Abstract
Studies show that spinal (intrathecal; i.t.) interleukin-10 (IL-10) gene therapy reverses neuropathic pain in animal models, and co-administration with the mannose receptor (MR; CD206) ligand D-mannose (DM) greatly improves therapeutic efficacy. However, the actions of endogenous IL-10 may be required for enduring pain control observed following i.t. IL-10 gene therapy, potentially narrowing the application of this non-viral transgene delivery approach. Here, we show that i.t. application of naked plasmid DNA expressing the IL-10 transgene co-injected with DM (DM/pDNA-IL-10) for the treatment of peripheral neuropathic pain in IL-10 deficient (IL-10 KO) mice results in a profound and prolonged bilateral pain suppression. Neuropathic pain is induced by unilateral sciatic chronic constriction injury (CCI), and while enduring relief of light touch sensitivity (mechanical allodynia) in both WT and IL-10 KO mice was observed following DM/pDNA-IL-10 co-therapy, transient reversal from allodynia was observed following i.t. DM alone. In stably pain-relieved IL-10 KO mice given DM/pDNA-IL-10, mRNA for the IL-10 transgene is detected in the cauda equina and ipsilateral dorsal root ganglia (DRG), but not the lumbar spinal cord. Further, DM/pDNA-IL-10 application increases anti-inflammatory TGF-β1 and decreases pro-inflammatory TNF mRNA in the ipsilateral DRG compared to allodynic controls. Additionally, DM/pDNA-IL-10 treated mice exhibit decreased spinal pro-inflammatory mRNA expression for TNF, CCL2 (MCP-1), and for the microglial-specific marker TMEM119. Similarly, DM/pDNA-IL-10 treatment decreases immunoreactivity for the astrocyte activation marker GFAP in lumbar spinal cord dorsal horn. Despite transient reversal and early return to allodynia in DM-treated mice, lumbar spinal cord revealed elevated TNF, CCL2 and TMEM119 mRNA levels. Both MR (CD206) and IL-10 receptor mRNAs are increased in the DRG following CCI manipulation independent of injection treatment, suggesting that pathological conditions stimulate upregulation and availability of relevant receptors in critical anatomical regions required for the therapeutic actions of the DM/pDNA-IL-10 co-therapy. Taken together, the current report demonstrates that non-viral DM/pDNA-IL-10 gene therapy does not require endogenous IL-10 for enduring relief of peripheral neuropathic pain and does not require direct contact with the spinal cord dorsal horn for robust and enduring relief of neuropathic pain. Spinal non-viral DM/pDNA-IL-10 co-therapy may offer a framework for the development of non-viral gene therapeutic approaches for other diseases of the central nervous system.
1. Introduction
Non-viral transgene delivery is one of the least efficient methods of gene transfer for therapeutic applications (Glover et al., 2005), but due to its improved safety profile and reduced cost burden, it has been pursued for the treatment of diseases of the central nervous system (CNS) (Jayant et al., 2016). While non-opioid treatments for the control of chronic neuropathic pain are limited, one promising avenue is the application of spinal non-viral interleukin-10 (IL-10) gene therapy, an approach previously demonstrated to provide enduring pain relief in a variety of animal models (Milligan et al., 2006b; Milligan et al., 2006a; Ledeboer et al., 2007; Sloane et al., 2009b; Sloane et al., 2009c; Soderquist et al., 2010b; Milligan et al., 2012; Dengler et al., 2014; Grace et al., 2017).
IL-10 is a powerful anti-inflammatory cytokine that pleiotropically inhibits the actions of many pro-inflammatory factors by mechanisms that include the destabilization of mRNA transcripts for the pro-inflammatory cytokines tumor necrosis factor (TNF) and interleukin-1β (IL-1β) (Moore et al., 2001; Lobo-Silva et al., 2016). Following peripheral nerve injury, a brief compensatory upregulation in IL-10 protein production is followed by decreased IL-10 expression below baseline levels in pain-relevant anatomic locations (Jancalek et al., 2010; Jancalek et al., 2011; Khan et al., 2015). Studies show that spinal non-viral IL-10 gene delivery in neuropathic animals produces pain relief through elevated spinal IL-10 production with corresponding reduction of pro-inflammatory mediators of pathological pain (Ledeboer et al., 2007; Sloane et al., 2009a; Soderquist et al., 2010a; Dengler et al., 2014). However, whether endogenous IL-10 is required for the long-lasting pain relief observed following i.t. spinal non-viral IL-10 gene therapy remains unknown. Additionally, the anatomical regions in the pain pathway necessary for IL-10 transgene expression that leads to pain relief are still unclear.
While a single large dose of naked plasmid DNA encoding the IL-10 transgene (pDNA-IL-10; 100μg) or repeated doses (100μg followed by ≥25μg within 3–72hrs) result in transient or enduring pain relief, respectively (Milligan et al., 2006a; Ledeboer et al., 2007; Sloane et al., 2009b), the doses used render these approached clinically unfeasible. A novel gene delivery formulation, whereby a single co-injection of as little as 1μg of naked pDNA-IL-10 with the immune cell adjuvant D-mannose (DM), a known mannose receptor-specific (MR; CD206) ligand, greatly improves the efficacy of spinal non-viral IL-10 gene therapy in rats, allowing for stable long lasting pain relief following a single i.t. injection of (Dengler et al., 2014). The MR is expressed by subpopulations of macrophages and dendritic cells, as well as by microvascular endothelial cells (Taylor et al., 2005). In the CNS, the MR is expressed by astrocytes, microglia, and some neurons (Burudi et al., 1999; Burudi and Regnier-Vigouroux, 2001), and in the PNS by Schwann cells (Baetas-da-Cruz et al., 2009). Increased MR expression is often associated with anti-inflammatory macrophages (Gordon, 2003). Macrophages and other trafficking lymphocytes (i.e. T cells), along with non-leukocytic resident cell types such as satellite glia, are present within the DRG following sciatic nerve injury and likely contribute to neuropathy (Hu et al., 2007; Hanani, 2015). Notably, MR expression is present on leukocytes (Martinez-Pomares, 2012), and MR-activation itself leads to anti-inflammatory signaling as well as transient pain relief (Dengler et al., 2014). However, the transcriptional regulation of critical pro- and anti-inflammatory cytokines and chemokines in the pain pathway following DM-mediated pDNA-IL-10 co-therapy is not known.
In the current report, we applied spinal non-viral DM/pDNA-IL-10 co-therapy to neuropathic wild type (WT) and IL-10 deficient mice (IL-10 KO mice). Sciatic nerve chronic constriction injury (CCI) was induced, an established mouse model of peripheral neuropathy, resulting in reliable pathological sensitivity to light touch known as allodynia (Bennett and Xie, 1988; Colleoni and Sacerdote, 2010; Jaggi et al., 2011). Both central and peripheral nervous tissues associated with the pain pathway were analyzed for IL-10 transgene mRNA, as well as transcriptional regulation of pro- vs. anti- inflammatory cytokine and chemokine mRNA and protein. The findings reported here support that DM acting as an immune adjuvant for improved spinal non-viral pDNA-IL-10 gene transfer provides a new strategy for gene therapeutics to treat chronic pain, with the potential for application to other chronic CNS diseases.
2. Materials and Methods
2.1 Animals
All experiments were performed using adult male mice (8–12 weeks of age). C57BL/6J (WT; RRID: IMSR_JAX:000664) or B6.129P2-Il10tm1Cgn/J (IL-10 KO; RRID: IMSR_JAX:002251) mice were purchased from Jackson Laboratories or bred in-house from breeders also purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were maintained in specific-pathogen free conditions confirmed negative for detection of Helicobacter spp. Prior to handling, all animals were acclimated to the mouse colony at the University of New Mexico (UNM) Health Sciences Center Animal Facility for a minimum of 7 days. Animals were housed in groups of 3–5 at 23° ± 2°C in light controlled rooms (12:12 light:dark; lights on at 6:00am) and fed standard rodent chow and water ad libitum. All procedures were approved by the Institutional Care and Use Committee (IACUC) of the UNM Health Sciences Center, conducted in accordance to the NIH Guidelines for the Care and Use of Laboratory Animals, and closely adhered to recommendations from the International Association for the Study of Pain for the use of animals in research.
2.2 Animal Model of Peripheral Neuropathy
A modification of the sciatic nerve chronic constriction injury (CCI) model developed by Bennett and Xie (Bennett and Xie, 1988) was used for application in the mouse (Costa et al., 2008; Martucci et al., 2008; Liu et al., 2017) and briefly described here. Under isoflurane anesthesia (induction at 3.0 followed by 2.0–2.5 vol.% in oxygen, 2.0L/min), the lower back and dorsal left thigh were shaved and then cleaned with diluted Bacti-Stat AE (EcoLab Health Care Division, Mississauga, Ontario, Canada), followed by water, and lastly swabbed with 70% EtOH that was allowed to air dry before proceeding. Using aseptic procedures, the left sciatic nerve was carefully isolated by gentle blunt dissection through the fascia between the gluteus superficialis and biceps femoris muscles. The exposed sciatic nerve was snuggly ligated with three segments of sterile 4-0 chromic gut suture (Ethicon; Cat#:635H) proximal to the nerve’s trifurcation and without pinching of the nerve. To allow enhanced malleability of the thick suture material thereby reducing the risk of unintended damage, segments of chromic gut material were briefly soaked in a bath of isotonic sterile saline (Hospira; Cat#:NDC 0404-4888-10) prior to application. Additionally, great attention was paid to keeping the sciatic nerve moist via regular irrigations with isotonic sterile saline. Sham surgery was identical to the CCI surgery but without nerve ligation. The overlying muscle was sutured closed with one 3-0 sterile silk suture (Ethicon; Cat#:K572H). The overlying skin was closed using two Reflex™ wound clips (Kent Scientific Corp.; Cat#:INS750344). Full recovery from anesthesia was observed within 10–15 minutes following surgery. At this time, mice that had undergone CCI showed minor ventroflexion of the ipsilateral hindpaw while Sham mice revealed no postural abnormalities. Animal body weights were monitored prior to and following surgery. One day after surgery, animals were monitored for wound condition, hindpaw health, and general activity level. If autotomy was present at any point during the experiments, mice were immediately euthanized (less than 1.0% of all mice).
2.3 Behavioral Assessment of Mechanical Allodynia
Hindpaw threshold responses to innocuous light mechanical touch were assessed in mice by adopting principles from the von Frey fiber test. The von Frey behavioral testing approach used in this report blends components of previously described von Frey behavioral assessment in rodents (Chaplan et al., 1994; Sommer and Schafers, 1998; Milligan et al., 2000; Bonin et al., 2014). Following habituation to the testing environment (30–45 minutes per day, 4 days), baseline (BL) responses were assessed as follows. A series of calibrated monofilaments were applied randomly to the plantar surface of left and right hindpaws for a maximum of 3.0s per application. A ≥30s interval was required between applications to the same mouse. The log stiffness of the nine monofilaments used is defined as log10 (grams x 10,000) with the following range of filaments having log stiffness values (the value in grams is given in parentheses) of 2.36 (0.022g), 2.44 (0.028g), 2.83 (0.068g), 3.22 (0.166g), 3.61 (0.407g), 3.84 (0.692g), 4.08 (1.202g), 4.17 (1.479g), and 4.31 (2.042g). The first monofilament applied was always 3.22 (0.166g). If a positive response (i.e. lifting, licking, or shaking of the paw) was observed, then the next weaker hair was applied. In contrast, a negative response indicated that the next stronger monofilament be applied to that hindpaw in the subsequent round. This testing sequence progressed for a total of six applications per hindpaw. Testing was stopped prematurely if a positive response was observed following application of the weakest filament (2.36). This testing paradigm requires far fewer hair applications (a maximum of 6 times) to a given paw as compared to the previous methods, thereby curbing artifacts caused by overstimulation. The total numbers of positive responses and negative responses at each of the tested monofilaments were used to calculate the absolute (50%) paw withdrawal threshold via the computer program PsychoFit (http://psych.colorado.edu/~lharvey; RRID: SCR_015381), as previously described (Milligan et al., 2000; Dengler et al., 2013). This software fits a Gaussian integral psychometric function to the observed withdrawal rates for each of the tested von Frey hairs using a maximum-likelihood fitting method (Milligan et al., 2000). The computed log stiffness threshold values were then used for subsequent statistical analyses, but graphical representations present data as stimulus intensity in grams. A considerable benefit of this new method of assessment and analysis is that all hairs tested, (i.e. the entire pattern of responses) collectively contribute to calculating the stimulus threshold, whereas prior approaches in mouse heavily depend on the observed response to the final hair applied (Sommer and Schafers, 1998; Bonin et al., 2014). All behavioral hindpaw-response assessments were performed within the first four hours of the light cycle to minimize physiological influences regulated by circadian rhythms.
Behavioral assessment was conducted at BL prior to and after surgery. Timepoints for behavioral assessment on Days post-surgery were carefully chosen to avoid disruption of behavioral responses that may result from frequent testing. For the long-duration timecourse study, behavioral assessment was conducted at BL, Days 3 and 5 post-surgery, and Days post-injection 1, 3, 5, 7, 10, 14, 17, 22, 26, 30, 35, and 40. In the short-duration studies that terminated prior to tissue collection for mRNA, protein, and immunohistochemical analysis, behavioral assessment occurred at BL, Days 3 and 5 post-surgery, and Days post-injection 1, 2, 3, 4, 5, 6, 7, 10, and 12. For immunohistochemical analysis, an additional control group of IL-10 KO CCI mice (N = 4) received no injection, and was behaviorally verified at BL, and Days post-surgery 3, 5, 7, 10, and 17. Pilot studies (data not shown) were conducted using this approach to validate hindpaw response thresholds demonstrated in previous reports (Sommer and Schafers, 1998; Shimoyama et al., 2002).
2.4 Preparation of Plasmid DNA
The plasmid vector pDNA-IL-10 (also called pTR2-CB-mIL10F129S) used in these studies (Fig. 1C) is the mouse IL-10 equivalent of a rat IL-10 plasmid that was fully described previously and is derived from an adeno-associated virus-2 (AAV-2) expression cassette (Milligan et al., 2005; Milligan et al., 2006b). It consists of a 5.9 kilobase circular plasmid DNA (pDNA) containing a transcriptional cassette consisting of a hybridized cytomegalovirus enhancer/chicken beta-actin promoter (CMV enh/CB pro) driving expression of the mouse Il10 gene containing a point mutation (mIL-10F129S), and a simian virus 40 (SV40) polyadenylation signal (SV40 poly(A)). The transcription cassette is flanked by 149 bp inverted terminal repeat (ITR) sequences. The plasmid backbone contains an ampicillin resistance (Ampr) gene, as well as components unique to the original AAV-2 expression cassette such an intervening sequence (IVS; intron). The control plasmid (pDNA-Ctrl) is an analogous plasmid cassette that instead drives expression of enhanced jellyfish green fluorescent protein (eGFP) (Milligan et al., 2006b). Plasmids were amplified in SURE2 Supercompetent Cells (Agilent Technologies; Cat#:200152) because the ITR elements are often deleted in conventional E. coli strains (Milligan et al., 2006b) resulting in reduced transgene expression. Plasmid DNA was isolated using an EndoFree Plasmid Giga Kit (Qiagen; Cat#:12391) according to manufacturer’s instructions. Purified endotoxin-free plasmids were resuspended in sterile Dulbecco’s PBS (1x) (Gibco; Cat#:14190-144) with 3% sucrose, aliquoted, and stored at −20°C.
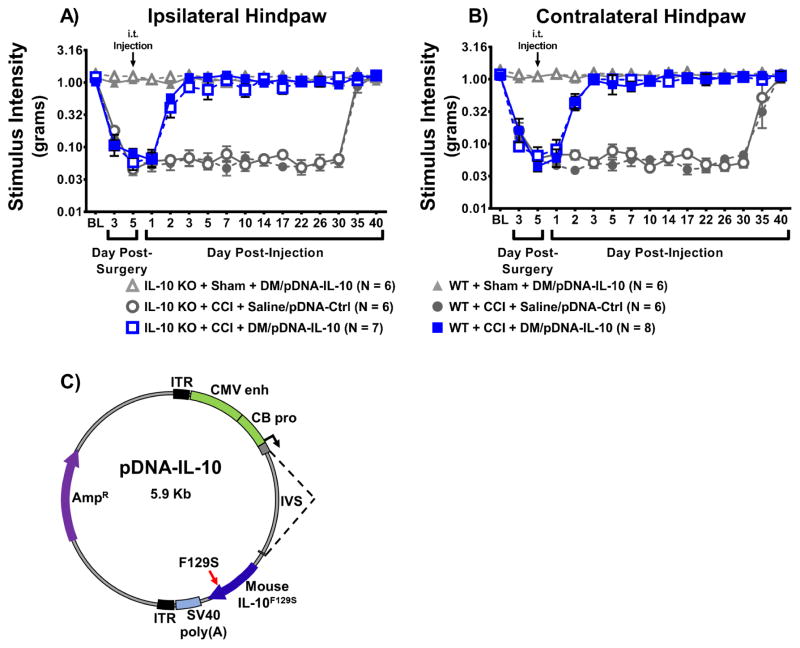
Figure 1. Intrathecal non-viral IL-10 gene therapy provides stable long-lasting relief of allodynia in mice and is independent of endogenous IL-10.
Absolute threshold behavioral responses for (A) ipsilateral and (B) contralateral hindpaws for WT and IL-10 KO mice are shown (N = 6–8 mice/group as indicated in figure legend). At baseline (BL), responses to low threshold mechanical stimuli were similar between all groups (ipsilateral, F(5, 33) = 1.01, P > 0.05; contralateral, F(5, 33) =1.31, P > 0.05). For data collected between BL through Day 5 post-surgery, a main effect of time (ipsilateral, F(2, 66) = 170.08, P < 0.001; contralateral, F(2, 66) = 134.35, P < 0.001) and an interaction between time and surgical manipulation (ipsilateral, F(2, 66) = 79.76, P < 0.001; contralateral, F(2, 66) = 64.04, P < 0.001) were observed. After behavioral assessment on Day 5 post-surgery, all mice received an intrathecal (i.t.) co-injection of plasmid DNA encoding interleukin-10 (pDNA-IL-10; 3μg in 7.5μL) with D-mannose (DM; 25μg in 3μL) vs. pDNA-Control (Ctrl; 3μg in 7.5μL) with isotonic sterile saline (saline; 3μL). Following injection, both WT and IL-10 KO CCI-mice given i.t. DM/pDNA-IL-10 reveal bilateral reversal to normal levels of hindpaw sensitivity (ipsilateral, F(2, 66) = 54.49, P < 0.001; contralateral, F(2, 66) = 34.34, P < 0.001). This reversal remains stable through Day 30 post-injection (ipsilateral, F(4.9, 162.5) = 0.432, P > 0.05; contralateral, F(4.9, 163.1) = 0.871, P > 0.05). Not surprisingly, control injected CCI-mice demonstrate bilateral spontaneous reversal from allodynia between Days 30–40 post-injection (ipsilateral, F(2, 66) = 141.30, P < 0.001; contralateral, F(1.5, 48.5) = 48.54, P < 0.001), with all groups experiencing similar normal levels of light touch sensitivity by Day 40 post-injection (ipsilateral, F(5, 33) = 0.81, P > 0.05; contralateral, F(5, 33) = 0.193, P > 0.05). (C) Plasmid map of the pDNA-IL-10 construct. The modified plasmid was derived from the expression cassette previously used for adeno-associated virus-2. pDNA-IL-10 is a 5.9 Kb plasmid with gene transcription driven by a chicken β-actin promoter (CB pro) hybridized with a cytomegalovirus enhancer (CMV enh). The expression cassette contains the transgene for mouse IL-10 with a point mutation (serine substitution for phenylalanine at amino acid 129) (IL-10F129S), and a viral SV40 polyadenylation signal (SV40 poly(A)), and is flanked by two inverted terminal repeat (ITR) sequences. The plasmid vector also contains an intervening sequence (IVS; intron) and an ampicillin-resistance (Ampr) gene.
2.5 Intrathecal Injections
Intrathecal (i.t.) injections were acutely administered under isoflurane anesthesia following behavioral assessment on Day 5 post-surgery and conducted as previously described (Hylden and Wilcox, 1980) with modification as indicated here. Injector units were constructed as follows. First a needle adaptor was created by inserting an intact sterile 27G x 0.5 in needle (PrecisionGlide, Becton Dickinson & Co.; Cat#:305109) into one end of a 30cm long segment of PE20 polyethylene tubing (Becton Dickinson & Co; Cat#:427406). At the opposite end, a second 27G needle with the needle hub removed was inserted such that the blunt end was inserted inside the tubing while leaving the beveled sharpened end available for lumbar puncture. These needle adaptors were placed in a sterile dry place until the time of use. At the time of injection, sterile isotonic saline (Hospira; Cat#: NDC 0404-4888-10) was used to fill the line as well as to check for leakage. Sterile isotonic saline was also used to fill a sterilized 50μL gastight calibrated syringe (Hamilton Co.; Cat#:CAL80901) which was then connected to the needle adaptor via the intact needle hub. Excess saline was expelled from the syringe leaving saline only within the tubing. The line was then loaded with the following four components in this order: (1) a 1μL air bubble is drawn into the tubing followed by (2) 3μg of plasmid DNA (in 7.5μL), (3) a second 1μL air bubble, and finally (4) either 25μg D-mannose (Sigma-Aldrich; Cat#:M6020) dissolved in 3μL of sterile isotonic saline or sterile isotonic sterile saline alone.
Under isoflurane anesthesia (induction at 2.5% followed by 1.5% volume in oxygen at 2.0 liters per minute), the mid- to lower-back was shaven and swabbed with 70% ethanol. The beveled sharpened end tip of the 27G needle of the injector unit was inserted percutaneously between lumbar vertebrae 5 and 6 (L5–L6). A tail flick was considered indicative of a successful i.t. puncture. Next, the i.t. injection bolus was slowly administered over the course of 30s. The total time required for each injection was ~3 minutes, excluding anesthesia induction. Following injection, all mice resumed motor activity consistent with that observed prior to i.t. injection.
2.6 Tissue Collection and Total RNA Isolation
Following behavioral assessment on Day 12 post-injection (Day 17 post-surgery) mice were deeply anesthetized (≥10min, 5% volume in oxygen at 2 liters per minute) followed by transcardial perfusion with ice-cold 0.1M phosphate buffered saline (pH = 7.40). The body was then placed on a frozen gel refrigerant pack (Glacier Ice, Pelton Shepherd Industries) previously maintained on dry ice. Rapid laminectomy followed by tissues dissection in the following order: lumbar spinal cord (L3–L6), contralateral lumbar (L3–L5) dorsal root ganglia (DRG), ipsilateral L3-5 DRG, cauda equina, and lastly ipsilateral sciatic nerve (~1cm). Samples were placed in DNase/RNase-free 1.5mL centrifuge tubes (VWR International; Cat#:47747-362), quickly frozen on dry ice, and then stored at −80°C for future analysis.
Total RNA was extracted from ipsilateral DRG, contralateral DRG, lumbar spinal cord, and cauda equina using the miRNeasy Mini Kit (Qiagen; Cat#:217004) per manufacturer’s instructions. Homogenizations were performed using a motorized VWR Disposable Pellet Mixer and cordless motor pestle system (VWR International; Cat#:47747-3). For ipsilateral sciatic nerves, special care was taken to process samples so that they could be analyzed for both RNA and protein. To accomplish this, tissues were placed in 100μL chilled 1x phosphate buffered saline (10x PBS diluted to 1x with DNase/RNase free water; Sigma-Aldrich; Cat#: P7059) and quickly chopped with scissors, and briefly homogenized with the motorized pestle system. Within 1.5min from initial chopping, 40% of the PBS-suspended homogenate was transferred to chilled Qiazol Lysis Reagent (Qiagen) and further homogenized. Total RNA was extracted using an miRNeasy Micro Kit (Qiagen; Cat#:217084) per manufacturer’s instructions but with single additional RPE and 80% EtOH wash steps in order to remove excess salts introduced by PBS. The remaining 60% of the PBS-suspended homogenate was pelleted at full speed for 1.5 minutes, the PBS aspirated, 35μL of protease inhibitor solution (MesoScale Discovery) added, frozen on dry ice, and finally stored at −80°C for future protein analysis (see section 2.7).
RNA concentration and quality was assayed by NanoDrop (Thermo Scientific). RNA samples were then diluted to a standardized RNA concentration for the following tissues: ipsilateral DRG (30ng/μL), contralateral DRG (30ng/μL), cauda equina (100ng/μL), and ipsilateral sciatic nerve (50ng/μL), and lumbar spinal cord (100ng/μL). Total RNA reversed transcribed to cDNA was 1,400ng for lumbar spinal cord, 1,000ng for cauda equina, and 525ng for ipsilateral sciatic nerve. For DRG, which had low total RNA yields due to the typically small DRG tissue samples, 390ng of total RNA were reverse transcribed for contralateral DRG, while 165ng and 390ng were used in two separate rounds of cDNA for ipsilateral DRG. Reverse transcription was performed for DRG, lumbar spinal cord, and sciatic nerve using a SuperScript™ IV VILO™ cDNA Synthesis Kit (Invitrogen; Cat#:11754250) per manufacturer’s instructions but required further optimization by extending the 42°C-incubation step to 2hr in order to improve cDNA yields from small samples such as the DRG. Reverse transcription was performed for cauda equina using a SuperScript IV First-Strand Synthesis System (Invitrogen; Cat#:18091050) per manufacturer’s instructions.
2.7 mRNA Analysis by Quantitative Real-Time PCR
Levels of mRNA expression were measured and analyzed as previously described (Mellios et al., 2014). The following dilution factors (indicated in parentheses) were applied to cDNA samples for assessment of transcripts of interest in given tissues: ipsilateral DRG (undiluted), contralateral DRG (undiluted), lumbar spinal cord (1:4), cauda equina (1:6), ipsilateral sciatic nerve (1:2.5). The following cDNA dilutions were used for assessment of the normalizer, mouse 18S rRNA: ipsilateral and contralateral DRG (1:40), lumbar spinal cord and cauda equina (1:200), ipsilateral sciatic nerve (1:100). Levels of mRNAs, as well as “Normalizer” 18S rRNA (Rn18s, Taqman Assay ID#: Mm03928990_g1), were assayed in triplicate via quantitative real-time PCR (qRT-PCR) with Taqman Gene Expression Assays (ThermoFisher Scientific). All selected mouse gene expression assays were identified by the manufacturer to be “best coverage” assays, unless otherwise noted, and to exclude detection of genomic DNA. mRNA levels were analyzed with the formula C = 2^CTNormalizer/2^CTTarget, as previously described (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008; Mellios et al., 2014).
The IL-10 Taqman Gene Expression Assay was selected to target sequences that span exons 1 and 2 (Il10; Taqman Assay ID#: Mm00439614_m1) in order to ensure that IL-10 transcripts detected in IL-10 KO mice truly represent exogenous transgene, versus the possible endogenous null mutant transcript. IL-10 KO mice possess a null copy of IL-10 in which a 500bp fragment of exon 1 (codons 5–55) has been replaced by a 24bp linker sequence, a neo expression cassette, and a termination codon (Kuhn et al., 1993). Therefore, any potential endogenous null IL-10 transcripts are predicted to be unrecognizable by the IL-10 Taqman Gene Expression Assay.
Because mannose receptor activation by DM is sufficient to improve non-viral IL-10 gene therapy (Dengler et al., 2014), mannose receptor gene expression was assayed (Mrc1; Taqman Assay ID#: Mm01329362_m1). Additionally, IL-10 transgene expression is known to limit the actions of many pro-inflammatory mediators (Moore et al., 2001; Milligan et al., 2012). Consequently, transcripts for the following pain-relevant pro-inflammatory cytokines were assessed: interleukin-1β (IL-1β, Il1b, Taqman Assay ID#: Mm00434228_m1) and tumor necrosis factor (TNF, Tnf, Taqman Assay ID#: Mm00443258_m1).
To address whether IL-10 deficient knockout mice possess compensatory changes in other anti-inflammatory pathways, the anti-inflammatory cytokine transforming growth factor β1 (TGF-β1, Tgfb1, Taqman Assay ID#: Mm01178820_m1) was evaluated. To investigate basal and post-CCI expression levels of the IL-10 receptor in IL-10 KO mice, transcript levels for IL-10R alpha (IL-10Rα, a.k.a. IL-10 R1; Il10ra, Taqman Assay ID#: Mm00434151_m1) were examined.
To assess whether actions of transgenic IL-10 may modulate microglia in the lumbar spinal cord, we tested the newly identified microglial specific marker (Bennett et al., 2016) transmembrane protein 119 (TMEM119, Tmem119, Taqman Assay ID#: Mm00525305_m1). The “best coverage” option was not available for the TMEM119 Taqman gene expression assay.
2.8 Multiplex Determination of Cytokine and Chemokine Expression
Ipsilateral sciatic nerve homogenates (see section 2.6) previously stored in a buffer with protease inhibitors (MesoScale Discovery) were kept on ice, further homogenized using a motorized homogenization system (VWR International; Cat#:47747-3), and subsequently sonicated. Tissue samples were centrifuged at 4,200 x g at 4°C for 10min to pellet cellular debris. Cellular lysates were collected from the supernatant and protein concentrations were determined by Quickstart™ Bradford Protein Assay (BioRad; Cat#:500-0201). Sciatic nerve protein expression levels were then determined using a V-PLEX™ immunoassay (MesoScale Discovery; detailed below) panel for quantification of the following cytokines and chemokines: TNF, IL-1β, IFN-γ, IL-6, CXCL1 (a.k.a. KC/GRO), and IL-12p70. All V-PLEX™ immunoassays were conducted according to manufacturer’s instructions.
The V-PLEX™ immunoassay is well validated for quantifying protein from small CNS tissue samples (Maxwell et al., 2015; Robinson et al., 2016; Noor et al., 2017). The methods are briefly described here. V-PLEX™ immunoassays apply electrochemiluminescence technology to precisely measure protein concentrations of multiple protein targets simultaneously with high sensitivity and reproducibility. Tissue lysates from experimental tissue samples, or calibrator (provided by kit), were loaded onto a ‘multi-spot’ plate. Each plate-well is pre-coated with antigen-specific ‘capture’ antibodies on independent spatially well-defined ‘spots’ that are in turn connected to a working electrode surface. Following incubation with protein lysates, immobilized proteins are next recognized by SULFO-TAG™-conjugated antigen-specific ‘detection’ antibodies. A Quickplex SQ120 Imager (MesoScale Discovery) was used to detect signal in each well in the plate via application of an electrical current to the plate electrodes and subsequent measurement of light intensity emitted by SULFO-TAG labeling. Where possible, 35ug total protein was loaded per well in duplicate, but due to limitations in tissue availability singlets were also accepted. For some samples, especially those from Sham mice, less than 35ug total protein was available, therefore all assay outputs were normalized by dividing the measured concentration for a specific analyte (i.e. 0.12pg TNF) for a given well by the total protein loaded to that same well, yielding units of (pg Analyte)/(μg Protein). In the two conditions, CCI+DM/pDNA-IL-10 and CCI+DM/pDNA-Ctrl, pairs of “low-protein” samples were combined once per condition to make a single N with a protein load of 35μg, yielding N = 6 and N = 8, respectively. The V-PLEX™ immunoassay system has high content validity and inter-assay variations less than 12% in our laboratory.
2.9 Tissue Preparation for Immunohistochemistry
Lumbar spinal cord tissue was collected from naïve IL-10 KO and WT mice (N = 3 mice/group), and from CCI-treated IL-10 KO mice (N = 3–4 mice/group) following behavioral assessment on Day 12 after intrathecal injection (Day 17 post-surgery). Samples were processed similarly as previously described (Dengler et al., 2014). Briefly, a lethal dose of pentobarbital (Fatal-Plus Solution, Vortech Pharmaceuticals, LTD.) was administered by intraperitoneal injection. Mice then underwent transcardial perfusion with 0.1M phosphate buffered solution (PBS; pH = 7.40) for 4min at 5mL/min, followed by room temperature 4% paraformaldehyde (PFA; pH = 7.40) (Sigma-Aldrich; Cat#:P6148) for 4min at 5mL/min, and finally ice cold 4% PFA for 4min at 5mL/min. Entire intact spinal vertebral columns (cervical 2 to coccygeal vertebrae) were collected and post-fixed 24hr in 4% PFA at 4°C. Columns were then washed 24hr in 0.1M PBS at 4°C and decalcified 4 weeks in 1.5L water containing 10% ethylenediaminetetraacetic acid (EDTA; Cat#:M101; VWR International) with 0.01% sodium azide (Sigma-Aldrich; Cat#:S2002) and 0.5% PFA with gentle consistent stirring atop a stir plate at room temperature. The method was used to allow examination for intact meninges and visualization of the subarachnoid matrix. The decalcification solution was changed every 5–7 days. Lumbar 3–6 (L3–L6) spinal vertebral column segments were paraffin processed according to previously described standard methods (Wilkerson et al., 2012a) and later sliced on a microtome, with 7μm tissue sections mounted on VWR VistaVision™ HistoBond® Adhesive Slides (VWR International; Cat#:16004) and stored in slide boxes at room temperature.
2.10 Immunohistochemistry
To investigate potential changes in lumbar dorsal horn spinal astrocytic activation following intrathecal non-viral IL-10 gene therapy, we analyzed the expression of the astrocyte marker glial fibrillary acidic protein (GFAP) in L4–L5 spinal segments, as previously described (Wilkerson et al., 2012a; Noor et al., 2017). Briefly, randomly selected paraffin-processed L4–L5 spinal cord tissue sections underwent deparaffinization in Hemo-De (Scientific Safety Solvents; Cat#: HD-150A) followed by rehydration via descending alcohols to 0.1M PBS (pH = 7.40). Antigen retrieval was applied by placing tissue slices in Nuclear Decloaker (BioCare Medical; Cat#: CB911M), a Tris-based buffer (pH = 9.50), and heating the samples in a conventional rice cooker (15min, ~94°C). After sufficient cooling, blocking was performed using 5% normal donkey serum (Jackson ImmunoResearch Labs; Cat#: 017-000-121, RRID: AB_2337258) (2hr, room temperature), followed by overnight primary antibody incubation at 4°C with 1:1,000 chicken anti-mouse GFAP (Abcam, Cat#: ab4674, RRID: AB_304558). The next day tissues were washed 4 × 3min in 0.1M PBS (pH = 7.40) followed by a 2hr incubation at room temperature with 1:200 donkey anti-chicken Alexafluor488-conjugated secondary antibody (Jackson ImmunoResearch Labs; Cat#: 703-545-155, RRID: AB_2340375). Slices were washed 4 × 3min in 0.1M PBS and then stained with 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich; Cat#:D9542) before coverslipping. All incubation steps were performed using a humidity chamber.
2.11 Microscope spectral imaging for immunofluorescent quantification
Image acquisition for spectral analysis was performed using the Nuance Multispectral Imaging System (PerkinElmer Inc.; RRID:SCR_015382) (Mansfield, 2014), as described previously (Wilkerson et al., 2012a; Dengler et al., 2014; Noor et al., 2017). Briefly, lumbar spinal cord dorsal horn images were obtained using a 20X objective with a Nikon TE-200 U inverted fluorescence microscope. Flat-field correction was applied in order to remove artifacts, including uneven field illumination, to produce a uniform illumination during image acquisition. Images of single-labeled control slides (one each for AF488 and DAPI) and a label-free (autofluorescence) slide were used to create a ‘spectral library’. Pure signals for each fluorophore were then computed by separating a known spectral profile (autofluorescence) from a ‘mixed’ spectrum profile (single labeled + autofluorescence). This allowed for the un-mixing of multi-labeled slides to obtain composite images containing only the labels of interest. Composite images were then used for further analysis using Slidebook 6 software (see Section 2.11).
2.12 Slidebook software image analysis
Composite images were analyzed using Slidebook 6 software (Intelligent Imaging Innovations; RRID: SCR_014300). To eliminate signals originating from artifact, an experimenter created a ‘threshold mask’ designed to limit the lowest-level fluorescent emissions in such a way that the final image file replicated that which was visible when the specimen was viewed through the microscope eyepiece, as described previously (Dengler et al., 2014). Additionally, for each image an individualized ‘area mask’ was created to include only the spinal cord dorsal horn gray matter in analyses, excluding surrounding white matter and peri-spinal blank space. The location of nuclei, as indicated by the DAPI channel image, for each slice was used to more accurately approximate the true borders of the dorsal horn when making the ‘area mask’. These masks were further refined to create a ‘final analysis mask’ for each slice that included only signal above the predetermined lower threshold (defined by the ‘threshold mask’) within the outlined region. ‘Fluorescence Intensity’ was calculated using the equation Fluorescence Intensity = (Sum Intensity)/(Area), where ‘Sum Intensity’ (the total signal measured by the ‘final analysis mask,’) is divided by ‘Area’ (the total area of the dorsal horn as indicated by the original ‘area mask,’ in microns squared [μm2]). The average Fluorescence Intensity of four slices (2 slice pairs taken ~140μm apart, mounted on a single slide) from one slide per animal was calculated to determine the value for each respective animal. Data from individual animals were analyzed as indicated in section 2.12, below.
2.13 Experimental Design and Statistical Analysis
To determine the efficacy of the DM/pDNA-IL-10 formulation in mice and to test whether endogenous IL-10 is required for long-lasting pain reversal, a complete timecourse of non-viral IL-10 efficacy for controlling allodynia was assessed in IL-10 KO mice compared to WT mice (N = 6–8 mice/group). In this long-duration timecourse, animals were followed behaviorally until Day 45 post-surgery (Day 40 post-injection), a timepoint by which pilot experiments (data not shown) indicated both IL-10 KO and WT mice reveal complete spontaneous reversal from CCI-induced allodynia. No tissues were collected.
All subsequent behavioral studies were performed using only IL-10 KO mice in order to (1) replicate initial observations, (2) to better understand the biodistribution of IL-10 transgene mRNA, (3) to examine the underlying mechanisms of long-lasting pain reversal independent of endogenous IL-10, and (4) to further explore the role of D-mannose in improved efficacy of non-viral transgene uptake and expression.
Behavioral results from the long-duration characterization were used to design a truncated timecourse such that tissues could be collected at a time when therapeutically treated neuropathic IL-10 KO mice had achieved stable reversal from allodynia (Day 12 post-injection; Day 17 post-surgery). In the first replication study (behavioral N = 6–12 mice/group), the following conditions were manipulated: surgery (Sham vs. CCI), plasmid DNA (pDNA-IL-10 vs. pDNA-Control), and adjuvant (DM vs. Saline). Collected tissues were analyzed by qRT-PCR (N = 5–8 mice/group). For ipsilateral sciatic nerve, samples from each animal were split for use in both qRT-PCR (Section 2.6) mRNA analysis and V-PLEX™ Immunoassay protein expression analysis (Section 2.7), and assessed typical injury-associated pro-inflammatory changes following peripheral nerve damage (Okamoto et al., 2001; Kleinschnitz et al., 2006; Uceyler et al., 2007). All corresponding tissues were collected from naïve WT (N = 3) and IL-10 KO (N = 2) mice to serve as IL-10 expression positive and negative controls, respectively, and to identify potential baseline transcriptional differences that may result from developmental IL-10 deficiency.
In a second study designed to replicate behavioral non-viral IL-10 efficacy for controlling allodynia, tissues were collected to assess changes in astrocytic activation markers at the level of the lumbar spinal cord. IL-10 KO mice given CCI-surgery were behaviorally verified and followed through Day 12 post-injection (N = 4 mice/group). On Day 12 post-injection (Day 17-post-surgery), the entire spinal cord enclosed within the vertebral column was collected from each mouse for subsequent immunohistochemical analysis. Decalcification and paraffin-embedding procedures were followed as described above. Prior work examining immunoreactive markers for spinal cord astrocytes following decalcification and paraffin-embedding procedures demonstrated through power analysis (Noor et al., 2017) and previously documented reports using similar methods (Wilkerson et al., 2012b; Wilkerson et al., 2012a; Noor et al., 2017) that N = 3 spinal cords per experimental condition was sufficient to yield reliable group differences. Therefore, to minimize unnecessary duplication, animal numbers were restricted to N = 3–4 per experimental condition.
All baseline behavioral data of hindpaw threshold responses were analyzed by One-way analysis of variance (ANOVA) using GraphPad PRISM version 7.02 (GraphPad Software Inc.; RRID:SCR_002798) to ensure no group differences were present at baseline, as well as to confirm complete spontaneous reversal from allodynia on Day 40 post-injection. Two-way repeated measures (RM) ANOVA using SPSS (IBM; RRID: SCR_002865) was performed for all other behavioral timepoint analyses. The assumption of sphericity for Two-way RM ANOVAs was assessed using Mauchly’s Test of Spericity (α = 0.05). If the assumption of sphericity was violated (P < 0.05), to protect against Type I errors, the reported degrees of freedom and p-values were adjusted using the conservative Greenhouse-Geisser correction. Data from microscope-acquired images reflecting immunoreactivity, relative mRNA transcript levels from qRT-PCR, and protein V-PLEX™ immunoassays were analyzed using One-way ANOVA with GraphPad PRISM.
To control the type I error rate during all multiple comparisons, Fisher’s LSD test (reported with adjusted P values) was applied for post hoc examination of possible group differences selected a priori. The threshold for statistical significance for all sets of multiple comparisons was set a priori to α = 0.05. All data are presented as the mean ± Standard Error of the Mean (SEM). For mRNA and protein analyses, within-group outliers where tested for by Grubbs’ Test using the GraphPad QuickCalc Outlier Calculator (https://graphpad.com/quickcalcs/grubbs1/) with α = 0.05.
3. Results
3.1 Intrathecal non-viral IL-10 gene therapy provides stable long-lasting relief of allodynia in mice, and is efficacious in the absence of endogenous IL-10
Previous investigations (Milligan et al., 2006b; Milligan et al., 2006a; Ledeboer et al., 2007; Sloane et al., 2009b; Soderquist et al., 2010b; Dengler et al., 2014) of non-viral IL-10 gene therapy were conducted in rodent models that were capable of expressing endogenous IL-10. Consequently, these studies were not able to unambiguously demonstrate whether enduring non-viral therapeutic efficacy relies on the actions of endogenous IL-10. To address this possibility, long-duration efficacy of i.t. non-viral IL-10 gene therapy was examined in IL-10 deficient mice, that is, IL-10 KO mice. In addition, D-mannose has previously been shown to improve the therapeutic efficacy of i.t. non-viral IL-10 gene delivery in rats for the treatment of allodynia (Dengler et al., 2014). However, the possibility that DM induces endogenous IL-10, acting as an immune adjuvant to enhance phagocytosis of pDNA-IL-10, suggests that endogenous IL-10 may be necessary for enduring pDNA-IL-10 efficacy. Therefore, the therapeutic efficacy DM/pDNA-IL-10 gene therapy in IL-10 KO vs. WT mice was examined in the current report.
Light mechanical touch assessed at BL revealed similar levels of hindpaw sensory threshold responses between all groups (Fig. 1A–D), which demonstrates that basal IL-10 is not required to maintain normal healthy sensory responses. Following surgery, CCI-treated WT and IL-10 KO mice develop clear bilateral allodynia by Day 3–5 post-surgery. All groups received an i.t. injection following behavioral assessment on Day 5 post-surgery (Fig 1A–B). By Day 2 post-injection, both IL-10 KO and WT CCI-treated mice given DM/pDNA-IL-10 reveal similar bilateral reversal of allodynia compared to CCI-treated mice given Saline/pDNA-IL-10, suggesting that possible additional actions of endogenous IL-10 in WT mice are not outwardly observable following gene therapy. Both WT and IL-10 KO CCI control groups given Saline/pDNA-Ctrl demonstrate spontaneous reversal of allodynia, with hindpaw responses similar to those observed in Sham-treated mice, on Day 35 post-injection (Day 40 post-surgery). However, both WT and IL-10 KO mice treated with CCI+DM/pDNA-IL-10 exhibit long-lasting and stable pain relief, never returning to allodynia.
Lastly, it is additionally important to note that the non-silent point mutation present in the mouse IL-10 gene (IL-10F129S) used in the current study and previously characterized in rat (Milligan et al., 2006b; Milligan et al., 2006a; Ledeboer et al., 2007; Sloane et al., 2009b; Soderquist et al., 2010b; Dengler et al., 2014) supports predicted IL-10 protein-receptor interaction. That is, IL-10 protein homodimerizes prior to binding to and activating the IL-10 receptor (Moore et al., 2001). The current report demonstrates that the IL-10F129S product does not require interaction with endogenous wild type IL-10 protein to achieve stable and enduring IL-10 transgene efficacy. This is the first demonstration that both non-viral IL-10 gene therapy and DM, the non-viral gene therapy adjuvant, are efficacious in mice and do not require endogenous IL-10.
3.2 The combination of DM/pDNA-IL-10 is necessary for long-lasting relief of allodynia
To better understand the effective relationship between pDNA-IL-10 and DM to induce long-lasting pain relief, i.t. DM/pDNA-IL-10 co-therapy was compared to various control conditions in neuropathic IL-10 KO mice (Fig. 2A–B). Threshold responses at BL were similar between all groups for both the ipsilateral and contralateral hindpaws, and following surgery, Sham-treated mice maintain normal levels of light touch sensitivity, replicating that observed in Fig. 1. CCI treated mice exhibit bilateral allodynia by Day 3 post-surgery, and CCI+Saline/pDNA-Ctrl-treated mice maintain stable allodynia through Day 12 post-injection. Surprisingly, CCI mice given DM with control plasmid DNA (lacking IL-10 transgene) exhibited delayed onset bilateral reversal from allodynia beginning on Day 3 post-injection. However, reversal of allodynia was transient, with increased hindpaw light touch sensitivity occurring by Day 6 post-injection and full allodynia by Day 12 post-injection. The data presented here support that DM-mediated relief of pathological light touch sensitivity is independent of IL-10. Overall, these data reveal that it is the combination of pDNA-IL-10 plus DM that is required for long-lasting relief of allodynia.
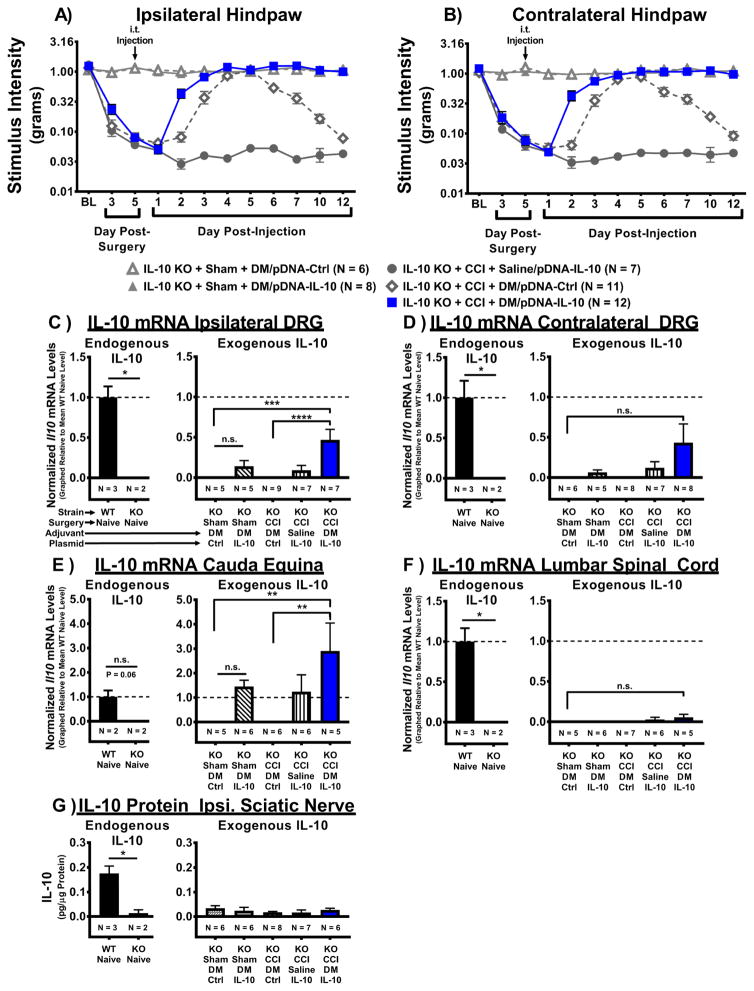
Figure 2. I.t. non-viral IL-10 gene therapy in IL-10 KO mice leads to expression of IL-10 transgene in pain-relevant lumbar dorsal root ganglia.
Absolute threshold behavioral responses for (A) ipsilateral and (B) contralateral hindpaws are shown. At baseline (BL), responses to low threshold mechanical stimuli were similar between all groups (ipsilateral, F(4, 39) = 1.39, P > 0.05; contralateral, F(4, 39) = 1.04, P > 0.05). For data collected between BL through Day 5 post-surgery a main effect of time (ipsilateral, F(1.7, 66.1) = 184.69, P < 0.001; contralateral, F(2, 78) = 186.41, P < 0.001) is observed. After behavioral assessment on Day 5 post-surgery, Sham- or CCI-treated mice received an i.t. co-injection of DM (25μg in 3μL) with either pDNA-IL-10 (3μg in 7.5μL) or pDNA-Ctrl (N = 6–12 mice/group). An additional control group consisted of CCI-treated mice that received i.t. saline with pDNA-IL-10 (N = 7). Following injection, a main effect of time (ipsilateral, F(6.0, 233.1) = 49.09, P < 0.001; contralateral, F(8, 312) = 46.2, P < 0.001) and an interaction of time and treatment (ipsilateral, F(23.9, 233.1) = 29.11, P < 0.001; contralateral, F(32, 312) = 25.13, P < 0.001) were observed. Comparisons between groups reveal that on Day 2 post-injection, CCI+DM/pDNA-IL-10-treated mice exhibit bilateral reversal from allodynia compared to mice lacking DM treatment (CCI+Saline/pDNA-IL-10) (ipsilateral and contralateral, P < 0.001). Surprisingly, on Day 3 post-injection, mice given DM and the control plasmid (CCI+DM/pDNA-Ctrl) reach levels of hindpaw sensitivity similar to Sham+DM/pDNA-IL-10-treated controls (ipsilateral and contralateral, P > 0.05). Beginning on Day 6 post-injection, CCI+DM/pDNA-Ctrl mice begin to return to bilateral allodynia (ipsilateral, F(3, 117) = 19.67, P < 0.001; contralateral, F(3, 117) = 9.73, P < 0.001), while mice treated with DM/pDNA-IL-10 never return to allodynia. (C–E) mRNA isolated from DRG and cauda equina tissues collected on Day 12 post-injection (Day 17 post-surgery) reveal IL-10 expression. IL-10 (Il10) mRNA levels were observed in WT Naïve positive controls (N = 3) but not in IL-10 KO Naïves (N = 2) for (C) ipsilateral, (D) contralateral lumbar DRG, and (F) lumbar spinal cord tissues (*P < 0.05), but not (E) cauda equina, despite a strong trend for positive expression (P = 0.062). IL-10 mRNA expression was not significantly different between Sham-treated conditions for DRG, cauda equina, and lumbar spinal cord (P > 0.05). (C) IL-10 transgene mRNA expression was significantly elevated in ipsilateral DRG of pain-relieved CCI+DM/pDNA-IL-10-treated IL-10 KO mice compared to IL-10 KO mice given either CCI+DM/pDNA-Ctrl or Sham+DM/pDNA-Ctrl (F(4, 28) = 7.72, P < 0.001). (D) No significant increase in IL-10 transgene mRNA levels were detected in contralateral DRG following i.t. injection (F(4, 29) = 2.20, P = 0.093). However, a priori comparison revealed that contralateral DRG from CCI-treated mice given DM co-treated with the IL-10 transgene contained significantly greater IL-10 mRNA levels than DRG from CCI-treated mice given DM with the control plasmid (P < 0.05) or Sham-treated mice with DM and the control plasmid (P < 0.05). (E) In the cauda equina, IL-10 transgene mRNA expression was greatly elevated in pain-relieved CCI+DM/pDNA-IL-10 KO mice compared to negative control conditions CCI+DM/pDNA-Ctrl and Sham/DM+pDNA-Ctrl (F(4, 23) = 4.19, P = 0.011). (G) IL-10 protein was detected in ipsilateral sciatic nerve (collected Day 12 post-injection) of WT Naïve but not IL-10 KO Naïve mice (P < 0.05), but no significant increase in IL-10 expression was detected in any i.t. injection condition (F(4, 27) = 0.65, P > 0.05). For mRNA and protein analyses, N = 5–7 mice/group. mRNA levels (mean ± SEM) are normalized to 18S rRNA and graphically presented relative to mean WT Naïve levels. For protein analyses, protein levels are presented as mean ± SEM. Post-hoc multiple comparisons via Fisher’s LSD (α = 0.05; *P < 0.05; **P < 0.01; ***P < 0.001).
3.3 I.t. non-viral IL-10 transgene mRNA is expressed in lumbar dorsal root ganglia
To investigate the IL-10 expression patterns that underlie i.t. non-viral IL-10 transgene-mediated pain relief in the absence of endogenous IL-10, nervous tissues were collected from behaviorally verified IL-10 KO mice (See section 3.2) on Day 12 post-injection (Day 17 post-surgery). This “midway” timepoint was chosen for tissue analysis because CCI+DM/pDNA-IL-10 mice are stably reversed from allodynia compared to all other CCI-treated groups (Fig. 2A–B). IL-10 transgene expression was examined in relevant “pain pathway” tissues, including ipsilateral and contralateral lumbar (L3-5) DRG, cauda equina, lumbar spinal cord, and ipsilateral sciatic nerve. Of note, the i.t. injections were performed such that pDNA was deposited into the spinal subarachnoid area at the level of the cauda equina (acute transcutaneous puncture between vertebral L5 and L6 interspinous processes). In IL-10 KO Naïve (no surgery and no injection) mice, IL-10 mRNA was undetectable in ipsilateral and contralateral DRG, but was present in samples from WT Naïve mice serving as positive controls (Fig 2C–D). In ipsilateral DRG (Fig. 2C), IL-10 transgene mRNA was significantly elevated in pain relieved IL-10 KO+CCI+DM/pDNA-IL-10 mice compared to chronically allodynic CCI+DM/pDNA-Ctrl controls and un-injured Sham controls. In contralateral DRG (Fig. 2D), IL-10 transgene expression was not significantly different across treatment groups. However, an a priori comparison revealed that contralateral DRG from CCI-treated mice given DM co-treated with the IL-10 transgene contained significantly greater IL-10 mRNA levels than DRG from CCI-treated mice given DM with the control plasmid (P < 0.05) or Sham-treated mice with DM and the control plasmid (P < 0.05). Similar findings in the cauda equina (Fig. 2E) revealed IL-10 transgene was significantly increased in pain-relieved CCI-treated mice given DM plus the IL-10 transgene compared to either CCI-treated mice given DM plus the control plasmid or Sham-treated mice given DM plus the control plasmid. While IL-10 mRNA levels were not significantly different between WT Naïve and IL-10 KO Naïve cauda equina samples, a strong trend for positive expression in WT animals was observed (P = 0.06). Surprisingly, IL-10 transgene mRNA was not detectable in lumbar spinal cord (Fig. 2F) of any experimental condition, though it was readily detectable from spinal cords of WT Naïve positive controls. As expected, IL-10 mRNA levels were not detected in ipsilateral sciatic nerve in any treatment condition, nor were IL-10 mRNA levels detected in the uninjured WT Naïve tissue (data not shown; WT Naïve vs. IL-10 KO Naïve, P = 0.226). Similarly, though IL-10 protein was detected in ipsilateral sciatic nerve tissue of WT Naïves (Fig. 2G), it was not observed in IL-10 KO sciatic nerve tissues from any treatment condition. These data demonstrate that long-duration relief of allodynia is likely mediated by long-term survival of the IL-10 plasmid and corresponding protein expression in the DRG. Additionally, non-viral gene therapeutic modulation of the lumbar DRG is sufficient to relieve bilateral light touch sensitivity following unilateral peripheral nerve damage.
3.4 IL-10 transgene mediates pain relief through anti-inflammatory changes in ipsilateral lumbar DRG
To further characterize the cytokine environment in the presence of elevated IL-10 transgene mRNA levels, the mRNA expression levels of pro- and anti-inflammatory cytokines in the ipsilateral and contralateral lumbar (L3–L5) DRG were assessed in tissues collected on Day 12 post-injection (See section 3.3). Interestingly, significantly greater mRNA levels for the pro-inflammatory TNF (Tnf) were observed in ipsilateral DRG of IL-10 KO Naïves compared to WT Naïves (Fig. 3A), but not in contralateral DRG (Fig. 3B). In addition, while TNF mRNA transcript levels were elevated in ipsilateral DRG following CCI, significantly less TNF mRNA expression was observed in pain-relieved mice treated with DM plus the IL-10 transgene compared to allodynic CCI-treated mice given Saline plus the IL-10 transgene (Fig. 3A). No differences in TNF mRNA levels were detected in un-injured contralateral DRG (Fig. 3B). Expression of mRNA for the pro-inflammatory cytokine IL-1β (Il1b) in ipsilateral DRG was similar across all conditions and was not different between WT Naïve and KO Naïve mice (Fig. 3E).
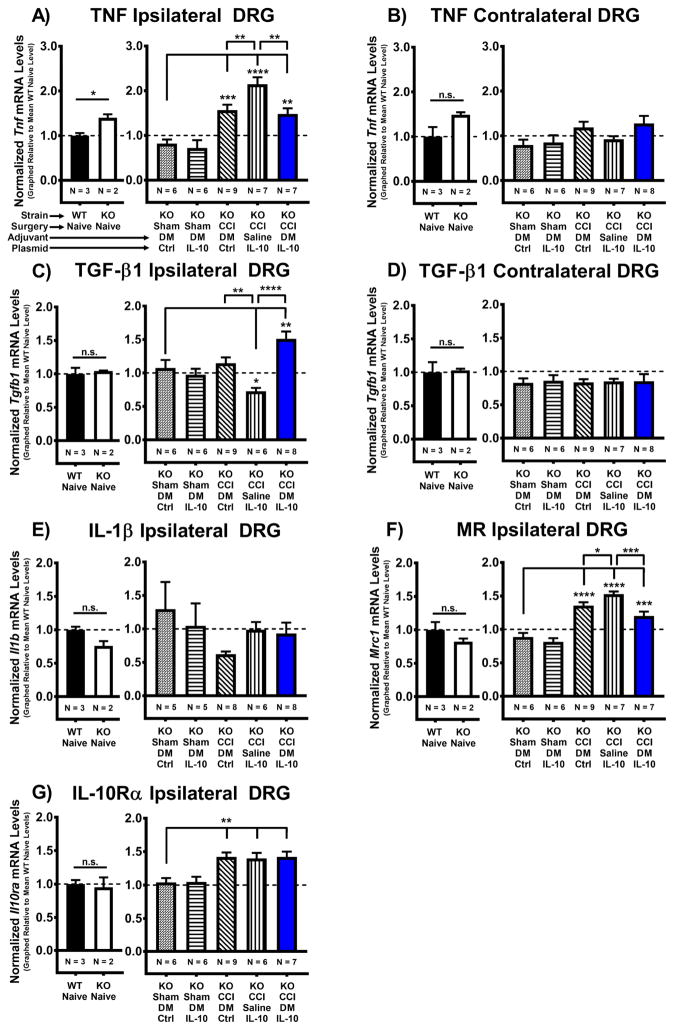
Figure 3. Therapeutic expression of IL-10 transgene mediates pain relief through anti-inflammatory changes in ipsilateral lumbar DRG.
mRNA was isolated from ipsilateral and contralateral Lumbar DRG (L3-5) on Day 12 post-injection (Day 17 post-surgery). (A–F) Baseline transcript levels were compared between WT Naïve (N = 3 mice) and IL-10 KO Naïve (N = 2 mice) groups. Transcript levels for TNF mRNA are significantly higher in ipsilateral DRG from IL-10 KO Naïves compared to WT Naïves (*P < 0.05), but not contralateral DRG (P > 0.05). Tgfb1, Il1b, Mrc1, and Il10ra mRNA transcript levels were not significantly different between WT and IL-10 KO Naïve groups in either ipsilateral or contralateral DRG (P > 0.05). (A) Post-surgical mRNA levels of pro-inflammatory cytokine TNF are greatly increased in ipsilateral lumbar DRG from all CCI conditions compared to Sham+DM/pDNA-Ctrl treated mice, (F(4, 30) = 17.17, P < 0.0001). However, TNF mRNA expression was significantly decreased in pain relieved CCI+DM/pDNA-IL-10 mice compared to allodynic CCI+Saline/pDNA-IL-10 controls. Interestingly, TNF mRNA expression was also significantly decreased in CCI+DM/pDNA-Ctrl mice that recently returned to allodynia as compared to CCI+Saline/pDNA-IL-10 controls. (B) No statistically significant difference was detected for TNF mRNA levels in contralateral DRG by 1-way ANOVA (F(4, 31) = 2.4, P > 0.05). (C) Transcript levels of the anti-inflammatory cytokine TGF-β1 (Tgfb1) are decreased in lumbar DRG collected from chronically allodynic mice (CCI+Saline/pDNA-IL-10) compared to Sham+DM/pDNA-Ctrl treated mice (F(4, 31) = 11.13, P < 0.0001). In contrast, pain relieved CCI+DM/pDNA-IL-10 mice exhibited elevated TGF-β1 mRNA expression compared to both Sham+DM/pDNA-Ctrl treated mice (F(4, 30) = 8.67, P < 0.0001). TGF-β1 mRNA levels were significantly decreased in chronically allodynic CCI+Saline/pDNA-IL-10 mice as compared to both CCI+DM/pDNA-Ctrl and pain-relieved CCI+DM/pDNA-IL-10 mice. (D) No statistically significant difference was detected for TGF-β1 mRNA levels in contralateral DRG (F(4, 31) = 0.03, P > 0.05). (E) No statistically significant difference was detected for pro-inflammatory cytokine IL-1β (Il1b) mRNA transcript levels in ipsilateral DRG (F(4, 27) = 1.36, P > 0.05). (F) Post-surgical mRNA levels for the mannose receptor (Mrc1; MR; CD206) are increased in ipsilateral lumbar DRG from all CCI conditions compared to Sham+DM/pDNA-Control treated mice, with MR transcript levels significantly decreased in pain-reversed CCI+DM/pDNA-IL-10 mice and recently allodynic CCI+DM/pDNA-Ctrl mice as compared to chronically allodynic CCI+Saline/pDNA-IL-10-treated mice (F(4, 30) = 29.48, P < 0.0001). (G) For IL-10 receptor alpha (Il10ra) mRNA levels, all CCI conditions exhibited significant increases compared to Sham+DM/pDNA-Ctrl mice (**P < 0.01) (F(4, 30) = 6.82, P < 0.001). N = 5–9 mice/group for treatment groups, as indicated on graphs. mRNA levels (mean ± SEM) are normalized to 18S rRNA and graphically presented relative to mean WT Naïve levels. Post-hoc multiple comparisons via Fisher’s LSD (α = 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Given existing evidence that the anti-inflammatory cytokine TGF-β1 relieves neuropathic pain in animal models and is increased by IL-10, the current study examined potential changes in DRG Tgfb1 mRNA levels as a consequence of DM/pDNA-IL-10 gene therapy. TGF-β1 mRNA expression is significantly elevated in ipsilateral DRG (Fig. 3C) of only pain-relieved CCI+DM/pDNA-IL-10 mice. Additionally, allodynic CCI+Saline/pDNA-IL-10 mice have significantly less TGF-β1 mRNA expression compared to both non-injured Sham controls and CCI+DM/pDNA-IL-10 mice. In contrast, no significant changes in TGF-β1 mRNA levels are observed in the contralateral DRG (Fig. 3D).
Several striking aspects of the behavioral response to the DM plus pDNA-IL-10 co-therapy are: 1) the dramatic improvement of enduring pain control over the individual treatment of DM or pDNA-IL-10, and 2) the complete, albeit transient, allodynic reversal following DM treatment alone (no IL-10 transgene). These results support that the mannose receptor (MR; CD206) is not simply promoting phagocytosis of surrounding material, but rather MR-mediated signaling is able to induce significant changes in the DRG microenvironment. Therefore, to confirm that the DRG environment is amenable to DM treatment strategies, mRNA levels for the mannose receptor (Mrc1) were assessed. In addition, alterations in IL-10 receptor (IL-10R) expression could exist in IL-10 KO mice thereby underlying unforeseen behavioral adaptations. Thus, comparisons of mRNA levels for IL-10Rα (a.k.a. IL-10R1; Il10ra), the IL-10R component responsible for binding IL-10, between WT and IL-10 KO Naïve mice, and between various gene therapy treatment groups were assessed in the ipsilateral lumbar DRG (Fig. 3F–G). While there were no significant differences for MR or IL-10Rα mRNA levels between WT and IL-10 KO Naïves, both IL-10R and MR mRNA expression was significantly elevated in the ipsilateral DRG following peripheral nerve injury (i.e. CCI). Surprisingly, though stably pain-relieved CCI+DM/pDNA-IL-10 mice had elevations in MR mRNA expression compared to Sham controls, their levels were significantly less than those observed in allodynic CCI+Saline/pDNA-IL-10 controls. Similarly, MR mRNA levels were also decreased in mice that had undergone transient pain reversal following DM treatment (CCI+DM/pDNA-IL-10). Together, these findings support that non-viral IL-10 transgene therapy acts at the level of the ipsilateral lumbar DRG to promote an anti-inflammatory environment and consequent relief of allodynia. Given that MR mRNA expression is elevated following CCI, these data further suggest that an initial pro-inflammatory environment may be beneficial for the “transgene adjuvant” effects of DM.
3.5 Transcriptional and protein characterization of the CCI-damaged sciatic nerve: i.t. non-viral gene therapy relieves pain in IL-10 KO mice despite an ongoing pro-inflammatory environment at the level of the sciatic nerve
Ipsilateral sciatic nerve collected on Day 12 post-injection (See section 3.3) was analyzed for typical injury-associated pro-inflammatory transcriptional changes following peripheral nerve damage (Fig. 4). There were no significant differences observed between WT Naïve and IL-10 KO Naïve mice for any mRNA transcript examined (P > 0.05). The transcript levels for the key pro-inflammatory cytokines TNF and IL-1β (Fig. 4A–B), and the pro-inflammatory chemokine CCL2 (Ccl2) (Fig. 4C) were significantly elevated in all IL-10 KO CCI conditions compared to Shams. To further assess typical immune cellular responses to sciatic nerve injury, markers for macrophages and T cells were examined. mRNA levels for the macrophage marker CD11b (Itgam) were significantly elevated compared to Sham-treated mice (Fig. 4D). Additionally, mRNA transcript levels for the general T cell marker CD3 (Cd3e) were elevated in CCI mice compared to Shams (Fig. 4E). mRNA transcript levels for the anti-inflammatory cytokine TGF-β1 were examined (Fig. 4F) as a possible compensatory cytokine in the absence of endogenous IL-10. Tgfb1 transcript expression was significantly elevated in all CCI conditions following injury.
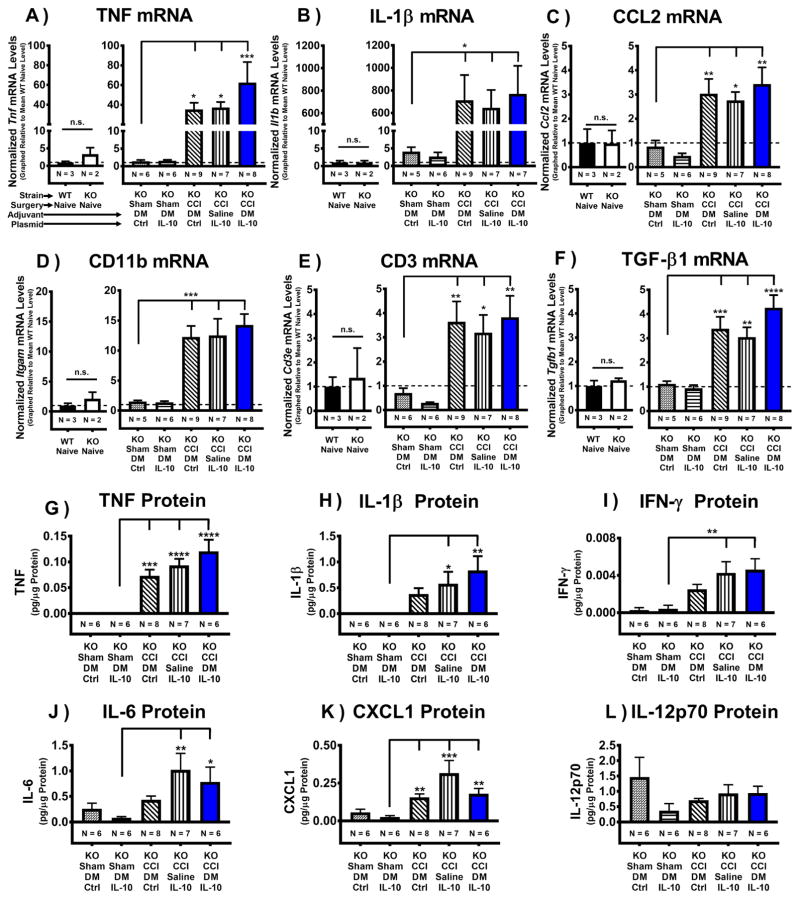
Figure 4. Pro-inflammatory characterization of the CCI-damaged sciatic nerve following i.t. non-viral IL-10 gene therapy in IL-10 KO mice.
mRNA was isolated from ipsilateral sciatic nerve (ipsi SCN) collected on Day 12 post-injection (Day 17 post-surgery) from previously behaviorally verified animals (Fig 3). (A–F) Baseline transcription levels are not significantly different between WT Naïve and IL-10 KO Naïve groups (N = 2–3 mice/group) for all targets (P > 0.05): Tnf, Il1b, Tgfb1, Ccl2, Itgam, and Cd3e. (A–B) Post-surgical mRNA levels of pro-inflammatory cytokines TNF (Tnf) and IL-1β (Il1b) are greatly increased in ipsi SCN from all CCI conditions compared to Sham+DM/pDNA-Control treated mice (F(4, 31) = 5.07, P < 0.01; F(4, 29) = 3.769, P = 0.0138, respectively). (C) mRNA levels of pro-inflammatory chemokine CCL2 (Ccl2) are greatly increased across all CCI conditions compared to Sham+DM/pDNA-Ctrl treated mice (F(4, 30) = 5.92, P < 0.01). (D) Following CCI surgery, the macrophage activation marker CD11b (itgam) is elevated in ipsi SCN, with CD11b mRNA levels significantly increased across all CCI conditions compared to Sham+DM/pDNA-Ctrl treated mice (F(4, 29) = 3.77, P < 0.05). (E) The general T cell marker CD3e (Cd3e) is elevated in sciatic tissue following CCI surgery, with CD3e mRNA expression significantly increased across all CCI conditions compared to Sham+DM/pDNA-Ctrl treated mice (F(4, 29) = 3.77, P < 0.05). (F) Post-surgical mRNA transcript levels of anti-inflammatory cytokine TGF-β1 are greatly increased across all CCI conditions compared to Sham+DM/pDNA-Ctrl treated mice (F(4, 31) = 11.13, P < 0.0001). N = 5–9 mice/group for treatment groups, as indicated on graphs. (G–L) Complementary protein analysis for ipsi SCN from the same animals previously assessed for mRNA analysis (A–F). Baseline protein levels are not significantly different between WT Naïve and IL-10 KO Naïve groups (N = 2–3 mice/group) for all targets (data not shown; P > 0.05): TNF, IL-1β, IFN-γ, IL-6, CXCL1, and IL-12p70. No significant differences were observed between Sham conditions (Sham+DM/pDNA-Ctrl and Sham+DM/pDNA-IL-10) for all protein targets (P > 0.05). (G) Pro-inflammatory cytokine TNF protein expression levels were elevated across all CCI conditions compared to Sham+DM/pDNA-IL-10 (F(4, 28) = 15.54, P < 0.0001). (H) In most CCI conditions, protein levels for the pro-inflammatory cytokine IL-1β are significantly increased in ipsi SCN compared to Sham+DM/pDNA-IL-10 (F(4, 28) = 4.33, P < 0.01). (I) Protein levels for the pro-inflammatory cytokine IFN-γ are greater in most CCI conditions compared to Sham+DM/pDNA-IL-10 (F(4, 28) = 5.97, P < 0.01). (J) IL-6, a pro-inflammatory cytokine, is significantly increased in ipsi SCN for most CCI conditions compared to Sham+DM/pDNA-IL-10 (F(4, 27) = 3.47, P < 0.05). (K) CXCL1 (a.k.a. KC/GRO), a neutrophil chemoattractant, is significantly elevated following CCI surgery compared to Sham+DM/pDNA-IL-10 (F(4, 27) = 6.45, P < 0.001). (L) No significant increases in IL-12p70, a pro-inflammatory cytokine downstream of CpG-activated TLR-9, were observed for any condition (F(4, 28) = 1.46, P > 0.05). For protein analyses, N = 6–8 mice/group as indicated, and protein levels are presented as mean ± SEM. For mRNA analyses, N = 5–9 mice/group as indicated, and mRNA levels (mean ± SEM) are normalized to 18S rRNA and graphically presented relative to mean WT Naïve levels. Post-hoc multiple comparisons via Fisher’s LSD (α = 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
While mRNA analysis provides strong evidence for the presence of key factors, their protein products could be quickly degraded resulting in little to no physiological impact. Consequently, complementary protein analysis provides a balanced approach to characterizing the damaged sciatic nerve. Here, multiplex protein analysis of injury-associated pro-inflammatory chemokines and cytokines in the sciatic nerve ipsilateral to the CCI surgery were examined in samples from the same animals previously assessed for injury-induced changes in mRNA (Fig. 4G–L). While no significant differences between WT Naïve and IL-10 KO Naïve nerves for any protein target examined were observed (data not shown; P > 0.05), not surprisingly, pro-inflammatory TNF protein levels were elevated in all CCI conditions (Fig. 4G). Additionally, the detected protein levels for the pro-inflammatory cytokines IL-1β, IFN-γ, and IL-6 were significantly elevated in both allodynic CCI+Saline/pDNA-IL-10 mice and non-allodynic CCI+DM/pDNA-IL-10 mice compared to Sham controls (Fig. 4H–J). CXCL1, a neutrophil chemoattractant molecule, was elevated in all CCI conditions compared to Sham controls (Fig. 4K). No differences in bioactive IL-12p70, a pro-inflammatory cytokine downstream of CpG-activated TLR-9, were observed (Fig. 4L).
Together, these mRNA and protein observations support that all IL-10 KO CCI animals experienced similar local responses to peripheral nerve damage regardless of i.t. gene therapy manipulation. Furthermore, the pain-suppressive actions of intrathecally delivered DM and IL-10 transgene co-therapy appear to occur in the DRG in IL-10 KO mice, despite a potent pro-inflammatory milieu at the level of the sciatic nerve.
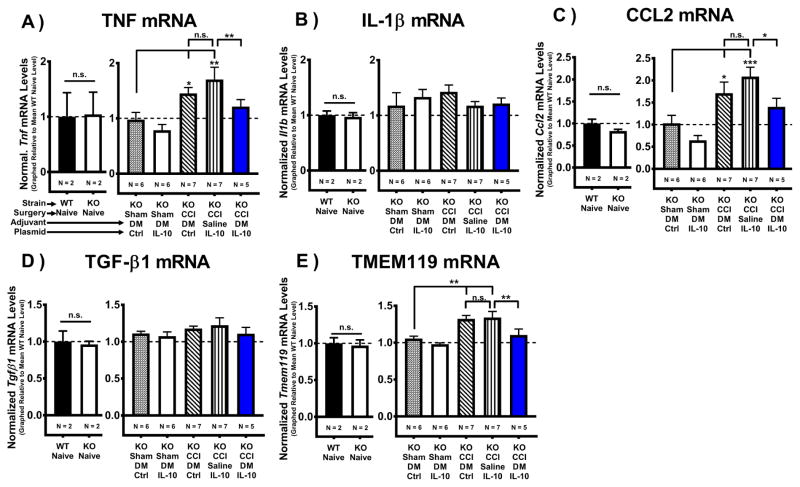
3.6 I.t. non-viral IL-10 gene therapy alters lumbar cytokines and microglial activation
To assess the spinal impact of DRG-mediated IL-10 transgene expression, mRNA levels were assessed in lumbar spinal cord tissue collected on Day 12 post-injection (See section 3.3). Levels of mRNA for the pro-inflammatory cytokine TNF (Tnf) (Fig. 5A) and the pro-inflammatory chemokine CCL2 (Ccl2) (Fig. 5C) were significantly elevated in allodynic mice given non-IL-10 control gene therapy, while spinal cords from pain-relieved IL-10 gene therapy-treated mice (CCI+DM/pDNA-IL-10) revealed a significant decrease in TNF and CCL2 mRNA compared to Sham-treated controls. Additionally, significant decreases for both targets were observed in pain-relieved mice compared to allodynic CCI+Saline/pDNA-IL-10 controls. (Fig. 5B) Surprisingly, no significant differences in pro-inflammatory cytokine IL-1β (Il1b) mRNA transcript levels were observed between any condition.
Figure 5. Intrathecal non-viral IL-10 gene therapy alters lumbar cytokines and decreases microglial activation.
Characterization of isolated cytokines and microglial-specific mRNA from the lumbar spinal cord collected on Day 12 after i.t. IL-10 gene therapy injection (Day 17 post-surgery). (A–F) Baseline transcription levels are not significantly different between WT Naïve and IL-10 KO Naïve groups (N = 2 mice/group) for all targets (P > 0.05): Tmem119, Tnf, Il1b, Ccl2, and Tgfb1. (A) mRNA transcript levels for the pro-inflammatory cytokine TNF (Tnf) and (C) the pro-inflammatory chemokine CCL2 (Ccl2) were elevated only in allodynic controls, with significantly less TNF and CCL2 mRNA expression observed in pain-relieved CCI+DM/pDNA-IL-10 vs. allodynic CCI+Saline/pDNA-IL-10 mice (TNF: F(4, 26) = 6.18, P < 0.01) (CCL2: F(4, 26) = 8.10, P < 0.0001). (B) No significant differences in mRNA transcript levels for the pro-inflammatory cytokine IL-1β (Il1b) were detected (F(4, 24) = 0.6927, P > 0.05). (D) No significant differences in mRNA levels for anti-inflammatory cytokine TGF-β1 (Tgfb1) were detected for any condition (F(4, 25) = 0.77, P > 0.05). (E) mRNA transcript levels for the microglial marker TMEM119 (Tmem119) were elevated in allodynic CCI controls, but not in pain-relieved CCI+DM/pDNA-IL-10 mice. Additionally, TMEM119 mRNA levels in pain-relieved CCI+DM/IL-10 treated mice were significantly less than for allodynic CCI+Saline/IL-10 mice (F(4, 26) = 8.24, P < 0.001). mRNA levels (mean ± SEM) are normalized to 18S rRNA and graphically presented relative to mean WT Naïve levels. Post-hoc multiple comparisons via Fisher’s LSD (α = 0.05; *P < 0.05; **P < 0.01; ***P < 0.001).
Interestingly, though mRNA levels for the anti-inflammatory cytokine TGF-β1 (Tgfb1) were found to be elevated only in ipsilateral DRG from pain-relieved DM/pDNA-IL-10 mice (Fig. 3D), no significant differences in Tgfb1 levels were observed in the lumbar spinal cord for any condition (Fig. 5D).
Spinal microglial activation is a critical component of pathologic pain (Hughes et al., 2009) but has not yet been examined following naked non-viral IL-10 gene therapy. A previously described novel transmembrane protein 119 (TMEM119) that identifies solely microglia (Bennett et al., 2016). Though TMEM119 has not been previously identified as a marker of microglial activation, we chose to examine changes in TMEM119 mRNA expression to assess whether spinal non-viral IL-10 gene therapy reduces pathological microglial activation. Examination of TMEM119 (Tmem119) mRNA (Fig. 5E) revealed spinal cords from allodynic CCI-treated mice given control gene therapy treatment (CCI+DM/Ctrl or CCI+Saline/IL-10) have significantly elevated Tmem119 transcript levels compared to Sham controls. However, Tmem119 mRNA expression is decreased in pain-relieved (CCI+DM/pDNA-IL-10) mice compared to allodynic CCI+Saline/pDNA-IL-10 controls. This evidence suggests that changes in expression of TMEM119 may serve as a useful marker of both pathologic microglial activation and a biomarker of therapeutic efficacy. Furthermore, these data support that non-viral DM/pDNA-IL-10 gene therapeutic actions at the level of the DRG lead to decreased microglial activation at the level of the lumbar spinal cord.
Together, these data support that the therapeutic actions of IL-10 transgene at the level of the ipsilateral DRG lead to decreased lumbar spinal microglial activation in parallel with decreased spinal production of classic pain-relevant pro-inflammatory cytokines and chemokines.
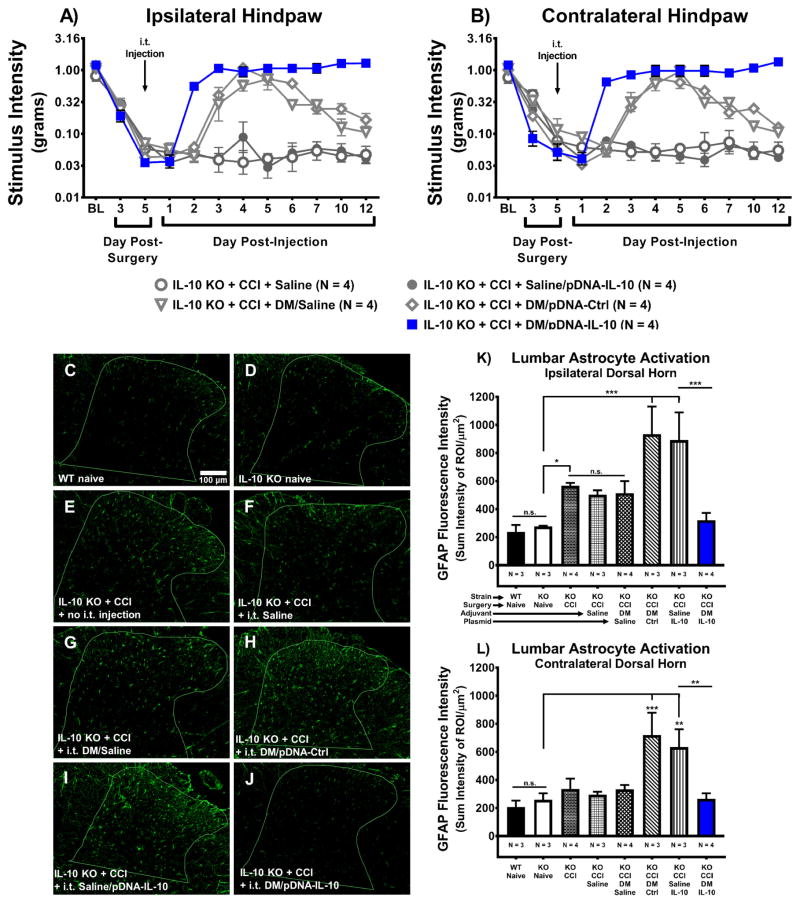
3.7 I.t. non-viral IL-10 gene therapy decreases astrocyte activation in the dorsal horn of the lumbar spinal cord
While lumbar spinal glial activation has been extensively examined in a variety of animal models (Garrison et al., 1991; Meller et al., 1994; Watkins et al., 1997; Colburn et al., 1999; Sweitzer et al., 1999; Hashizume et al., 2000; Milligan et al., 2001; Watkins and Maier, 2002), the underlying glial mechanisms at the level of the lumbar spinal cord following naked non-viral IL-10 gene therapy were previously unknown. It has not yet been characterized whether subarachnoid IL-10 gene therapy that alleviates allodynia also reduces L3–L5 dorsal horn astrocyte activation. Therefore, the current study aimed to examine whether subarachnoid IL-10 gene therapy that alleviates allodynia in behaviorally verified mice also reduces L3–L5 dorsal horn astrocyte activation as indicated by immunoreactive levels of glial fibrillary acidic protein (GFAP). GFAP is an astrocytic protein that increases in expression as astrocyte activation increases. At BL prior to surgery and i.t. injection, all mice revealed similar responses to light mechanical touch (Fig. 6A–B). CCI-treated IL-10 KO mice develop clear bilateral allodynia by Day 3 post-surgery. All groups received an i.t. injection following behavioral assessment on Day 5 post-surgery. On Day 2 post-injection, mice given DM/pDNA-IL-10 reveal bilateral reversal of allodynia compared to CCI mice treated with various control transgene injections. As before, IL-10 KO mice treated with CCI+DM/pDNA-IL-10 exhibit stable pain relief for the remainder of the timecourse. Mice given i.t. DM/Saline or i.t. DM/pDNA-Ctrl reveal transient pain reversal beginning on Day 3 post-injection, and returning to allodynia beginning on Day 6 post-injection. The additional control group of un-injected IL-10 KO CCI mice (N = 4) was behaviorally verified and revealed bilateral allodynia on Day 17 post-surgery with the following stimulus intensity thresholds (mean ± SEM) 0.032g ± 0.004g and 0.048g ± 0.009g for the left and right hindpaws, respectively (data not shown).
Figure 6. Intrathecal non-viral IL-10 gene therapy decreases astrocyte activation in the lumbar spinal cord dorsal horn.
Absolute threshold behavioral responses for (A) ipsilateral and (B) contralateral hindpaws are shown. Again replicating our prior results illustrated in Figs. 1 and 2, BL responses to low threshold mechanical stimuli were similar between all groups (ipsilateral, F(4, 15) = 1.25, P > 0.05; contralateral, F(4, 15) = 48.84, P < 0.001). For data collected between BL through Day 5, a main effect of time (ipsilateral, F(2, 30) = 306.14, P < 0.001; contralateral, F(2, 30) = 221.66, P < 0.001) was observed, again replicating our prior data (Figs. 1 and 2). After behavioral assessment on Day 5 post-surgery, all mice received an i.t. co-injection of pDNA-IL-10 (3μg in 7.5μL) with DM (25μg in 3μL) vs. DM/pDNA-Control, pDNA-IL-10 with saline, DM with saline, or saline alone. Following injection, a main effect of time (ipsilateral, F(8, 120) = 35.817, P < 0.001; contralateral, F(8, 120) = 42.82, P < 0.001) and an interaction of time and treatment (ipsilateral, F(32, 120) = 9.81, P < 0.001; contralateral, F(32, 120) = 13.53, P < 0.001) were revealed. Comparisons between groups show that on Day 2 post-injection, CCI+DM/pDNA-IL-10-treated mice exhibit bilateral reversal from allodynia (ipsilateral, P < 0.001; contralateral, P < 0.001) compared to CCI+Saline/pDNA-IL-10-treated control mice, and these mice never return to allodynia. Surprisingly, on Day 3 post-injection, DM/Saline and DM/pDNA-Ctrl treated mice reverse from allodynia (ipsilateral, P < 0.001; contralateral, P < 0.001) but on Day 6 post-injection both control groups begin to return to bilateral allodynia (ipsilateral, F(3, 45) = 6.27, P < 0.01; contralateral, F(3, 45) = 5.62, P < 0.01). These transient effects of DM on pain thresholds was previously observed in data from separate experiments represented in Fig. 2. N = 4 mice/group for all behavioral conditions. (C–J) Representative images (20x objective) of GFAP-stained lumbar spinal cord dorsal horn sections from tissues collected on Day 12 post-injection that were part of the image analysis. (K–L) Image analysis of acquired GFAP-stained images of ipsilateral and contralateral lumbar spinal cord dorsal horn revealed a main effect of gene therapy treatment (F(7, 19) = 6.71, P < 0.001; F(7, 19) = 5.64, P < 0.01, respectively). Acquired GFAP-stained images of contralateral lumbar spinal cord dorsal horn are not shown, as the images appear highly similar to those from the ipsilateral lumbar spinal cord. Statistical analyses reveal no significant ipsilateral or contralateral differences in GFAP fluorescence intensity between WT Naïve and IL-10 KO Naïve tissues. IL-10 KO+CCI un-injected mice exhibit heightened ipsilateral GFAP fluorescence intensity compared to IL-10 KO Naïves and are not significantly different than CCI+Saline or CCI+ DM/Saline conditions. Bilaterally, GFAP immunoreactive fluorescence intensity levels are greater in IL-10 KO+CCI+DM/pDNA-Ctrl mice and CCI+Saline/pDNA-IL-10 mice compared to IL-10 KO Naïve mice, but do not differ significantly from each other. Pain relieved IL-10 gene therapy treated mice (IL-10 KO+CCI+DM/pDNA-IL-10) reveal significantly less GFAP fluorescence intensity compared to allodynic gene therapy controls (IL-10 KO+CCI mice treated with either DM/pDNA-Ctrl or Saline/pDNA-IL-10), and are not significantly greater than IL-10 KO Naïves. Bar graphs represent mean ± SEM. Superimposed outlines in images (C–J) demarcate the unique dorsal horn regions of interest for each slice. For immunohistochemical analyses, N = 3–4 mice per group, as indicated. Post-hoc multiple comparisons via Fisher’s LSD (α = 0.05; *P < 0.05; **P < 0.01; ***P < 0.001).
Following behavioral assessment on Day 12 post-injection, lumbar spinal tissues were collected and assessed for GFAP immunoreactivity (IR) by immunohistochemistry. Representative photomicrographs (Fig. 6C–J) of ipsilateral lumbar dorsal horn are shown. As expected, GFAP IR is present at low levels in Naïve conditions, but notably elevated in most CCI conditions. Importantly, GFAP IR for pain relieved CCI+DM/pDNA-IL-10 mice reveals GFAP IR levels similar to those observed for Naïve mice. Analysis of (Fig. 6K) ipsilateral and (Fig. 6L) contralateral (contralateral lumbar dorsal horn images not shown) lumbar dorsal horn GFAP IR quantification show no significant difference between WT and IL-10 KO Naïves. However, significantly more GFAP fluorescence intensity is observed in the ipsilateral dorsal horn following CCI surgery, with no significant difference between CCI alone, CCI+Saline, and CCI+DM/Saline groups. Curiously, the greatest levels of GFAP IR in the spinal cord were observed bilaterally in control therapy treated CCI-mice (DM/pDNA-Ctrl and Saline/pDNA-IL-10), which are also significantly greater than those observed in pain-relieved DM plus IL-10 gene co-therapy treated mice (CCI+DM/pDNA-IL-10).
Several key comparisons, were predicted, a priori, to be significantly different. Unpaired t-test revealed differences between CCI+DM/pDNA-IL10 vs. CCI alone (P < 0.001) and CCI+Saline (P < 0.05). Conversely, GFAP IR levels for CCI+DM/pDNA-IL-10 mice are strikingly similar (not significantly different) to Naïve conditions. The heightened GFAP IR observed in DM/pDNA-Ctrl and Saline/pDNA-IL-10 treated mice supports the possibility that the IL-10 transgene does not influence GFAP IR. Rather, it is hypothesized that pDNA itself (either lacking or encoding IL-10) at the DRG creates additional pro-inflammatory signaling downstream of TLR-9 activation via unmethylated CpGs present in the plasmid vector (Soderquist et al., 2009). Indeed, pDNA treatment leads to increased production of pro-inflammatory mediators (Soderquist et al., 2009). Thus, elevated DRG pro-inflammatory drive results in elevated spinal GFAP. Though pain-relieved DM/pDNA-IL-10 treated mice were also exposed to unmethylated CpGs, these mice additionally received D-mannose. That is, MR activation, in conjunction with IL-10 transgene expression, increases TGF-β1 mRNA transcription in the DRG resulting in greater anti-inflammatory drive from the DRG with a consequent reduction of the pro-inflammatory drive to the spinal cord. Thus, reduced spinal astrocyte activation following DM/pDNA-IL-10 co-therapy is a predicted result despite the presence of CpG DNA.
Therefore, these data support that i.t. non-viral gene therapy leading to DRG expression of IL-10 transgene modulates pro-inflammatory factors in the lumbar spinal cord dorsal horn, supporting an anti-inflammatory spinal milieu.
4. Discussion
While i.t. non-viral IL-10 gene therapy is efficacious for the treatment of chronic pain in several rat models of peripheral neuropathy (Milligan et al., 2006b; Milligan et al., 2006a; Ledeboer et al., 2007; Sloane et al., 2009b; Sloane et al., 2009a; Milligan et al., 2012; Dengler et al., 2014), the current data provide new insight into underlying mechanisms by which a unique non-viral vector formulation results in robust and enduring pain control. Here, the unique combination of delivering D-mannose (DM) with non-viral plasmid DNA encoding the IL-10 transgene (DM/pDNA-IL-10) unambiguously demonstrates that endogenous IL-10 is not required for enduring and profound pain reversal following DM/pDNA-IL-10 co-therapy. Additionally, the biodistribution of transgene IL-10 mRNA expression following i.t. DM/pDNA-IL-10 co-therapy in IL-10 deficient mice is robustly observed in the ipsilateral DRG and cauda equina, but not in the lumbar spinal cord. The corresponding pain-associated pro-inflammatory cytokine TNF mRNA expression is decreased in pain-relieved mice given DM/pDNA-IL-10 co-therapy compared to allodynic controls. In contrast, the anti-inflammatory cytokine TGF-β1 mRNA expression is increased in the DRG of gene therapy-treated pain-reversed animals. Robust mRNA changes observed in the DRG of neuropathic mice prompted examination of mRNA levels for the mannose receptor (MR) and the IL-10 receptor (IL-10Rα), both of which are significantly upregulated following peripheral nerve damage, supporting their availability and action for DM/pDNA-IL-10 co-therapy in treatment of pathological conditions.
IL-10 transgene mRNA expression is predominantly present in the DRG and not the lumbar spinal cord, while dramatic pain-related cytokine changes are observed in the lumbar spinal cord. Specifically, mRNA levels for TNF and the pro-inflammatory chemokine CCL2, but not IL-1β or TGF-β1, are upregulated in spinal cords of allodynic CCI mice. In contrast, pain-relieved mice given DM/pDNA-IL-10 co-therapy have spinal levels of TNF and CCL2 mRNA no different from uninjured Sham controls. Upon examination of the lumbar spinal mRNA expression for the microglial-specific transmembrane protein 119 (TMEM119), previously reported to upregulate upon increased microglial activity (i.e. proliferation) (Bennett et al., 2016), the activation state of microglia is significantly reduced in DM/pDNA-IL-10-treated pain-reversed mice. Similarly, immunohistochemical analysis for changes in astrocyte activation in the dorsal horn of the spinal cord reveal a profound reduction in GFAP immunoreactivity in DM/pDNA-IL-10 treated pain-reversed mice. Importantly, cytokine mRNA and protein levels from the injured mouse sciatic nerve reveal equivalently strong upregulation of pro-inflammatory cytokines, as well as general monocyte and T cell involvement regardless of injection formulation. Thus, these data support that i.t. non-viral DM/pDNA-IL-10 co-therapy provides stable and long-lasting relief from peripheral neuropathic pain through anti-inflammatory actions at the level of the DRG and consequent anti-inflammatory actions at the level of the lumbar spinal cord.
Endogenous IL-10 is not required for efficacy of therapeutic mouse IL-10 gene containing a known non-silent point mutation: mIL-10F129S
We show for the first time that the known point mutation present in the mouse IL-10 (mIL-10F129S) transgene expressed by the pDNA-IL-10 used in these studies (Fig 1E) does not require endogenous IL-10 to exert IL-10 action. IL-10 monomers associate as homodimers prior to binding with the interleukin-10 receptor; IL-10Rα (a.k.a. IL-10R1) (Moore et al., 2001). Though this point mutation lies outside the IL-10 dimer’s known receptor binding region, it may still interfere with IL-10 homodimerization resulting in the possibility that the IL-10F129S protein could require wildtype IL-10 for adequate dimerization, receptor binding, and downstream anti-inflammatory function. However, preliminary in vitro work using macrophages and B cells suggest IL-10F129S may result in greater anti-inflammatory actions than wildtype IL-10 alone (Sloane et al., 2009b). While it is currently unknown whether pDNA-IL-10F129S lacks the requirement for dimerization, the findings in the current report demonstrate two critical issues: that 1) wildtype IL-10/IL-10F129S heterodimerization is not required for IL-10F129S efficacy, and 2) exogenous mIL-10F129S produces profound stable and long-lasting pain suppressive effects in the absence of endogenous wildtype IL-10.
The immune system has built in anti-inflammatory redundancy
An interesting behavioral finding in the current report is that loss of endogenous IL-10 may not lead to dramatic susceptibility to injury-induced pain pathology. This is surprising given that IL-10 deficiency and IL-10R mutations observed in humans and animal models (i.e. IL-10 KO mice and IL-10R KO mice) are associated with increased risk of enterocolitis (Kuhn et al., 1993; Ward et al., 1996; Spencer et al., 1998). In these cases, absence of IL-10 signaling leads to loss of intestinal mucosal immune homeostasis and consequent enhanced intestinal inflammation in response to the presence of gut microbiota (Shouval et al., 2014). In the current report, no sensory differences were observed between WT and IL-10 KO mice at BL, which is consistent with previous findings that IL-10 KO mice do not differ in their basal light touch sensitivity thresholds (da Silva et al., 2015; Siqueira Mietto et al., 2015; Krukowski et al., 2016), though there is some evidence that uninjured IL-10 KO mice may differ in their thermal nociceptive response (Tu et al., 2003). There are mixed reports regarding intensity and duration of allodynia in IL-10 KO mice following various models of injury (da Silva et al., 2015; Siqueira Mietto et al., 2015; Krukowski et al., 2016). For example, in a paclitaxel model of peripheral neuropathic pain, WT and IL-10 KO mice exhibited both a similar onset of hindpaw mechanical allodynia and a similar peak intensity of allodynia. However, the duration of the timecourse of allodynia was extended in paclitaxel-treated IL-10 KO compared to their WT counterparts (Krukowski et al., 2016). In a model of intramuscular carrageenan, no differences in IL-10 KO vs. WT were observed for allodynic intensity or timecourse duration in response to non-noxious cutaneous or muscular stimulation (da Silva et al., 2015). In a sciatic nerve crush model, the onset and duration of allodynia in WT vs. IL-10 KO mice was similar, though IL-10 KO mice were significantly more allodynic on the last day (Day 28 post-surgery) of the reported timecourse (Siqueira Mietto et al., 2015). Data from the current report indicate a similar onset, intensity, and duration of allodynia following peripheral nerve damage in IL-10 KO mice compared to WT mice, and both WT and IL-10 KO CCI mice given control gene therapy remain stably responsive throughout the entire timecourse. However, it is possible that the intensity of allodynia could be greater in IL-10 KO mice relative to WT mice, as the behavioral assessment assay may be limited in detecting maximal hindpaw sensitivity.
The similar levels of allodynia observed between IL-10 KO and WT mice following CCI may be a result of the functional redundancy that occurs within the immune system. Compensatory anti-inflammatory mechanisms could be engaged, which reduces the enhanced allodynia one would have predicted in an IL-10 KO model of CCI. While the data reported here reveal a potent pro-inflammatory mRNA and protein environment at the level of the sciatic nerve, congruent with previous characterizations of WT mice following sciatic CCI (Okamoto et al., 2001; Kleinschnitz et al., 2006; Uceyler et al., 2007), a clear concurrent upregulation of anti-inflammatory TGF-β1 mRNA was observed in the ipsilateral DRG of pain relieved DM/pDNA-IL-10 co-therapy treated mice.
One possible mechanism by which i.t. non-viral DM/pDNA-IL-10 co-therapy generates enduring pain suppression is via consequent elevated TGF-β1 expression in the DRG. TGF-β1 and IL-10 have bidirectional regulation, as prior work demonstrates astrocytes previously stimulated in vitro with lipopolysaccharide (LPS; a potent TLR-4 agonist) can be redirected by incubation with IL-10 to produce TGF-β (Norden et al., 2014). Additionally, IL-10 prevents enterocolitis through increased TGF-β production (Fuss et al., 2002). In turn, TGF-β promotes IL-10 upregulation in macrophages (Maeda et al., 1995) and is known to induce differentiation of IL-10-producing regulatory T cells (iTregs) and T regulatory type 1 cells (Tr1) (Kleinewietfeld and Hafler, 2014). Furthermore, TGF-β1 non-viral gene therapy administered intranasally for treatment of enterocolitis promotes beneficial effects via enhanced production of IL-10 (Kitani et al., 2000). In line with the anti-inflammatory function of TGF-β, prior work reveals that i.t. recombinant TGF-β1 attenuates neuropathic pain following partial ligation of the sciatic nerve in rats (Echeverry et al., 2009). However, in contrast to IL-10, which exerts predominantly anti-inflammatory actions (Saxena et al., 2015), TGF-β actions are determined by the nearby cellular and receptor milieu (Bottner et al., 2000). For example, TGF-β can act as either a dynamic tumor promoter or a tumor suppressor, thereby making the modulation of TGF-β alone a challenging and unpredictable therapeutic approach (Bottner et al., 2000; Colak and Ten Dijke, 2017). The possibility that the IL-10 transgene works synergistically with TGF-β1 for control of allodynia while potentially attenuating the untoward effects of TGF-β upregulation warrants further investigation.
Gene therapeutic modulation of the ipsilateral DRG is sufficient for enduring relief of bilateral allodynia
It is increasingly apparent in the literature that cytokine changes at the level of the DRG are deeply involved in the modulation of sensory information and the development of neuropathic pain (Krames, 2015). Therefore, it is noteworthy in this report that IL-10 transgene mRNA expression was detected in the ipsilateral DRG but not in the lumbar spinal cord where centrally projecting nociceptive terminals are communicating to pain projection neurons. What is most striking about the current data is that anti-inflammatory cytokine expression in the DRG is sufficient to drive anti-inflammatory changes at the level of the lumbar spinal cord (i.e. decreased mRNA for TNF and CCL2). The spinal anti-inflammatory bias following DM/pDNA-IL-10 co-therapy is further supported by decreased lumbar mRNA levels of the microglial marker TMEM119 (upregulates upon activation/proliferation), concurrent with bilateral decreases in GFAP IR in the lumbar spinal cord dorsal horn. In combination, the data reveal a profound reduction of lumbar spinal cord GFAP IR following DM/IL-10 combination therapy, but with less striking mRNA decreases in spinal TNF and CCL2 and DRG TNF, suggesting that the role of other factors critical for ongoing astrocyte activation may generate greater effects on spinal pain processing. Most importantly, the pro- vs. anti-inflammatory rebalancing in the lumbar spinal cord is reflected in the behavioral responses of stably pain-relieved mice treated with non-viral IL-10 gene therapy. These findings further suggest that the protein product of the IL-10 transgene does not require direct contact with pain projection neurons in the dorsal spinal cord, but rather, interaction with cell bodies in the DRG alone is sufficient to induce pain relief.
There are several potential mechanisms that IL-10 expression at the DRG leads to changes in neuronal signaling. For instance, several key mediators of neuropathic pain, such as TNF, increase neuronal excitability (Kagan et al., 1992; Watkins and Maier, 2005; Leung and Cahill, 2010; Grace et al., 2014; Ji et al., 2016). The current report demonstrates decreased TNF mRNA expression in the ipsilateral DRG of pain relieved DM/pDNA-IL-10 treated mice. This, in conjunction with synergistic IL-10/TGF-β1 actions, may lead to a decrease in TNF protein actions resulting in decreased neuronal excitability. While prior reports document neuronal IL-10R1 is present in the DRG and direct activation of neuronal IL-10R1 may help mediate decreases in pathologic neuronal signaling (Shen et al., 2013; Alvarez et al., 2017), it is speculated in the current report that immune- and glial cell-derived IL-10R1 is predominantly responsible for the anti-allodynic effect because neurons do not produce the cytokines measured here (i.e. TNF, IL-10 and TGF-β1). Currently, the source of pain-controlling TGF-β1 and IL-10 is thought to be from glial and immune cells following IL-10R1 activation. However, whether IL-10R1 activation on DRG neurons is required for the beneficial effects of DM/pDNA-IL-10 co-therapy will provide an intriguing avenue of future study.
In the current report, we demonstrate that unilateral DRG IL-10 transgene expression is sufficient for relief of bilateral allodynia. Our behavioral observations in the context of absent contralateral DRG pro-inflammatory cytokine changes support that bilateral relief is mediated through events occurring at the level of the lumbar spinal cord. Unfortunately, the mechanisms that underlie allodynia contralateral to the side of injury, clinically referred to as “mirror pain,” are not completely understood (Huang and Yu, 2010), though spinal glial activation is strongly implicated. For instance, work by Spataro et al. revealed that astrocyte communication via gap junctions may be critical for contralateral allodynia (Spataro et al., 2004). They elegantly demonstrated that i.t. administration of the gap junction decoupler carbenoxolone relieves contralateral, but not ipsilateral, mechanical allodynia following sciatic CCI in rats. They further argue that: 1) astrocytic gap junctions far outnumber those found on neurons, 2) though astrocytes are slow to activate, gap junctions are quickly upregulated (within 45 minutes post-nerve injury) and dynamically modifiable, and 3) astrocytes possess extensive gap junction-mediated glial connectivity throughout the spinal cord (Spataro et al., 2004). A competing hypothesis is that contralateral allodynia results from diffusion of ipsilaterally released pro-inflammatory cytokines through cerebrospinal fluid to act on contralateral pain-relevant anatomic structures (Milligan et al., 2001; Cheng et al., 2014). Alternatively, neurons of the ipsilateral spinal cord dorsal horn may directly communicate with neurons, and likely glia, in the contralateral spinal cord dorsal horn (Fitzgerald, 1982; Fitzgerald and Woolf, 1982). In the current report, the astrocyte activation marker GFAP revealed bilateral upregulation in the spinal cord dorsal horn in chronically bilaterally allodynic CCI animals. Interestingly, the presence of DM/pDNA-IL-10 co-therapy in bilaterally pain-relieved animals was sufficient to reduce bilateral CCI-induced GFAP IR to basal levels. These findings support a role for the actions of spinal astrocytes in the production and resolution of contralateral allodynia. Though mRNA levels for the microglial activation marker TMEM119 were decreased in pain-relieved mice compared to allodynic controls, we cannot draw conclusions about laterality as both ipsilateral and contralateral spinal cord segments were analyzed together as single mRNA samples. However, the literature so far demonstrates that microglia are not dramatically altered in the contralateral spinal cord, as assessed by common markers of microglial activation (Ji et al., 2013).
Changes in TMEM119 expression as a possible marker of microglial activation
It is well understood that nervous tissue insults, such as damage to peripheral nerves, lead to CNS “microgliosis,” a condition that describes microglial proliferation as well as changes in microglial morphology, gene expression profiles, and behavior (Calvo and Bennett, 2012). Additionally, microglial proliferation has been clearly linked to neuropathic pain pathology (Liu et al., 2000; Inoue and Tsuda, 2009; Zhuo et al., 2011). While increased microglial activation can occur without proliferation (Nimmerjahn et al., 2005; Chen et al., 2012), increased microglial proliferation does not occur without stimulation, reflecting the diverse responses microglia generate under healthy or pathological conditions (Ulland et al., 2015).
TMEM119 was recently identified as a microglial specific marker in the mouse and human CNS (Bennett et al., 2016; Satoh et al., 2016). Work by Satoh et al. reveal TMEM119 mRNA levels are elevated in microglia from humans with Alzheimer’s disease. Further, they report that TMEM119 exhibits a positive correlation with Iba-1 mRNA expression (Satoh et al., 2016), a microglial/monocyte marker known to increase in the CNS under pathological conditions (Echeverry et al., 2008). Another recent report used TMEM119 to distinguish microglia and macrophages in lesions found in postmortem brain samples from humans diagnosed with multiple sclerosis (Zrzavy et al., 2017). They identified TMEM119 expression in activated microglia present within early active lesions. Such lesions are known to have increased microglial populations and are associated with demyelination, oxidative injury, and antigen presentation (Zrzavy et al., 2017). However, Bennett et al. report increased immunoreactivity for Iba-1 (a widely-used marker for microglial activation) but not TMEM119 in the CNS following either sciatic nerve crush injury or LPS-induced systemic inflammation (Bennett et al., 2016). Additionally, Satoh et al. did not find an association between TMEM119 immunoreactivity and markers of “M1” classical activation (CD80) or “M2” alternative activation (CD163), speculated to reflect neuroprotective conditions (Satoh et al., 2016). However, it is worth considering that the applicability of the M1/M2 polarization paradigm to microglia is still under dispute (Ransohoff, 2016). Taken together, TMEM119 may not readily reflect the activation state of diversely functional microglia, changes in TMEM119 mRNA levels can be an indicator of a shift from homeostatic microglial behavior.
The current report provides evidence that TMEM119 mRNA is elevated in the lumbar spinal cord following sciatic CCI, which is consistent with prior reports of increased expression of microglial markers following peripheral nerve damage (Eriksson et al., 1993; Echeverry et al., 2008). Further work must be performed to determine whether increases in TMEM119 mRNA levels reliably predict increases in TMEM119 protein expression and whether these increases positively correlate with other markers of microglial activation.
Mannose receptor structure, expression, and activation
MR is a c-type lectin receptor commonly associated with endocytosis and pathogen recognition, and possesses an extracellular cysteine rich domain (CR; binds sulfated oligosaccharides), a fibronectin II domain (FNII; binds collagen fragments), and carbohydrate recognition domain (CRD; binds mannose, fucose, and N-acetyl-D-glucosamine) (Martinez-Pomares, 2012; Sedaghat et al., 2014). MR is expressed by macrophages, dendritic cells, and microvascular endothelial cells (Taylor et al., 2005), as well as in the CNS by astrocytes, microglia, some neurons (Burudi et al., 1999; Burudi and Regnier-Vigouroux, 2001), and in the PNS by Schwann cells (Baetas-da-Cruz et al., 2009). Increased MR expression is often associated with anti-inflammatory macrophages (Gordon, 2003). It is important to note that macrophages are known to infiltrate the DRG following sciatic CCI (Hu et al., 2007).
While partial ligation of the sciatic nerve in WT mice increases MR in the ipsilateral DRG, (Komori et al., 2011), the data in the current report offer critical new information because elevated MR mRNA in the ipsilateral DRG of all CCI conditions occurs independently of IL-10 actions. Additionally, it is interesting to note that CCI mice given the MR agonist (DM/pDNA-IL-10 and DM/pDNA-Ctrl) exhibit significantly less elevation in MR mRNA expression compared to CCI mice that did not receive the MR agonist (Saline/pDNA-IL-10). For example, a recent report by Xu et al. demonstrated in vivo that intravenous mannose treatment in a mouse model of acute lung injury induced by intratracheal instillation of LPS dose-dependently attenuates LPS-induced decreases in MR mRNA expression in lung homogenates 3 hours following mannose administration (Xu et al., 2015). Additionally, in vitro mannose pre-treatment of RAW 264.7 macrophages prevents LPS-induced decreases in MR mRNA levels while also dose-dependently increasing MR protein levels as compared to unstimulated cells (Xu et al., 2015). The same group demonstrated increased MR protein expression by primary alveolar macrophages 16 hours after ex vivo stimulation with DM plus LPS compared to LPS alone (Xu et al., 2010). Lastly, monocyte-derived dendritic cells differentiated in the presence of glycosylated glycosylated mucins (tumor-derived MR agonist) for a continuous 7 day exposure exhibit increased MR expression at the cell surface compared to controls (Rughetti et al., 2005). Taken together, these data suggest that acute and chronic exposure to MR agonists increase both MR protein and mRNA expression, suggesting MR agonists do not overtly generate tolerance.
Curiously, DRG from chronically allodynic Saline/pDNA-IL-10-treated mice revealed the highest MR expression levels, suggesting that both the pro-inflammatory or anti-inflammatory peripheral immune cell response to nerve injury can be identified by local alterations in MR levels. Though MR upregulation is commonly considered a phenotypic marker of alternatively activated “M2” macrophages (Gordon, 2003; Martinez and Gordon, 2014), the applicability of classically activated “M1” vs. “M2” macrophage phenotypes to the ipsilateral DRG following sciatic nerve injury has not been fully established. Furthermore, while macrophages are known to infiltrate the DRG following sciatic CCI (Hu et al., 2007), the possible contribution of other cell types within the DRG following sciatic CCI, such as satellite glial cells and additional trafficking leukocytes (i.e. T cells), in modulating both MR expression and the “balance” between M1 vs. M2 factors is not well understood. Therefore, changes in MR expression reported here are interpreted with the DRG cytokine profile on balance. Specifically, chronically allodynic Saline/pDNA-IL-10 mice express the greatest MR and TNF mRNA levels while simultaneously expressing the lowest TGF-β1 mRNA levels. In contrast, pain-relieved CCI+DM/pDNA-IL-10 mice express significantly elevated IL-10, MR, and TGF-β1 mRNA levels with low TNF mRNA expression. In future studies aimed at distinguishing the biochemical effects of IL-10 gene therapy from DM, analysis of CCL2 in DRG may be useful to elucidate the mechanisms by which DM alone generates transient pain reversal.
Given that the lumbar spinal cord, for which no IL-10 transgene was identifiable, exhibited substantial decreases in key inflammatory markers, the M1-to-M2 changes within the DRG suggest that DM/pDNA-IL-10 alters the ability of injured primary sensory neurons to relay pathological pain information to spinal pain projection neurons. That is, DM/pDNA-IL-10 actions at the level of the DRG diminish the salience of incoming peripheral nociceptive signals, with the level of MR expression potentially serving as a useful biomarker of the degree of influence.
Non-viral naked plasmid gene therapy for treatment of pathological pain
Non-viral gene therapies provide promising avenues for the treatment of CNS diseases (Jayant et al., 2016), and methods using naked plasmid DNA have been previously explored for treatment of pathological pain. Yao et al. demonstrated pain relief following a single i.t. injection of naked pDNA (25μg) encoding human interleukin-2 in CCI-treated Sprague-Dawley rats. However, anti-nociceptive effects disappeared 5–6 days post-injection (Yao et al., 2002). More recently, Hu et al. demonstrated about ~2 weeks of inflammatory pain reversal in mice following i.t. or intramuscular (i.m.) delivery of naked pDNA (20μg) encoding the human proenkaphalin gene. While promising, their pain model exhibits >3 weeks of allodynia, making the value of ~2 weeks of pain relief unclear. Milligan et al. were the first to explore i.t. delivery of naked pDNA-IL-10 for relief of peripheral neuropathic pain following CCI in rats. While a single i.t. injection of pDNA-IL-10 (100μg) provides only ~2 days of pain relief, an appropriately timed second i.t. injection (100μg) produces long-lasting pain reversal for greater than 40 days (Milligan et al., 2006b). Later work by Sloane et al. further optimized this repeated injection paradigm, revealing that an initial i.t. pDNA injection (100μg) induces a sensitization which when followed by a smaller plasmid load (25ug pDNA-IL-10) provides greater than 3 months of pain relief (Sloane et al., 2009b). Non-viral naked pDNA-IL-10 gene therapy was further improved by Dengler et al., who demonstrated that a single i.t. co-injection of D-mannose (DM) with naked pDNA-IL-10 (25ug) provides greater than 90 days of pain relief in CCI-treated Sprague-Dawley rats (Dengler et al., 2014). The current report demonstrates that co-injection of DM with just 3μg of naked pDNA-IL-10 in neuropathic mice leads to enduring pain relief for the remainder of the full behavioral timecourse. Notably, data in the current report reveal spontaneous recovery from CCI allodynia occurs sooner in mice than in rats (45 days). Especially given its long-lasting pain suppression profile, these intriguing findings support that non-viral IL-10 gene therapy formulated with DM is the preferred non-viral naked pDNA approach for treatment of neuropathic pain conditions. Future investigations may support use of this unique formulation in treatment of other pain conditions, and perhaps other diseases of the CNS.
Naked plasmid DNA co-injected with D-mannose is a safe, inexpensive, and easy to use
Naked plasmid DNA must face numerous extra- and intracellular obstacles to achieve transgene expression (Glover et al., 2005). To address this, both physical and chemical (e.g. carrier-based) methods have been utilized in an effort to improve the efficacy of non-viral gene therapy (Jayant et al., 2016). Physical methods of gene delivery, such as electroporation, gene gun, ultrasound, or magnetofection, sidestep the numerous obstacles faced by naked pDNA, but are often both costly and labor intensive (Oliveira et al., 2017). Carrier-based methods of non-viral gene therapy, such as polyethynlamine (PEI) pDNA complexes, liposomes, and PLGA microparticles, work to shield pDNA from cellular degradation and aid in cell entry (Slivac et al., 2017), but are not without their disadvantages. For instance, in addition to traditionally low efficacy and transient expression, PEI exhibits moderate toxicity, liposomes can have a low-to-moderate inflammatory response, and many polymer-based methods require surface modification for improved targeting (Jayant et al., 2016). The current report supports that DM co-therapy provides a safe, inexpensive, and easy alternative for improving pDNA uptake and expression.
Role of MR-activation in non-viral gene therapy
The current report supports that MR activation alone transiently attenuates pathologic sensitivity to light touch stimulation by an IL-10-independent mechanism, but it is unclear how MR activation leads to changes in intracellular signaling. Though we show that this mechanism is IL-10 independent, it is likely that MR-mediated pain relief arises from the potential MR role in anti-inflammatory immunomodulation (Nigou et al., 2001; Pathak et al., 2005; Zhang et al., 2005; Gazi and Martinez-Pomares, 2009; Dengler et al., 2014). Many studies of MR signaling are performed under inflammatory states, with MR-activation associated with enhanced pro-inflammatory signaling (Fernandez et al., 2005; Lopez-Herrera et al., 2005; Tachado et al., 2007). However, the “mode” of MR signaling may be dependent on the cellular activation state, with certain phenotypes leading to MR-activated anti-inflammation (Martinez-Pomares, 2012; Hussell and Bell, 2014). For instance, in vitro dendritic cell cultures treated with “activating” anti-MR monoclonal antibodies potently increase IL-10 and IL-1 receptor antagonist (IL-1Ra) secretion with concurrent decreases in IL-1β and TNF production (Chieppa et al., 2003). In line with these findings, MR-activation has also been repeatedly demonstrated to prevent LPS-induced acute lung injury (Zhang et al., 2004; Zhang et al., 2005; Xu et al., 2008; Xu et al., 2015) through downstream activation of the anti-inflammatory transcription factor peroxisome proliferator activated receptor gamma (PPARγ) and decreased production of TNF (Xu et al., 2015). Furthermore, in vitro pretreatment with DM prior to LPS stimulation of RAW 264.7 mouse macrophage cells reduces release of pro-inflammatory TNF, IL-1β, and nitric oxide, while increasing the secretion of IL-10 (Dengler et al., 2014). Zhou et al. recently reported that bone marrow derived monocytes from MR-deficient Mrc1−/− mice reveal greater mRNA levels for pro-inflammatory “M1” phenotypic markers (IL-1β, IL-6, and nitric oxide synthase-2) following LPS-challenge, and lower mRNA levels for markers of an “M2” phenotype (Fizz1, Ym1, and Arginase-1) (Zhou et al., 2017). They further demonstrated that overexpression of miR-511-3p, an intronic miRNA encoded by both mouse and human Mrc1/MRC1 and transcriptionally co-regulated with Mrc1 in macrophages, reverses the M1 bias and instead promotes an anti-inflammatory M2 phenotype. Moreover, Mrc1−/− macrophages accumulate cockroach allergens in vesicles not associated with the lysosomal compartment (Zhou et al., 2017), which is reminiscent of prior studies illustrating ways MR activation in the presence of intracellular pathogens is appropriated to activate anti-inflammatory signaling with delayed phagosome-lysosome fusion (Shibata et al., 1997; Astarie-Dequeker et al., 1999; Shimada et al., 2006; Garcia-Aguilar et al., 2016). These MR-dependent delays in phagosome-lysosome fusion offer a possible mechanism by which MR activation improves transgene expression, and is worthy of future investigation. The behavioral evidence of transient anti-allodynia reported in the current study supports MR-mediated anti-inflammatory effects, and it is reasonable to imagine that MR itself may serve as a novel target for the development of pain therapeutics.
As MR-targeting for enhanced non-viral gene therapy is explored for other CNS pathologies, consideration must be taken to ensure availability of MR prior to application of this DM/pDNA approach. While MR is widely expressed in a variety of cell types, there is some evidence that priming by pro-inflammatory processes may be beneficial to this non-viral approach. For instance, significant levels of IL-10 transgene were observed in ipsilateral, but not contralateral, DRG of CCI+DM/pDNA-IL-10 mice. Furthermore, it is possible that the non-significant levels of IL-10 transgene expression in the ipsilateral DRG of Sham+DM/pDNA-IL-10 treated mice are linked to expression of only basal levels of MR. While the current report further validates targeting MR activation to facilitate efficacious non-viral transgene uptake and expression for treatment of neuropathic pain, further investigation is needed to assess the translatability of this gene therapeutic approach for other CNS pathologies.
Exploration of MR-targeting therapeutics
D-mannose, the MR agonist applied here, is an inexpensive and commonly available dietary supplement (Hu et al., 2016) used in a phase 3 clinical trial for prevention of recurrent urinary tract infections (#NCT01808755, clinicaltrials.gov) (Porru et al., 2014). N-acetyl-D-glucosamine is another MR ligand examined in an early phase 1 clinical trial to improve wound healing of venous stasis ulcers (#NCT00720239, clinicaltrials.gov) (Kelechi et al., 2012). The FDA recently approved the use of the MR-targeting agent γ-Tilmanocept, also known as [99mTc]-DTPA-mannosyl-dextran, for imaging of sentinel lymph nodes in solid tumor staging (Vera et al., 2001; Azad et al., 2015). Mannitol is another FDA approved mannose derivative considered an osmotic diuretic, though whether it may interact with MR on leukocytes or vascular endothelial cells is not yet known.
Depending on the pain etiology, such as for peripheral neuropathic pain or inflammatory pain, activation of MR by small molecules like D-mannose could be explored for therapeutic treatment, especially for patients that would not be good candidates for intrathecal gene therapy. Furthermore, targeting MR could be harnessed to improve the anti-inflammatory response for treatment of diseases where inflammation has become pathologic.
Conclusion
In summary, our findings provide evidence that the spinal non-viral DM/pDNA-IL-10 gene delivery for chronic neuropathic pain does not require endogenous IL-10 for enduring pain relief, with uptake and expression of the IL-10 transgene predominantly in the DRG. Reliable and significant anti-inflammatory changes in the DRG and the lumbar spinal cord are observed as a consequence of this safe non-viral gene therapy approach. MR-mediated improvements in transgene efficacy and transient pain relief are IL-10-independent, findings that support further investigation into potential MR-activated intracellular cascades and their consequent modification in the presence or absence of anti-inflammatory cytokines. This therapy may provide a framework upon which other non-viral gene therapy approaches can be adopted to treat central nervous system disease that extend beyond chronic pain.
Highlights.
Intrathecal non-viral IL-10/D-mannose gene therapy acts without endogenous IL-10
Intrathecal IL-10 transgene is expressed in the DRG but not the lumbar spinal cord
Intrathecal IL-10 transgene expression induces an anti-inflammatory DRG environment
Transgene induced DRG cytokines drive anti-inflammation in the lumbar spinal cord
MR actions produce transient pain reversal that does not require IL-10
Acknowledgments
The authors sincerely thank Dr. Karin Westlund High, PhD, for use of University of New Mexico School of Medicine (UNM SOM) Dept. of Anesthesiology & Critical Care Medicine laboratory space to perform RNA extractions. We greatly appreciate Dr. Michael L. Paffett, PhD, of the University of New Mexico Cancer Research and Treatment Center Fluorescence Microscopy Shared Resource for sharing his expertise in microscope spectral imaging. We also thank Tamara Howard, MS, of the UNM SOM Dept. of Cell Biology and Physiology for her invaluable guidance in tissue processing and immunohistochemistry, and Nathan W. Harris, BS, for technical assistance with injections and surgical procedures.
This work was supported by National Institute of Health grants DA018156-10, AA023051, AA022534-01, by dedicated Health Research funds from the UNM SOM, Research Funds from the UNM SOM Dept. of Anesthesiology & Critical Care Medicine, and funding from the Science and Technology Corporation (STC) at UNM; Gap Fund Project and New Mexico Angels Project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Alvarez P, Bogen O, Green PG, Levine JD. Nociceptor interleukin 10 receptor 1 is critical for muscle analgesia induced by repeated bouts of eccentric exercise in the rat. Pain. 2017;158:1481–1488. doi: 10.1097/j.pain.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarie-Dequeker C, N’Diaye EN, Le Cabec V, Rittig MG, Prandi J, Maridonneau-Parini I. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect Immun. 1999;67:469–477. doi: 10.1128/iai.67.2.469-477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad AK, Rajaram MV, Metz WL, Cope FO, Blue MS, Vera DR, Schlesinger LS. gamma-Tilmanocept, a New Radiopharmaceutical Tracer for Cancer Sentinel Lymph Nodes, Binds to the Mannose Receptor (CD206) J Immunol. 2015;195:2019–2029. doi: 10.4049/jimmunol.1402005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetas-da-Cruz W, Alves L, Pessolani MC, Barbosa HS, Regnier-Vigouroux A, Corte-Real S, Cavalcante LA. Schwann cells express the macrophage mannose receptor and MHC class II. Do they have a role in antigen presentation? J Peripher Nerv Syst. 2009;14:84–92. doi: 10.1111/j.1529-8027.2009.00217.x. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113:E1738–1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Bories C, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 2014;10:26. doi: 10.1186/1744-8069-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottner M, Krieglstein K, Unsicker K. The transforming growth factor-betas: structure, signaling, and roles in nervous system development and functions. J Neurochem. 2000;75:2227–2240. doi: 10.1046/j.1471-4159.2000.0752227.x. [DOI] [PubMed] [Google Scholar]
- Burudi EM, Regnier-Vigouroux A. Regional and cellular expression of the mannose receptor in the post-natal developing mouse brain. Cell Tissue Res. 2001;303:307–317. doi: 10.1007/s004410000311. [DOI] [PubMed] [Google Scholar]
- Burudi EM, Riese S, Stahl PD, Regnier-Vigouroux A. Identification and functional characterization of the mannose receptor in astrocytes. Glia. 1999;25:44–55. doi: 10.1002/(sici)1098-1136(19990101)25:1<44::aid-glia5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Calvo M, Bennett DLH. The mechanisms of microgliosis and pain following peripheral nerve injury. Experimental Neurology. 2012;234:271–282. doi: 10.1016/j.expneurol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 2012;32:11706–11715. doi: 10.1523/JNEUROSCI.0730-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CF, Cheng JK, Chen CY, Lien CC, Chu D, Wang SY, Tsaur ML. Mirror-image pain is mediated by nerve growth factor produced from tumor necrosis factor alpha-activated satellite glia after peripheral nerve injury. Pain. 2014;155:906–920. doi: 10.1016/j.pain.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, Introna M, Allavena P. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- Colak S, Ten Dijke P. Targeting TGF-beta Signaling in Cancer. Trends Cancer. 2017;3:56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- Colleoni M, Sacerdote P. Murine models of human neuropathic pain. Biochim Biophys Acta. 2010;1802:924–933. doi: 10.1016/j.bbadis.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPARgamma receptors and neurotrophic factors. Pain. 2008;139:541–550. doi: 10.1016/j.pain.2008.06.003. [DOI] [PubMed] [Google Scholar]
- da Silva MD, Bobinski F, Sato KL, Kolker SJ, Sluka KA, Santos AR. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol. 2015;51:19–31. doi: 10.1007/s12035-014-8790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler EC, Alberti LA, Bowman BN, Kerwin AA, Wilkerson JL, Moezzi DR, Limanovich E, Wallace JA, Milligan ED. Improvement of spinal non-viral IL-10 gene delivery by D-mannose as a transgene adjuvant to control chronic neuropathic pain. Journal of neuroinflammation. 2014;11:92. doi: 10.1186/1742-2094-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler EC, Liu J, Kerwin A, Torres S, Olcott CM, Bowman BN, Armijo L, Gentry K, Wilkerson J, Wallace J, Jiang X, Carnes EC, Brinker CJ, Milligan ED. Mesoporous silica-supported lipid bilayers (protocells) for DNA cargo delivery to the spinal cord. J Control Release. 2013;168:209–224. doi: 10.1016/j.jconrel.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain. 2008;135:37–47. doi: 10.1016/j.pain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Echeverry S, Shi XQ, Haw A, Liu H, Zhang ZW, Zhang J. Transforming growth factor-beta1 impairs neuropathic pain through pleiotropic effects. Mol Pain. 2009;5:16. doi: 10.1186/1744-8069-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson NP, Persson JK, Svensson M, Arvidsson J, Molander C, Aldskogius H. A quantitative analysis of the microglial cell reaction in central primary sensory projection territories following peripheral nerve injury in the adult rat. Experimental brain research. 1993;96:19–27. doi: 10.1007/BF00230435. [DOI] [PubMed] [Google Scholar]
- Fernandez N, Alonso S, Valera I, Vigo AG, Renedo M, Barbolla L, Crespo MS. Mannose-containing molecular patterns are strong inducers of cyclooxygenase-2 expression and prostaglandin E2 production in human macrophages. J Immunol. 2005;174:8154–8162. doi: 10.4049/jimmunol.174.12.8154. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Alterations in the ipsi- and contralateral afferent inputs of dorsal horn cells produced by capsaicin treatment of one sciatic nerve in the rat. Brain Res. 1982;248:97–107. doi: 10.1016/0006-8993(82)91151-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Woolf CJ. The time course and specificity of the changes in the behavioural and dorsal horn cell responses to noxious stimuli following peripheral nerve capsaicin treatment in the rat. Neuroscience. 1982;7:2051–2056. doi: 10.1016/0306-4522(82)90119-1. [DOI] [PubMed] [Google Scholar]
- Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- Garcia-Aguilar T, Espinosa-Cueto P, Magallanes-Puebla A, Mancilla R. The Mannose Receptor Is Involved in the Phagocytosis of Mycobacteria-Induced Apoptotic Cells. J Immunol Res. 2016;2016:3845247. doi: 10.1155/2016/3845247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565:1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- Gazi U, Martinez-Pomares L. Influence of the mannose receptor in host immune responses. Immunobiology. 2009;214:554–561. doi: 10.1016/j.imbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 2005;6:299–310. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Loram LC, Christianson JP, Strand KA, Flyer-Adams JG, Penzkover KR, Forsayeth JR, van Dam AM, Mahoney MJ, Maier SF, Chavez RA, Watkins LR. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain Behav Immun. 2017;59:49–54. doi: 10.1016/j.bbi.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanani M. Role of satellite glial cells in gastrointestinal pain. Front Cell Neurosci. 2015;9:412. doi: 10.3389/fncel.2015.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine (Phila Pa 1976) 2000;25:1206–1217. doi: 10.1097/00007632-200005150-00003. [DOI] [PubMed] [Google Scholar]
- Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun. 2007;21:599–616. doi: 10.1016/j.bbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Hu X, Shi YN, Zhang P, Miao M, Zhang T, Jiang B. d-Mannose: Properties, Production, and Applications: An Overview. Compr Rev Food Sci F. 2016;15:773–785. doi: 10.1111/1541-4337.12211. [DOI] [PubMed] [Google Scholar]
- Huang D, Yu B. The mirror-image pain: an unclered phenomenon and its possible mechanism. Neurosci Biobehav Rev. 2010;34:528–532. doi: 10.1016/j.neubiorev.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Hughes TS, Langer SJ, Johnson KW, Chavez RA, Watkins LR, Milligan ED, Leinwand LA. Intrathecal injection of naked plasmid DNA provides long-term expression of secreted proteins. Mol Ther. 2009;17:88–94. doi: 10.1038/mt.2008.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57:1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- Jaggi AS, Jain V, Singh N. Animal models of neuropathic pain. Fundamental & clinical pharmacology. 2011;25:1–28. doi: 10.1111/j.1472-8206.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- Jancalek R, Dubovy P, Svizenska I, Klusakova I. Bilateral changes of TNF-alpha and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. Journal of neuroinflammation. 2010;7:11. doi: 10.1186/1742-2094-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancalek R, Svizenska I, Klusakova I, Dubovy P. Bilateral changes of IL-10 protein in lumbar and cervical dorsal root ganglia following proximal and distal chronic constriction injury of peripheral nerve. Neurosci Lett. 2011;501:86–91. doi: 10.1016/j.neulet.2011.06.052. [DOI] [PubMed] [Google Scholar]
- Jayant RD, Sosa D, Kaushik A, Atluri V, Vashist A, Tomitaka A, Nair M. Current status of non-viral gene therapy for CNS disorders. Expert Opin Drug Deliv. 2016;13:1433–1445. doi: 10.1080/17425247.2016.1188802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013;154:S10–S28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan BL, Baldwin RL, Munoz D, Wisnieski BJ. Formation of ion-permeable channels by tumor necrosis factor-alpha. Science. 1992;255:1427–1430. doi: 10.1126/science.1371890. [DOI] [PubMed] [Google Scholar]
- Kelechi TJ, Mueller M, Hankin CS, Bronstone A, Samies J, Bonham PA. A randomized, investigator-blinded, controlled pilot study to evaluate the safety and efficacy of a poly-N-acetyl glucosamine-derived membrane material in patients with venous leg ulcers. Journal of the American Academy of Dermatology. 2012;66:e209–215. doi: 10.1016/j.jaad.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Khan J, Ramadan K, Korczeniewska O, Anwer MM, Benoliel R, Eliav E. Interleukin-10 levels in rat models of nerve damage and neuropathic pain. Neurosci Lett. 2015;592:99–106. doi: 10.1016/j.neulet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Kitani A, Fuss IJ, Nakamura K, Schwartz OM, Usui T, Strober W. Treatment of experimental (Trinitrobenzene sulfonic acid) colitis by intranasal administration of transforming growth factor (TGF)-beta1 plasmid: TGF-beta1-mediated suppression of T helper cell type 1 response occurs by interleukin (IL)-10 induction and IL-12 receptor beta2 chain downregulation. J Exp Med. 2000;192:41–52. doi: 10.1084/jem.192.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259:231–244. doi: 10.1111/imr.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Hofstetter HH, Meuth SG, Braeuninger S, Sommer C, Stoll G. T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Exp Neurol. 2006;200:480–485. doi: 10.1016/j.expneurol.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Komori T, Morikawa Y, Inada T, Hisaoka T, Senba E. Site-specific subtypes of macrophages recruited after peripheral nerve injury. Neuroreport. 2011;22:911–917. doi: 10.1097/WNR.0b013e32834cd76a. [DOI] [PubMed] [Google Scholar]
- Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. 2015;18:24–32. doi: 10.1111/ner.12247. discussion 32. [DOI] [PubMed] [Google Scholar]
- Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A. CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci. 2016;36:11074–11083. doi: 10.1523/JNEUROSCI.3708-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, Watkins LR. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. Journal of neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Rudin M, Kozlova EN. Glial cell proliferation in the spinal cord after dorsal rhizotomy or sciatic nerve transection in the adult rat. Experimental brain research. 2000;131:64–73. doi: 10.1007/s002219900273. [DOI] [PubMed] [Google Scholar]
- Liu L, Yin Y, Li F, Malhotra C, Cheng J. Flow cytometry analysis of inflammatory cells isolated from the sciatic nerve and DRG after chronic constriction injury in mice. J Neurosci Methods. 2017 doi: 10.1016/j.jneumeth.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. Balancing the immune response in the brain: IL-10 and its regulation. Journal of neuroinflammation. 2016;13:297. doi: 10.1186/s12974-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Herrera A, Liu Y, Rugeles MT, He JJ. HIV-1 interaction with human mannose receptor (hMR) induces production of matrix metalloproteinase 2 (MMP-2) through hMR-mediated intracellular signaling in astrocytes. Biochim Biophys Acta. 2005;1741:55–64. doi: 10.1016/j.bbadis.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Maeda H, Kuwahara H, Ichimura Y, Ohtsuki M, Kurakata S, Shiraishi A. TGF-beta enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J Immunol. 1995;155:4926–4932. [PubMed] [Google Scholar]
- Mansfield JR. Multispectral imaging: a review of its technical aspects and applications in anatomic pathology. Vet Pathol. 2014;51:185–210. doi: 10.1177/0300985813506918. [DOI] [PubMed] [Google Scholar]
- Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martucci C, Trovato AE, Costa B, Borsani E, Franchi S, Magnaghi V, Panerai AE, Rodella LF, Valsecchi AE, Sacerdote P, Colleoni M. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain. 2008;137:81–95. doi: 10.1016/j.pain.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Maxwell JR, Denson JL, Joste NE, Robinson S, Jantzie LL. Combined in utero hypoxia-ischemia and lipopolysaccharide administration in rats induces chorioamnionitis and a fetal inflammatory response syndrome. Placenta. 2015;36:1378–1384. doi: 10.1016/j.placenta.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33:1471–1478. doi: 10.1016/0028-3908(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Mellios N, Woodson J, Garcia RI, Crawford B, Sharma J, Sheridan SD, Haggarty SJ, Sur M. beta2-Adrenergic receptor agonist ameliorates phenotypes and corrects microRNA-mediated IGF1 deficits in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2014;111:9947–9952. doi: 10.1073/pnas.1309426111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Penzkover KR, Soderquist RG, Mahoney MJ. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation. 2012;15:520–526. doi: 10.1111/j.1525-1403.2012.00462.x. discussion 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Soderquist RG, Malone SM, Mahoney JH, Hughes TS, Langer SJ, Sloane EM, Maier SF, Leinwand LA, Watkins LR, Mahoney MJ. Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biol. 2006a;2:293–308. doi: 10.1017/S1740925X07000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, Cruz PE, Chacur M, Spataro L, Wieseler-Frank J, Hammack SE, Maier SF, Flotte TR, Forsayeth JR, Leinwand LA, Chavez R, Watkins LR. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain. 2005;1:9. doi: 10.1186/1744-8069-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, Flotte TR, Johnson KW, Mahoney MM, Chavez RA, Leinwand LA, Watkins LR. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006b;126:294–308. doi: 10.1016/j.pain.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Noor S, Sanchez JJ, Vanderwall AG, Sun MS, Maxwell JR, Davies S, Jantzie LL, Petersen TR, Savage DD, Milligan ED. Prenatal alcohol exposure potentiates chronic neuropathic pain, spinal glial and immune cell activation and alters sciatic nerve and DRG cytokine levels. Brain Behav Immun. 2017;61:80–95. doi: 10.1016/j.bbi.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Fenn AM, Dugan A, Godbout JP. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–895. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol. 2001;169:386–391. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- Oliveira AV, Rosa da Costa AM, Silva GA. Non-viral strategies for ocular gene delivery. Materials science & engineering C, Materials for biological applications. 2017;77:1275–1289. doi: 10.1016/j.msec.2017.04.068. [DOI] [PubMed] [Google Scholar]
- Pathak SK, Basu S, Bhattacharyya A, Pathak S, Kundu M, Basu J. Mycobacterium tuberculosis lipoarabinomannan-mediated IRAK-M induction negatively regulates Toll-like receptor-dependent interleukin-12 p40 production in macrophages. J Biol Chem. 2005;280:42794–42800. doi: 10.1074/jbc.M506471200. [DOI] [PubMed] [Google Scholar]
- Porru D, Parmigiani A, Tinelli C, Barletta D, Choussos D, Franco CD, Bobbi V, Bassi S, Miller O, Gardella B, Nappi R, Spinillo A, Rovereto B. Oral D-mannose in recurrent urinary tract infections in women: a pilot study. Journal of Clinical Urology. 2014;7:208–213. [Google Scholar]
- Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- Robinson S, Winer JL, Berkner J, Chan LAS, Denson JL, Maxwell JR, Yang Y, Sillerud LO, Tasker RC, Meehan WP, Mannix R, Jantzie LL. Imaging and serum biomarkers reflecting the functional efficacy of extended erythropoietin treatment in rats following infantile traumatic brain injury. Journal of neurosurgery Pediatrics. 2016;17:739–755. doi: 10.3171/2015.10.PEDS15554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rughetti A, Pellicciotta I, Biffoni M, Backstrom M, Link T, Bennet EP, Clausen H, Noll T, Hansson GC, Burchell JM, Frati L, Taylor-Papadimitriou J, Nuti M. Recombinant tumor-associated MUC1 glycoprotein impairs the differentiation and function of dendritic cells. J Immunol. 2005;174:7764–7772. doi: 10.4049/jimmunol.174.12.7764. [DOI] [PubMed] [Google Scholar]
- Satoh J, Kino Y, Asahina N, Takitani M, Miyoshi J, Ishida T, Saito Y. TMEM119 marks a subset of microglia in the human brain. Neuropathology. 2016;36:39–49. doi: 10.1111/neup.12235. [DOI] [PubMed] [Google Scholar]
- Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad AR. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74:27–34. doi: 10.1016/j.cyto.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sedaghat B, Stephenson R, Toth I. Targeting the mannose receptor with mannosylated subunit vaccines. Curr Med Chem. 2014;21:3405–3418. doi: 10.2174/0929867321666140826115552. [DOI] [PubMed] [Google Scholar]
- Shen KF, Zhu HQ, Wei XH, Wang J, Li YY, Pang RP, Liu XG. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol. 2013;247:466–475. doi: 10.1016/j.expneurol.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Metzger WJ, Myrvik QN. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J Immunol. 1997;159:2462–2467. [PubMed] [Google Scholar]
- Shimada K, Takimoto H, Yano I, Kumazawa Y. Involvement of mannose receptor in glycopeptidolipid-mediated inhibition of phagosome-lysosome fusion. Microbiol Immunol. 2006;50:243–251. doi: 10.1111/j.1348-0421.2006.tb03782.x. [DOI] [PubMed] [Google Scholar]
- Shimoyama M, Tanaka K, Hasue F, Shimoyama N. A mouse model of neuropathic cancer pain. Pain. 2002;99:167–174. doi: 10.1016/s0304-3959(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Shouval DS, Ouahed J, Biswas A, Goettel JA, Horwitz BH, Klein C, Muise AM, Snapper SB. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv Immunol. 2014;122:177–210. doi: 10.1016/B978-0-12-800267-4.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira Mietto B, Kroner A, Girolami EI, Santos-Nogueira E, Zhang J, David S. Role of IL-10 in Resolution of Inflammation and Functional Recovery after Peripheral Nerve Injury. J Neurosci. 2015;35:16431–16442. doi: 10.1523/JNEUROSCI.2119-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivac I, Guay D, Mangion M, Champeil J, Gaillet B. Non-viral nucleic acid delivery methods. Expert Opin Biol Ther. 2017;17:105–118. doi: 10.1080/14712598.2017.1248941. [DOI] [PubMed] [Google Scholar]
- Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier SF, Johnson KW, Chavez R, Watkins LR, Leinwand L, Milligan ED, Van Dam AM. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav Immun. 2009a;23:92–100. doi: 10.1016/j.bbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane E, Langer S, Jekich B, Mahoney J, Hughes T, Frank M, Seibert W, Huberty G, Coats B, Harrison J, Klinman D, Poole S, Maier S, Johnson K, Chavez R, Watkins LR, Leinwand L, Milligan E. Immunological priming potentiates non-viral anti-inflammatory gene therapy treatment of neuropathic pain. Gene Ther. 2009b;16:1210–1222. doi: 10.1038/gt.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane EM, Soderquist RG, Maier SF, Mahoney MJ, Watkins LR, Milligan ED. Long-term control of neuropathic pain in a non-viral gene therapy paradigm. Gene Ther. 2009c;16:470–475. doi: 10.1038/gt.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderquist RG, Milligan ED, Harrison JA, Chavez RA, Johnson KW, Watkins LR, Mahoney MJ. PEGylation of interleukin-10 for the mitigation of enhanced pain states. J Biomed Mater Res A. 2010a;93:1169–1179. doi: 10.1002/jbm.a.32611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderquist RG, Milligan ED, Sloane EM, Harrison JA, Douvas KK, Potter JM, Hughes TS, Chavez RA, Johnson K, Watkins LR, Mahoney MJ. PEGylation of brain-derived neurotrophic factor for preserved biological activity and enhanced spinal cord distribution. J Biomed Mater Res A. 2009;91:719–729. doi: 10.1002/jbm.a.32254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderquist RG, Sloane EM, Loram LC, Harrison JA, Dengler EC, Johnson SM, Amer LD, Young CS, Lewis MT, Poole S, Frank MG, Watkins LR, Milligan ED, Mahoney MJ. Release of plasmid DNA-encoding IL-10 from PLGA microparticles facilitates long-term reversal of neuropathic pain following a single intrathecal administration. Pharm Res. 2010b;27:841–854. doi: 10.1007/s11095-010-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Schafers M. Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–162. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR. Spinal gap junctions: potential involvement in pain facilitation. J Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- Tachado SD, Zhang J, Zhu J, Patel N, Cushion M, Koziel H. Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2. J Leukoc Biol. 2007;81:205–211. doi: 10.1189/jlb.1005580. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Gordon S, Martinez-Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005;26:104–110. doi: 10.1016/j.it.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Tu H, Juelich T, Smith EM, Tyring SK, Rady PL, Hughes TK., Jr Evidence for endogenous interleukin-10 during nociception. J Neuroimmunol. 2003;139:145–149. doi: 10.1016/s0165-5728(03)00126-7. [DOI] [PubMed] [Google Scholar]
- Uceyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun. 2007;21:553–560. doi: 10.1016/j.bbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Ulland TK, Wang Y, Colonna M. Regulation of microglial survival and proliferation in health and diseases. Semin Immunol. 2015;27:410–415. doi: 10.1016/j.smim.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera DR, Wallace AM, Hoh CK, Mattrey RF. A synthetic macromolecule for sentinel node detection: (99m)Tc-DTPA-mannosyl-dextran. J Nucl Med. 2001;42:951–959. [PubMed] [Google Scholar]
- Ward JM, Anver MR, Haines DC, Melhorn JM, Gorelick P, Yan L, Fox JG. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiological reviews. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. Journal of internal medicine. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71:225–235. doi: 10.1016/s0304-3959(97)03369-1. [DOI] [PubMed] [Google Scholar]
- Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Kuhn MN, Zvonok AM, Thakur GA, Makriyannis A, Milligan ED. Immunofluorescent spectral analysis reveals the intrathecal cannabinoid agonist, AM1241, produces spinal anti-inflammatory cytokine responses in neuropathic rats exhibiting relief from allodynia. Brain Behav. 2012a;2:155–177. doi: 10.1002/brb3.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Armijo LM, Kuhn MN, Thakur GA, Makriyannis A, Milligan ED. Intrathecal cannabilactone CB(2)R agonist, AM1710, controls pathological pain and restores basal cytokine levels. Pain. 2012b;153:1091–1106. doi: 10.1016/j.pain.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Xie Q, Shen Y, Lu G, Yao H, Chen Y, Zhou J. Involvement of mannose receptor in the preventive effects of mannose in lipopolysaccharide-induced acute lung injury. Eur J Pharmacol. 2010;641:229–237. doi: 10.1016/j.ejphar.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Xu XL, Xie QM, Shen YH, Jiang JJ, Chen YY, Yao HY, Zhou JY. Mannose prevents lipopolysaccharide-induced acute lung injury in rats. Inflamm Res. 2008;57:104–110. doi: 10.1007/s00011-007-7037-y. [DOI] [PubMed] [Google Scholar]
- Xu XL, Zhang P, Shen YH, Li HQ, Wang YH, Lu GH, Zhou JY. Mannose prevents acute lung injury through mannose receptor pathway and contributes to regulate PPARgamma and TGF-beta1 level. Int J Clin Exp Pathol. 2015;8:6214–6224. [PMC free article] [PubMed] [Google Scholar]
- Yao MZ, Wang JH, Gu JF, Sun LY, Liu H, Zhao ZQ, Liu XY. Interleukin-2 gene has superior antinociceptive effects when delivered intrathecally. Neuroreport. 2002;13:791–794. doi: 10.1097/00001756-200205070-00011. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhu J, Imrich A, Cushion M, Kinane TB, Koziel H. Pneumocystis activates human alveolar macrophage NF-kappaB signaling through mannose receptors. Infect Immun. 2004;72:3147–3160. doi: 10.1128/IAI.72.6.3147-3160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tachado SD, Patel N, Zhu J, Imrich A, Manfruelli P, Cushion M, Kinane TB, Koziel H. Negative regulatory role of mannose receptors on human alveolar macrophage proinflammatory cytokine release in vitro. J Leukoc Biol. 2005;78:665–674. doi: 10.1189/jlb.1204699. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Do DC, Ishmael FT, Squadrito ML, Tang HM, Tang HL, Hsu MH, Qiu L, Li C, Zhang Y, Becker KG, Wan M, Huang SK, Gao P. Mannose receptor modulates macrophage polarization and allergic inflammation through miR-511-3p. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Wu G, Wu L-J. Neuronal and microglial mechanisms of neuropathic pain. Molecular Brain. 2011;4:31–31. doi: 10.1186/1756-6606-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain. 2017 doi: 10.1093/brain/awx113. [DOI] [PMC free article] [PubMed] [Google Scholar]