Abstract
[Purpose] Neuromuscular activity has been evaluated in patellofemoral pain syndrome but movement velocity has not been considered. The aim was to determine differences in onset latency of hip and knee muscles between individuals with and without patellofemoral pain syndrome during a single leg squat, and whether any differences are dependent on movement velocity. [Subjects and Methods] Twenty-four females with patellofemoral pain syndrome and 24 healthy females participated. Onset latency of gluteus maximus, anterior and posterior gluteus medius, rectus femoris, vastus medialis, vastus lateralis and biceps femoris during a single leg squat at high and low velocity were evaluated. [Results] There was an interaction between velocity and diagnosis for posterior gluteus medius. Healthy subjects showed a later posterior gluteus medius onset latency at low velocity than high velocity; and also later than patellofemoral pain syndrome subjects at low velocity and high velocity. [Conclusion] Patellofemoral pain syndrome subjects presented an altered latency of posterior gluteus medius during a single leg squat and did not generate adaptations to velocity variation, while healthy subjects presented an earlier onset latency in response to velocity increase.
Keywords: Patellofemoral joint, Electromyography, Gluteus medius
INTRODUCTION
Patellofemoral pain syndrome (PFPS) is one of the most frequent pathologies in the clinic, accounting for 25–40% of knee injuries in the sports field1, 2). It is more common among runners3) and young people4), and is 2 to 3 times more frequent in women1). Although the cause is unclear, PFPS may be related to altered patellar alignment, increased patellar pressure, and alteration of tissue homeostasis due to joint overload4, 5). This may be the consequence of poor dynamic patellar stability, or an excessive medial rotation and femoral adduction in weight-bearing activities6, 7) caused by an altered neuromuscular control at the knee and hip joints8).
Electromyographic (EMG) studies have related PFPS to altered muscle amplitude (activity intensity) and onset latency (muscle reaction time) of hip and knee muscle during running and ascending-descending of stairs8,9,10). This neuromuscular control alteration would increase hip medial rotation and adduction during weight-bearing activities, e.g. single leg squat (SLS), increasing the risk of knee joint dysfunctions5, 6). Recently, gluteus medius (Gmed) muscle has received particular attention for its role in lower limb kinematics and functional differentiation of its fibers during weight-bearing activities11,12,13). Semciw et al.11) showed differences in the EMG amplitude between the anterior gluteus medius (Gma) and posterior gluteus medius (Gmp) during gait and clam exercise, indicating that posterior fibers would have a major role in abduction and external rotation stabilization.
Single leg squat is a useful clinical test related to dynamic valgus and lower limb kinematics14, 15). In this context, Crossley et al.12) relate a poor SLS performance to delayed onset latency of anterior gluteus medius (Gma) and posterior gluteus medius (Gmp). Nevertheless, the investigators did not examine the magnitude of Gmed onset latency during the test and did not include PFPS subjects in the study. Recently, O’Sullivan et al.16) compared the activity of the three Gmed compartments between healthy and PFPS subjects during the execution of 4 common exercises (wall press, pelvic drop, step-up-and-over and single leg squat), showing no differences in muscle amplitude between fibers or groups. Nakagawa et al.17) showed a lower activation amplitude of Gmed muscle in PFPS subjects during a SLS, although the authors did not consider functional differentiation Gmed fibers in their study. Despite this, there are no reports about Gma and Gmp onset latency in patellofemoral pain females.
These differences between studies may correspond to kinematic factors not considered during the performance of a SLS. Previous reports have shown that movement velocity generates greater EMG activation of trunk muscles18) in healthy subjects, and changes in muscle onset latency in subjects with upper limb pathology19). However, there are no reports on the influence of SLS performance velocity on lower limb neuromuscular control in healthy or PFPS subjects, since the authors only evaluate this task at a single velocity. Therefore, the aim of the present study was to determine (1) differences in onset latency of hip and knee muscles between individuals with and without PFPS during the eccentric phase of a SLS, and (2) whether any difference in these variables is dependent on SLS performance velocity. Our hypothesis is that healthy and PFPS subjects present differences in onset latency of hip and knee muscles during a SLS, and that performance velocity influences these differences.
SUBJECTS AND METHODS
The present investigation consisted of a case-control study. The sample consisted of young females recruited through advertisements and direct communication. A sample size calculation for independent groups was performed using a power of 0.8 and an alpha of 0.05 based on the mean and standard deviation (35.2 ± 32.3) of the gluteus medius onset latency9). Considering a loss percentage of 20%, 24 healthy and PFPS participants were recruited. Anthropometric variables, including Q angle, are shown in Table 1. All participants read and signed the informed consent approved by the Bioethics Committee of the University of Talca (approval number 069).
Table 1. Anthropometric variables.
| Variables | PFPS (Mean ± SD) | Healthy (Mean ± SD) |
|---|---|---|
| Age (yrs) | 20.7 ± 1.6 | 21.0 ± 1.1 |
| Height (cm) | 150.1 ± 3.4 | 150 ± 5 |
| Weight (kg) | 56.8 ± 4.4 | 56.6 ± 5.9 |
| Body Max Index (kg/cm2) | 22.6 ± 1.8 | 22.6 ± 1.7 |
| Q angle (degrees) | 12 ± 2 | 14 ± 1 |
PFPS: patellofemoral pain syndrome; SD: standard deviation.
The inclusion criteria were normal BMI (18.5−24.9), unilateral or bilateral knee pain located specifically around the patella, for at least 1 month with an average pain level of 3 cm on a 10 cm visual analogue scale and have an insidious onset of symptoms unrelated to a traumatic incident, and pain in at least two of the following activities: prolonged sitting, climbing stairs, squatting, running, kneeling, hopping/jumping1). Subjects were excluded in case present history of surgery for the spine or lower extremities, neurologic alterations any other orthopaedic condition observed by the evaluator. The healthy group consisted of subjects with no traumatic or surgical history of the lower limbs, with anthropometric characteristics similar to the PFPS group.
Both groups were evaluated in a single session, which consisted of a surface EMG (sEMG) evaluation during a SLS. Muscle onset latency of the GMax, Gma, Gmp, rectus femoris (RF), vastus lateralis (VL), vastus medialis (VM) and biceps femoris (BF), and variables were low velocity (LV) and high velocity (HV) were measured during a SLS execution.
The sEMG signal was acquired with a Delsys TrignoTM Wireless sEMG System and recorded with the Delsys EMGworks Acquisition 4.2.0 (Delsys Inc., Boston, MA, USA). The electrodes were made of silver (99.9%) and had an inter-electrode distance of 10 mm. The electrodes consist of 4 bars of rectangular-shaped 99% silver (27 × 37 × 15 mm), with a distance between the electrodes of 10 mm. A bandpass filter was used (4th order, zero delay, Butterworth filter with frequencies between 20 and 450 Hz), common mode rejection ratio (CMRR) >80 dB, and signal to noise ratio <0.75 mV RMS. The sEMG was sampled at 2,000 Hz, using a 16-bit analog-digital converter. Hip acceleration was measured with a triaxial accelerometer (Delsys Inc., Boston, MA, USA) integrated into the electrode.
The symptomatic leg was evaluated in the PFPS group for those subjects with unilateral pain, the most symptomatic for those with bilateral knee pain. Dominant leg was evaluated in the healthy group. The dominant leg was defined as the preferred leg to kick a ball. Prior to evaluation, all subjects performed a 5 minutes warm-up on a cycle ergometer according previously reported protocols16). The skin was shaved and cleaned with alcohol, and the electrodes were placed on GMax, RF, VL, VM and BF according to SENIAM recommendations20). The Gma electrode was located at a distance of 50% between the anterior superior iliac spine (ASIS) and the greater trochanter; the Gmp electrode was placed at a distance of 33% between the posterior iliac and the major trochanter, according to a previous study13).
The participants of both groups received instructions on how to properly perform the SLS. They were asked to fold their arms across their chest, stand on the evaluated limb and perform a single leg squat in a fluid and controlled way, reaching approximately 60° knee flexion. sEMG activity was recorded in the descent or eccentric phase, with 3 SLS repetitions for each execution speed in order to decrease the inter-individual variability21). High velocity SLS was performed in 1 second, and low velocity SLS performed in 3 seconds. Performance velocities were selected based on SLS performance from previous studies12) and were determined by the beats of a metronome, pointing the beginning and ending of the SLS. EMG basal signal was collected in single leg position during 2 s, to avoid fatigue and balance loss, and the movement initiation was recorded by a triaxial accelerometer integrated to the EMG electrode located in the middle third of the anterior thigh of the standing leg. SLS initiation was determined on the inflection of the acceleration curve (Fig. 1).
Fig. 1.
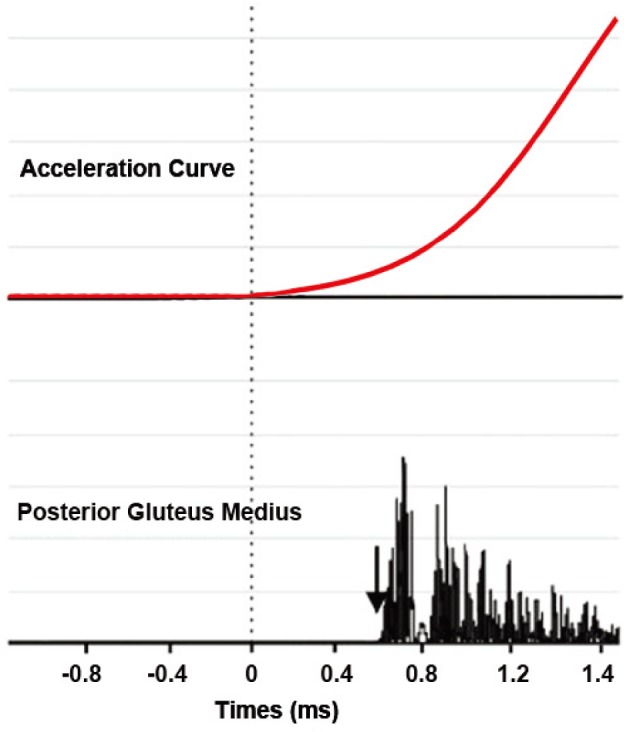
Rectified and filtered EMG signal of the posterior gluteus medius onset lantecy muscles during a single leg squat of one subject. Dotted line shows time zero, which corresponds with accelerometry inflection. The black arrows show the onset latency for each muscle.
To avoid muscle fatigue every repetition was followed by rest periods of 30 s before the next SLS, and high and low velocity were performed alternately. In case of loss of alignment or bad movement performance, the data was excluded and an additional repetition was performed respecting resting times. It was considered bad movement performance if loss of balance, trunk misalignment or inability to adapt movement velocity were observed during SLS execution.
For data processing EMG work analysis software 4.0 (Delsys Inc., Boston, MA, USA) was used. A full wave rectification of all raw electromyographic signals was performed and a 50 Hz low pass filter was used2). Onset latency of hip and knee was calculated relative to the accelerometer activity and corresponds to the time when the EMG activity surpassed a threshold of at least 3 standard deviations above the resting mean activity of a 200 ms window prior to the initiation of the SLS8, 22,23,24), and remained above this threshold for 25 milliseconds. All onsets were visually confirmed since movement artifact could be misinterpreted as muscle activity22) (Fig. 1)
The statistical software SPSS (version 22.0 for Windows, IBM Inc., IL, USA) was used. A t-test for independent samples was performed to determine differences between groups in weight, height, BMI and age. All data are presented as mean and standard deviation. An alpha level <0.05 was considered in all statistical tests. The Shapiro-Wilk test and Levene’s test were applied to calculate the normality and homogeneity of variance, respectively.
For each muscle onset latency an analysis of variance (ANOVA) of repeated measures of two factors was performed: one factor between subjects of two levels (diagnostic; PFPS and healthy) and 1 intra-subject factor of two levels (velocity; HV and LV). A Bonferroni corrected t-test was performed to compare between pairs.
RESULTS
Independent t-test results for weight, height, BMI, age and Q angle show no differences between groups (p>0.05). Muscle onset latency met the criteria of normality and homogeneity of variances. The means and standard deviations for each variable are shown in Table 2. There was a significant interaction between velocity and diagnostic factors only for Gmp onset latency (F=20.85; df=1; p<0.001). Post-hoc analysis showed differences between PFPS and Healthy subjects during a SLS, characterized by a significantly later Gmp activity of PFPS group at LV (p<0.05) and HV (p<0.05) (Table 3). The healthy group showed a significantly later Gmp onset latency at LV SLS than a HV SLS (p<0.05), while there was no significant differences between LV and HV SLS in the PFPS group (p>0.05). The remaining comparisons of Gmp onset latency did not show significant differences (Table 3).
Table 2. Descriptive statistics for muscle onset latency between groups and velocities.
| Muscle Onset (ms) | PFPS (Mean ± SD) | Healthy (Mean ± SD) | ||
|---|---|---|---|---|
| Low velocity | High velocity | Low velocity | High velocity | |
| Gluteus maximus | −518.7 ± 437.2 | −202.0 ± 339.0 | −462.5 ± 688.2 | −213.2 ± 330.2 |
| Anterior gluteus medius | −524.5 ± 669.6 | −195.2 ± 356.8 | −206.2 ± 457.5 | −136.2 ± 215.4 |
| Posterior gluteus medius | −657.4 ± 689.4 | −265.8 ± 456.1 | 753.7 ± 1,117.5 | −230.7± 485.7 |
| Rectus femoris | 15.9 ± 422.9 | 19.9 ± 263.1 | 78.7 ± 247.9 | −41.7 ± 123.9 |
| Vastus medialis | 32.4 ± 371.9 | −70.4 ± 224.0 | 226.2 ± 299.8 | −66.7 ± 155.4 |
| Vastus lateralis | 11.1 ± 434.4 | −61.8 ± 274.3 | 175.3 ± 335.5 | −44.7 ± 176.2 |
| Biceps femoris | −28.4 ± 693.8 | 2.5 ± 397.9 | 778.3 ±1,441.6 | 346.0 ± 662.6 |
PFPS: patellofemoral pain syndrome; SD: standard deviation.
Table 3. Multiple pairwise comparison of posterior gluteus medius onset latency between groups and velocities.
| Pairwise comparison | Mean difference | 95% CI of difference | |
|---|---|---|---|
| Healthy LV v/s Healthy HV | 984.4* | 366.9 | 1,601.9 |
| Healthy LV v/s PFPS LV | −1,411.1* | −2,028.7 | 793.6 |
| Healthy LV v/s PFPS HV | −1,019.5* | 1,637 | 402 |
| Healthy HV v/s PFPS LV | −426.7 | −1,044.2 | 190.8 |
| Healthy HV v/s PFPS HV | −35 | −652.6 | 582.4 |
| PFPS LV v/s PFPS HV | −391.6 | −1,009.1 | 225.9 |
PFPS: patellofemoral pain syndrome; LV: low velocity; HV: high velocity; CI: confidence interval. *p<0.05.
DISCUSSION
The main results of the present study are: (1) subjects with and without PFPS present differences in Gmp onset latency during the SLS performance at LV; and (2) healthy individuals show an earlier onset latency at higher velocity performance of the SLS, while PFPS individuals do not present changes in their strategy. Despite this, PFPS individuals presented an earlier Gmp activation than healthy individuals at LV, regardless of movement velocity.
Our findings show that subjects with PFPS showed an earlier Gmp onset (−657.4 ms) during a SLS, while healthy subjects showed a later activation of Gmp (753.7 ms). Previous reports showed a delayed Gmed activation in PFPS subjects during running9) and the stairs ascent-descent8, 10). Nevertheless, during SLS the evaluated limb is always in loading position, which differs from loading transition tasks. Kim et al.25) showed an early Gmed onset latency of 330 ± 133 ms prior to single leg transition, pointing that Gmed is already active before SLS movement initiation, specifically Gma providing trunk and pelvis stability11). Additionally, during a SLS hip flexion angle increases progressively (up to 60°), increasing the Gmp contribution as hip external rotator and abductor11). Considering that and our results, Gmp would activate later during the SLS to stabilize hip flexion and internal femoral rotation11), showing an altered neuromuscular Gmp activity in PFPS subjects. Previous studies show altered cortical representation of back muscles in chronic pain subjects, characterized poor muscle differentiation during movement26). In line with this, the earlier onset latency of Gmp in PFPS subjects could correspond to the inability to differentiate his function as external rotator, working with Gma on pelvic stability during single stance position.
Only Crossley et al.12) evaluated the muscle onset latency of the different portions of Gmed during SLS, reporting an early activation of Gma (−46 ± 144 ms) and Gmp (−23 ± 76 ms) during the SLS in healthy subjects. These results differ from those of the present study, probably because the muscle onset latency reported by Crossley et al.12) was determined by the visual detection method during a step up, but not during SLS, which could be considered imprecise and unreliable based on the evaluator criterion rather than on quantitative variables22, 24). To our knowledge, this is the first study to report Gma and Gmp muscle onset during SLS performance and therefore the observed results could better represent the neuromuscular control strategy during this movement.
The present study showed that the increase in performance velocity of the SLS changes the neuromuscular control strategy in healthy subjects, from a later (753.7 ± 1,117.5 ms) to an earlier (−230.7 ± 485.7) muscle activation of the Gmp. In contrast, PFPS subjects did not modify their neuromuscular strategy, presenting an early onset latency of Gmp in both LV (−657.4 ± 689.4) and HV SLS performance (−265.8 ± 456.1).
The first reports involving movement velocity and EMG activity establish that stabilizing muscles exhibit earlier activation at high velocity movement27), consistent with the Gmp neuromuscular strategy shown by healthy subjects in the present study. Additionally, reports on the effect of velocity on ascending and descending stairs establish that increasing velocity generates major angular moments and torques, particularly at the hip level28). It is possible that the increased SLS velocity generates a pre-activation of the Gmp in healthy subjects in order to control the greater angular torque during higher velocity movements. While PFPS subjects would not be able to generate this strategy due to the altered hip motor coordination and knee muscles inhibition29, 30). Our results show a nonsignificant tendency to a rectus femoris preactivation in healthy subjects at HV, not observed in PFPS subjects. In this context, it is possible that Gmp early activity in PFPS presents as a compensatory strategy to knee neuromuscular inhibition. Differences between healthy and PFPS women are only present at LV movement, which could suggest that clinical differences in this test become evident when asking the subjects to reduce the movement velocity.
An important limitation is the use of sEMG instead of fine-wire to measure Gmed activity. Gluteus medius, specifically Gmp, would be positioned bellow GMax muscle, leading to a high risk of Crosstalk11, 12). However, fineware is an invasive method and does not properly reflect the behavior of a muscle as a whole during an activity. In the present study, we tried to minimize potential of crosstalk by respecting previous recommendations and protocols to measure the different portions of Gmed13, 16). In addition, the present study considered muscle onset latency as the activation time relative the movement initiation measured through accelerometry, unlike previous studies that have used primary motor activity as a reference27). We believe that this better represents the neuromuscular strategy during SLS, since the quadriceps is not active in the initial stages of the motion, but in late stages of deceleration31). In summary, the present study demonstrated that subjects with PFPS present differences in hip neuromuscular activity compared to healthy subjects during SLS, and that these differences can be influenced by movement velocity. Increasing SLS velocity could allow better differentiation between healthy and PFPS subjects.
Conflict of interest
The authors declare that they have no competing interests.
REFERENCES
- 1.Boling M, Padua D, Marshall S, et al. : Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports, 2010, 20: 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dye SF: The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res, 2005, (436): 100–110. [DOI] [PubMed] [Google Scholar]
- 3.Taunton JE, Ryan MB, Clement DB, et al. : A retrospective case-control analysis of 2002 running injuries. Br J Sports Med, 2002, 36: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall R, Barber Foss K, Hewett TE, et al. : Sport specialization’s association with an increased risk of developing anterior knee pain in adolescent female athletes. J Sport Rehabil, 2015, 24: 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collado H, Fredericson M: Patellofemoral pain syndrome. Clin Sports Med, 2010, 29: 379–398. [DOI] [PubMed] [Google Scholar]
- 6.Powers CM: The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther, 2010, 40: 42–51. [DOI] [PubMed] [Google Scholar]
- 7.Souza RB, Draper CE, Fredericson M, et al. : Femur rotation and patellofemoral joint kinematics: a weight-bearing magnetic resonance imaging analysis. J Orthop Sports Phys Ther, 2010, 40: 277–285. [DOI] [PubMed] [Google Scholar]
- 8.Aminaka N, Pietrosimone BG, Armstrong CW, et al. : Patellofemoral pain syndrome alters neuromuscular control and kinetics during stair ambulation. J Electromyogr Kinesiol, 2011, 21: 645–651. [DOI] [PubMed] [Google Scholar]
- 9.Willson JD, Kernozek TW, Arndt RL, et al. : Gluteal muscle activation during running in females with and without patellofemoral pain syndrome. Clin Biomech (Bristol, Avon), 2011, 26: 735–740. [DOI] [PubMed] [Google Scholar]
- 10.Cowan SM, Crossley KM, Bennell KL: Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med, 2009, 43: 584–588. [DOI] [PubMed] [Google Scholar]
- 11.Semciw AI, Pizzari T, Murley GS, et al. : Gluteus medius: an intramuscular EMG investigation of anterior, middle and posterior segments during gait. J Electromyogr Kinesiol, 2013, 23: 858–864. [DOI] [PubMed] [Google Scholar]
- 12.Crossley KM, Zhang WJ, Schache AG, et al. : Performance on the single-leg squat task indicates hip abductor muscle function. Am J Sports Med, 2011, 39: 866–873. [DOI] [PubMed] [Google Scholar]
- 13.O’Sullivan K, Smith SM, Sainsbury D: Electromyographic analysis of the three subdivisions of gluteus medius during weight-bearing exercises. Sports Med Arthrosc Rehabil Ther Technol, 2010, 2: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ugalde V, Brockman C, Bailowitz Z, et al. : Single leg squat test and its relationship to dynamic knee valgus and injury risk screening. PM R, 2015, 7: 229–235, quiz 235. [DOI] [PubMed] [Google Scholar]
- 15.Kagaya Y, Fujii Y, Nishizono H: Association between hip abductor function, rear-foot dynamic alignment, and dynamic knee valgus during single-leg squats and drop landings. Journal of Sport and Health Science, 4 (2015) 182e187. [Google Scholar]
- 16.O’Sullivan K, Herbert E, Sainsbury D, et al. : No difference in gluteus medius activation in women with mild patellofemoral pain. J Sport Rehabil, 2012, 21: 110–118. [DOI] [PubMed] [Google Scholar]
- 17.Neumann DA, Moriya E, Maciel C, et al. : Kinesiology of the hip: a focus on muscular actions. J Orthop Sports Phys Ther, 2010, 40: 82–94. [DOI] [PubMed] [Google Scholar]
- 18.Van Damme BB, Stevens VK, Van Tiggelen DE, et al. : Velocity of isokinetic trunk exercises influences back muscle recruitment patterns in healthy subjects. J Electromyogr Kinesiol, 2013, 23: 378–386. [DOI] [PubMed] [Google Scholar]
- 19.Roy JS, Moffet H, McFadyen BJ: Upper limb motor strategies in persons with and without shoulder impingement syndrome across different speeds of movement. Clin Biomech (Bristol, Avon), 2008, 23: 1227–1236. [DOI] [PubMed] [Google Scholar]
- 20.Hermens HJ, Freriks B, Disselhorst-Klug C, et al. : Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol, 2000, 10: 361–374. [DOI] [PubMed] [Google Scholar]
- 21.Winter D: Biomechanics and motor control of human movement, 4th ed. University of Waterloo. John Wiley & Sons, Inc., 2009. [Google Scholar]
- 22.Hodges PW, Bui BH: A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol, 1996, 101: 511–519. [DOI] [PubMed] [Google Scholar]
- 23.Bolgla LA, Malone TR, Umberger BR, et al. : Reliability of electromyographic methods used for assessing hip and knee neuromuscular activity in females diagnosed with patellofemoral pain syndrome. J Electromyogr Kinesiol, 2010, 20: 142–147. [DOI] [PubMed] [Google Scholar]
- 24.Méndez-Rebolledo G, Gatica-Rojas V, Martinez-Valdes E, et al. : The recruitment order of scapular muscles depends on the characteristics of the postural task. J Electromyogr Kinesiol, 2016, 31: 40–47. [DOI] [PubMed] [Google Scholar]
- 25.Kim D, Unger J, Lanovaz JL, et al. : The relationship of anticipatory gluteus medius activity to pelvic and knee stability in the transition to single-leg stance. PM R, 2016, 8: 138–144. [DOI] [PubMed] [Google Scholar]
- 26.Tsao H, Danneels LA, Hodges PW: ISSLS prize winner: smudging the motor brain in young adults with recurrent low back pain. Spine, 2011, 36: 1721–1727. [DOI] [PubMed] [Google Scholar]
- 27.Hodges PW, Richardson CA: Relationship between limb movement speed and associated contraction of the trunk muscles. Ergonomics, 1997, 40: 1220–1230. [DOI] [PubMed] [Google Scholar]
- 28.Lewis J, Freisinger G, Pan X, et al. : Changes in lower extremity peak angles, moments and muscle activations during stair climbing at different speeds. J Electromyogr Kinesiol, 2015, 25: 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brindle TJ, Mattacola C, McCrory J: Electromyographic changes in the gluteus medius during stair ascent and descent in subjects with anterior knee pain. Knee Surg Sports Traumatol Arthrosc, 2003, 11: 244–251. [DOI] [PubMed] [Google Scholar]
- 30.Cowan SM, Bennell KL, Hodges PW, et al. : Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome. Arch Phys Med Rehabil, 2001, 82: 183–189. [DOI] [PubMed] [Google Scholar]
- 31.Dionisio VC, Almeida GL, Duarte M, et al. : Kinematic, kinetic and EMG patterns during downward squatting. J Electromyogr Kinesiol, 2008, 18: 134–143. [DOI] [PubMed] [Google Scholar]


