Abstract
Objective
We investigated whether RA increases risk for chronic obstructive pulmonary disease (COPD) or asthma independent of factors occurring before RA onset or mediating these respiratory morbidities after diagnosis, such as cigarette smoking.
Methods
Within the prospective Nurses’ Health Study (n=121,701 women; 1976–2014), we identified an incident RA cohort and matched each woman with RA to 10 comparators without RA by age and year at index date of RA diagnosis, excluding women with COPD or asthma at baseline. Data were obtained through biennial questionnaires and medical records. We used marginal structural models to determine the independent effect of RA on incident COPD or asthma adjusting for confounders and time-varying mediators through inverse probability weighting.
Results
We identified 843 women with RA, matched to 8,399 comparators without RA. Mean age was 59.8 years and mean follow-up after index date was 18.6 years (SD 9.0) for women with RA, and 18.8 years (SD 9.5) for comparators. We identified 68 (8.1%) incident COPD and 40 (4.7%) asthma cases among women with RA, and 459 (5.5%) COPD and 268 (3.2%) asthma cases among comparators. RA was associated with increased risk of COPD (HR 1.52, 95%CI 1.17–1.97) and asthma (HR 1.55, 95%CI 1.11–2.16) compared to comparators adjusted for the matching factors of age and calendar year at index date. After further adjustment for confounders and time-varying mediators occurring after index date, including smoking, RA was significantly associated with COPD (HR 1.68, 95%CI 1.36–2.07), but not asthma (HR 1.11, 95%CI 0.59–2.09) compared to non-RA comparators. Women with seropositive RA (HR 1.60, 95%CI 1.17–2.19) and seronegative RA (HR 1.62, 95%CI 1.09–2.40) had similar increased risk for COPD compared to non-RA comparators.
Conclusion
In this prospective cohort study, RA was associated with increased risk for incident COPD, independent of lifestyle confounders and mediators after diagnosis, including smoking.
Keywords: rheumatoid arthritis, epidemiology, causal inference, COPD, asthma, respiratory
The lungs have an important role in rheumatoid arthritis (RA), both in terms of pathogenesis and clinical outcomes (1–3). Inflammation in the bronchiolar mucosa may be an initial trigger for immune tolerance breakdown that leads to the formation RA-related autoantibodies, perhaps years prior to the clinical onset of RA (4–9). Individuals with genetic RA susceptibility, such as HLA-DRB1 shared epitope alleles, and environmental triggers, such as cigarette smoking, may be particularly prone to immune dysregulation occurring in the airways and pulmonary interstitium among other anatomic sites (10, 11). Among patients diagnosed with RA, interstitial lung disease (ILD) has been long recognized as an extra-articular disease manifestation with serious morbidity and mortality (12). Respiratory mortality is increasingly being recognized as a significant contributor to the excess total mortality for patients with RA compared to the general population (13–15). In particular, patients with seropositive RA have three-fold increased risk for respiratory mortality compared to those without RA (14, 15).
While some of the excess respiratory mortality risk in RA may be attributed to smoking or ILD, RA may be associated with excess diseases of the airways (1–3, 7). Recent studies have investigated the association of RA with common obstructive lung diseases, specifically chronic obstructive pulmonary disease (COPD, composed of emphysema and chronic bronchitis) and asthma (Table 1). A recent meta-analysis of four retrospective studies reported that patients with RA had relative risk for COPD of 1.99 (95%CI 1.61–2.45) (16). RA was significantly associated with increased risk of subsequently being diagnosed with both COPD and asthma using a large nationwide Taiwanese administrative dataset, but data on smoking and other lifestyle variables were unavailable (17, 18). A US population-based study associated RA with increased incidence of a composite of obstructive lung diseases compared to controls sampled from the general population, but did not investigate COPD and asthma separately (19). Moreover, a possible differential effect of RA serologic phenotypes on risk of COPD or asthma was not previously investigated. Smoking is strongly related to development of both anti-citrullinated protein antibodies (ACPA). ACPA may develop more frequently among smoking-related conditions such as COPD even among patients without RA (20–22). However, it is unclear whether there is a differential effect for development of obstructive lung disease between seropositive and seronegative RA. Since prior studies investigating RA and COPD/asthma risk had retrospective designs, findings may have been limited due to inadequate measures of confounding factors and lack of appropriate comparison group. Therefore, we performed a prospective cohort study with detailed measures of possible confounders/mediators in a sample of women both with and without RA.
Table 1.
Summary of prior studies investigating the association between rheumatoid arthritis and obstructive lung diseases.
| First author (Year) (Reference) | Study design | Population Number of cases /comparators | RA method of identification | Outcome; method of identification | Smoking adjustment | Results | Comments |
|---|---|---|---|---|---|---|---|
| COPD | |||||||
| Hemminki (2011) (47) | Retrospective cohort | Sweden Not reported | Billing code | COPD; billing code | No | SIR 2.57 (95% CI 2.21–2.99) | Evaluated many autoimmune diseases for risk of COPD/lung cancer; observed results compared to expected number by socioeconomic strata |
| Nannini (2013) (19) | Retrospective cohort | USA, Olmsted County, Minnesota 594 RA/596 matched comparators | 1987 ACR criteria | Obstructive lung disease*; clinical testing/physician diagnosis | Yes; never/past/current at baseline | HR 1.54 (95% CI 1.01–2.34) | Incident RA matched to comparators by age and sex; also adjusted for alcoholism; subset of non-smokers had HR of 1.98 (95% CI 0.73–5.40); rheumatoid factor positivity had increased risk |
| Ursum (2013) (48) | Retrospective cohort | Netherlands 3,356 RA/6,708 matched controls | Billing code for “Inflammatory arthritis” | COPD; billing code | No | HR 1.8 (95% CI 1.4–2.3) | Incident inflammatory arthritis matched to controls by age and sex; other comorbid disorders also investigated |
| Shen (2014) (17) | Retrospective cohort | Taiwan 28,725 RA/114,900 matched comparators | Billing code | COPD; billing code | No | HR 1.85 (95% CI 1.70–2.01) | Adjusted for age, sex, and comorbidities |
| Ungprasert (2016) (16) | Meta-analysis | - | - | COPD | - | RR 1.99 (1.61–2.45) | Meta-analyzed results of 4 studies (references 17, 19, 47, 48) |
| Doss (2017) (41) | Phenome-wide association study | USA, Vanderbilt University Medical Center 2,199 RA (1,382 seropositive vs. 817 seronegative) | Electronic medical record algorithm | Chronic airway obstruction; billing code | No | Seropositive RA: OR 2.2 (95% CI 1.5–3.4) vs. seronegative RA | Hypothesis-generating study comparing disease associations in seropositive vs. seronegative RA |
|
| |||||||
| Asthma | |||||||
| Ursum (2013) (48) | Retrospective cohort | Netherlands 3,356 RA/6,708 matched controls | Billing code for “Inflammatory arthritis” | Asthma; billing code | No | HR 1.4 (95% CI 1.1–1.8) | Incident inflammatory arthritis matched to controls by age and sex; other comorbid disorders also investigated |
| Shen (2014) (18) | Retrospective cohort | Taiwan 27,602 RA/82,806 matched comparators | Billing code | Asthma; billing code | No | HR 2.07 (95% CI 1.89–2.26) | Adjusted for age, sex, and comorbidities |
| Lai (2015) (49) | Retrospective cohort | Taiwan 170,570 patients with allergic diseases/170,238 matched comparators | Billing code | Incident RA; billing code | No | Asthma: HR 1.67 (95% CI 1.32–2.62) for incident RA vs. no asthma | Exposures were asthma, allergic rhinitis, and atopic dermatitis; incident RA was the outcome; allergic rhinitis was also associated with RA (HR 1.62, 95% CI 1.33–1.98) |
Obstructive lung disease was a composite outcome comprised of COPD, asthma, bronchiectasis, and interstitial lung disease. The majority of cases were COPD.
ACR, American College of Rheumatology; CI, confidence interval; COPD, chronic obstructive lung disease; HR, hazard ratio; OR, odds ratio; RA, rheumatoid arthritis; RR, risk ratio; SIR, standardized incidence ratio.
The known association of smoking with both RA risk and respiratory outcomes presents methodologic challenges (20, 23, 24). Since smoking could be on the causal pathway between RA and COPD or asthma, epidemiologic causal inference methods accounting for a potential mediating effect are necessary. Traditional epidemiologic methods for adjustment are inappropriate to use for variables that are mediators and may produce spurious results. Therefore, in this study we used statistical methods suitable for variables, such as smoking, that can be both confounders and mediators depending on whether the behavior occurred before or after an index event such as RA diagnosis.
We aimed to investigate the association between RA and the common obstructive lung diseases of COPD and asthma in a prospective study, independent of confounders or time-varying mediators occurring before or after RA diagnosis. We hypothesized that RA is associated with increased risk of COPD and asthma independent of confounders and time-varying mediators including smoking. In secondary analyses, we investigated whether risk for COPD and asthma differed between seropositive and seronegative RA phenotypes.
METHODS
Study population
We performed a matched prospective cohort study within the Nurses’ Health Study (NHS), a closed cohort of 121,701 female registered nurses in the United States aged 30–55 years at baseline in 1976. Participants completed questionnaires at baseline and every two years during follow-up to provide data on sociodemographics, behaviors, diet, medications, and diseases. More than 90% of women have returned questionnaires each cycle (15). All aspects of this study were approved by the Partners HealthCare Institutional Review Board.
Identification of incident RA
Since we were interested in following women throughout the entire course of RA, we excluded women with prevalent RA or other connective tissue diseases (CTD) diagnosed prior to the baseline of the NHS in 1976. Medical records were obtained for participants that reported a diagnosis of RA or other CTD during follow-up and screened positive on a questionnaire (25). These records were independently reviewed by two rheumatologists to confirm RA according to accepted classification criteria (26, 27). Data on RA characteristics at the time of diagnosis, including date of diagnosis and serologic status were obtained by medical record review. The dates of RA diagnosis in this analysis ranged from June 1, 1976 to May 31, 2012. We defined seropositive as positive rheumatoid factor (RF) (available since baseline) or anti-cyclic citrullinated peptide (anti-CCP) (clinically available since the early 2000s). Since clinical laboratory assays utilized varied by site and year, positive RF or anti-CCP tests were considered positive as being greater than the upper limit of normal as obtained on medical records. For this study, women who self-reported COPD or asthma prior to or on the date of RA diagnosis were excluded from the analysis.
Matched comparators without RA
We matched each woman with RA with up to ten non-RA comparators in the NHS based on age (within 12 months) and calendar year to form a comparison cohort with analogous follow-up to the RA cohort. We defined the index date for matching as the date of RA diagnosis. Women in the NHS were eligible to be a comparator if they had never reported RA, other CTD, COPD, and asthma prior to or on the index date. We did not match on other lifestyle or clinical variables to maximize sample size and maintain otherwise expected differences between women with RA and comparators without RA that could be adjusted for using multivariable analyses. For women diagnosed with RA late in the NHS follow-up, there may not have been exactly 10 comparators available for every woman with RA.
Identification of incident COPD and asthma
Women self-reported COPD and asthma and date of diagnosis on biennial questionnaires from 1976 to 2014. Data on FEV1, chest imaging, and symptoms were extracted from the medical record for a subset of women who reported COPD or asthma from 1988–2000 (28). Self-report in the NHS from these health care professionals had positive predictive values ranging from 79–92%, depending on the subtype of COPD or asthma identified using a supplemental questionnaire (28). Therefore, we defined incident self-reported COPD or incident self-reported asthma from 1976–2014 as outcomes for this analysis. The distinction between asthma and COPD is difficult to precisely define in an older population even if specialized clinical procedures, such as methacholine challenge testing, are available (29). Since we relied on self-report and older onset asthma is relatively uncommon compared to incident COPD, women who reported both asthma and COPD were considered as COPD for all analyses.
Covariates
We considered covariates based on associations with RA risk and COPD or asthma based on prior literature (30–34). Baseline covariates were assessed on the questionnaire two years prior to index date (i.e., before RA was diagnosed). In multivariable models, covariates were updated after baseline every two years until the end of follow-up, outcome, or censoring.
Information on smoking was self-reported on the mailed questionnaires administered every two years since 1976 and previously validated as accurate (35). On the initial NHS questionnaire, participants reported whether they were current smokers or had ever smoked in the past and the age at which they began to smoke. Current smokers reported the number of cigarettes smoked per day, and past smokers reported the age at which they stopped smoking and number of cigarettes smoked per day before quitting. On each subsequent questionnaire, participants reported whether they were never, current, or past smokers as well as the number of cigarettes smoked per day. We calculated pack-years (years of smoking multiplied by packs of cigarettes per day) for past and current smokers. Since women were surveyed repeatedly during the NHS follow-up, we were able to prospectively update time-varying smoking status as well as the timing and accrual of pack-years. At the baseline for analysis (two years prior to index date), we categorized cumulative smoking as: never, >0 to 10, 10.1 to 20, and >20 pack-years. After index date, we considered smoking as time-varying smoking status, categorized as never, past, or current to capture smoking behavior changes that might occur after index date that would not be reflective on a cumulative smoking measure such as pack-years.
Annual household income was based on updated home address and US Census tract-level data as a proxy for socioeconomic status (<$40K or ≥$40K USD). Physical activity was measured starting in 1980 with a validated survey and converted into continuous weekly hours of moderate/vigorous activity (36). Region of the US was based on reported residence. Menopausal status, postmenopausal hormone use (PMH), and aspirin use (a surrogate measure for preventive care that may result in healthier behaviors and more frequent contact with health care providers; women with RA were previously reported to be prescribed aspirin more frequently than those without RA (37)) were self-reported. Body mass index (BMI) was calculated based on self-reported weight and height and considered as a continuous variable or categorized according to the World Health Organization as underweight (<18.5 kg/m2), normal (18.5 to 24.9 kg/m2), overweight (25 to 29.9 kg/m2), or obese (≥30 kg/m2) (38). Dietary factors were assessed using semi-quantitative food frequency questionnaires assessing 133 dietary items in 1980, 1984, 1986, and every four years until 2010. Participants were classified into quartiles of the 2010 Alternate Healthy Eating Index, which classifies diet quality as healthy or unhealthy based on 11 foods/nutrients/alcohol groups using methods previously described (39).
Although data on RA disease activity and disease-modifying antirheumatic drugs (DMARDs) were not available throughout follow-up, participants were not yet diagnosed with RA or treated with DMARDs at the baseline period two years prior to index date the timepoint when other covariates were assessed. Comparators without RA or other CTD would not be expected to be prescribed DMARDs and would not have any RA disease activity so comparisons concerning these factors could not be pursued even if available.
Statistical analysis
We reported descriptive baseline statistics based on RA or comparator status. Since smoking is a strong risk factor for both RA as well as the subsequent respiratory outcomes, we used marginal structural modeling (MSM) as a method to adjust for confounders as well as time-varying mediators, such as smoking, on the causal pathway between the exposure and outcome. Alternative methods of multivariable adjustment, such as Cox regression, are appropriate for adjusting for confounders, but not mediators on the causal pathway. We restricted the sample to non-smokers or light smokers (<10 pack-years) at baseline to investigate these subgroups. We reported descriptive frequencies and proportions of incident COPD and asthma cases according to RA or comparator status in never or light smokers. There were too few incident COPD and asthma cases to perform further statistical analyses.
Exposures of interest were all RA, seropositive RA, and seronegative RA, each compared to matched comparators. For the subgroup analyses of seropositive or seronegative RA, we restricted the analysis to only those comparators that were matched to women in each respective RA serologic phenotype. The outcomes of interest were COPD and asthma as separate outcomes. Dates of outcomes all occurred after the index date. For this study, follow-up accrued after index date. Participants were censored at outcome, loss to follow-up, self-reported CTD (for comparators only), death, or end of follow-up.
We used MSM to calculate the inverse probability of exposure and censoring weights conditioning on baseline (i.e., time-fixed) and time-varying covariates in each two-year interval of follow-up, using methods as previously published (40). Weighting is a method to equalize observed data that may otherwise be biased related to an imbalance in the distribution of covariates between exposure groups and differential censoring. For example, informative censoring due to loss of follow-up varies across subgroups related to covariate and exposure data and could introduce bias. Weighting by the inverse probability of censoring can correct this imbalance of loss to follow-up and other censoring variables such as death. The product of the inverse probability of exposure and censoring weights is the stabilized weight (SW), which is the summary for how well the model performs and the mean SW is ideally close to 1 at each follow-up time point. Using the SW corrects imbalances between covariates and exposure groups as well as possible informative censoring. At baseline and each interval of follow-up, the covariates for the primary exposure (RA vs. comparator status) were weighted by the SW such that all covariates were balanced in this pseudopopulation. We truncated 5% of the SW such that a rare observation would not contribute disproportionately to the study pseudopopulations. We plotted the distribution of the SW, exposures, and covariates over follow-up time to examine the balance between the RA and comparator pseudopopulations. Pooled logistic regression models weighted by the SW were used to estimate the association between RA and outcomes and approximate hazard ratios (HR) and 95%CI.
The first MSM adjusted only for the matching factors of year of index date and age using a conditional analysis without other covariates to estimate hazard ratios (HR) and 95% confidence intervals (CI) for COPD, and then for asthma. Since we were particularly interested in evaluating the relationship between RA and COPD/asthma independent of smoking, we performed MSM adjusting for the confounding and mediating effect of smoking in addition to the matching factors. Since smoking pack-years is known to be associated with RA risk, we considered categories of pre-index smoking at baseline (never, >0 to 10, 10 to 20, and >20 pack-years) to adjust for this confounding effect prior to RA diagnosis or comparator status. After index date, we considered smoking status (never, past, current) as a time-varying mediator. Thus, in this MSM, we adjusted for the confounding effect of smoking occurring before RA diagnosis and the mediating effect of smoking occurring after RA diagnosis. We only considered the income covariate at baseline in models since the meaning of the income cutoff could change with calendar time. Since women with incident RA and their comparators had the same index date for matching, there was no differential effect of calendar time on income at baseline between those with RA and comparators. In the final multivariable MSM, we additionally included dietary quality, BMI, physical activity, menopausal status and PMH use, and aspirin use as time-varying covariates.
To graphically display the cumulative COPD or asthma-free survival adjusted for potential confounders and mediators, we weighted the observed survival by the stabilized weight from MSM models during each interval of follow-up (41). Alternative methods of displaying survival over time, such as Kaplan-Meier curves, only evaluate observed survival according to exposure status and are unable to adjust for confounders and mediators. We tested for a difference in the curves using the pooled logistic regression from the MSM model testing for exposure status (all/seropositive/seronegative RA) for each outcome.
We considered a two-sided p value <0.05 as statistically significant. All analyses were performed using SAS v9.3 (Cary, NC).
RESULTS
Among 121,701 women in the NHS, we identified 843 women with incident RA during follow-up without any prior report of COPD or asthma. We matched them to 8.399 comparators without RA, other CTD, COPD, or asthma at the index date of RA diagnosis. Among the 843 women with RA, 518 (61.4%) were seropositive and 325 (38.6%) were seronegative. Baseline characteristics at the questionnaire cycle two years prior to index date are shown in Table 2. Mean age at index date for both women with RA and matched comparators was 59.8 years. At baseline, 64.1% of women who were diagnosed with RA were ever smokers compared to 54.8% of matched comparators. Women subsequently diagnosed with RA were also heavier smokers than comparators, with 45.4% of women with RA having ≥10 pack-years at index date compared to 36.6% of comparators. Slightly more women with RA were overweight or obese (50.1%) than comparators (46.6%). There were other slight differences between RA and comparator status in other factors as well, including physical activity, menopausal status and postmenopausal hormone use, and aspirin use.
Table 2.
Baseline characteristics two years prior to index date of RA diagnosis for women with incident RA (n=843) and matched comparators (n=8,399) in the Nurses’ Health Study.
| Rheumatoid arthritis (n=843) | Comparators* (n=8,399) | |
|---|---|---|
|
|
||
| Mean age, years (SD)** | 59.8 (10.0) | 59.8 (10.0) |
| Annual household income, % | ||
| <$40K | 11.5 | 12.1 |
| ≥$40K | 88.5 | 87.9 |
| Region, % | ||
| West | 31.6 | 32.6 |
| Midwest | 14.2 | 13.0 |
| Mid-Atlantic | 21.6 | 21.5 |
| New England | 16.4 | 16.8 |
| Southeast | 14.0 | 12.1 |
| Smoking status, % | ||
| Never | 35.6 | 44.1 |
| Past | 43.8 | 36.1 |
| Current | 20.3 | 18.7 |
| Smoking pack-years at baseline, % | ||
| 0 | 35.6 | 44.1 |
| >0 to 10 | 16.4 | 16.9 |
| 10.1 to 20 | 11.6 | 9.9 |
| >20 | 33.8 | 26.7 |
| Body mass index categories, % | ||
| Underweight (<18.5 kg/m2) | 2.1 | 2.1 |
| Normal (18.5–24.9 kg/m2) | 47.8 | 51.4 |
| Overweight (25.0–29.9 kg/m2) | 32.2 | 29.6 |
| Obese (≥30.0 kg/m2) | 17.9 | 17.0 |
| Physical activity hours/week, % | ||
| 0 | 6.6 | 12.7 |
| 0.01 to 0.9 | 24.2 | 21.9 |
| 1 to 3.49 | 21.8 | 19.7 |
| 3.5 to 5.9 | 27.8 | 25.7 |
| ≥6 | 10.3 | 10.9 |
| Alternate Healthy Eating Index (quartiles), % | ||
| Q1 – Least healthy | 19.9 | 18.8 |
| Q2 | 23.8 | 19.8 |
| Q3 | 22.5 | 19.7 |
| Q4 – Most healthy | 23.1 | 20.3 |
| Menopausal status and postmenopausal hormone use | ||
| Premenopausal | 26.6 | 30.4 |
| Postmenopausal and never PMH use | 26.3 | 27.9 |
| Postmenopausal and past PMH use | 22.8 | 18.6 |
| Postmenopausal and current PMH use | 22.9 | 19.5 |
| Aspirin use, % | 43.4 | 35.8 |
Each woman with incident rheumatoid arthritis occurring during follow-up of the Nurses’ Health Study was matched to up to 10 women without rheumatoid arthritis or connective tissue disease by age and calendar year at the index date of RA diagnosis.
Age is reported as of index date.
Missing data not shown.
CTD, connective tissue disease; PMH, postmenopausal hormone use; RA, rheumatoid arthritis; SD, standard deviation.
Women with RA were followed for a total of 15,716 person-years after diagnosis (mean 18.6 [SD 9.0] years per participant). Comparators were followed for a total of 157,768 person-years (mean 18.8 [SD 9.5] years). After index date, there were 68 (8.1%) incident COPD cases reported among women with RA and 459 (5.5%) reported among comparators. Forty (4.7%) women with RA reported a new diagnosis of asthma after RA diagnosis compared to 268 (3.2%) of comparators who reported asthma after index date (Table 3). When restricted to never smokers, 3.0% (9/300) of women with RA developed COPD compared to 2.3% (85/3,700) of comparators. When restricted to never or light (<10 pack-years), a higher proportion of women with RA developed COPD (19/438, 4.3%) than comparators (128/5,115, 2.5%).
Table 3.
Frequencies of incident COPD and asthma after index date during follow-up in the Nurses’ Health Study (1976–2014) for women with RA and matched comparators.
| Rheumatoid arthritis | Comparators | |
|---|---|---|
| Entire analyzed study sample | ||
| COPD, cases/n (%) | 68/843 (8.1%) | 459/8,399 (5.5%) |
| Asthma, cases/n (%)* | 40/843 (4.7%) | 268/8,399 (3.2%) |
| Among never smokers at index date | ||
| COPD, cases/n (%) | 9/300 (3.0%) | 85/3,700 (2.3%) |
| Asthma, cases/n (%)* | 14/300 (4.7%) | 114/3,700 (3.1%) |
| Among never or <10 pack-years at index date | ||
| COPD, cases/n (%) | 19/438 (4.3%) | 128/5,115 (2.5%) |
| Asthma, cases/n (%)* | 19/438 (4.3%) | 163/5,115 (3.2%) |
| Follow-up after index date, person-years** | 15,716 | 157,768 |
| Mean follow-up after index date per participant, years (SD)** | 18.6 (9.0) | 18.8 (9.5) |
Women who reported both asthma and COPD during follow-up were classified as COPD for all analyses.
Follow-up is reported for the entire analyzed study sample.
COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Association of RA with incident COPD
Table 4 shows the associations of RA with COPD and asthma with comparator status as the reference group. For all women with RA, the HR for incident COPD was 1.52 (95%CI 1.17–1.97) adjusted only for the matching factors of age and year of index date. When adjusting for smoking before and after index date using MSM, RA was still significantly associated with COPD compared to comparators (HR 1.43, 95%CI 1.09–1.87). After adjusting for additional time-varying covariates, including BMI, dietary quality, menopausal status and PMH use, physical activity, income, and aspirin use, the association of RA with incident COPD remained statistically significant (HR 1.68, 95%CI 1.36–2.07). In multivariable analyses stratified by serostatus, both seropositive RA (HR 1.74, 95%CI 1.36–2.23) and seronegative RA (HR 1.62, 95%CI 1.09–2.40) were significantly associated with increased COPD risk compared to matched comparators. Figure 1 shows worsened COPD-free survival for all RA (p<0.001), seropositive RA (p<0.001) and seronegative RA (p=0.017) compared to non-RA in the weighted cumulative survival curves, adjusted for the same factors as in the multivariable MSM.
Table 4.
Hazard ratios for COPD and asthma for women with RA serologic phenotypes compared to matched comparators, adjusted for time-varying confounders and mediators using marginal structural models.
| COPD | Asthma | |
|---|---|---|
|
| ||
| HR (95% CI) | HR (95% CI) | |
| All RA | ||
| Age-adjusted* | 1.52 (1.17–1.97) | 1.55 (1.11–2.16) |
| Smoking-adjusted** | 1.43 (1.09–1.87) | 1.48 (1.05–2.08) |
| Multivariable adjusted*** | 1.68 (1.36–2.07) | 1.15 (0.82–1.61) |
|
| ||
| Seropositive RA | ||
| Age-adjusted* | 1.60 (1.17–2.19) | 1.38 (0.90–2.13) |
| Smoking-adjusted** | 1.44 (1.04–2.00) | 1.33 (0.86–2.05) |
| Multivariable adjusted*** | 1.74 (1.36–2.23) | 1.11 (0.75–1.66) |
|
| ||
| Seronegative RA | ||
| Age-adjusted* | 1.41 (0.89–2.23) | 1.88 (1.10–3.23) |
| Smoking-adjusted** | 1.47 (0.91–2.39) | 1.72 (0.99–2.98) |
| Multivariable adjusted*** | 1.62 (1.09–2.40) | 1.11 (0.59–2.09) |
Comparator status is the reference in all models.
The all RA analyses included 843 women with all RA and 8,399 matched comparators. The seropositive RA analyses included 518 women with seropositive RA and 5,163 matched comparators. The seronegative RA analyses included 325 women with seronegative RA and 3,236 matched comparators.
Adjusted for matching factors (age and calendar year) at index date.
Adjusted for matching factors (age and calendar year) at index date, pre-index smoking pack-years (0, >0–10, 10.1–20, >20), and time-varying smoking status (never, past, current).
Adjusted for matching factors (age and calendar year) at index date as well as baseline factors of annual family income (<$40K, ≥$40K) and pre-index smoking pack-years (0, >0–10, 10.1–20, >20) as well as time-varying factors of smoking status (never, past, current), body mass index (continuous, kg/m2), Alternate Healthy Eating Index (quartiles), menopausal status and PMH use (premenopausal, postmenopausal and never PMH use, postmenopausal and past PMH use, postmenopausal and current PMH use), physical activity (continuous, METs/week), and aspirin use (yes/no) using marginal structural models.
COPD, chronic obstructive pulmonary disease; PMH, postmenopausal hormones; RA, rheumatoid arthritis.
Figure 1.
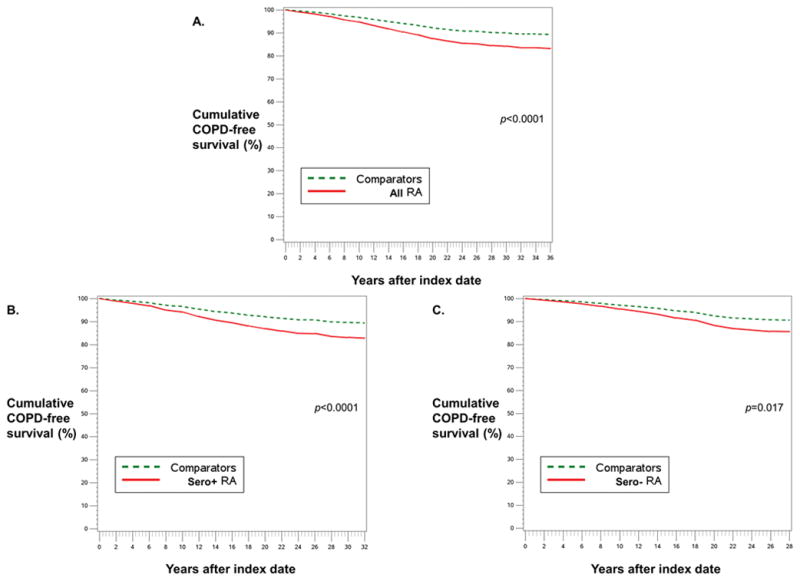
Weighted cumulative survival curves for COPD among women with RA (solid red line) and matched comparators (dashed green line). A) All RA, B) Seropositive RA, and C) Seronegative RA, each vs. matched comparators. Adjusted for covariates listed in multivariable model in Table 3.
Association of RA with incident asthma
In models adjusted for only age and year of index date, RA was significantly associated with increased risk of asthma (HR 1.55, 95%CI 1.11–2.16). However, when adjusted for time-varying confounders and mediators using MSM, RA was no longer significantly associated with incident asthma (HR 1.15, 95%CI 0.82–1.61). In models stratified by RA serostatus, there was a statistically significant association of seronegative RA with incident asthma in the age and year-adjusted model (HR 1.88, 95%CI 1.10–3.23), which was no longer statistically significant after adjusting for time-varying confounders and mediators (HR 1.11, 95%CI 0.59–2.09). For seropositive RA, there was no association with asthma in either the age- and year-adjusted model (HR 1.38, 95%CI 0.90–2.13) or the multivariable model (HR 1.11, 95%CI 0.75–1.66). Figure 2 shows similar the weighted asthma-free cumulative survival curve for all RA (p=0.43) compared to comparator status.
Figure 2.
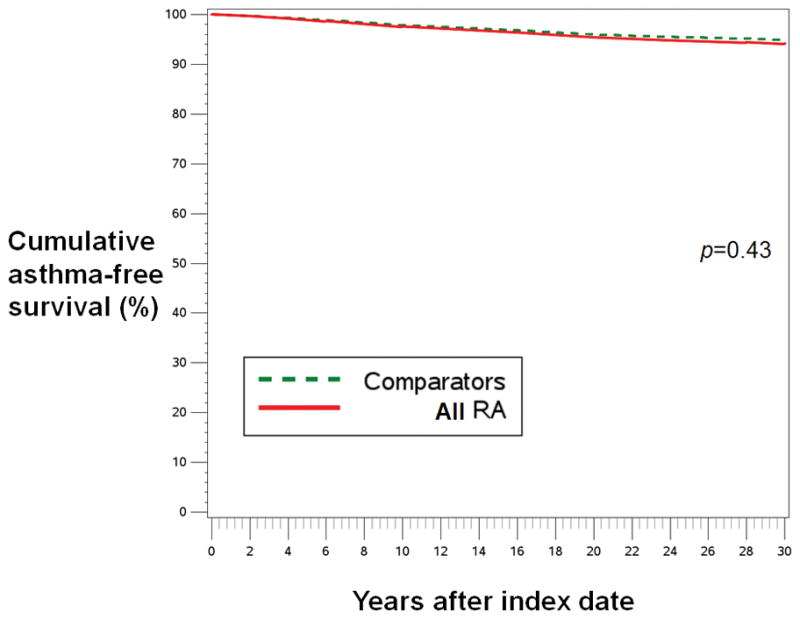
Weighted cumulative survival curves for asthma among women with all RA (solid red line) and matched comparators (dashed green line). Results were similar for seropositive RA vs. matched comparators (p=0.60) and seronegative RA vs. matched comparators (p=0.75, curves not shown). Adjusted for covariates listed in multivariable model in Table 3.
DISCUSSION
In this large study with up to 38 years of prospective follow-up, we found that women with RA had a 68% elevated risk of COPD compared to women without RA, independent of smoking and other lifestyle factors occurring before or after RA diagnosis, using MSM to adjust for confounding and mediation by smoking and other covariates. Conversely, we found no association of RA with asthma risk after adjusting for confounders and mediators. These results suggest that RA-specific factors such as chronic inflammation may increase COPD risk independent of smoking and other lifestyle factors. These results are particularly pertinent since patients with RA are at markedly elevated risk for respiratory mortality. Since COPD is a common chronic lung disease, these results add to prior literature suggesting that a portion of the excess respiratory mortality in RA could be due to increased risk of COPD (15, 19, 42).
Airways may be involved in the pathogenesis of RA (1, 3, 7). Immune tolerance breakdown leading to the formation of RA-related autoantibodies may occur specifically at bronchiolar respiratory mucosa (1, 43). Induced sputum from unaffected RA first-degree relatives have shown IgA RF and ACPA suggesting that these autoantibodies are produced locally in the lung prior to the serum presence of RA-related autoantibodies (10). In other studies of newly diagnosed patients with RA prior to medications, lung biopsies and fluid obtained by bronchoalveolar lavage showed increases in lymphocyte aggregates that produce ACPA in lung parenchyma and airways (5, 6). These local anatomic lesions may pre-dispose patients with RA to bronchiolar dysfunction that results in obstructive lung disease independent of the effect of cigarette smoking. After RA diagnosis, patients with RA may be pre-disposed to develop COPD from a variety of factors including systemic inflammation, side effects of DMARDs such as methotrexate, as well as parenchymal and airway inflammation (44). In a recent non-biased approach to identify clinical subphenotypes of RA by a phenome-wide association study, patients with RA with anti-fibrinogen autoantibodies on a research ACPA assay were at increased risk for bronchiolitis, which may manifest as an obstructive lung disease similar to COPD (45). Patients with COPD are known to have persistent airway inflammation even among distant former and never smokers (46). Up to one-quarter of patients with COPD are never smokers who presumably have had significant exposure to second-hand smoke or other inhalants (47). Over 7% of patients with COPD without RA were reported to have the presence of serum ACPA in a cross-sectional study (22). Finally, previous studies have suggested that there may be an autoimmune component to COPD independent of smoking since multiple autoimmune diseases have also been associated with subsequent COPD development (48).
These results add to the literature implicating RA and risk of COPD (Table 1). A recent meta-analysis of four retrospective studies associated RA with increased risk for incident COPD (RR 1.99, 95%CI 1.61–2.45) (16). However, our study is the first prospective study to investigate the effect of RA on subsequent COPD and asthma while also controlling for the mediating effect of smoking occurring after RA diagnosis. A large nationwide Taiwanese administrative database study associated RA with increased risk for COPD (HR 1.85, 95%CI 1.70–2.01) (17). However, data on smoking and other lifestyle variables were unavailable and both RA and COPD were defined using billing codes. A large Swedish register study investigated 29 autoimmune diseases, including RA, with COPD and lung cancer and found that RA as well as other autoimmune diseases were associated with COPD (48). However, this study compared the observed incidence rate to the expected incidence rate based on age, sex, region, and period to obtain standardized incidence ratio (SIR for RA: 2.57, 95%CI 2.21–2.97), so could not account for lifestyle factors such as smoking (48). Finally, a previous US population-based study also evaluated the association of RA with a composite measure of obstructive lung diseases composed of mostly COPD as well as asthma, bronchiectasis, and ILD. Unlike our study, imaging and pulmonary function tests were available to define these respiratory outcomes as well as richer data on RA characteristics such as DMARD use. In that study, RA had a HR of 1.54 (95%CI 1.01–2.34) for the composite definition of obstructive lung disease compared to controls, adjusted for age, sex, smoking (categorized as never, past, or current), and alcoholism at the index date of RA diagnosis (19). That study estimated that 6.3% of excess RA mortality was explained by this increased risk of obstructive lung disease (19). Our study was able to adjust for confounders and time-varying mediators occurring after index date to define the independent effect of RA on subsequent COPD. Therefore, our results suggest that RA is associated with increased COPD risk independent of the mediating effect of smoking.
We hypothesized that RA would be associated with increased asthma risk based on previous studies (Table 1). A Dutch study using electronic medical record data showed that patients who presented with inflammatory arthritis had 1.4-fold increased risk for asthma compared to matched controls, but data on smoking were unavailable (49). A large nationwide study in Taiwan using billing claims data showed that RA was associated with 2-fold increased risk for asthma compared to matched comparators (18). Both of these retrospective studies included all ages and relied on billing codes and had no data available on lifestyle factors that might have affected the relationship. In our study, we also found a statistically significant association of RA with asthma in the model adjusted for age and year of index date. However, after adjusting for time-varying covariates, this association of RA with asthma was no longer statistically significant, suggesting that other lifestyle factors such as smoking may have explained the initial association, rather than RA-specific factors. A previous study among younger adults using allergic diseases as the exposure and RA as the dependent outcome found that those with asthma had 67% increased risk for RA than matched comparators (50). While we were unable to detect a relationship between RA and asthma, this may have been due to relatively older ages at RA onset among women that were included in our study.
Another explanation for our results could be that patients with RA and COPD share common risk factors other than smoking. Since our analyses were also adjusted for dietary quality, BMI, and physical activity occurring before and after RA diagnosis, these factors are less likely to explain the association of RA and COPD that we report. While we did not have genetics available for this study, currently described genetic factors for RA and COPD have little overlap so it is unlikely that shared genetics explains this association (51, 52). A previous study examined whether pulmonary dysfunction was associated with subsequent development of RA and did not find and association between COPD and subsequent RA risk (53). Another nationwide Taiwanese study reported that asthma was associated with increased subsequent RA risk (50). Factors other than smoking are known to increase risk for COPD. In particular, citrullination is a biologic process important in both RA and COPD that might explain the observed increased risk of COPD for patients with RA (54). Future studies are needed to investigate shared environmental or genetic factors and RA-specific factors such as citrullination, autoimmunity, and systemic inflammation that might further explain the respiratory burden of RA.
Strengths of our study included large sample size with lengthy follow-up (mean of nearly 20 years after RA diagnosis) and repeated measures of important time-varying factors related to both RA and the respiratory outcomes, in particular smoking. Since we identified incident RA during follow-up of the NHS according to accepted classification criteria, we were able to prospectively follow women before and after validated RA diagnosis. We were therefore able to collect detailed data on important covariates prospectively without differential recall bias as in case-control studies). Since we performed the study within the NHS, we also had a large pool of potential comparators without RA that had prospective data collected identically to women with RA. We were therefore able to perform a prospective matched cohort study with all participants beginning follow-up for respiratory outcomes as of the index date.
Our study does have some limitations to consider. While we collected RA disease characteristics near the time of diagnosis through medical record review, we did not have RA-specific data on disease activity, progression, and treatment occurring during follow-up. Since we were interested in comparing RA to non-RA, we would have been unable to analyze these characteristics in this study since non-RA comparators would have no treatment for RA or disease activity so adjustment could not have occurred. Future studies performed among RA cohorts with longitudinal follow-up are needed to investigate the effects of disease activity and DMARDs on respiratory outcomes such as COPD and asthma. There were not a sufficient number of outcomes to perform analyses among never or light smokers to understand these relationships in a cohort without substantial cigarette smoke exposure. While we had detailed data available on many covariates including smoking, residual confounding is always possible. To maximize the number of outcomes, we relied on self-reported COPD and asthma, which might result in misclassification. A previous validated study showed PPV of 79–90% for self-reported COPD and asthma in this cohort of nurses (28). A subset of women with self-reported COPD and asthma returned a supplemental questionnaire in 1998 and 2000 to gather more data on their COPD and asthma. Unfortunately, there were not enough incident COPD or asthma cases among patients with RA to analyze this subset. There may have been some misclassification of asthma and COPD. Since late-onset asthma is relatively uncommon, and smoking-related COPD may carry some stigma, it is more likely that women who reported asthma may have had COPD. Since we still found a statistically significant association of RA with COPD, it is unlikely that this misclassification of the outcome explains the results. However, it is possible we were unable to detect a true association of RA with asthma due to misclassification or the inclusion of older women in this study. Since the NHS is focused on epidemiology for relatively common chronic diseases, ILD was never assessed on questionnaires. It is possible that women with RA and ILD may have reported COPD or asthma instead. We found similar results of increased COPD risk for seropositive and seronegative RA. Since we relied on clinical testing of RA-related autoantibodies, women diagnosed with RA prior to its routine use did not have anti-CCP tested. Some women in the earlier years of the cohort may have been misclassified as seronegative based only on RF. Therefore, it is still possible that there may be differences in COPD or asthma risk related to anti-CCP positivity that our study could not detect. While the NHS was performed throughout the US, it only included well-educated women who were healthy enough to be working at baseline of the study in 1976. It is unclear how generalizable these results might be to men, other populations, or different time periods as the composition of cigarettes changed. However, our results associating RA with increased risk for COPD are similar to a recent meta-analysis (16).
In conclusion, women with RA were at increased risk for subsequently being diagnosed with COPD, but had similar risk for being diagnosed with asthma compared to women without RA followed in this same cohort during up to 38 years of prospective follow-up. Further, this association was not explained by lifestyle factors such as smoking occurring before or after RA diagnosis. These results suggest that RA-specific factors may pre-dispose patients to developing COPD independent of smoking. Since COPD is a common chronic respiratory disease, the increased COPD risk among RA patients may contribute to the excess respiratory mortality for RA beyond that of smoking or ILD. Screening and early treatment for respiratory diseases among patients with RA may help decrease the respiratory burden of RA.
Acknowledgments
Funding/Support: This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases to Dr. Sparks under award number K23 AR069688. This work also supported by the National Institutes of Health under award numbers K24 AR052403, R01 AR049880, P30 AR070253, P30 AR069625, R01 AR049880, K24 AR066109, P60 AR047782, and UM1 CA186107. Dr. Sparks and Dr. Barbhaiya are supported by the Scientist Development award from the Rheumatology Research Foundation. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank the participants of the NHS for their dedicated participation in this longitudinal study as well as the NHS staff members at the Channing Division of Network Medicine (Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual contact, and all authors approved the final version to be published. Dr. Sparks had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sparks, Lin, Karlson
Acquisition of data. Sparks, Camargo, Barbhaiya, Tedeschi, Costenbader, Karlson
Analysis and interpretation of data. Sparks, Lin, Camargo, Barbhaiya, Tedeschi, Costenbader, Raby, Choi, Karlson
References
- 1.Kelmenson LB, Demoruelle MK, Deane KD. The Complex Role of the Lung in the Pathogenesis and Clinical Outcomes of Rheumatoid Arthritis. Curr Rheumatol Rep. 2016;18(11):69. doi: 10.1007/s11926-016-0618-4. [DOI] [PubMed] [Google Scholar]
- 2.Chatzidionisyou A, Catrina AI. The lung in rheumatoid arthritis, cause or consequence? Curr Opin Rheumatol. 2016;28(1):76–82. doi: 10.1097/BOR.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 3.Sparks JA, Karlson EW. The Roles of Cigarette Smoking and the Lung in the Transitions Between Phases of Preclinical Rheumatoid Arthritis. Curr Rheumatol Rep. 2016;18(3):15. doi: 10.1007/s11926-016-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64(6):1756–61. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynisdottir G, Olsen H, Joshua V, Engstrom M, Forsslund H, Karimi R, et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis. 2016;75(9):1722–7. doi: 10.1136/annrheumdis-2015-208216. [DOI] [PubMed] [Google Scholar]
- 6.Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 2014;66(1):31–9. doi: 10.1002/art.38201. [DOI] [PubMed] [Google Scholar]
- 7.Klareskog L, Catrina AI. Autoimmunity: lungs and citrullination. Nat Rev Rheumatol. 2015;11(5):261–2. doi: 10.1038/nrrheum.2015.38. [DOI] [PubMed] [Google Scholar]
- 8.Sparks JA, Chang SC, Deane KD, Gan RW, Kristen Demoruelle M, Feser ML, et al. Associations of Smoking and Age With Inflammatory Joint Signs Among Unaffected First-Degree Relatives of Rheumatoid Arthritis Patients: Results From Studies of the Etiology of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(8):1828–38. doi: 10.1002/art.39630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Hair MJ, Landewe RB, van de Sande MG, van Schaardenburg D, van Baarsen LG, Gerlag DM, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis. 2013;72(10):1654–8. doi: 10.1136/annrheumdis-2012-202254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65(10):2545–54. doi: 10.1002/art.38066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim K, Jiang X, Cui J, Lu B, Costenbader KH, Sparks JA, et al. Interactions between amino acid-defined major histocompatibility complex class II variants and smoking in seropositive rheumatoid arthritis. Arthritis Rheumatol. 2015;67(10):2611–23. doi: 10.1002/art.39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.England BR, Sayles H, Michaud K, Caplan L, Davis LA, Cannon GW, et al. Cause-Specific Mortality in Male US Veterans With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68(1):36–45. doi: 10.1002/acr.22642. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez A, Icen M, Kremers HM, Crowson CS, Davis JM, 3rd, Therneau TM, et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J Rheumatol. 2008;35(6):1009–14. [PMC free article] [PubMed] [Google Scholar]
- 15.Sparks JA, Chang SC, Liao KP, Lu B, Fine AR, Solomon DH, et al. Rheumatoid Arthritis and Mortality Among Women During 36 Years of Prospective Follow-Up: Results From the Nurses’ Health Study. Arthritis Care Res (Hoboken) 2016;68(6):753–62. doi: 10.1002/acr.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungprasert P, Srivali N, Cheungpasitporn W, Davis JM., III Risk of incident chronic obstructive pulmonary disease in patients with rheumatoid arthritis: A systematic review and meta-analysis. Joint Bone Spine. 2016;83(3):290–4. doi: 10.1016/j.jbspin.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Shen TC, Lin CL, Chen CH, Tu CY, Hsia TC, Shih CM, et al. Increased risk of chronic obstructive pulmonary disease in patients with rheumatoid arthritis: a population-based cohort study. QJM. 2014;107(7):537–43. doi: 10.1093/qjmed/hcu027. [DOI] [PubMed] [Google Scholar]
- 18.Shen TC, Lin CL, Wei CC, Tu CY, Li YF. The risk of asthma in rheumatoid arthritis: a population-based cohort study. QJM. 2014;107(6):435–42. doi: 10.1093/qjmed/hcu008. [DOI] [PubMed] [Google Scholar]
- 19.Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken) 2013;65(8):1243–50. doi: 10.1002/acr.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Giuseppe D, Discacciati A, Orsini N, Wolk A. Cigarette smoking and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther. 2014;16(2):R61. doi: 10.1186/ar4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokkonen H, Brink M, Hansson M, Lassen E, Mathsson-Alm L, Holmdahl R, et al. Associations of antibodies against citrullinated peptides with human leukocyte antigen-shared epitope and smoking prior to the development of rheumatoid arthritis. Arthritis Res Ther. 2015;17:125. doi: 10.1186/s13075-015-0638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Esquide V, Gomara MJ, Peinado VI, Gomez Puerta JA, Barbera JA, de Canete JD, et al. Anti-citrullinated peptide antibodies in the serum of heavy smokers without rheumatoid arthritis. A differential effect of chronic obstructive pulmonary disease? Clin Rheumatol. 2012;31(7):1047–50. doi: 10.1007/s10067-012-1971-y. [DOI] [PubMed] [Google Scholar]
- 23.Forey BA, Thornton AJ, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med. 2011;11:36. doi: 10.1186/1471-2466-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaakkola MS, Jaakkola JJ. Effects of environmental tobacco smoke on the respiratory health of adults. Scand J Work Environ Health. 2002;28(Suppl 2):52–70. [PubMed] [Google Scholar]
- 25.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 26.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 27.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 28.Barr RG, Herbstman J, Speizer FE, Camargo CA., Jr Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155(10):965–71. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
- 29.Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, et al. Asthma in the elderly: Current understanding and future research needs--a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol. 2011;128(3 Suppl):S4–24. doi: 10.1016/j.jaci.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503e1–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 31.Bengtsson C, Malspeis S, Orellana C, Sparks JA, Costenbader KH, Karlson EW. Menopausal factors are associated with seronegative RA in large prospective cohorts: results from the Nurses’ Health Studies. Arthritis Care Res (Hoboken) 2017 doi: 10.1002/acr.23194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu B, Hiraki LT, Sparks JA, Malspeis S, Chen CY, Awosogba JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis. 2014;73(11):1914–22. doi: 10.1136/annrheumdis-2014-205459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varraso R, Fung TT, Barr RG, Hu FB, Willett W, Camargo CA., Jr Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86(2):488–95. doi: 10.1093/ajcn/86.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gan WQ, Man SF, Postma DS, Camp P, Sin DD. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2006;7:52. doi: 10.1186/1465-9921-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84(7):1086–93. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Solomon DH, Karlson EW, Curhan GC. Cardiovascular care and cancer screening in female nurses with and without rheumatoid arthritis. Arthritis Rheum. 2004;51(3):429–32. doi: 10.1002/art.20418. [DOI] [PubMed] [Google Scholar]
- 38.de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64(4):650–8. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 39.Hu Y, Sparks JA, Malspeis S, Costenbader KH, Hu FB, Karlson EW, et al. Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann Rheum Dis. 2017 doi: 10.1136/annrheumdis-2016-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Westreich D, Cole SR, Tien PC, Chmiel JS, Kingsley L, Funk MJ, et al. Time scale and adjusted survival curves for marginal structural cox models. Am J Epidemiol. 2010;171(6):691–700. doi: 10.1093/aje/kwp418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doss J, Mo H, Carroll RJ, Crofford LJ, Denny JC. Phenome-Wide Association Study of Rheumatoid Arthritis Subgroups Identifies Association Between Seronegative Disease and Fibromyalgia. Arthritis Rheumatol. 2017;69(2):291–300. doi: 10.1002/art.39851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demoruelle MK, Solomon JJ, Fischer A, Deane KD. The lung may play a role in the pathogenesis of rheumatoid arthritis. Int J Clin Rheumtol. 2014;9(3):295–309. doi: 10.2217/ijr.14.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antoniou KM, Walsh SL, Hansell DM, Rubens MR, Marten K, Tennant R, et al. Smoking-related emphysema is associated with idiopathic pulmonary fibrosis and rheumatoid lung. Respirology. 2013;18(8):1191–6. doi: 10.1111/resp.12154. [DOI] [PubMed] [Google Scholar]
- 45.Liao KP, Sparks JA, Hejblum BP, Kuo IH, Cui J, Lahey LJ, et al. Phenome-wide association study of autoantibodies to citrullinated and non-citrullinated epitopes in rheumatoid arthritis. Arthritis Rheumatol. 2016 doi: 10.1002/art.39974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutgers SR, Postma DS, ten Hacken NH, Kauffman HF, van Der Mark TW, Koeter GH, et al. Ongoing airway inflammation in patients with COPD who do not currently smoke. Thorax. 2000;55(1):12–8. doi: 10.1136/thorax.55.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139(4):752–63. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemminki K, Liu X, Ji J, Sundquist K, Sundquist J. Subsequent COPD and lung cancer in patients with autoimmune disease. Eur Respir J. 2011;37(2):463–5. doi: 10.1183/09031936.00070410. [DOI] [PubMed] [Google Scholar]
- 49.Ursum J, Nielen MM, Twisk JW, Peters MJ, Schellevis FG, Nurmohamed MT, et al. Increased risk for chronic comorbid disorders in patients with inflammatory arthritis: a population based study. BMC Fam Pract. 2013;14:199. doi: 10.1186/1471-2296-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai NS, Tsai TY, Koo M, Lu MC. Association of rheumatoid arthritis with allergic diseases: A nationwide population-based cohort study. Allergy Asthma Proc. 2015;36(5):99–103. doi: 10.2500/aap.2015.36.3871. [DOI] [PubMed] [Google Scholar]
- 51.Hobbs BD, de Jong K, Lamontagne M, Bosse Y, Shrine N, Artigas MS, et al. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat Genet. 2017;49(3):426–32. doi: 10.1038/ng.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506(7488):376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergstrom U, Jacobsson LT, Nilsson JA, Berglund G, Turesson C. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford) 2011;50(11):2005–13. doi: 10.1093/rheumatology/ker258. [DOI] [PubMed] [Google Scholar]
- 54.Kilsgard O, Andersson P, Malmsten M, Nordin SL, Linge HM, Eliasson M, et al. Peptidylarginine deiminases present in the airways during tobacco smoking and inflammation can citrullinate the host defense peptide LL-37, resulting in altered activities. Am J Respir Cell Mol Biol. 2012;46(2):240–8. doi: 10.1165/rcmb.2010-0500OC. [DOI] [PubMed] [Google Scholar]


