Abstract
Background
Resistin, an adipokine with inflammatory properties, has been associated with plaque vulnerability. Vascular smooth muscle cells (VSMCs) and macrophages (MΦ) are the major cellular components in advanced atherosclerotic plaques and interdependently affect plaque stability. The purpose of this study was to examine the effects of resistin on the interactions of VSMCs and MΦ using co-culture systems.
Methods
Human monocytes were differentiated into MΦ. VSMCs were grown and starved prior to co-culture condition. Indirect co-culture was performed by treating MΦ with resistin at 10 ng/ml for 24 h with/without εV1-2, a selective PKCε inhibitor. MΦ supernatants were then used to treat VSMCs for 24 h. Direct co-culture was performed by culturing MΦ and VSMCs together for 24–48 hours. Cultures were evaluated for changes in proliferation, apoptosis, and gene expression of apoptosis, proliferation, and inflammation-associated genes.
Results
MΦ induced VSMC proliferation, which was further exaggerated in resistin-treated macrophages in the indirect co-culture model. Resistin also upregulated cyclin D1 and PCNA via PKCε in the indirect co-culture. Augmented proliferation was further confirmed in the direct co-culture model, particularly at increased macrophage ratios. However, resistin treatment induced apoptosis in the presence of direct cell-cell interactions. Along with the shift to apoptosis, expressions of caspase 3 and caspase 8 were upregulated. The expression of NFkB-1 and NFkB-2 was similar in direct and indirect co-cultures.
Conclusions
Resistin promotes a shift from proliferation to apoptosis in VSMCs and macrophage co-culture systems with cellular composition similar to that found in vulnerable regions of plaques. PKCε mediates the effects of resistin, suggesting that PKCε may represent a therapeutic strategy in resistin-associated atherosclerotic complications.
Keywords: resistin, macrophage, proliferation, apoptosis, vascular smooth muscle cell, protein kinase C epsilon
INTRODUCTION
Plaque instability represents major concerns among patients with atherosclerotic cardiovascular disease. Studies have reported that clinical events, including stroke and myocardial infraction, are the result of plaque rupture rather than luminal narrowing.1–4 Plaque vulnerably is thought to be the result of core thinning and necrotic core formation.5–7 Thin-capped plaques in coronary and carotid arteries are usually considered more susceptible to rupture. 8, 9 In addition, increased infiltration of inflammatory macrophages and presence of necrotic cores—composed of abundant apoptotic cells—are thought to contribute to plaque vulnerability.
Vascular smooth muscle cells (VMSCs) and macrophages are major cellular drivers of atherosclerotic plaque development. VSMC have migrative, proliferative, and matrix-producing properties that contribute to intima thickening and fibrous cap formation.10 On the other hand, macrophages are plastic cells that can change between a pro-inflammatory (M1) and tissue-repairing (M2) state. Studies suggest that M1 macrophages are more abundant in rupture-prone plaque areas.11
Resistin is an adipokine secreted from macrophages that has pro-inflammatory properties. We have found that resistin is associated with increased inflammatory profiles in patients undergoing carotid surgery interventions.12 In addition, our in vitro studies show that resistin promotes an M1-like phenotype in macrophages, and that it induces VSMC dysfunction.12, 13 We also demonstrate that protein kinase C epsilon (PKCε) is an important upstream modulator of resistin action in VSMCs and macrophages, mediating downstream signals that lead to exacerbated cellular dysfunction and inflammation. However, our work thus far has only focused on single cell types.
In this study, we address for the first time how physiological levels of resistin affect VSMC and macrophages in the presence of cell-to-cell communication and interactions. We use indirect and direct co-culture models to investigate the effects of resistin in the presence of paracrine (cellular secretions) and direct cell-cell contact. Since cellular apoptosis and inflammation drive detrimental changes of plaque stability, we concentrated on cellular proliferation, inflammation, and death.
MATERIALS AND METHODS
Cell culture: growth and differentiation
Human coronary artery smooth muscle cells (HCASMC) or VSMCs from Genlantis were used at passages 5 to 8 for experiments. Peripheral blood mononuclear cells (PBMCs) were collected from buffy coats of healthy control male donors (Stanford Blood Center), age >20 using the Ficoll-Paque density gradient method. Then, monocytes were isolated using negative magnetic sorting (Stemcell Technologies). Population purity was assessed with flow cytometry by conjugating a sample of sorted cells to CD14-FITC antibody (Invitrogen). Cells were differentiated to macrophages during a 7-day culture in RPMI media supplemented with 10% FBS and 100 ng/ml of macrophage colony stimulating factor (PeproTech). Effective macrophage differentiation was confirmed by immunocytochemistry as described below. Differentiated macrophages were then treated with resistin. All macrophage cultures (including untreated control) had their media removed after the differentiation period and washed twice with PBS. Then, these were treated with/without respective modulators in media containing 0.5% FBS based on our findings that this concentration of FBS alone has minimal effect on VSMC proliferation or apoptosis.
Cell treatments
Human recombinant resistin (PeproTech) was used to treat cells. We chose a 10 ng/ml resistin concentration based on a previous study by us suggesting that this concentration promotes pro-inflammatory macrophage polarization.12 The PKCε-specific inhibitor εV1-2 (from Dr. Mochly-Rosen Laboratory, Stanford University) was used at 1 μM concentration, 13 with a 30 min pre-treatment prior to the addition of resistin.
VSMC and macrophage co-culture systems
Indirect co-culture: VSMCs were seeded in 6-well plates and starved for 24 hours. Differentiation media was removed from macrophage cultures, and the cultures were washed twice with PBS. Then these were treated with resistin, with or without εV1-2, for 24 hours in media containing 0.5% FBS. Conditioned media (CM) from macrophages was collected, centrifuged to remove residual debris, and used to treat the starved VSMCs. Our recent review proposed that co-culture systems with VSMC:MΦ ratios of 1:4–1:5 potentially represent scenarios found in vulnerable/thrombotic stages of atherosclerotic plaques.14 Hence, this indirect co-culture was performed at a 1:4 VSMC:MΦ ratio (2.5×105 VSMCs were treated with CM from 1×106 MΦ) for 24 hours. VSMCs were then assessed proliferation or gene expression changes. Direct co-culture: After respective VSMC starvation or macrophage differentiation, cells were cultured together in media containing 0.5% FBS. Several VSMC:MΦ ratios were assessed prior to testing treatments, which included 1:1, 1:2, 1:4. 1:6,1:8, and 1:10. Based on these results and in our previous report, 14 we used the 1:4 ratio for the cell treatments. Co-culture was performed for 24 or 48 hours, and cells were assessed for proliferation, apoptosis, and gene expression.
Real-time polymerase chain reaction (RT-PCR)
Total RNA from cultures was isolated using TRIzol according to standard protocols. SYBR green PCR master mix was used for real time PCR. Human primers are listed in Table 1. RT-PCR was performed in a Mastercycler RT-PCR detection system (Eppendorf, Westbury, NY). The relative level of target gene in each group was normalized against internal housekeeping gene GAPDH using the calculation formula of 2ΔCT(GAPDH-target). The target gene levels in drug treated groups were further normalized against the control group.
Table 1.
PCR primers used for amplification
| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | 5′-TGCACCACCAACTGCTTAGC-3′ | 5′-GGCATGGACTGTGGTCATGAG-3′ |
| CYCLIN D1 | 5′-GCTGCGAAGTGGAAACCATC-3′ | 5′-CCTCCTTCTGCACACATTTGAA-3′ |
| PCNA | 5′-CCTGCTGGGATATTAGCTCCA-3′ | 5′-CAGCGGTAGGTGTCGAAGC-3′ |
| CASPASE 3 | 5′-TGGTATTGAAGCAGACAGTGGA -3′ | 5′-GGAGTAGTAGCCTGGAGCAGTAGA-3′ |
| CASPASE 8 | 5′-ATTTGGCTGGCATCATCTGT-3′ | 5′-ACTGCTTCCCTGGCTTTTG-3′ |
| NF-KB1(P50) | 5′-CCTGGATGACTCTTGGGAAA-3′ | 5′-TCAGCCAGCTGTTTCATGTC-3′ |
| NF-KB2(P52) | 5′-GAACAGCCTTGCATCTAGCC-3′ | 5′-TTTTCAGCATGGATGTCAGC-3′ |
BrdU proliferation assay
The BrdU (5-bromo-2′-deoxyuridine) incorporation assay was used to detect proliferation changes. BrdU (Thermo Fisher Scientific) at 10 μM was added at the start of the co-culture for incorporation into newly synthesized DNA during the co-culture period. Cells were then processed for flow cytometry and labeled with APC-conjugated anti-BrdU antibody (Affymetrix). Flow cytometry was performed using the BD LSR II Flow Cytometer, following standard protocols and manufacturer’s recommendations, with 10000 events per replicate.
Apoptosis assay
The Annexin V-FITC apoptosis detection kit was used (Sigma-Aldrich) in the direct co-culture according to the manufacturer’s instructions. Cells were processed for flow cytometry and they were probed with FITC-conjugated anti-Annexin V antibody. Flow cytometry was performed using the BD LSR II Flow Cytometer, following standard protocols and manufacturer’s recommendations, with 10000 events per replicate.
Statistical analysis
All in vitro experiments were performed independently at least four times in duplicate. Results are expressed as the mean +SEM. Statistical analyses were performed with the GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA) software package. Data sets were tested for parametric or non-parametric analysis, and the appropriate statistical test was selected. Data comparing inhibitor effects were analyzed using two-factor ANOVA followed by Tukey’s test. Statistical significance was considered if the p-value was <0.05.
RESULTS
Indirect co-culture promoted VSMC proliferation, and resistin further increased proliferation via PKCε
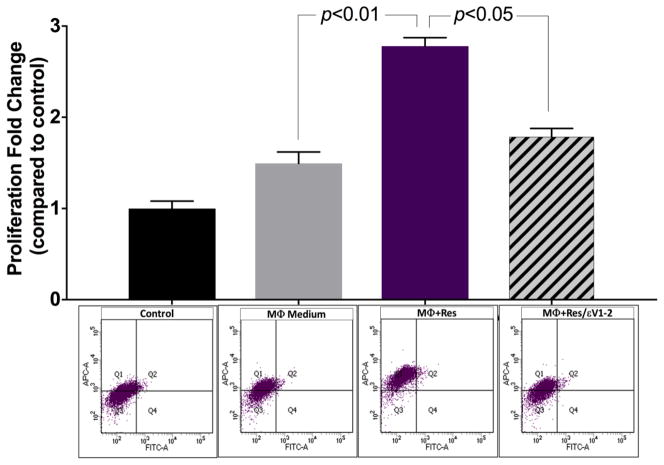
CM from untreated macrophages slightly increased VSMC proliferation but the effect was more pronounced when macrophages were treated with resistin (Figure 1). Resistin treatment significantly increased VSMC proliferation (p<0.01). Inhibiting PKCε in macrophages, with εV1-2, prior to resistin treatment decreased the effects elicited in VMSCs (p<0.05).
Fig. 1. Indirect co-culture promoted VSMC proliferation, and resistin further increased proliferation via PKCε.
Cell proliferation analysis was completed using the BrdU incorporation assay. Flow cytometry was performed 24 hours after treatment with conditioned media of: untreated control macrophages (MΦ Medium); macrophages treated with resistin (MΦ+Res); and macrophages treated with εv1-2+resistin (MΦ+Res/εv1-2). Treatment groups were compared to control group, and data are shown as mean + SEM.
Macrophage secretions upregulated gene expression of proliferation and inflammation markers in VSMCs
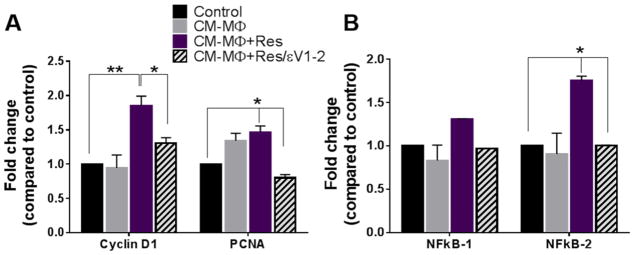
Increased expressions of the proliferating cell nuclear antigen (PCNA) and cyclin D1 have been identified as markers of proliferation, indicating cell cycle transition to the S phase, whereas activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) is an indicator cellular inflammation state. 15–17 In this study, we observed that CM from resistin-treated macrophages significantly increased expressions of cyclin D1 (p<0.01) and PCNA (p<0.05) (Figure 2A), as well as NFkB-2 (p<0.05) in VSMCs (Figure 2B). These effects were diminished when PKCε was inhibited, returning gene expression to levels similar to the control.
Fig. 2. Macrophage secretions upregulated gene expression of proliferation and inflammation markers in VSMCs.
Gene expression was assessed in VSMCs 24 hours after respective treatments using RT-PCR. Conditioned media from macrophages treated with resistin (MΦ+Res) significantly increased cyclin D1, PCNA (A) and NFkB-2 expression (B), which were prevented by εv1-2. Data are shown as mean + SEM, *p <0.05 and **p<0.01.
Direct co-culture stimulated proliferation, but resistin induced apoptotic transformation
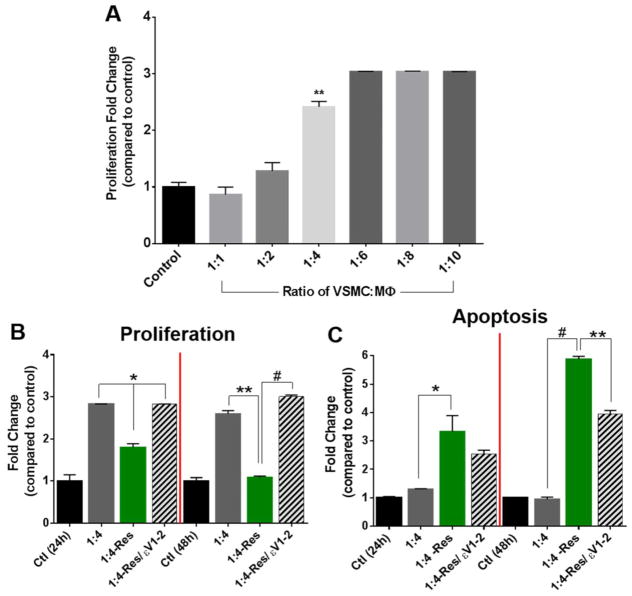
We first examined various ratios between macrophages and VSMCs in direct co-culture. Macrophages (without resistin treatment) promoted the overall proliferative state of the co-culture. A significant increase in proliferation was observed at the 1:4 VSMC:macrophage ratio (p<0.01), and it leveled up at the 1:6 ratio (Figure 3A). Based on these results, we chose the VSMC:macrophage ratio of 1:4 for the remaining direct co-culture experiments. When resistin was added to the co-culture, the number of proliferative cells decreased after 24 hours, and it continued to decrease after 48 hours. On the other hand, the number of apoptotic cells increased, and this shift was mediated by PKCε (Figure 3B–C).
Fig. 3. Direct co-culture stimulated proliferation, but resistin induced apoptotic transformation.
(A) Various ratios of untreated VSMC:macrophage were tested in direct co-culture using the BrdU incorporation assay to asses changes in proliferative state after 24 hours. Then, treatment groups were tested at the 1:4 VSMC-to-macrophage ratio for changes in proliferation (B) and apoptosis (C) after 24 or 48 hours, using the BrdU incorporation assay or the Annexin V-FITC apoptosis assay, respectively. Data are shown as mean + SEM, *p <0.05, **p<0.01, and # p<0.001.
Direct cell-to-cell interaction allowed resistin to upregulate of apoptotic markers
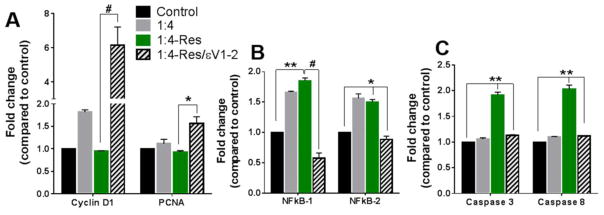
The shift from a proliferative to an apoptotic state was observed at the transcriptional level. After 48 hours of co-culture with resistin, the gene expression of the proliferation markers did not change (Figure 4A), and the expression of inflammation markers was similar to that in indirect co-culture (Figure 4B). However, the apoptosis markers caspase 3 and caspase 8 were upregulated (Figure 4C). The role of PKCε in such shift was further confirmed.
Fig. 4. Direct cell-to-cell interaction allowed resistin to upregulate of apoptotic markers.
Gene expression was assessed 48 hours after respective treatments in the 1:4 VSMC-to-macrophage co-culture using RT-PCR. (A) Proliferation markers. (B) Inflammation markers. (C) Apoptosis markers. Data are shown as mean + SEM, *p <0.05, **p<0.01, and # p<0.001.
DISCUSSION
Resistin is an inflammatory adipokine secreted from macrophages that has been associated with cardiovascular risk factors. Particularly, it has been found elevated in patients with acute coronary syndrome,18 associated with myocardium injury, 19 and linked to increased risk of myocardial infarction.20 However, the specific mechanism and cellular changes linking these clinical observations, relevant to plaque vulnerability and rupture, remain uncertain.
Co-culture systems composed of VSMCs and macrophages are useful platforms to study atherosclerotic plaque development. VSMC are important drivers of plaque formation by contributing to plaque thickening through migration, proliferation, and matrix-producing activities.21 These properties also make them vital in maintaining plaque stability. VSMC apoptosis has been linked to vulnerability,7 and cap thinning in plaques is thought to result from excessive cell death, proteolytic activity, and inflammation.22, 23 Macrophages, on the other hand, drive the inflammatory response in plaques by secreting pro-inflammatory cytokines, reactive oxygen species, and prompting the expression of co-stimulatory molecules.
As these cell types play key roles in plaque formation and vulnerability, we set to address how physiological levels of resistin (10 ng/ml) may affect VSMC and macrophage interactions. First, we show that resistin increased VSMC proliferation in the indirect co-culture system and promoted cyclin D1 and PCNA upregulation, markers of proliferation. These events were presumably the result of paracrine secretions triggered by the resistin treatment in macrophages. These observations are consistent with our recent study showing that resistin polarizes macrophages towards a pro-inflammatory state via PKCε, prompting increased secretion of inflammatory cytokines,12 which may be responsible for the increased proliferation induced in VSMCs.
Second, we show that un-stimulated macrophages increase proliferation in direct co-culture (Figure 3A), even when the number is increased to ratios representative of vulnerable plaques.14 However, when resistin is added as stimuli in direct co-culture, cells shift from proliferative to apoptotic state (Figure 3B). In addition, gene expression of proliferative markers was decreased while caspase 3 and caspase 8, apoptotic markers, were upregulated. The dichotomous effects of resistin on macrophage-VSMC co-culture systems underscores the complicity of athero-genesis and atheroprogression. We have previously shown that resistin leads to VSMC dysfunction by activating NADPH oxidase and ROS production, and that it induces a pro-inflammatory phenotype in macrophages via TLR4, and that PKCε interferes in these processes.12, 13 We believe that these cascades of events are responsible for the shift from proliferation to apoptosis observed in the direct-co-culture. Studies have shown that macrophage-mediated VSMC apoptosis requires cell-to-cell junctions,24 and that activated human monocyte-derived macrophages are capable of inducing VSMC apoptosis through Fas-Fas ligand (Fas-L) interactions.25, 26 Boyle et al. have shown that human macrophage-induced VSMC apoptosis requires nitric oxide enhancement of Fas/Fas-L interactions.24 We previously observed that resistin at a physiological concentration of 10ng/ml activates nitric oxide synthase (NO) in VSMCs.13 It is possible that direct cell-cell contact promotes Fas/Fas-L interaction in the direct co-culture system and that resistin-induced NO activation further enhanced the Fas/Fas-L interaction, which led to apoptosis.
Overall, our study shows that resistin produces differential physiological effects in the presence and absence of cell-cell interactions, highlighting the importance of considering multicellular systems when studying key cellular processes in atherosclerosis. Admittedly, the specific source of apoptotic cells is not clear and cell-tissue interaction was not investigated. Nonetheless, this is the first time that the effects of resistin were examined in the presence of cell-cell interaction. Also, for the first time, we show that PKCε is an important mediator in apoptotic signals between VSMC and macrophages. Our study suggests that PKCε may represent a target for resistin-induced plaque changes.
Acknowledgments
Sources of financial support: National Institutes of Health (grant number R01NS070308) and United States Department of Veterans Affairs (grant number I01BX001398).
Financial support: This work was supported by National Institutes of Health [grant number R01NS070308] and the United States Department of Veterans Affairs [grant number I01BX001398].
We thank Dr. Daria Mochly-Rosen (Stanford University) for providing the PKCε inhibitor and activator utilized in this investigation.
Footnotes
Presented at the 12th Annual Academic Surgical Congress in Las Vegas, NV February 7–9, 2017
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–71. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 2.Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 1997;17:1859–67. doi: 10.1161/01.atv.17.10.1859. [DOI] [PubMed] [Google Scholar]
- 3.Spagnoli LG, Mauriello A, Sangiorgi G, Fratoni S, Bonanno E, Schwartz RS, et al. Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA. 2004;292:1845–52. doi: 10.1001/jama.292.15.1845. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M, Paganini-Hill A, Martin A, Cosgrove M, Toole JF, Barnett HJ, et al. Carotid plaque pathology: Thrombosis, ulceration, and stroke pathogenesis. Stroke. 2005;36:253–7. doi: 10.1161/01.STR.0000152336.71224.21. [DOI] [PubMed] [Google Scholar]
- 5.Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: Role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69:377–81. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Björkerud S, Björkerud B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. The American journal of pathology. 1996;149:367. [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–80. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 8.Narula J, Nakano M, Virmani R, Kolodgie FD, Petersen R, Newcomb R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. 2013;61:1041–51. doi: 10.1016/j.jacc.2012.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hitchner E, Zayed MA, Lee G, Morrison D, Lane B, Zhou W. Intravascular ultrasound as a clinical adjunct for carotid plaque characterization. Journal of vascular surgery. 2014;59:774–80. doi: 10.1016/j.jvs.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newby AC, Zaltsman AB. Fibrous cap formation or destruction—the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res. 1999;41:345–60. [PubMed] [Google Scholar]
- 11.Stöger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos, Chris M, Biessen EA, et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012 doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Zuniga MC, Raghuraman G, Hitchner E, Weyand C, Robinson W, Zhou W. PKC-epsilon and TLR4 synergistically regulate resistin-mediated inflammation in human macrophages. Atherosclerosis. 2017 doi: 10.1016/j.atherosclerosis.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghuraman G, Zuniga MC, Yuan H, Zhou W. PKCε mediates resistin-induced NADPH oxidase activation and inflammation leading to smooth muscle cell dysfunction and intimal hyperplasia. Atherosclerosis. 2016;253:29–37. doi: 10.1016/j.atherosclerosis.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuniga MC, White SLP, Zhou W. Design and utilization of macrophage and vascular smooth muscle cell co-culture systems in atherosclerotic cardiovascular disease investigation. Vascular Medicine. 2014;19:394–406. doi: 10.1177/1358863X14550542. [DOI] [PubMed] [Google Scholar]
- 15.Kurki P, Vanderlaan M, Dolbeare F, Gray J, Tan E. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986;166:209–19. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- 16.Resnitzky D, Gossen M, Bujard H, Reed SI. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–79. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tak PP, Firestein GS. NF-kappaB: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lubos E, Messow CM, Schnabel R, Rupprecht HJ, Espinola-Klein C, Bickel C, et al. Resistin, acute coronary syndrome and prognosis results from the AtheroGene study. Atherosclerosis. 2007;193:121–8. doi: 10.1016/j.atherosclerosis.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Chu S, Ding W, Li K, Pang Y, Tang C. Plasma resistin associated with myocardium injury in patients with acute coronary syndrome. Circulation Journal. 2008;72:1249–53. doi: 10.1253/circj.72.1249. [DOI] [PubMed] [Google Scholar]
- 20.Weikert C, Westphal S, Berger K, Dierkes J, Mohlig M, Spranger J, et al. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. The Journal of Clinical Endocrinology & Metabolism. 2008;93:2647–53. doi: 10.1210/jc.2007-2735. [DOI] [PubMed] [Google Scholar]
- 21.Newby AC, Zaltsman AB. Fibrous cap formation or destruction--the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res. 1999;41:345–60. [PubMed] [Google Scholar]
- 22.Cicha I, Worner A, Urschel K, Beronov K, Goppelt-Struebe M, Verhoeven E, et al. Carotid plaque vulnerability: A positive feedback between hemodynamic and biochemical mechanisms. Stroke. 2011;42:3502–10. doi: 10.1161/STROKEAHA.111.627265. [DOI] [PubMed] [Google Scholar]
- 23.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278:483–93. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle JJ, Bowyer DE, Weissberg PL, Bennett MR. Human blood-derived macrophages induce apoptosis in human plaque-derived vascular smooth muscle cells by fas-ligand/fas interactions. Arterioscler Thromb Vasc Biol. 2001;21:1402–7. doi: 10.1161/hq0901.094279. [DOI] [PubMed] [Google Scholar]
- 25.Geng YJ, Henderson LE, Levesque EB, Muszynski M, Libby P. Fas is expressed in human atherosclerotic intima and promotes apoptosis of cytokine-primed human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17(10):2200–8. doi: 10.1161/01.atv.17.10.2200. [DOI] [PubMed] [Google Scholar]
- 26.Imanishi T, Han DK, Hofstra L, Hano T, Nishio I, Liles WC, Gorden AM, Schwartz SM. Apoptosis of vascular smooth muscle cells is induced by Fas ligand derived from monocytes/macrophage. Atherosclerosis. 2002;161(1):143–51. doi: 10.1016/s0021-9150(01)00631-1. [DOI] [PubMed] [Google Scholar]