficacy of anti-PD1/PDL1 blockade based on mechanism of action.
Tumors with a low mutation rate (very likely associated with fewer neaontigens) such as pancreas and prostate, are poorly immunogenic and basically resistant to anti-PD1 agents [27]. Hence, sensitivity to anti-PD-1 therapy would likely be enhanced by therapies that may contribute to release tumor antigens, including radiotherapy, virotherapy and some chemotherapies [28]. On the other hand, autologous cancer vaccination strategies that prime adaptive immune responses with TAAs can enhance the sensitivity to anti-PD1 therapy [29]. Neoantigen vaccination approaches [30] may work even better although they are currently limited by MHC restriction.
Antigen presentation and T cell stimulation can be enhanced in several ways. Promoting IFNγ release in the tumor microenvironment by intratumoral delivery of oncolytic virus (ref) or RNA adjuvants [31] may increase the expression of class I MHC, which is required for T cell antitumor responses and usually down-regulated by tumors. Since cytokines such as VEGF and TGF-β play a key role in the suppression of DC function in the tumor microenvironment, agents that neutralize their actions could work synergistically with anti-PD1/PDL1 therapy [32]. Agonistic monoclonal antibodies that target immunostimulatory molecules such as CD40 or CD137 may also improve the effector functions of DC, and their combination with anti-PD1 agents were synergistic in models of melanoma and other tumors [33] [34]. Finally, oncolytic viruses may enhance the activity of DC.
PDL1 is not the only immunosuppressive factor in the tumor microenvironment. Treg stand out among the immunosuppressive cells of the tumor niche. Elevated Foxp3+/CD8+ cell ratios are commonly associated with resistance to anti-PD1 therapy [35]. Anti-CTLA4 agents deplete tumor-associated Treg via an FcγR-dependant mechanism in preclinical models, and enhance the efficacy of tumor-specific T cell-mediated anti-tumor immunity [36]. The combination of anti-CTLA4 and anti-PD1 therapy is highly synergistic in experimental melanoma and results in the highest rates of objective remissions in patients with advanced melanoma (58% vs. 19% for anti-CTLA4 alone and 44% for anti-PD1 alone) [37]. This comes at the price of increased toxicity with 36% of patients with melanoma having to discontinue the combination in comparison with 7% of the patients receiving nivolumab monotherapy. Regarding liver toxicity, the proportion of patients with increased ALT was 3.8% for nivolumab, 3.9% for ipilimumab and 17.6% for the combination [38]. The results of the combination of these two checkpoint inhibitors in HCC are expected soon. Anti-OX40 monoclonal antibodies may also be relevant at selectively depleting tumor-associated Treg [39], and the combination with anti-PD1 is synergistic in animal models of cancer resistant to anti-PD1 therapy [40]. In combination with anti-PD1/PDL1 therapy, anti-TIM3 agents may help deplete Treg [41], and avoid T cell exhaustion. Indeed, anti-TIM3 or anti-LAG3 in combination with anti-PD1 have demonstrated synergistic effects in several pre-clinical models.
Finally, studies comparing immune cell infiltrates within tumors before and after treatment with anti-PD1 therapy showed that patients that responded poorly to therapy contained significantly fewer tumor-associated effector memory T cells than responsive patients [42]. Strategies that may expand this subset of T cells and protect them from exhaustion would result in promising combination therapies.
Cell based therapies
Different types of cell-based therapies are being tested for the treatment of patients with HCC. Most experience exist for the treatment with cytokine-induced killer cells (CIK). CIK are characterized by the coexpression of CD3 and CD56. They can be generated by expanding human peripheral blood mononuclear cells in the presence of interferon-γ (IFN-γ), anti-CD3 and IL-2. Lee and colleagues conducted a randomized controlled trial in 230 HCC patients the adjuvant setting (post-surgical resection, RFA and ethanol injection). They were able to demonstrate an increase in PFS from 30 to 44 months upon treatment with CIK [43]. A few much smaller studies tested the use of dendritic cells as a potential cancer vaccine in HCC. While no definite conclusions on the clinical efficacy of this approach can be drawn these type of treatments appear to be safe in general [44] [45]. More recently and mainly based on studies in hematological malignancies adoptive T cell therapies using genetically engineered T cells have gained a lot of interest. Two different approaches are currently being developed for patients with HCC. Autologous T cells are either being transduced with a chimeric antigen receptor (CAR) or T cell receptor (TCR). In either cases T cells recognize specific antigens to be expressed on tumors but not on healthy tissue. CARs enable highly specific targeting of antigen in an MHC-independent fashion. CARs are formed from a combination of antibody-derived or ligand-derived domains and TCR domains. In contrast TCR transduced T cells, which also recognize a specific antigenic peptide, are MHC restricted [46]. Glypican 3 is a target frequently used for antigen specific responses in HCC [47]. Preclinical data using a CAR T cells against Glypican 3 have been published [48] and two clinical trial using Glypican 3 targeting CAR T cell approaches have either already been started or are about to be launched (NCT02723942 NCT02932956). A few investigators also test AFP directed therapies [49], also one needs to point out that AFP can also be expressed on healthy tissue and it is not clear how tumor specific such therapy will be. In summary, the field of cancer immunotherapy for HCC has never been as exciting as it is now. Results from the first large randomized phase III trial are expected to be published in 2018. A number of different combination therapies are being evaluated and novel cell based therapies will hopefully be effective in this difficult to treat disease.
Key points.
In the past, attempts to enhance antitumor immune responses in hepatocellular carcinoma by vaccination strategies or with cytokine-induced killer cells have been too weak to produce significant and consistent clinical benefit.
Over the last decade, identification and increasing knowledge of the role of immune checkpoint molecules has fostered the development of a new class of therapeutic agents.
Monoclonal antibodies targeting CTLA-4 and PD-1 or PD-L1 can effectively help overcome the mechanisms of immune evasion in a wide spectrum of human cancers.
In patients with advanced hepatocellular carcinoma, immune stimulation by means of CTLA-4 blockade with Tremelimumab has provided strong signals of antitumor efficacy in pilot clinical trials.
Nivolumab, an agent that stimulates the immune response through PD-1 blockade, has shown unequivocal signs of efficacy in a large phase 2 trial that recruited mainly patients refractory or intolerant to the standard of care sorafenib.
PD-1 or PD-L1 blockade now serves as the backbone of a number of combination regimes that are being tested as first or second line therapies in phase 2 and phase 3 trials.
Acknowledgments
TFG is supported by the Intramural Research Program of the NIH, NCI. BS is supported by EC FP7 Project Cancer Vaccine development for Hepatocellular Carcinoma – HEPAVAC (Grant Nr. 602893); EC H2020 Project Immunology and Immunotherapy of cancer: strengthening the translational aspect - HepaMUT (Grant Nr. AC16/00165); and project PI16/01845, integrated in Plan Estatal de I+D+I 2013–2016 and co-financed by ISCIII-Subdireccion General de Evaluacion y Fomento de la investigacion and Fondo Europeo de Desarrollo Regional (FEDER).
Footnotes
Conflicts of Interest
Bruno Sangro has received consulting and/or lecture fees from Adaptimmune, Astra Zeneca, Bayer Healthcare, Bristol-Myers-Squibb, Medimmune and Onxeo.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 3.Greten TF, Manns MP, Korangy F. Immunotherapy of hepatocellular carcinoma. J Hepatol. 2006;45:868–78. doi: 10.1016/j.jhep.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Greten TF, Manns MP, Korangy F. Immunotherapy of HCC. Rev Recent Clin Trials. 2008;3:31–9. doi: 10.2174/157488708783330549. [DOI] [PubMed] [Google Scholar]
- 5.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy Science. 2013;342:1432–3. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 6.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nature Reviews Gastroenterology & Hepatology. 2015;12:681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 7.Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the Generation Z of Negative Checkpoint Regulators. Front Immunol. 2015;6:418. doi: 10.3389/fimmu.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 9.Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59:567–79. doi: 10.1002/hep.26694. [DOI] [PubMed] [Google Scholar]
- 10.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 11.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res. 2014;2:393–8. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3--potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 13.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–8. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–9. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 16.Sangro B, Gomez-Martin C, la Mata de M, Inarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–8. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–51. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprinzl MF, Galle PR. Current progress in immunotherapy of hepatocellular carcinoma. J Hepatol. 2017;66:482–4. doi: 10.1016/j.jhep.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Shi F, Shi M, Zeng Z, Qi R-Z, Liu Z-W, Zhang J-Y, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–96. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 20.Gao Q, Wang X-Y, Qiu S-J, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 21.Wang B-J, Bao J-J, Wang J-Z, Wang Y, Jiang M, Xing M-Y, et al. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17:3322–9. doi: 10.3748/wjg.v17.i28.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.el-khoueiry AB, Sangro B, yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017 doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crocenzi TS, el-khoueiry AB, yau T, Melero I, Sangro B, Kudo M, et al. Nivolumab in Sorafenib-Naive and - Experienced Patients With Advanced Hepatocellular Carcinoma: CheckMate 040 Study. American Society of Clinical Oncology. 2017 doi: 10.1016/j.annonc.2023.12.008. n.d. [DOI] [PubMed] [Google Scholar]
- 24.Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–83. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Donnell JS, Long GV, Scolyer RA, Teng MWL, Smyth MJ. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treatment Reviews. 2017;52:71–81. doi: 10.1016/j.ctrv.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168:707–23. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 28.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emens LA. Cancer vaccines: on the threshold of success. Expert Opin Emerg Drugs. 2008;13:295–308. doi: 10.1517/14728214.13.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buonaguro L HEPAVAC Consortium. Developments in cancer vaccines for hepatocellular carcinoma. Cancer Immunol Immunother. 2016;65:93–9. doi: 10.1007/s00262-015-1728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bald T, Landsberg J, Lopez-Ramos D, Renn M, Glodde N, Jansen P, et al. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov. 2014;4:674–87. doi: 10.1158/2159-8290.CD-13-0458. [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5:2963–70. [PubMed] [Google Scholar]
- 33.Zippelius A, Schreiner J, Herzig P, Muller P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol Res. 2015;3:236–44. doi: 10.1158/2326-6066.CIR-14-0226. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Paulete AR, Cueto FJ, Martinez-Lopez M, Labiano S, Morales-Kastresana A, Rodriguez-Ruiz ME, et al. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov. 2016;6:71–9. doi: 10.1158/2159-8290.CD-15-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngiow SF, Young A, Jacquelot N, Yamazaki T, Enot D, Zitvogel L, et al. A Threshold Level of Intratumor CD8+ T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1. Cancer Res. 2015;75:3800–11. doi: 10.1158/0008-5472.CAN-15-1082. [DOI] [PubMed] [Google Scholar]
- 36.Wolchok JD, Saenger Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. The Oncologist. 2008;13(Suppl 4):2–9. doi: 10.1634/theoncologist.13-S4-2. [DOI] [PubMed] [Google Scholar]
- 37.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92:475–80. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 40.Guo Z, Wang X, Cheng D, Xia Z, Luan M, Zhang S. PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS ONE. 2014;9:e89350. doi: 10.1371/journal.pone.0089350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, et al. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol Res. 2016;4:194–203. doi: 10.1158/2326-6066.CIR-15-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Lee J-H, Lim Y-S, Yeon JE, Song T-J, Yu SJ, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 44.Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, la Rocha de P, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817–25. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]
- 45.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, et al. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2008;49:124–32. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 46.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–81. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho M, Kim H. Glypican-3: a new target for cancer immunotherapy. Eur J Cancer. 2011;47:333–8. doi: 10.1016/j.ejca.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20:6418–28. doi: 10.1158/1078-0432.CCR-14-1170. [DOI] [PubMed] [Google Scholar]
- 49.Sun L, Guo H, Jiang R, Lu L, Liu T, He X. Engineered cytotoxic T lymphocytes with AFP-specific TCR gene for adoptive immunotherapy in hepatocellular carcinoma. Tumour Biol. 2016;37:799–806. doi: 10.1007/s13277-015-3845-9. [DOI] [PubMed] [Google Scholar]
- 50.Llovet JM, Decaens T, Raoul J-L, Boucher E, Kudo M, Chang C, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. Journal of Clinical Oncology. 2013;31:3509–16. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 51.Zhu AX, Kudo M, Assenat E, Cattan S, Kang Y-K, Lim HY, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. Jama. 2014;312:57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 52.Zhu AX, Park JO, Ryoo B-Y, Yen C-J, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. The Lancet Oncology. 2015;16:859–70. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]