Abstract
Objective
Mounting evidence indicates that stress influences the experience of pain. Exposure to psychosocial stress disrupts bi-directional communication pathways between the central nervous system and peripheral immune system, and can exacerbate the frequency and severity of pain experienced by stressed subjects. Repeated social defeat (RSD) is a murine model of psychosocial stress that recapitulates the immune and behavioral responses to stress observed in humans, including activation of stress-reactive neurocircuitry and increased pro-inflammatory cytokine production. It is unclear, however, how these stress-induced neuroimmune responses contribute to increased pain sensitivity in mice exposed to RSD. Here we used a technique of regional analgesia with local anesthetics in mice to block the development of mechanical allodynia during RSD. We next investigated the degree to which pain blockade altered stress-induced neuroimmune activation and depressive-like behavior.
Methods
Following development of a mouse model of regional analgesia with discrete sensory blockade over the dorsal-caudal aspect of the spine, C57BL/6 mice were divided into experimental groups and treated with Ropivacaine (0.08%), Liposomal Bupivacaine (0.08%), or Vehicle (0.9% NaCl) prior to exposure to stress. This specific region was selected for analgesia because it is the most frequent location for aggression-associated pain due to biting during RSD. Mechanical allodynia was assessed 12 hours after the first, third, and sixth day of RSD after resolution of the sensory blockade. In a separate experiment, social avoidance behavior was determined after the sixth day of RSD. Blood, bone marrow, brain, and spinal cord were collected for immunological analyses after the last day of RSD in both experiments following behavioral assessments.
Results
RSD increased mechanical allodynia in an exposure-dependent manner that persisted for at least one week following cessation of the stressor. Mice treated with either Ropivacaine or Liposomal Bupivacaine did not develop mechanical allodynia following exposure to stress, but did develop social avoidance behavior. Neither drug affected stress-induced activation of monocytes in the bone marrow, blood, or brain. Neuroinflammatory responses developed in all treatment groups, as evidenced by elevated IL-1β mRNA levels in the brain and spinal cord after RSD.
Conclusions
In this study, psychosocial stress was associated with increased pain sensitivity in mice. Development of mechanical allodynia with RSD was blocked by regional analgesia with local anesthetics, Ropivacaine or Liposomal Bupivacaine. Despite blocking mechanical allodynia, these anesthetic interventions did not prevent neuroimmune activation or social avoidance associated with RSD. These data suggest that stress-induced neuroinflammatory changes are not associated with increased sensitivity to pain following RSD. Thus, blocking peripheral nociception was effective in inhibiting enhanced pain signaling without altering stress-induced immune or behavioral responses.
Introduction
Numerous clinical studies indicate that psychological stress increases susceptibility to experience pain and exacerbates existing pain (Ashkinazi and Vershinina 1999, Turner, Jensen et al. 2002, Greco, Rudy et al. 2004, Nicholson and Martelli 2004, DeLeo 2006). Unfortunately, it is unclear exactly how pain is initiated or amplified by stress. Clinical and preclinical data indicate that enhanced neuroimmune activation elicits adaptive changes in the nervous system that can contribute to exaggerated pain sensation (Maier and Watkins 2003, Ji and Strichartz 2004, Tsuda, Inoue et al. 2005, Campbell and Meyer 2006). For example, activation of peripheral nociceptors initiates central immune signaling that enhances neuronal excitability, leading to increased pain sensitivity (Griffis 2011). Inflammatory mediators including IL-1β play a critical role in driving this response (Maier and Watkins 2003, Tsuda, Inoue et al. 2005, Griffis 2011). For instance, IL-1β has been shown to directly modulate excitatory synaptic transmission at central terminals, which is associated with enhanced pain responses (Kawasaki, Zhang et al. 2008, Yan and Weng 2013, Grace, Hutchinson et al. 2014). Furthermore, in response to noxious stimuli, peripheral immune cells have the capacity to traffic to the central nervous system (CNS) and promote an inflammatory environment, thus leading to exaggerated pain responses (Milligan and Watkins 2009, Grace, Rolan et al. 2011). Therefore, activation of pain pathways is associated with enhanced neuroimmune signaling, which may underlie the pathophysiology of exaggerated pain symptoms.
Related to these findings, psychosocial stress disrupts homeostatic communication pathways between the CNS and peripheral innate immune system, leading to dysregulated and heightened neuroinflammation (Wohleb, McKim et al. 2014, Reader, Jarrett et al. 2015, Weber, Godbout et al. 2017). Repeated social defeat (RSD) is a rodent model of psychosocial stress that recapitulates many of the human immune and behavioral responses to stress (Wohleb, McKim et al. 2014, Reader, Jarrett et al. 2015, Weber, Godbout et al. 2017). For example, exposure to RSD increases the production and release of Ly6Chi monocytes into circulation that exhibit a pro-inflammatory gene expression profile similar to that observed in CD14+/CD16− peripheral monocytes found in chronically stressed humans (Powell, Sloan et al. 2013). Furthermore, RSD promotes the recruitment of Ly6Chi monocytes to stress-responsive brain regions, where they differentiate into macrophages and propagate inflammatory signaling (Wohleb, Powell et al. 2013). Notably, these neuroimmune responses are associated with the development of anxiety-like behavior and social avoidance following stress (Ramirez, Niraula et al. 2016, McKim, Weber et al. 2017).
It is apparent that exposure to either psychosocial stress or painful stimuli is associated with enhanced neuroimmune signaling and increased production of inflammatory mediators that ultimately result in behavioral alterations. Therefore, understanding the mechanism that mediates the relationship between stress and the development of an altered response to pain may lead to novel therapeutic strategies for the management of human chronic pain states. Ropivacaine and Liposomal Bupivacaine are long-lasting local anesthetics that are extensively used for intraoperative anesthesia and postoperative analgesia (Kuthiala and Chaudhary 2011). The role of local anesthetics in modulating immune function has been previously described (Colucci, Puig et al. 2013), but the effect of local anesthetics on stress-induced pain sensitization and neuroimmune activation is unknown.
Here we developed a model of regional analgesia in mice using repeated, low-volume, subcutaneous injections of Ropivacaine or Liposomal Bupivacaine to block sensation of pain during repeated social stress. We assessed the effects of Ropivacaine and Liposomal Bupivacaine on the development of mechanical allodynia and social avoidance in mice following exposure to RSD. Furthermore, we determined whether either drug treatment affected neuroimmune responses to stress, including increased Ly6Chi monocytes in circulation, enhanced myelopoiesis in the bone marrow, recruitment of brain macrophages, and augmented IL-1β expression in the brain and spinal cord. Taken together, our results show that blocking peripheral nociception is effective in preventing increased pain signaling without blocking immune or behavioral responses associated with stress.
Methods
Mice
Male C57BL/6 (6–8 weeks old) and male CD-1 (12 months, retired breeders) mice were purchased from Charles River Breeding Laboratories (Wilmington, MA), and allowed to acclimate to their surroundings for 7–10 days prior to experiments. Resident C57BL/6 mice were housed in cohorts of three and aggressor CD-1 mice were individually housed. All mice were housed in 11.5″× 7.5″ × 6″ polypropylene cages. Rooms were maintained at 21°C under a 12-h light-dark cycle (lights on at 0600) with ad libitum access to water and rodent chow. All procedures were in accordance with the National Institutes of Health Guidelines and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
Repeated Social Defeat
Mice were subjected to repeated social defeat (RSD) stress as previously described (McKim, Weber et al. 2017). In brief, an aggressive male intruder CD-1 mouse was introduced into cages of established male cohorts (3 per cage) of C57BL/6 mice for 2 hours between 17:00 and 19:00 for six consecutive nights. During each cycle, submissive behavior (e.g., upright posture, fleeing, and crouching) was observed to ensure defeat of the resident mice. A new intruder was introduced if an attack on the resident mice was not initiated within the first 5–10 minutes, or if the intruder was defeated by any of the resident mice. At the end of the 2 h period, the intruder was removed and the residents were left undisturbed until the following day when the paradigm was repeated. To avoid habituation, different intruders were used on consecutive nights. As described previously in studies with RSD, inter-male aggression observed during each cycle resulted in minor tissue damage inflicted by the intruder mouse. The mice were monitored at least twice daily for any indication of distress or illness. Mice that were injured or moribund were removed from the study. Consistent with previous studies using RSD (Sawicki, McKim et al. 2015, McKim, Niraula et al. 2016, McKim, Patterson et al. 2016), less than 5% of mice met the early removal criteria. Control mice were left undisturbed in their home cages. All social behavior and biological measures were obtained 12 h after the final cycle. This time point was selected because sympathetic nervous system and hypothalamic-pituitary-adrenal axis activation returns to baseline by 12 hours after the final cycle (Wohleb, Powell et al. 2013).
Pain Behavior
Tactile mechanical sensitivity was analyzed by measuring threshold responses to a calibrated von Frey rigid tip (IITC Life Science Inc., Woodland Hills, CA). Mice were placed on a mesh platform in a clear compartment (8 cm × 12 cm × 5.5 cm) that allows unrestrained exploration, locomotion, and grooming. The rigid tip was applied to the mid-line of the plantar surface of the right hind paw to determine the smallest force that repeatedly elicits withdrawal of the hind paw from the tip. A lower withdrawal threshold in grams (g) is indicative of increased pain sensitivity or mechanical allodynia. Baseline measurements were performed 24 h prior to RSD exposure. Subsequent behavioral testing for mechanical allodynia was completed 12 h after the first, third, and final day of RSD (n = 6 per group).
Social Interaction
Social avoidance was determined as previously described (McKim, Weber et al. 2017). In Trial 1 (empty), an experimental mouse was placed into the arena with an empty wire mesh cage, and activity was recorded for 2.5 min. In Trial 2 (social), an unfamiliar CD-1 mouse was placed in the wire mesh cage, the experimental mouse was placed in the arena, and activity was recorded for 2.5 min. Activity in the social avoidance behavior test was video recorded and analyzed using Noldus EthoVision XT Software. Behavioral testing for social avoidance was determined in a separate experiment 12 hours after the sixth day of RSD (n = 6 per group).
Ropivacaine Treatment
0.2% Ropivacaine (NAROPIN, Fresenius Kabi USA) was diluted in saline (0.9% NaCl) for a final concentration of 0.08%. A total of 400 microliters of Ropivacaine was administered immediately prior to each RSD exposure through two to four injections with a 26G needle over the dorsal-caudal aspect of the spine. This dose of Ropivacaine prevented vocalization in response to repeated electric shocks delivered to the insensate area, thus indicating successful sensory blockade. This region was selected because it is the most frequent location for aggression-associated pain due to biting during RSD.
Liposomal Bupivacaine Treatment
1.3% Liposomal Bupivacaine (EXPAREL, Pacira Pharmaceuticals, Inc.) was diluted in saline (0.9% NaCl) for a final concentration of 0.08%. A total of 400 microliters of Liposomal Bupivacaine was administered immediately prior to each RSD exposure through two to four injections with a 21G needle over the dorsal-caudal aspect of the spine. This dose of Liposomal Bupivacaine prevented vocalization in response to repeated electric shocks delivered to the insensate area, thus indicating successful sensory blockade.
Behavioral Assessments for Sensory and Motor Blockade
Animals were exposed to a standard train-of-four stimulus (70 mV applied through 22G 50 mm stimulating needles (Pajunk®, Norcross, GA) applied to the dorsal-caudal aspect of the spine. To ensure successful blockade of nociceptive responses and duration of pain blockade, lack of vocalization was confirmed with noxious electrical stimuli during model development for each drug. Confirmation of motor activity was determined following six consecutive days of injections (saline, Ropivacaine, Liposomal Bupivacaine) using the open field testing apparatus. In brief, mice were placed in the center of the test apparatus (40 × 40 × 25 cm Plexiglas box) and activity was recorded for 5 min. Locomotor activity and total distance traveled were recorded and analyzed using an automated system (VersaMax, AccuScan Instruments, Omnitech Electronics Inc., Columbus, OH) as previously described (Lieblein-Boff, McKim et al. 2013) (n = 6 per group).
Isolation of Cells from Bone Marrow and Blood
Tissues were collected immediately following CO2 asphyxiation. Whole blood was collected with EDTA-lined syringes by cardiac puncture and red blood cells were lysed. Bone marrow was collected from the femur and flushed out with ice-cold HBSS. Tissue samples were washed with HBSS, filtered through a 70-μm nylon cell strainer, and then the total number of cells was determined with a BD Coulter Particle Count and Size Analyzer (Beckman Coulter, Brea, CA) (n = 6 per group).
Isolation of CD11b+ Cells from Brain and Spinal Cord
CD11b+ cells were isolated from whole brain homogenates as previously described (McKim, Weber et al. 2017). In brief, brains and spinal cords were passed through a 70-μm nylon cell strainer and centrifuged at 600 × g for 6 min. Supernatants were removed and cell pellets were re-suspended in 70% isotonic Percoll (GE-Healthcare). A discontinuous Percoll density gradient was layered as follows: 50, 35, and 0% isotonic Percoll. The gradient was centrifuged for 20 min at 2000 × g and cells were collected from the interphase between the 70 and 50% Percoll layers. These cells were referred to as enriched CD11b+ cells based on previous studies demonstrating that viable cells isolated by Percoll density gradient yields >90% CD11b+ cells (Wohleb, Powell et al. 2013) (n = 6 per group).
Flow Cytometry
Labeling of cell surface antigens was performed as previously described (McKim, Weber et al. 2017). In brief, Fc receptors were blocked with anti-CD16/CD32 antibody (eBioscience; catalog number 553142). Cells were washed and then incubated with the appropriate antibodies (CD45, CD11b eBioscience; Ly6C, BD Biosciences) for 1 h at 4°C. Cells were washed and then re-suspended in FACS buffer for analysis. Cell numbers were estimated using counting beads (BD Biosciences). Nonspecific binding was assessed using isotype-matched antibodies. Antigen expression was determined using a Becton-Dickinson FACSCalibur four-color cytometer (BD Biosciences). Data were analyzed using FlowJo software (Tree Star) and positive labeling for each antibody was determined based on isotype stained controls (n = 6 per group).
RNA Isolation and Real-Time PCR
For brain tissue analyses, a 1-mm coronal brain section that included the cortex, hippocampus, striatum, and hypothalamus was removed and immediately flash frozen in liquid nitrogen. For spinal cord analyses, the cervical, thoracic, and lumbar regions were dissected from the spinal cord and immediately flash frozen in liquid nitrogen. RNA was isolated using tri-reagent/isopropanol precipitation, and RNA concentration was determined by NanoPhotomtery (Implen, Munich, Germany). RNA (1.2 µg) was reverse transcribed to cDNA using an RT-RETROscript kit (Ambion, ThermoFisher, Waltham, MA). For Percoll-enriched microglia, the PrepEase kit (USB, CA) was used to isolate RNA according to the manufacturer’s instructions. Real-time quantitative PCR was performed using the Applied Biosystems Assay-on-Demand Gene Expression protocol (Foster City, CA). Experimental cDNA was amplified by real-time PCR where a target cDNA and reference cDNA (glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (FAM) and a 3′ quencher dye (non-fluorescent quencher). Florescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems). Relative gene expression was analyzed using the ΔΔCT method and results were expressed as fold difference from GAPDH (n = 6 per group).
Statistical Analysis
All data are expressed as treatment means ± standard error of the mean (SEM). Individual data points more than two standard deviations above and below the mean were counted as outliers, and were excluded in the subsequent analyses. To determine significant main effects and interactions between main factors, data were analyzed using one- or two-way ANOVA using GraphPad Prism Statistical Software (San Diego, CA). In the event of a main effect of experimental treatment, differences between group-means were evaluated by an F-protected t-test. Post hoc analyses are graphically presented in figures. Threshold for statistical significance was set at p<0.05.
Results
Repeated social defeat caused social avoidance and mechanical allodynia
Previous studies indicate that RSD is associated with the development of depressive-like behavior, as measured by social avoidance (Ramirez, Niraula et al. 2016, McKim, Weber et al. 2017). Here mice were exposed to six days of RSD and social avoidance behavior was determined 12 hours after the final day of stress. Social avoidance was determined using an interaction paradigm with a social target as described previously (Ramirez, Niraula et al. 2016, McKim, Weber et al. 2017). Time spent in the interaction zone was significantly decreased in mice exposed to RSD when an unfamiliar CD-1 mouse was introduced in the social trial (Figure 1A, p<0.05). Furthermore, time spent in the corners was significantly increased in mice exposed to RSD during the social trial (Figure 1B, p<0.05). Taken together, these results confirm previous findings (McKim, Weber et al. 2017) and demonstrate that six days of RSD promoted the development of social avoidance behavior. The next objective was to determine whether resident mice develop increased pain sensitivity following exposure to RSD. In a separate experiment, mice were exposed to six days of RSD and mechanical allodynia was assessed 12 hours after the first, third, and sixth day of stress (Figure 1C). To determine whether mechanical allodynia persisted over time, behavior was also evaluated one week following termination of the stressor. Mechanical allodynia was quantified by measuring threshold responses to a calibrated von Frey rigid tip. Prior to stress, each group exhibited comparable baseline withdrawal thresholds. Exposure to RSD induced mechanical allodynia throughout the testing period (Stress × Time Interaction; F(4, 90)=6.014, p=0.0002). Post hoc analysis revealed increased mechanical allodynia after three days of RSD that was further increased by six days (Figure 1C, p<0.05). Notably, mechanical allodynia associated with RSD persisted for at least one week after stress cessation (Figure 1C, p<0.05). These findings demonstrated that RSD was associated with increased pain sensitivity that persisted for at least a week after cessation of stressor.
Figure 1. Repeated social defeat caused mechanical allodynia and social avoidance.
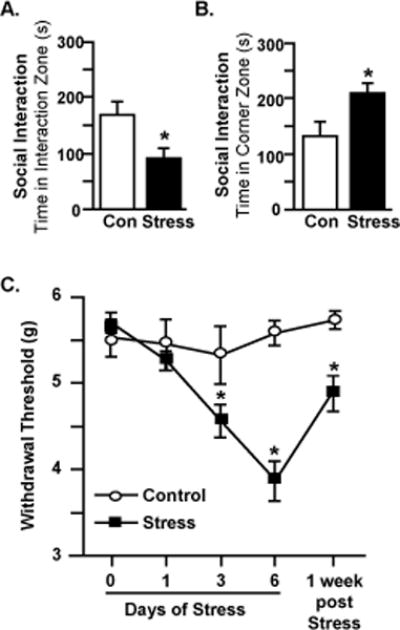
Male C57BL/6 mice were subjected to six days of repeated social defeat (Stress) or left undisturbed as controls (Con). Mice were tested for social avoidance 12 hours after the sixth day of stress. Time spent in the A) interaction zone and B) corner zone are shown. In a separate experiment, mice were tested for mechanical allodynia prior to stress exposure and 12 hours after the first, third, and sixth day of RSD. Additionally, mice were tested for mechanical allodynia one week following termination of stress. C) Withdrawal threshold of mechanical stimulation to the hind paw using the von Frey behavior test. Bars represent average ± SEM. Means with (*) are significantly different from Con (p<0.05).
Ropivacaine and Liposomal Bupivacaine blocked sensory processing without affecting motor behavior
A model of regional analgesia was developed in C57BL/6 mice using repeated low-volume subcutaneous injections of Ropivacaine (0.08%) and Liposomal Bupivacaine (0.08%). In this experimental design, mice received either vehicle or drug every day prior to stress exposure (Figure 2A). In a subset of animals, localized analgesia and duration (2 and 8 hrs for Ropivacaine and Liposomal Bupivacaine, respectively) was confirmed by absence of vocalization (i.e., chirping sounds) to noxious electrical stimulus (Figures 2B&C). Locomotion testing in animals receiving drug or vehicle immediately after the blocks were placed confirmed no compromise in motor function after successful sensory blockade (data not shown). Locomotion testing was repeated after six daily injections of local anesthetic and did not affect locomotor activity or total distance travelled in the open-field testing apparatus (Figures 2D&E). Moreover, six consecutive days of RSD plus daily drug injections did not affect total distance travelled (Figures 2F&G). Representative open-field plots confirming motor activity are shown for Vehicle, Ropivacaine and Liposomal Bupivacaine (Figure 2H). Collectively, these results verify that Ropivacaine and Liposomal Bupivacaine block sensory processing without affecting motor behavior.
Figure 2. Ropivacaine and Liposomal Bupivacaine blocked sensory processing without affecting motor behavior.
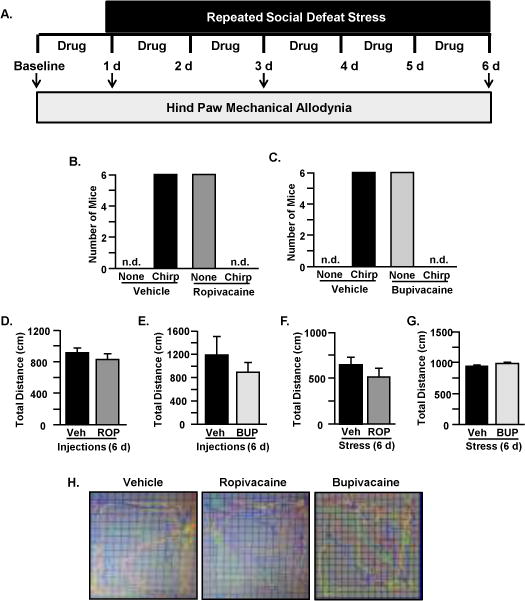
A) Experimental Design is shown. Male C57BL/6 mice were tested for mechanical allodynia prior to exposure to stress (Baseline) and 12 hours after the first, third, and sixth day of stress. Either Ropivacaine or Liposomal Bupivacaine (Drug) was injected prior to each stress exposure. To confirm blockade of sensory processing, lack of vocalization (i.e., no chirping) to electrical noxious stimuli was measured in mice treated with B) Ropivacaine and C) Liposomal Bupivacaine. Total distance traveled was not affected by six days of injections with D) Ropivacaine or E) Liposomal Bupivacaine. Furthermore, total distance was not affected by six days of stress and injections with F) Ropivacaine or G) Liposomal Bupivacaine. H) Representative open-field plots of mice injected with Vehicle, Ropivacaine, and Liposomal Bupivacaine are shown to confirm motor activity. Bars represent average ± SEM.
Ropivacaine analgesia prevented mechanical allodynia during repeated social defeat without affecting immune and behavioral responses to stress
To determine whether local analgesia during RSD would prevent pain sensitization, Ropivacaine or vehicle was administered every day prior to stressor exposure. Mechanical allodynia was measured by the von Frey behavior test and analyzed by two-way ANOVA at baseline and 12 hours after the first, third, and final day of stress (Figure 3A). Mechanical allodynia was observed after one day of RSD (main effect of Stress; F(1, 19)=80.43, p<0.0001). Similar to Figure 1, mechanical allodynia continued to be observed following three and six days of RSD exposure. Notably, development of mechanical allodynia with RSD was reversed by Ropivacaine after three days of RSD (Stress × Drug Interaction; F(1, 19)=18.21, p=0.0004) and six days of RSD (Stress × Drug Interaction; F(1, 19)=152.3, p<0.0001). Therefore, stressed subjects provided Ropivacaine analgesia maintained normal pain sensitivity thresholds while mice without analgesia developed abnormal pain responses in a dose-dependent manner following RSD.
Figure 3. Ropivacaine prevented mechanical allodynia during repeated social defeat without affecting immune and behavioral responses to stress.
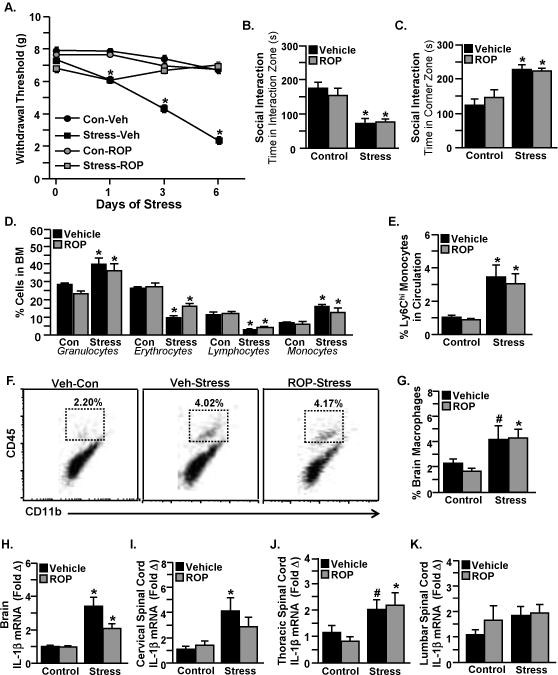
Male C57BL/6 mice were administered Ropivacaine (ROP) or Vehicle and subjected to six days of repeated social defeat (Stress) or left undisturbed as controls (Con). Mice were tested for mechanical allodynia prior to RSD exposure and 12 hours after the first, third, and sixth day of stress. A) Withdrawal threshold of mechanical stimulation to the hind paw using the von Frey behavior test. In a separate experiment, mice were tested for social avoidance 12 hours after the sixth day of stress. Time spent in the B) interaction zone and C) corner zone are shown. Following behavioral testing, samples (blood, bone marrow, brain, spinal cord) were collected for cell or mRNA analyses. D) Quantification of granulocytes, erythrocytes, lymphocytes, and monocytes in the bone marrow. E) Percentage of Ly6Chi monocytes in circulation. F) Representative flow bivariate dot plots of CD11b and CD45 labeling of Percoll-enriched cells from the brain. G) Percentage of CD11b+/CD45hi monocytes/macrophages in the brain. IL-1β mRNA levels in the H) brain, I) cervical spinal cord, J) thoracic spinal cord, and K) lumbar spinal cord are shown. Bars represent average ± SEM. Means with (*) are significantly different from (p<0.05), and means with (#) tended to be different (p<0.1) from the corresponding control mice, according to F-protected post analysis.
As shown in Figures 1A&B and in previous studies (Ramirez, Niraula et al. 2016, McKim, Weber et al. 2017), RSD promotes depressive-like behavior that can be measured in the social avoidance behavior test. Therefore, we determined whether stress-induced social avoidance behavior could be prevented in mice exposed to RSD with Ropivacaine. In a separate experiment, mice were exposed to six days of RSD and social avoidance behavior was determined 12 hours after the final day of stress using an interaction paradigm with a social target. There was a main effect of stress on time spent in the interaction zone (main effect of Stress; F(1, 19)=24.76, p<0.0001). Post hoc analysis revealed that there was no difference in the time spent in the interaction zone in mice exposed to RSD and treated with vehicle or Ropivacaine when an unfamiliar CD-1 mouse was introduced in the social trial (Figure 3B, p<0.05). Furthermore, there was a main effect of stress on time spent in the corner zone (main effect of Stress; F(1,19)=24.76, p<0.0001). Post hoc analysis revealed that there was no difference in the time spent in the corners in mice exposed to RSD and treated with Vehicle or Ropivacaine during the social trial (Figure 3C, p<0.05).
Previous studies indicate that RSD enhances myelopoiesis and promotes the production and egress of monocytes from the bone marrow (Engler, Bailey et al. 2004). Notably, monocytes derived from the bone marrow that are released into circulation can traffic to the brain and influence behavior (Powell, Sloan et al. 2013). Thus, we determined whether Ropivacaine affected stress-induced alterations in hematopoiesis in the bone marrow (Figure 3D) and the release of monocytes into circulation (Figure 3E). Mice were exposed to six days of RSD and samples (bone marrow and blood) were collected 14 hours after the final day of stress for flow cytometry analyses. RSD increased the production of granulocytes (main effect of Stress; F(1, 42)=17.29, p=0.0002) and monocytes (main effect of Stress; F(1, 41)=54.70, p<0.0001) that was not affected by Ropivacaine. Furthermore, Ropivacaine had no effect on the RSD-induced reduction in erythrocytes (main effect of Stress; F(1, 42)=68.21, p<0.0001) and lymphocytes (main effect of Stress; F(1, 42)=46.02, p<0.0001). Consistent with these results, stressed mice showed increased Ly6Chi monocytes in circulation regardless of Ropivacaine treatment (main effect of Stress; F(1, 42)=21.62, p<0.0001). Taken together, these findings indicate that Ropivacaine did not affect the peripheral immune responses to RSD.
We previously showed that RSD promoted the trafficking of bone marrow-derived monocytes to the brain to augment inflammatory cytokine signaling (Wohleb, McKim et al. 2014, Reader, Jarrett et al. 2015, Weber, Godbout et al. 2017). Therefore, we next determined the effects of Ropivacaine on monocyte trafficking to the brain and neuroinflammatory signaling. Here mice were exposed to six days of RSD and samples (brain and spinal cord) were collected 14 hours after the last day of stress for enumeration of monocytes by flow cytometry, or pro-inflammatory cytokine gene expression by PCR analyses. Figure 3F shows representative bivariate dot plots of CD11b and CD45 labeling for brain macrophages (CD11b+/CD45high) for the different experimental groups. As shown in Figure 3G, Ropivacaine did not alter the RSD-induced recruitment of brain monocytes (main effect of stress; F(1, 41)=9.228, p=0.0041). We recently demonstrated that monocyte-derived IL-1β is a key cytokine response that links immune activation to behavioral alterations during stress (McKim, Weber et al. 2017). Therefore, we determined whether Ropivacaine attenuated the gene expression of IL-1β in the brain (Figure 3H). RSD increased brain IL-1β mRNA expression that was not affected by Ropivacaine (main effect of stress; F(1, 41)=29.37, p<0.0001). Although Ropivacaine did not alter inflammatory signaling in the brain, it was plausible that spinal cord inflammation was affected. Therefore, we next determined mRNA levels of IL-1β in the cervical, thoracic, and lumbar regions of the spinal cord (Figures 3I–K). RSD increased IL-1β mRNA expression in the cervical spinal cord (main effect of Stress; F(1, 11)=7.293, p=0.0206) and thoracic spinal cord (main effect of Stress; F(1, 18)=8.758, p=0.0084) that was not attenuated by Ropivacaine. IL-1β mRNA levels in the lumbar spinal cord remained unchanged during RSD. Collectively, these results demonstrate that central immune responses to stress remain intact in mice treated with Ropivacaine.
Liposomal Bupivacaine analgesia prevented mechanical allodynia during repeated social defeat without altering stress-induced immune and behavioral responses
Many of the innate immune responses to RSD (i.e., release of monocytes into circulation and trafficking of monocytes to the brain) persist for longer than two hours (Wohleb, McKim et al. 2014, Reader, Jarrett et al. 2015, Weber, Godbout et al. 2017). Since Ropivacaine inhibits sensory processing in C57BL/6 mice for approximately two hours (i.e., during the duration of RSD), it was plausible that a longer-acting pain blockade would be more effective in altering stress-induced neuroimmune activation. Therefore, we developed a model of regional analgesia using the local anesthetic Liposomal Bupivacaine, which blocks sensory processing in C57BL/6 mice for up to eight hours. Liposomal Bupivacaine or vehicle was administered every day prior to stress exposure and mechanical allodynia was assessed 12 hours after the first, third, and final day of stress (Figure 4A). As described above, here we again ran a two-way ANOVA to assess Drug × Stress interactions for each time point with Liposomal Bupivacaine. Similar to Ropivacaine, the effect of RSD on increasing mechanical allodynia was reversed by Liposomal Bupivacaine following exposure to three days of RSD (Stress × Drug Interaction; F(1, 18)=55.71, p<0.0001) and six days of RSD (Stress × Drug Interaction; F(1, 18) =114.1, p<0.0001). Thus, similar to Ropivacaine, Liposomal Bupivacaine analgesia was associated with maintenance of normal pain thresholds in mice exposed to repeated social defeat stress.
Figure 4. Liposomal Bupivacaine prevented mechanical allodynia during repeated social defeat without altering stress-induced immune and behavioral responses.
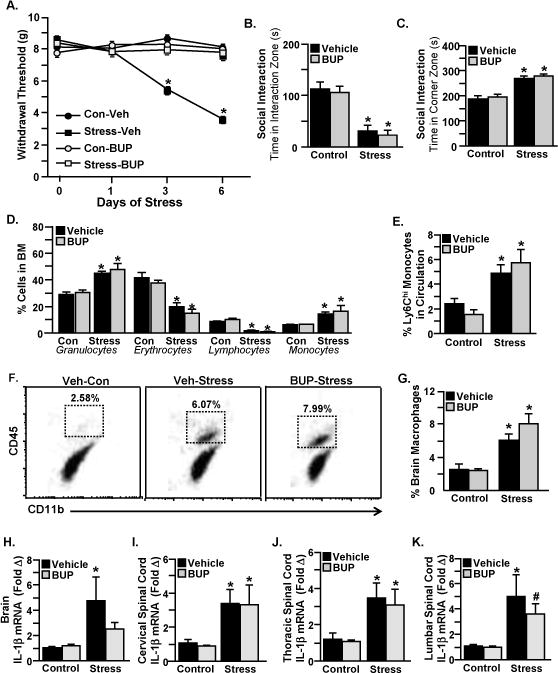
Male C57BL/6 mice were administered Liposomal Bupivacaine (BUP) or Vehicle and subjected to six days of repeated social defeat (Stress) or left undisturbed as controls (Con). Mice were tested for mechanical allodynia prior to RSD exposure and 12 hours after the first, third, and sixth day of stress. A) Withdrawal threshold of mechanical stimulation to the hind paw using the von Frey behavior test. In a separate experiment, mice were tested for social avoidance 12 hours after the sixth day of stress. Time spent in the B) interaction zone and C) corner zone are shown. Following behavioral testing, samples (blood, bone marrow, brain, spinal cord) were collected for cell or mRNA analyses. D) Quantification of granulocytes, erythrocytes, lymphocytes, and monocytes in the bone marrow. E) Percentage of Ly6Chi monocytes in circulation. F) Representative flow bivariate dot plots of CD11b and CD45 labeling of Percoll-enriched cells from the brain. G) Percentage of CD11b+/CD45hi monocytes/macrophages in the brain. IL-1β mRNA levels in the H) brain, I) cervical spinal cord, J) thoracic spinal cord, and K) lumbar spinal cord are shown. Bars represent average ± SEM. Means with (*) are significantly different from (p<0.05), and means with (#) tended to be different (p<0.1) from the corresponding control mice, according to F-protected post analysis.
We next investigated whether Liposomal Bupivacaine would prevent the development of depressive-like behavior after stress. As described above, mice were exposed to six days of RSD and social avoidance behavior was determined in a separate experiment 12 hours after the final day of stress. Stressed mice spent less time in the interaction zone when an unfamiliar CD-1 mouse was introduced in the social trial (Figure 4B), and this was not prevented by Liposomal Bupivacaine (main effect of Stress; F(1, 20)=42.49, p<0.0001). Furthermore, mice exposed to RSD spent more time in the corner zone (Figure 4C), and this was unaffected by Liposomal Bupivacaine (main effect of Stress; F(1, 20)=42.49, p<0.0001).
Next, we determined whether a longer-acting pain blockade would affect the peripheral production (Figure 4D) or release (Figure 4E) of bone marrow-derived, inflammatory monocytes during RSD. Thus, mice were exposed to six days of RSD and samples (bone marrow and blood) were collected 14 hours after the final day of stress for flow cytometry analyses. Similar to Ropivacaine, Liposomal Bupivacaine did not prevent the RSD-induced production of granulocytes (main effect of Stress; F(1, 18)=31.41, p<0.0001) or monocytes (main effect of Stress; F(1, 18)=13.39, p=0.0018). Moreover, RSD reduced erythrocytes (main effect of Stress; F(1, 18)=40.04, p<0.0001) and lymphocytes (main effect of Stress; F(1, 18)=121.1, p < 0.0001), and this was not affected by Liposomal Bupivacaine. Consistent with these results, mice exposed to RSD exhibited increased Ly6Chi monocytes in circulation despite Liposomal Bupivacaine treatment (main effect of Stress; F (1, 18) = 19.70, p=0.0003). Taken together, these results indicate that a longer-acting pain blockade with Liposomal Bupivacaine did not alter peripheral innate immune responses to stress.
We next determined whether monocyte trafficking to the brain and neuroinflammatory signaling were affected by a longer-acting pain blockade. As described above, mice were exposed to six days of RSD and samples (brain and spinal cord) were collected 14 hours after the last day of stress for flow cytometry or PCR analyses. Figure 4F shows representative bivariate dot plots of CD11b and CD45 labeling for macrophages (CD11b+/CD45high) for the different experimental groups. RSD increased the percentage of inflammatory monocytes that traffic to the brain (Figure 4G), and this was not affected by Liposomal Bupivacaine (main effect of stress; F(1, 18)=27.48, p<0.0001). There was a main effect of stress on the RSD-induced increase in brain IL-1β mRNA expression (main effect of Stress; F(1, 18)=6.170, p=0.0231). Notably, post hoc analysis revealed that Liposomal Bupivacaine prevented the RSD-induced increase in brain IL-1β gene expression (Figure 4H). We next determined the mRNA levels of IL-1β in the cervical, thoracic, and lumbar regions of the spinal cord (Figures 4I–K). The RSD-induced increase in IL-1β mRNA expression in the cervical spinal cord (main effect of Stress; F(1, 18)=10.72, p=0.0042), thoracic spinal cord (main effect of Stress; F(1, 18)= 11.37, p=0.0034), and lumbar spinal cord (main effect of Stress; F(1, 18)=11.63, p= 0.0031) were all unaffected by Liposomal Bupivacaine treatment. Collectively, these findings demonstrate that a longer-acting pain blockade with Liposomal Bupivacaine did not affect central immune responses to stress.
Discussion
This study assessed the effects of regional analgesia with local anesthetics Ropivacaine and Liposomal Bupivacaine on neuroimmune responses and behavior following exposure to repeated social stress. We first showed that psychosocial stress was associated with increased pain sensitivity. To further understand the mechanism, we developed a technique of regional analgesia with local anesthetics to block peripheral pain signaling during repeated social defeat (RSD) stress. We showed that stressed subjects provided Ropivacaine or Liposomal Bupivacaine analgesia maintained normal pain sensitivity thresholds while mice without analgesia developed abnormal pain responses in a dose-dependent manner following RSD. Furthermore, mice treated with either drug maintained neuroimmune responses and depressive-like behavior associated with stress. These data indicate that blocking peripheral nociception is effective in inhibiting enhanced pain signaling without altering stress-induced innate immune or depressive-like behavioral responses. In other words, preemptive analgesia prevents the development of abnormal pain processing associated with RSD through a mechanism not mediated by the immune system.
One of our main findings here was that increased pain sensitivity observed after RSD could be attenuated by Ropivacaine and Liposomal Bupivacaine analgesia during stress. Numerous clinical studies indicate that psychological stress contributes to the onset and progression of pain. For example, chronically stressed individuals exhibit lower pain thresholds in tests of tactile sensitivity (Ashkinazi and Vershinina 1999) and pressure pain (Persson, Hansson et al. 2000). Moreover, psychological stress prior to surgery prolongs pain symptoms during postoperative recovery (Mathews and Ridgeway 1981, Kiecolt-Glaser, Page et al. 1998). Therefore, understanding the mechanism that mediates the relationship between stress and the development of pain is critical in the treatment and management of human pain states.
Both Ropivacaine and Liposomal Bupivacaine are classified as long-acting local anesthetics that reversibly inhibit nerve impulses to cause a prolonged sensory or motor blockade (Kuthiala and Chaudhary 2011). It is important to note that Ropivacaine and Liposomal Bupivacaine each have distinct pharmacokinetic properties that dictate their duration of action (Kuthiala and Chaudhary 2011). Therefore, during model development, it was critical to determine the length of anesthetic action for each drug. Due to its shorter half-life, Ropivacaine was effective in providing a sensory blockade for approximately two hours (i.e., the duration of one cycle of RSD), as determined by absence of vocalization to electrical noxious stimuli. Liposomal Bupivacaine, on the other hand, prevented sensory processing for up to eight hours. Thus, it was plausible that a longer-acting local anesthetic might have different immunological and behavioral outcomes following exposure to social stress. However, our findings here indicate that there was no difference in the immune and behavioral responses to stress between Ropivacaine and Liposomal Bupivacaine.
Previous studies indicate that some local anesthetics may have immunological properties aside from direct anesthetic activity (Colucci, Puig et al. 2013). For example, local anesthetics may influence immune cell functionality (Heine, Jaeger et al. 2000) and secretion patterns of pro- and anti-inflammatory cytokines (Zura, Kozmar et al. 2012). Therefore, we investigated the effects of Ropivacaine and Liposomal Bupivacaine on immune responses to stress. Our previous studies show that RSD is associated with activation of stress-reactive neurocircuitry and neuroinflammatory events including cytokine production (Wohleb, McKim et al. 2014, Reader, Jarrett et al. 2015, Sawicki, McKim et al. 2015, Weber, Godbout et al. 2017). Moreover, RSD promotes increased production and release of bone marrow-derived inflammatory Ly6Chi monocytes that have increased trafficking capacity, elevated pro-inflammatory gene expression and are insensitive to the inhibitory effects of glucocorticoids (Wohleb, McKim et al. 2014, Reader, Jarrett et al. 2015, Weber, Godbout et al. 2017). Following exposure to RSD, mice treated with either Ropivacaine or Liposomal Bupivacaine showed no difference in the peripheral immune responses to RSD. For example, neither Ropivacaine nor Liposomal Bupivacaine affected enhanced myelopoiesis in the bone marrow after RSD. Moreover, neither drug prevented the stress-induced increase in inflammatory Ly6Chi monocytes in circulation. We previously demonstrated that increased production of monocytes in the bone marrow and their subsequent accumulation in circulation was dependent on activation of the sympathetic nervous system (Wohleb, McKim et al. 2014, Reader, Jarrett et al. 2015, Weber, Godbout et al. 2017). Therefore, we interpret our findings here to indicate that blocking peripheral nociception does not alter stress-induced sympathetic signaling to peripheral immune organs.
We next investigated whether regional analgesia with local anesthetics altered central immune responses to stress. RSD is associated with the recruitment of peripherally-derived Ly6Chi monocytes to stress-responsive brain regions, where they differentiate into macrophages and propagate inflammatory cytokine signaling (McKim, Weber et al. 2017). This significant finding has translational relevance because increased brain macrophage accumulation is seen in chronically-stressed individuals (Cole, Hawkley et al. 2011, Heidt, Sager et al. 2014) and in patients suffering from post-traumatic stress disorder (Skarpa, Rubesa et al. 2001). Here we found that mice treated with either Ropivacaine or Liposomal Bupivacaine still showed increased macrophages in the brain following exposure to RSD. Consistent with the fact that increased macrophage accumulation alters neuroinflammatory signaling, the mRNA expression of pro-inflammatory cytokine IL-1β remained elevated in the brain after RSD in mice treated with either drug. Furthermore, IL-1β mRNA levels were increased in all regions of the spinal cord (i.e., cervical, thoracic, and lumbar) after RSD despite drug treatment. These data suggest that blocking peripheral nociception does not alter central (brain or spinal cord) immune signaling during stress. Since pain information is transmitted to the brain via ascending tracts originating in the spinal cord, it was surprising that neither drug affected inflammatory cytokine signaling in the spinal cord. However, it is possible that spinal cord inflammation reflects the enhanced inflammatory response to RSD that is not altered by Ropivacaine or Liposomal Bupivacaine. Thus, we conclude that IL-1β is not associated with the development of mechanical allodynia in the RSD model, despite its critical role in promoting RSD-induced behavioral changes and immune cell trafficking. Numerous studies demonstrating a link between inflammation and pain posit IL-1β as a potent pro-nociceptive agent in the periphery and CNS (Ren and Torres 2009). However, despite significant elevations in IL-1β, the development of persistent or abnormal pain states can be attenuated by various interventions including antagonism of Nerve Growth Factor (Safieh-Garabedian, Poole et al. 1995) or alpha-adrenergic receptor (Safieh-Garabedian, Poole et al. 2002). These findings suggest that IL-1β has indirect and direct mechanisms contributing to pain behavior. Based on our findings, peripheral pain signaling, rather than an increase in systemic IL-1β levels, was associated with the development of abnormal pain processing after stress.
Another key finding was that mice treated with either Ropivacaine or Liposomal Bupivacaine still developed social avoidance following exposure to stress. As described previously, RSD is associated with the accumulation of macrophages within stress-responsive brain regions (McKim, Weber et al. 2017). This is significant because these brain regions are involved in regulating fear/anxiety and depressive behaviors (LeDoux 2003). Moreover, neuroinflammatory cytokine signaling is argued to be involved in the development of stress-related psychiatric disorders, including depression (Levine, Barak et al. 1999, Miller, Chen et al. 2009, Miller and Raison 2016). This is especially relevant here because mounting clinical data indicate that individuals suffering from chronic pain also suffer from depression (Bair, Robinson et al. 2003). Previous studies have shown that RSD promotes depressive-like behavior, as measured by social avoidance (Ramirez, Niraula et al. 2016, McKim, Weber et al. 2017). Notably, social avoidance behavior is prevented by treatments that affect neuronal interpretation of stress, including anxiolytics and antidepressants (Ramirez, Shea et al. 2015, Ramirez, Niraula et al. 2016, Ramirez and Sheridan 2016). Our findings here demonstrate that regional analgesia with local anesthetics does not impair neuronal interpretation of RSD.
Ropivacaine and Liposomal Bupivacaine block nerve conduction by preventing increased membrane permeability to sodium ions that allow normal axonal relay of nerve impulses (Kuthiala and Chaudhary 2011). This mechanism is non-specific, thus capable of impacting myelinated, unmyelinated, sensory, motor, and autonomic nerves alike, with varying onset and effectiveness of nerve conduction blockade depending upon method of delivery and pharmacokinetic parameters, both of which are impacted by the concentration and dose of local anesthetic, among others (Kuthiala and Chaudhary 2011). Due to the regional sensory blockade of afferent nociceptive impulses performed in our study, we believe our data support the notion that peripheral-to-central communication of pain during stress is necessary for the development of increased pain sensitivity. As shown in our data, all groups of mice developed similar immune, inflammatory, and behavioral changes following RSD despite treatment with local anesthetics. The only difference between stressed mice that received regional analgesia with Ropivacaine and Liposomal Bupivacaine versus stressed mice that received vehicle treatment, was the prevention of mechanical allodynia after RSD in the animals treated with local anesthetics. Mice that did not receive Ropivacaine or Liposomal Bupivacaine developed abnormal pain responses following RSD. These findings demonstrate the critical importance of peripheral-to-central neuronal relay of nociception on the development of abnormal pain processing in stressed animals.
In conclusion, our findings indicate that blocking peripheral nociception is effective in inhibiting enhanced pain signaling without altering stress-induced innate immune or behavioral responses. We showed that repeated social stress in mice was associated with increased mechanical allodynia that persisted over time. Furthermore, we used a technique of regional analgesia with local anesthetics to block pain signaling during stress. We showed that mice treated with either Ropivacaine or Liposomal Bupivacaine did not develop mechanical allodynia during RSD, despite maintenance of stress-induced neuroimmune responses and depressive-like behavior. Taken together, these results show that blocking peripheral nociception to a painful stimulus in stressed subjects is effective for maintenance of normal pain responses following stress; however, immune activation and abnormal behavioral responses associated with stress persist.
Highlights.
Psychosocial stress increased pain sensitivity in mice
Regional analgesia with local anesthetics blocked stress-induced pain responses
Anesthetic interventions did not alter neuroimmune responses after stress
Acknowledgments
This study was supported by National Institute of Health (NIMH) grants R01-MH-093473 and R01-MH-093472 to JFS. MDW and BLJ were supported by NIDCR Training Grant T32-DE014320. CMS was supported by F30-DE026075.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- Ashkinazi I, Vershinina EA. Pain sensitivity in chronic psychoemotional stress in humans. Neurosci Behav Physiol. 1999;29(3):333–337. doi: 10.1007/BF02465346. [DOI] [PubMed] [Google Scholar]
- Bair MJ, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, et al. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108(7):3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci D, et al. Influence of anaesthetic drugs on immune response: from inflammation to immunosuppression. OA Anaesthetics. 2013;3(21) [Google Scholar]
- DeLeo JA. Basic science of pain. J Bone Joint Surg Am. 2006;88(Suppl 2):58–62. doi: 10.2106/JBJS.E.01286. [DOI] [PubMed] [Google Scholar]
- Engler H, et al. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 2004;148(1–2):106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Grace PM, et al. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, et al. Peripheral immune contributions to the maintenance of central glial activation underlying neuropathic pain. Brain Behav Immun. 2011;25(7):1322–1332. doi: 10.1016/j.bbi.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Greco CM, et al. Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: a randomized controlled trial. Arthritis Rheum. 2004;51(4):625–634. doi: 10.1002/art.20533. [DOI] [PubMed] [Google Scholar]
- Griffis CA. Neuroimmune activation and chronic pain. AANA J. 2011;79(1):31–37. [PubMed] [Google Scholar]
- Heidt T, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J, et al. Anaesthesia with propofol decreases FMLP-induced neutrophil respiratory burst but not phagocytosis compared with isoflurane. Br J Anaesth. 2000;85(3):424–430. doi: 10.1093/bja/85.3.424. [DOI] [PubMed] [Google Scholar]
- Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;2004(252):reE14. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, et al. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28(20):5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, et al. Psychological influences on surgical recovery. Perspectives from psychoneuroimmunology. Am Psychol. 1998;53(11):1209–1218. doi: 10.1037//0003-066x.53.11.1209. [DOI] [PubMed] [Google Scholar]
- Kuthiala G, Chaudhary G. Ropivacaine: A review of its pharmacology and clinical use. Indian J Anaesth. 2011;55(2):104–110. doi: 10.4103/0019-5049.79875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4–5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, et al. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40(4):171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Lieblein-Boff JC, et al. Neonatal E. coli infection causes neuro-behavioral deficits associated with hypomyelination and neuronal sequestration of iron. J Neurosci. 2013;33(41):16334–16345. doi: 10.1523/JNEUROSCI.0708-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain Behav Immun. 2003;17(Suppl 1):S125–131. doi: 10.1016/s0889-1591(02)00079-x. [DOI] [PubMed] [Google Scholar]
- Mathews A, Ridgeway V. Personality and surgical recovery: a review. Br J Clin Psychol. 1981;20(Pt 4):243–260. doi: 10.1111/j.2044-8260.1981.tb00525.x. [DOI] [PubMed] [Google Scholar]
- McKim DB, et al. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. J Neurosci. 2016;36(9):2590–2604. doi: 10.1523/JNEUROSCI.2394-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, et al. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol Psychiatry. 2016;79(10):803–813. doi: 10.1016/j.biopsych.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, et al. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson K, Martelli MF. The problem of pain. J Head Trauma Rehabil. 2004;19(1):2–9. doi: 10.1097/00001199-200401000-00002. [DOI] [PubMed] [Google Scholar]
- Persson AL, et al. Pressure pain thresholds and electromyographically defined muscular fatigue induced by a muscular endurance test in normal women. Clin J Pain. 2000;16(2):155–163. doi: 10.1097/00002508-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Powell ND, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110(41):16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, et al. GABAergic modulation with classical benzodiazepines prevent stress-induced neuroimmune dysregulation and behavioral alterations. Brain Behav Immun. 2016;51:154–168. doi: 10.1016/j.bbi.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, et al. Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain Behav Immun. 2015;46:212–220. doi: 10.1016/j.bbi.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, Sheridan JF. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive-like behaviors. Brain Behav Immun. 2016;57:293–303. doi: 10.1016/j.bbi.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader BF, et al. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015;289:429–442. doi: 10.1016/j.neuroscience.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60(1):57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieh-Garabedian B, et al. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115(7):1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieh-Garabedian B, et al. The role of the sympathetic efferents in endotoxin-induced localized inflammatory hyperalgesia and cytokine upregulation. Neuropharmacology. 2002;42(6):864–872. doi: 10.1016/s0028-3908(02)00028-x. [DOI] [PubMed] [Google Scholar]
- Sawicki CM, et al. Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience. 2015;302:151–164. doi: 10.1016/j.neuroscience.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarpa I, et al. Changes of cytolytic cells and perforin expression in patients with posttraumatic stress disorder. Croat Med J. 2001;42(5):551–555. [PubMed] [Google Scholar]
- Tsuda M, et al. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28(2):101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Turner JA, et al. Catastrophizing is associated with pain intensity, psychological distress, and pain-related disability among individuals with chronic pain after spinal cord injury. Pain. 2002;98(1–2):127–134. doi: 10.1016/s0304-3959(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Weber MD, et al. Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology. 2017;42(1):46–61. doi: 10.1038/npp.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, et al. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2014;8:447. doi: 10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, et al. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33(34):13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Weng HR. Endogenous interleukin-1beta in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic N-methyl-D-aspartic acid receptors. J Biol Chem. 2013;288(42):30544–30557. doi: 10.1074/jbc.M113.495465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zura M, et al. Effect of spinal and general anesthesia on serum concentration of pro-inflammatory and anti-inflammatory cytokines. Immunobiology. 2012;217(6):622–627. doi: 10.1016/j.imbio.2011.10.018. [DOI] [PubMed] [Google Scholar]


