Abstract
Background
Inflammation plays a role in mood and behavior that may be relevant to identifying risk factors and treatment for depression and other stress-related illnesses. The purpose of this study was to examine whether fluctuations in inflammation following a mild immune stimulus were associated with changes in daily reported features of depression for up to a week in a healthy sample of young adults.
Methods
Forty one undergraduate students completed daily diaries of mood, feelings of social disconnection, sleep, and physical symptoms for one week before and after receiving the seasonal influenza vaccine. Circulating plasma interleukin-6 (IL-6) was measured via blood samples taken immediately before and one day after vaccination.
Results
There was a significant increase in circulating IL-6 from pre- to post-intervention (p = .008), and there was significant variability in the magnitude of IL-6 change. Greater increases in IL-6 were associated with greater mood disturbance on post-vaccine days, specifically depressed mood and cognitive symptoms.
Conclusions
Minor increases in inflammation were associated with corresponding increases in features of depression, and these associations occurred in the absence of any physical symptoms. The influenza vaccine could be used to probe causal relationships with a high degree of ecological validity, even in high-risk and vulnerable populations, to better understand the role of inflammation in the pathogenesis of depression.
Keywords: inflammation, interleukin-6, IL-6, depression, mood, influenza vaccine, sickness behavior
Introduction
The innate immune system is the body’s first response to injury and pathogen exposure, and its effectiveness depends upon the mobilization of inflammatory cytokines (Iwasaki and Medzhitov, 2015). Inflammatory processes can lead to sickness behavior, or the short-term loss of appetite, sleepiness, withdrawal from normal social activities, fever, aching joints, and fatigue (Dantzer, 2001; Dantzer and Kelley, 2007). Prolonged inflammation has been implicated in the development of stress-related psychiatric disorders (Raison et al., 2006), including the pathophysiology of depression (Dantzer et al., 2008; Irwin, 2002; Slavich and Irwin, 2014).
Experimental paradigms that induce inflammation have provided the strongest evidence that inflammation can elicit features of depression. In animal models, inducing inflammation can cause changes in locomotor activity, social behavior, cognition, and anhedonic behaviors (Aubert, 1999; Kubera et al., 2011; Wohleb et al., 2015; Yirmiya and Goshen, 2011). Likewise, causal inferences can be drawn from experimental models in humans (Capuron et al., 2002; Eisenberger et al., 2010b; Wichers et al., 2007). The two primary models for inducing inflammation in healthy individuals are endotoxin administration and typhoid vaccination. Endotoxin injection causes a marked increase in peripheral markers of inflammation (Martich et al., 1993), as well as depressed mood, cognitive disturbance, and increased feelings of social disconnection (Eisenberger et al., 2010b; Moieni et al., 2015; Reichenberg et al., 2001). On average, the endotoxin paradigm leads to an 100 pg/mL increase in IL-6 (Eisenberger et al., 2009), which resolves within 6-hours of administration (Eisenberger et al., 2009; Martich et al., 1993). This paradigm enables measurement of robust inflammatory effects on short-term cognitive and behavioral outcomes. However, the elevations in circulating inflammatory markers induced using endotoxin are far greater than those observed among depressed individuals (Andrei et al., 2007; Häfner et al., 2011; Howren et al., 2009), or levels elicited by stress (Steptoe et al., 2007), one of the key predictors of depression.
The typhoid vaccine has also been used to interrogate the causal role inflammation plays in the neural and behavioral underpinnings of depression, and induces approximately 1.0 pg/mL increases in IL-6 at 3 hours post-injection (Brydon et al., 2008; Harrison et al., 2009b). Individuals who exhibit the largest inflammatory response to typhoid vaccination demonstrate degradations in mood, increases in fatigue and confusion, and slowed reactions times on cognitive tasks (Brydon et al., 2008; Harrison et al., 2009a; Strike et al., 2004).
It is important to note that studies using both endotoxin and the typhoid vaccine have focused on the within-subject associations between the magnitude of inflammatory responses and changes in mood or activity with neural substrates involved in emotion regulation and reward-processing (Brydon et al., 2008; Eisenberger et al., 2009; Harrison et al., 2009a, 2009b; Wright et al., 2005). Yet, studies using the typhoid vaccine and endotoxin have limited their inquiry to the hours immediately following vaccine exposure. Whether fluctuations in inflammation exert sustained effects on mood and behavior that go beyond the laboratory remains unanswered. In the present study, we examined the association between a mild inflammatory stimulus and multiple domains of sickness behavior and features of depression including mood, sleep, feelings of social disconnection, and physical symptoms for up to a week, using daily diaries to assess sickness behavior and features of depression in the context of participants’ typical day-to-day experiences.
The influenza vaccine results in a mild inflammatory response. Increases in IL-6 following the influenza vaccine can be seen as early as 60 minutes following vaccination (Edwards et al., 2006a). A significant increase in IL-6 following the influenza vaccine is consistently observed 1- (Carty et al., 2006; Christian et al., 2011; Tsai et al., 2005), and 2-days post-vaccination (Christian et al., 2011). This inflammatory response may resolve as early as 3-days post-vaccination (Tsai et al., 2005), and is no longer observed 7 days following vaccination (Christian et al., 2011; Tsai et al., 2005). In fact, one study provides evidence that the inflammatory response to the influenza vaccine resolved within 3 days (Tsai et al., 2005). Thus, use of the influenza vaccine as an inflammatory stimulus enables measurement of daily change in subjective mood and behavior over longer periods of time, and the gradual increase in circulating inflammatory markers over days may be a more useful model for clarifying the role of inflammation in the pathogenesis of depression (See Slavich and Irwin, 2014 for review). Yet, among the published studies of inflammatory responses to influenza vaccine, no studies have examined changes in mood and behavior as a function of the inflammatory response.
The purpose of this study was to examine the association between inflammatory responses to influenza vaccination and daily reports of mood, sleep, social disconnection, and physical symptoms in a healthy, young adult sample. Consistent with the approach used in the endotoxin and typhoid studies, we focused on individual differences in IL-6 responses to vaccination (Brydon et al., 2008; Eisenberger et al., 2009; Slavich et al., 2010). Given the time course and expected magnitude of the inflammatory response to influenza vaccination, we used daily diaries to capture subtle variations in correlates of depression for 1 week before and after the vaccine. We hypothesized that greater increases in IL-6 from immediately before to 1-day post-vaccine would be associated with increases in depressed mood, cognitive symptoms, tension, fatigue, and social disconnection. We also hypothesized that greater increases in IL-6 would be associated with decreases in subjective sleep quality and positive affect.
Method
Participants
Recruitment procedures included posting flyers on the university campus during the Fall quarter in 2015 and 2016. Participants completed a phone interview to determine study eligibility. Inclusion criteria for the study were undergraduate students (ages 18–22) who had not yet received the influenza vaccine that year. Participants were excluded if they were allergic to eggs, were currently using any medications (e.g., steroids, antidepressants) or substances (e.g., tobacco products) known to affect the immune system, or had any influenza or upper respiratory symptoms, current depression, anxiety, any major medical condition (e.g., diabetes, asthma). Forty six individuals were enrolled in the study, 3 participants dropped from the study due to upper respiratory infection between study enrollment and vaccine administration, and 2 participants were dropped from analyses due to inability to complete the blood draw. Therefore all subsequent analyses represent complete data from 41 individuals. See Table 1 for characteristics of the study sample.
Table 1.
Sample characteristics and pre- and post-vaccine inflammation.
| M (SD) | % (n) | |
|---|---|---|
| Age | 18.49 (0.75) | |
| Female | 73.2 (30) | |
| BMI | 24.08 (3.84) | |
| Race/Ethnicity1 | ||
| White | 41.5 (17) | |
| Asian | 58.5 (24) | |
| Hispanic/Latino | 22.0 (9) | |
| Maternal Education | ||
| Some high school | 14.6 (6) | |
| High school diploma | 22.0 (9) | |
| Bachelor’s | 31.7 (13) | |
| Graduate | 31.7 (13) | |
| IL-6 (pg/ml) | ||
| Baseline | 1.14 (.95) | |
| 1-day post-vaccine | 1.47 (1.22) | |
| Δ IL-6 | 0.33 (0.75) | |
Groups are not mutually exclusive
Procedures
Participants first came to the lab to complete informed consent, behavioral measures, and daily diary training. For the next 14 days, participants completed 3–5 minute, online daily diaries assessing sickness behavior and features of depression, including mood, energy, sleep, feelings of social disconnection, and physical symptoms. Participants received a link to this survey each evening at 8:00pm, and were instructed to complete each diary before bedtime. Participants who did not complete the daily diary by midnight each day received a reminder at 6:00am to complete the diary upon waking. Diaries completed after 9:00am the following day were considered invalid. We had an overall 97.0% completion rate of valid daily diaries across 41 participants, or 557 complete and valid diaries. The majority of participants (78.0%, n = 32) completed all 14 diaries; the fewest daily diaries any participant completed was 10 (n = 3).
Approximately 1 week following study enrollment, participants returned to the laboratory to provide a blood sample. Research staff then escorted participants to the campus health center where they received the influenza vaccine. The influenza vaccine varied depending on year of participation. The 2015/2016 vaccine was trivalent and included A/California/7/2009 (H1N1) pdm09-like virus, which had been in flu vaccines since 2009, A/Switzerland/9715293/2013 (H3N2)-like virus, and B/Phuket/3073/2013, which were both new in Fall 2015. The 2016/2017 influenza vaccine was also trivalent and consisted of A/California/7/2009 (H1N1) pdm09-like virus, A/Hong Kong/4801/2014 (H3N2)-like virus, and B/Brisbane/60/2008-like virus (B/Victoria lineage). The following day participants returned to the laboratory to provide a second blood sample and completed behavioral measures. The post-vaccine blood draw varied between 21 and 29 hours after the vaccination, Mean Vaccine Delay = 24:35, SD Vaccine Delay = 2:10. Participants continued to complete daily diaries for the remainder of the 14-day assessment period. This study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All study procedures were approved by the UCLA Institutional Review Board, and all participants provided informed consent. Participants were compensated up to $200 for their time, including up to $40 for each laboratory visit, and up to $30 for completing all 14 possible daily diaries.
Measures
Mood
Mood was measured via daily diary using the 15-item Profile of Mood States (POMS-15), which has been previously used in daily diary research (Cranford et al., 2006; Shrout et al., 2006). These items comprised 5 sub-domains: Depressed mood (sad, hopeless, discouraged), Vigor (vigorous, cheerful, lively), Tension (anxious, uneasy, on edge), Anger (angry, resentful, annoyed), Fatigue (fatigued, worn out, exhausted). Three additional items were added to assess cognitive symptoms using items from the POMS-Confusion subscale (unable to concentrate, forgetful, confused) (McNair et al., 1981). Four additional items were included to assess positive affect (enthusiastic and interested (Watson et al., 1988; Watson and Clark, 1999), content and grateful (Fredrickson et al., 2003)). Participants were asked to indicate the degree to which they experienced each mood state that day and responded to each item according to a 5-point Likert scale from 1 = not at all to 5 = extremely. For multi-item diary scales, reliability coefficients ranging from 0 to 1 for between-person (RKF) and day-to-day within-person change (RC) were computed using methods described for daily diary scales (Cranford et al., 2006). For all POMS mood subscales, RKF > .96. RC for Depressed mood, Anxiety, Anger, Vigor, and Fatigue subscales were all between .65 – .87, and were comparable to prior work (Cranford et al., 2006). RC for Confusion was .47.
Social disconnection
Social disconnection was measured via a 12-item scale reflecting the degree to which participants felt disconnected from other people that day. Participants responded according to a 5-point Likert scale from 1 = not at all, and 5 = very much so. These items were used in a previous study linking endotoxin-induced changes in inflammatory markers with subjective feelings of social disconnection (Eisenberger et al., 2010b; Moieni et al., 2015). Reliability for social disconnection, RKF = .97, RC = .36.
Sleep disturbance and sleep quantity
Subjective sleep quality was measured on the daily diary using one modified item from the Pittsburgh Sleep Quality Inventory (Buysse et al., 1989), similar to that used in previous diary studies (Kane et al., 2014). Participants rated their sleep quality for the previous night on a 4-point Likert scale from 1 = very good to 4 = very bad. Participants also indicated the time they went to bed each night and woke each morning for a measure of sleep quantity. Reliability coefficients RKF and RC cannot be computed for these single-item measures of sleep disturbance and sleep quantity, however the test-retest reliability for the measure of sleep disturbance across diary days was good, ICC = .76 (Cappelleri et al., 2009).
Physical symptoms
Participants reported physical symptoms they experienced in the past 24 hours including feeling sick, headache, chills, fever, muscle/joint aches or pains. For each symptom, participants could select Absent, Mild, Moderate, or Severe on a scale from 1 to 4. The chosen physical symptoms were taken from past studies using daily diaries to examine subjective symptoms following a viral challenge (Cohen et al., 2006). Reliability for physical symptoms was RKF = .95, RC = .56.
Inflammation
Interleukin-6 (IL-6) was used to assess within-subject change in inflammation following vaccination in order to optimize comparison of our results with existing literature. IL-6 is reliably elevated in depressed patients (Dowlati et al., 2010), and the majority of studies that have demonstrated an inflammatory response to the influenza vaccine have selected IL-6 as the inflammatory marker of interest (Carty et al., 2006; Christian et al., 2011; Edwards et al., 2006a; Segerstrom et al., 2012; Tsai et al., 2005). Furthermore, studies using typhoid vaccination and endotoxin have demonstrated an association between IL-6 and mood/behavior (Eisenberger et al., 2010a; Harrison et al., 2009a; Wright et al., 2005). Blood samples for IL-6 were collected between 8:21am and 12:45pm (MeanBlood Draw 1 = 9:59, SDBlood Draw 1 = 1:04) by venipuncture into EDTA tubes, placed on ice, centrifuged for acquisition of plasma, and stored at −80 C for subsequent batch testing. Samples were assayed in duplicate using a high sensitivity ELISA (R&D Systems, Minneapolis, Minn) at the UCLA Inflammatory Biology Core. The range of detection for this assay was 0.20 mg/L to 10.0 pg/mL, and there were no undetectable values in the sample. Intra- and inter-assay CVs were < 9%.
Data Analysis
We fit linear mixed models predicting mood, social disconnection, sleep disturbance, and physical symptoms as a function of our variable of interest, the interaction between change in IL-6 and time. This allowed us to test whether average mood, social disconnection, and sleep differed before and after the vaccine based on the magnitude of IL-6 change from pre- to post-vaccine. Change in IL-6 (ΔIL-6) was computed by subtracting IL-6 concentrations immediately prior to the vaccine from IL-6 concentrations 1-day after the vaccination, such that higher values indicate a greater increase in circulating IL-6. Predictors in each of these models included: study day (1–14), time (a dichotomous variable where 0 = pre-vaccine and 1 = post-vaccine), baseline IL-6 (each participant’s pre-vaccination IL-6), ΔIL-6 (a post-diction term that accounts for any association between mood/sleep on pre-vaccine days and change in IL-6), and our variable of interest, Time*ΔIL-6 (the association between change in IL-6 and mood/sleep on post-vaccine days). There is evidence that body mass index (BMI) and sex contribute to differences in circulating markers of inflammation (O’Connor et al., 2009) as well as the way the immune system responds to challenges such as an immunization or a psychological stressor (Edwards et al., 2006b; Fish, 2008; Kitahara et al., 2014; Sun et al., 2012). Therefore, all models controlled for BMI and sex. Whether participants enrolled in the 2015 or 2016 cohort1 of the study was also included as a covariate. For all analyses, a significance level of 95% or p < .05 should be considered reliable, although variations in significance up to p < .10 are presented to indicate any possible statistical trends that may inform efforts to replicate and extend these results.
Results
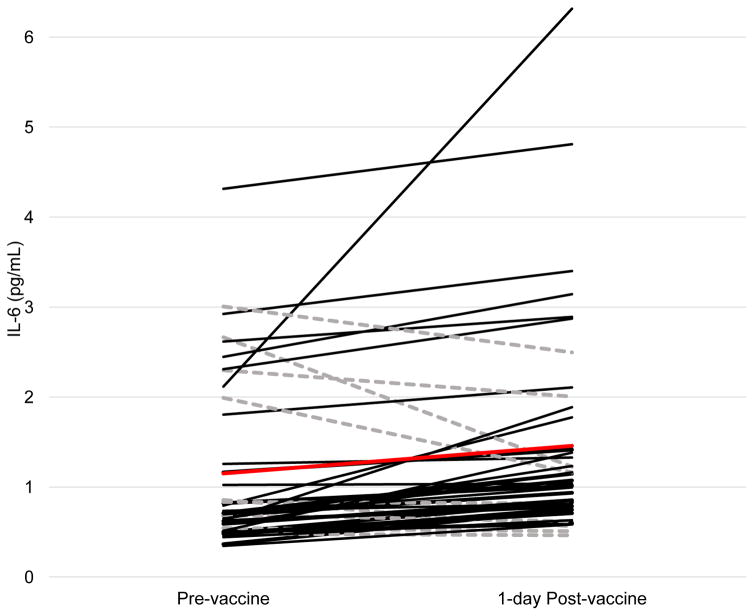
Participants in this study were healthy, ethnically diverse undergraduate students who were representative of their university undergraduate population. See Table 1 for detailed characteristics of the study sample. From pre- to post-vaccine participants demonstrated a significant increase in IL-6 from baseline to post-vaccination, t(41) = -2.77, p = .008. Overall, 33 participants (80.5%) showed an increase in IL-6, and the mean change in IL-6 was 0.33 pg/mL (SD = 0.75; range = −1.44–4.202), d = 0.45. See Figure 1 for IL-6 at pre- and post-vaccine for all participants.
Figure 1. Change in IL-6 from pre- to one-day post-vaccine administration.
IL-6 at pre- and post-vaccine for all participants. Black lines indicate participants with an increase in IL-6 from pre- to post-vaccine (n = 32), dashed grey lines indicate participants who did not show an increase in IL-6 (n = 9), and the red line indicates the mean change in IL-6 across the sample.
Inflammation before and after influenza vaccination and daily diary reported mood
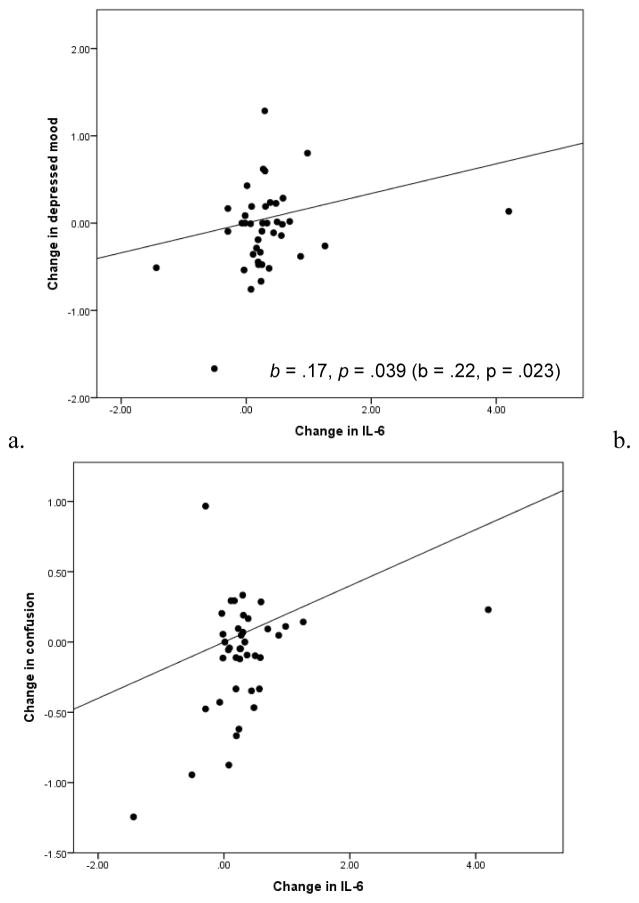
Table 2 provides descriptive statistics for all daily diary measures on pre-vaccine days. Reports of depressed mood on pre-vaccine days were low. Female participants reported more depressed mood than male participants, p = .020. Consistent with our hypotheses, larger increases in IL-6 from pre- to post-vaccine were associated with greater reported depressed mood on post-vaccine days, p = .039. While daily reported confusion was also generally low in the sample on pre-vaccine days, individuals demonstrating larger increases in IL-6 from pre- to post-vaccine reported greater confusion on post-vaccine days, p = .003. There were no significant associations between change in IL-6 and anger, tension, fatigue, vigor, or positive affect. However, individuals with higher IL-6 at the pre-vaccine blood draw reported lower average daily positive affect, p = .044. See Table 3 for unstandardized fixed effects of time and IL-6 on all sickness behavior and features of depression measured in the daily diary.
Table 2.
Mean (SD) of mood, sleep, social disconnection, and physical symptoms on days prior to influenza vaccine.
| M (SD) | |
|---|---|
| Mood | |
| Depressed mood | 1.44 (0.71) |
| Anger | 1.39 (0.62) |
| Tension | 1.49 (0.76) |
| Fatigue | 2.28 (1.12) |
| Vigor | 2.69 (0.83) |
| Confusion | 1.60 (0.68) |
| Positive affect | 3.21 (0.83) |
| Sleep | |
| Sleep Disturbance | 1.73 (0.69) |
| Sleep Quantity | 7.12 (1.93) |
| Social Disconnection | 1.82 (0.60) |
| Physical Symptoms | 1.13 (0.20) |
Table 3.
Unstandardized coefficient estimates in models predicting daily diary reported measures of affective well-being by inflammatory increase following influenza vaccine (Time* ΔIL-6)
| Depressed mood |
Anger | Tension | Fatigue | Vigor | Confusion | Positive affect |
Sleep Disturbance |
Sleep Quantity |
Social Disconnection |
Physical symptoms |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | |
| Intercept | 1.24(0.15)*** | 1.17 (0.12)*** | 1.31(0.17)*** | 2.25(0.28)*** | 2.77 (0.19)*** | 1.36(0.16)*** | 3.08(0.21)*** | 1.77 (0.13)*** | 6.78(0.35)*** | 1.89 (0.14)*** | 1.09 (0.04)*** |
| Study Day | −0.01 (0.01) | 0.01(0.01) | −0.003(0.01) | −0.05(0.02)** | 0.01(0.1) | −0.01 (0.01) | 0.02(0.01) | −0.004 (0.01) | 0.14(0.03)*** | −0.01 (0.09) | −0.002(0.003) |
| BMI | 0.001(0.02) | −0.001(0.01) | 0.002(0.02) | −0.02(0.03) | 0.02(0.02) | 0.02 (0.02) | 0.03(0.03) | −0.03 (0.02) | −0.03(0.04) | −0.01 (0.02) | −0.001(0.01) |
| Sex | 0.30 (0.13)* | 0.14(0.011) | 0.20(0.17) | 0.29(0.27) | 0.12(0.20) | 0.25 (0.16) | 0.25(0.21) | −0.03 (0.13) | −0.11(0.34) | −0.22 (0.15) | 0.12 (0.04)* |
| Cohort | 0.16 (0.11) | 0.18(0.09)+ | 0.16(0.15) | 0.17(0.23) | −0.35(0.17)* | 0.28(0.14)+ | −0.27(0.18) | −0.03 (0.11) | −0.28(0.29) | 0.30 (0.13)* | −0.06 (0.04) |
| Time | −0.06 (0.09) | −0.13(0.08) | −0.08(0.09) | 0.08(0.13) | −0.06(0.11) | −0.09 (0.08) | −0.17(0.10) | −0.01 (0.11) | −1.00(0.28)*** | 0.04 (0.07) | 0.03 (.03) |
| Baseline IL−6 | 0.01 (0.06) | −0.01(0.06) | −0.07(0.09) | −0.06(0.13) | −0.19(0.10)+ | −0.06 (0.08) | −0.22(0.10)* | 0.19 (0.06)** | −0.29(0.17)+ | 0.06 (0.07) | 0.07(0.02)** |
| ΔIL−6 | −0.17(0.09)+ | −0.08(0.08) | −0.16(0.11) | −0.31(0.17)+ | −0.01(0.12) | −0.24(0.10)* | −0.01(0.12) | 0.05 (0.08) | −0.04(0.21) | −0.12 (0.09) | −0.02 (0.03) |
| Time* ΔIL-61 | 0.17 (0.08)* | 0.03(0.06) | 0.10(0.07) | 0.08(0.12) | −0.10(0.09) | 0.20(0.06)** | −0.13(0.09) | 0.13 (0.08)+ | 0.14(0.20) | 0.11 (0.06)+ | 0.002(0.02) |
p <.001,
p < .01,
p < .05,
p < .10 (non-significant)
Indicates key parameter of interest.
Inflammation before and after influenza vaccination and daily diary reported social disconnection
Participants in this study generally reported low feelings of social disconnection on pre-vaccine study days. Individuals demonstrating the largest change in IL-6 from pre- to post-vaccine also reported a non-significant increase in feelings of social disconnection on post-vaccine days, p = .072.
Inflammation before and after influenza vaccination and daily diary reported sleep
Participants in this study slept an average of 7.12 hours on pre-vaccine days. Change in IL-6 was not associated with changes in sleep duration from pre- to post-vaccine, p = .64. Participants in this study reported their average sleep quality as either Very good or Good on pre-vaccine study days. Participants in the study with high pre-vaccine IL-6 reported greater daily sleep disturbance, p = .004, and individuals demonstrating a larger increase in IL-6 from pre- to post-vaccine also reported non-significant increases in sleep disturbance on post-vaccine days, p = .10.
Inflammation before and after influenza vaccination and daily diary reported physical symptoms
Change in inflammation from pre- to post-vaccine was not associated with physical symptoms, p = .69. Daily reports of physical symptoms were very infrequent or Absent on the response scale on pre-vaccine days. Change in IL-6 from pre- to post-vaccine was not associated with post-vaccine physical symptoms, however participants with higher pre-vaccine IL-6 reported more pre-vaccine physical symptoms, p = .002.
Discussion
This study documents associations between mild fluctuations in the proinflammatory cytokine IL-6 and features of depression in a sample of healthy, young adults. The majority of participants in this sample demonstrated an increase in IL-6 from immediately before to 1-day after flu vaccine exposure, and while this change was mild, participants varied considerably in the degree of inflammatory change. Consistent with our hypotheses, larger increases in IL-6 were associated with greater depressed mood and greater confusion in the week following vaccination. These findings align with previous research and with the hypothesized role of inflammation in depression (Dantzer et al., 2008; Dantzer and Kelley, 2007) as well as established experimental models in both animals and humans. Notably, some of the initial observations in preclinical studies were that inflammation interferes with cognitive functioning (Aubert et al., 1995). Further, inflammatory challenge studies in humans using endotoxin (Eisenberger et al., 2009; Reichenberg et al., 2001) and typhoid (Brydon et al., 2008; Harrison et al., 2009a), as well as observational studies (Gimeno et al., 2009; Motivala et al., 2005) have documented associations between inflammation and both cognitive symptoms of depression and sleep disturbance. Our findings extend this literature by demonstrating increases in depressed mood and cognitive symptoms following a mild inflammatory stimulus using a naturalistic and ecologically valid approach.
Further, the results of this study support the use of influenza vaccination as a translational model for interrogating well-established preclinical observations on the role of mild inflammatory changes in depressive symptoms. This model could be used to interrogate sensitivity to these changes in high-risk and vulnerable populations including children, adolescents, older adults, pregnant women, and patients with chronic illness. Inflammatory immune cells and cytokines, including IL-6, can cross the blood-brain interface and activate microglia (Banks et al., 1995; Wohleb et al., 2015). Activation of microglia causes local inflammation which mediates anxiety-like behavior, and primes the immune system for a greater inflammatory response to future stressors (Reader et al., 2015). Repetition of these processes in animals, such as through chronic stress, has been linked to anhedonia, sleep disturbance, and maintenance of anxiety-like behavior (Wohleb et al., 2015). Affective correlates of mild fluctuations in pro-inflammatory cytokines, as demonstrated in this study, may be an indicator of affective sensitivity to increases in peripheral inflammation and thus potentially a neurobiological risk factor for depression.
Contrary to our hypotheses, larger increases in IL-6 were not associated with decreases in daily positive affect or increases in fatigue. Given the subtle changes in both inflammation and daily diary reports of features of depression, we may have lacked power to detect these effects that have been observed in other experimental studies (Eisenberger et al., 2010b; Harrison et al., 2009b; Moieni et al., 2015). It is possible that the effect of inflammation on positive affect and fatigue occurs acutely (i.e., within hours), and is not effectively observed using a daily diary assessment. Indeed, preclinical models have shown that acute inflammatory states interfere with the opioid receptors in the neural structures involved in reward processes that would underlie positive affect (Eisenberger et al., 2010a; Narita et al., 2004). It is also possible that the effect of inflammation on positive affect and fatigue is only observed at higher levels of inflammation than seen here. We did find that higher IL-6 at baseline (immediately prior to vaccination) was associated with lower average daily positive affect. In previous studies, higher trait positive affect has been linked to lower circulating markers of inflammation, IL-6 and CRP, in both adolescents and adults (Chiang et al., 2015; Stellar et al., 2015; Steptoe et al., 2008). A similar pattern was observed for sleep disturbance, such that higher IL-6 at baseline was associated with greater daily reported sleep disturbance. Sleep and inflammation are intimately-linked physiological processes; sleep problems relate to greater inflammation, and interventions that improve sleep are associated with reductions in inflammatory processes (Irwin et al., 2016; Irwin and Opp, 2017). Therefore, further interrogation of dose, magnitude, and time course of inflammatory challenge is necessary. Some domains of sickness behavior and features of depression may be more sensitive to acute inflammatory signaling, while others may be induced by chronic, sustained elevations in inflammation. These differences further emphasize the value of using different paradigms to examine the role of inflammation in mood and behavior.
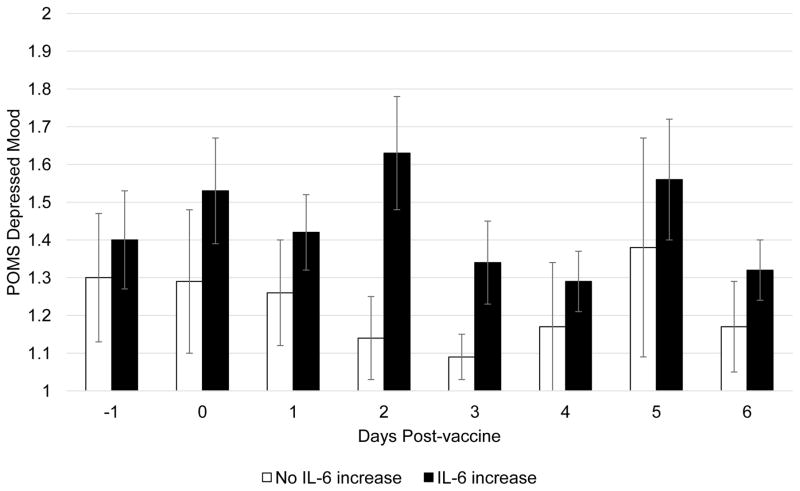
An important strength of this study was the use of daily diaries to capture within-subject changes in mood, and the interrogation of multiple domains that relate to depression (e.g., depressed mood, cognitive symptoms, poor sleep quality, positive affect, social disconnection). These findings should be considered preliminary due to the small sample size. Given the number of past studies that have demonstrated an inflammatory response following influenza vaccination (Carty et al., 2006; Christian et al., 2011; McDade et al., 2015; Posthouwer et al., 2004; Tsai et al., 2005) and the within-subject hypotheses of the present study, we did not include a placebo or waitlist control group and therefore no causal conclusions can be drawn. We also did not measure antibody response to the influenza vaccine, and therefore our data cannot speak to the role of change in IL-6 in the potential effectiveness of the vaccine. This study was also conducted in a small, convenience sample of young adults. Further investigations using this paradigm in larger samples are needed. Previous studies have shown that experimentally induced inflammation impacts feelings of social disconnection and sleep disturbance. In this study, the associations between IL-6 response and both of these constructs were in the expected direction but were non-significant. Larger studies would enable us to understand whether these effects only occur following a more robust inflammatory stimulus (e.g., endotoxin) or are subtle effects that require more power to detect. A larger study would facilitate the generalizability of this study’s observations as well as identify important moderators of inflammatory responses to influenza vaccination including BMI, sex, and exposure to early life stress (Kuhlman et al., 2017; O’Connor et al., 2009). Finally, we chose to measure the inflammatory response to the influenza vaccine 1 day after vaccination when an inflammatory response would most likely be observable (Carty et al., 2006; Tsai et al., 2005), however one study in pregnant women demonstrated that the inflammatory response to influenza vaccine is higher 2-days post-vaccination than 1-day post-vaccine (Christian et al., 2011). It is possible that some individuals’ IL-6 continued to rise beyond our post-vaccine IL-6 measurement. Indeed, there was a modest association between the magnitude of the IL-6 response and the length of the delay between the vaccine and post-vaccine blood draw, r = .27, p = .08; in other words, IL-6 continued to increase across the day following vaccination. Figure 3 clearly demonstrates that differences in depressed mood between participants with and without an increase in IL-6 are most prominent on the 2nd and 3rd day after vaccination. It will be informative for future studies to determine individual differences in the time course of the inflammatory response to the influenza vaccine as it relates to changes in mood and behavior.
Figure 3. Daily reported depressed mood for participants who did and did not demonstrate an inflammatory response to the flu vaccine.
Daily mean (SE) of depressed mood following vaccine administration by participants demonstrating an increase in IL-6 from immediately before to 1-day after the vaccine.
The results of this study provide preliminary evidence that mild, within-subject increases in IL-6 following influenza vaccination are associated with increases in features of depression, specifically depressed mood and cognitive symptoms. These findings are encouraging evidence that the annual influenza vaccine can be used to interrogate the effects of mild changes in inflammation on mood and behavior in a wide range of populations. The flu vaccine meets risk/benefit ratio criteria by including the tangible benefit of increased protection against influenza infection while also being familiar to most people, recommended annually, cost effective, and widely accessible. Using the influenza vaccine paradigm may help to identify risk factors for depression in high-risk populations and inform psychological and pharmacological interventions targeting inflammation in the pathogenesis of depression.
Figure 2. Change in depressed mood and confusion over week following vaccine by IL-6 response.
Within-subject change in (a) depressed mood and (b) confusion from pre-vaccine to post-vaccine days by within-subject change in IL-6. Estimates of fixed effects for the Time*ΔIL-6 term in each model are presented in the bottom right corner of each panel.
Highlights.
Participants demonstrated a significant increase in IL-6 from immediately before to 1-day after influenza vaccine.
Individuals vary in the magnitude of IL-6 change following influenza vaccine.
Larger increases in IL-6 were associated with corresponding increases in depressed mood and confusion.
The annual influenza vaccine may be a useful tool for investigating the role of inflammation in mood and behavior in a wide range of populations.
Acknowledgments
This research would not be possible if not for the support of the faculty and staff at the UCLA Cousins Center for Psychoneuroimmunology, and the participants who generously gave their time. The composition of this manuscript was made possible by the NIMH (T32MH015750 and K08MH112773 awarded to Dr. Kuhlman).
Footnotes
We observed significant differences between cohorts such that individuals in the 2016 cohort reported more symptoms of confusion, p = .050, less vigor, p = .049, greater feelings of social disconnection, p = .026, and change in IL-6 from pre- to post-vaccine, p < .001, than participants in the 2015 cohort. For this reason, the fixed effect of cohort was included as a covariate in all models.
One participant demonstrated an extreme increase in IL-6 from pre- to post-vaccine, ΔIL-6 = 4.20 pg/mL and was determined to be an outlier. Winsorizing the inflammatory response of this participant does not change the pattern of results. See Figure 2 for fixed effects of primary results with and without winsorizing this participant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrei AM, Fraguas R, Telles RMS, Alves TCTF, Strunz CMC, Nussbacher A, Rays J, Iosifescu DV, Wajngarten M. Major Depressive Disorder and inflammatory markers in elderly patients with heart failure. Psychosomatics. 2007;48:319–324. doi: 10.1176/appi.psy.48.4.319. https://doi.org/10.1176/appi.psy.48.4.319. [DOI] [PubMed] [Google Scholar]
- Aubert A. Sickness and behaviour in animals: A motivational perspective. Neurosci Biobehav Rev. 1999;23:1029–1036. doi: 10.1016/s0149-7634(99)00034-2. https://doi.org/10.1016/S0149-7634(99)00034-2. [DOI] [PubMed] [Google Scholar]
- Aubert A, Vega C, Dantzer R, Goodall G. Pyrogens specifically disrupt the acquisition of a task involving cognitive processing in the rat. Brain Behav Immun. 1995;9:129–148. doi: 10.1006/brbi.1995.1013. https://doi.org/10.1006/brbi.1995.1013. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. https://doi.org/doi:10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. https://doi.org/10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, McDermott AM, Sadosky AB, Petrie CD, Martin S. Psychometric properties of a single-item scale to assess sleep quality among individuals with fibromyalgia. Health Qual Life Outcomes. 2009;7:54. doi: 10.1186/1477-7525-7-54. https://doi.org/10.1186/1477-7525-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-α in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. https://doi.org/10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Carty CL, Heagerty P, Nakayama K, McClung EC, Lewis J, Lum D, Boespflug E, McCloud-Gehring C, Soleimani BR, Ranchalis J, Bacus TJ, Furlong CE, Jarvik GP. Inflammatory response after influenza vaccination in men with and without carotid artery disease. Arterioscler Thromb Vasc Biol. 2006;26:2738–2744. doi: 10.1161/01.ATV.0000248534.30057.b5. https://doi.org/10.1161/01.ATV.0000248534.30057.b5. [DOI] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, Almeida DM, Irwin MR, Seeman TE, Fuligni AJ. Socioeconomic status, daily affective and social experiences, and inflammation during adolescence. Psychosom Med. 2015;77:256–266. doi: 10.1097/PSY.0000000000000160. https://doi.org/10.1097/PSY.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Iams JD, Porter K, Glaser R. Inflammatory responses to trivalent influenza virus vaccine among pregnant women. Vaccine. 2011;29:8982–8987. doi: 10.1016/j.vaccine.2011.09.039. https://doi.org/10.1016/j.vaccine.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Alper CM, Doyle WJ, Treanor JJ, Turner RB. Positive emotional style predicts resistance to illness after experimental exposure to rhinovirus or influenza a virus. Psychosom Med. 2006;68:809–815. doi: 10.1097/01.psy.0000245867.92364.3c. https://doi.org/10.1097/01.psy.0000245867.92364.3c. [DOI] [PubMed] [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, Bolger N. A procedure for evaluating sensitivity to within-person change: Can mood measures in diary studies detect change reliably? Pers Soc Psychol Bull. 2006;32:917–929. doi: 10.1177/0146167206287721. https://doi.org/10.1177/0146167206287721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: Where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. https://doi.org/10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. https://doi.org/10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. https://doi.org/10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in Major Depression. Biol Psychiatry, Cortical Inhibitory Deficits in Depression. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. https://doi.org/10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Burns VE, Reynolds T, Carroll D, Drayson M, Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav Immun. 2006a;20:159–168. doi: 10.1016/j.bbi.2005.07.001. https://doi.org/10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Burns VE, Ring C, Carroll D. Sex differences in the interleukin-6 response to acute psychological stress. Biol Psychol. 2006b;71:236–239. doi: 10.1016/j.biopsycho.2005.06.006. https://doi.org/10.1016/j.biopsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010a;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. https://doi.org/10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010b;24:558–563. doi: 10.1016/j.bbi.2009.12.009. https://doi.org/10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. NeuroImage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. https://doi.org/10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737. doi: 10.1038/nri2394. https://doi.org/10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crisis? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. J Pers Soc Psychol. 2003;84:365. doi: 10.1037//0022-3514.84.2.365. https://doi.org/10.1037/0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GDO, Rumley A, Marmot MG, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39:413–423. doi: 10.1017/S0033291708003723. https://doi.org/10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner S, Emeny RT, Lacruz ME, Baumert J, Herder C, Koenig W, Thorand B, Ladwig KH. Association between social isolation and inflammatory markers in depressed and non-depressed individuals: Results from the MONICA/KORA study. Brain Behav Immun. 2011;25:1701–1707. doi: 10.1016/j.bbi.2011.06.017. https://doi.org/10.1016/j.bbi.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009a;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. https://doi.org/10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009b;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. https://doi.org/10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. https://doi.org/10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Irwin MR. Psychoneuroimmunology of depression: Clinical implications. Brain Behav Immun. 2002;16:1–16. doi: 10.1006/brbi.2001.0654. https://doi.org/10.1006/brbi.2001.0654. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. https://doi.org/10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Opp MR. Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. 2017;42:129–155. doi: 10.1038/npp.2016.148. https://doi.org/10.1038/npp.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. https://doi.org/10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane HS, Slatcher RB, Reynolds BM, Repetti RL, Robles TF. Daily self-disclosure and sleep in couples. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2014;33:813–822. doi: 10.1037/hea0000077. https://doi.org/10.1037/hea0000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara CM, Trabert B, Katki HA, Chaturvedi AK, Kemp TJ, Pinto LA, Moore SC, Purdue MP, Wentzensen N, Hildesheim A, Shiels MS. Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2014;23:2840–2849. doi: 10.1158/1055-9965.EPI-14-0699-T. https://doi.org/10.1158/1055-9965.EPI-14-0699-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744–759. doi: 10.1016/j.pnpbp.2010.08.026. https://doi.org/10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Kuhlman KR, Chiang JJ, Horn S, Bower JE. Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neurosci Biobehav Rev. 2017;80:166–184. doi: 10.1016/j.neubiorev.2017.05.020. https://doi.org/10.1016/j.neubiorev.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martich GD, Boujoukos AJ, Suffredini AF. Response of man to endotoxin. Immunobiology. 1993;187:403–416. doi: 10.1016/S0171-2985(11)80353-0. https://doi.org/10.1016/S0171-2985(11)80353-0. [DOI] [PubMed] [Google Scholar]
- McDade TW, Borja JB, Kuzawa CW, Perez TLL, Adair LS. C-reactive protein response to influenza vaccination as a model of mild inflammatory stimulation in the Philippines. Vaccine. 2015;33:2004–2008. doi: 10.1016/j.vaccine.2015.03.019. https://doi.org/10.1016/j.vaccine.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF, et al. Profile of mood states. Educational and industrial testing service; San Diego, CA: 1981. [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: Implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. https://doi.org/10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. https://doi.org/10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Narita M, Kishimoto Y, Ise Y, Yajima Y, Misawa K, Suzuki T. Direct evidence for the involvement of the mesolimbic κ-Opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology. 2004;30:111–118. doi: 10.1038/sj.npp.1300527. https://doi.org/10.1038/sj.npp.1300527. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. https://doi.org/10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthouwer D, Voorbij HAM, Grobbee DE, Numans ME, van der Bom JG. Influenza and pneumococcal vaccination as a model to assess C-reactive protein response to mild inflammation. Vaccine. 2004;23:362–365. doi: 10.1016/j.vaccine.2004.05.035. https://doi.org/10.1016/j.vaccine.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. https://doi.org/10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: Monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015;289:429–442. doi: 10.1016/j.neuroscience.2015.01.001. https://doi.org/10.1016/j.neuroscience.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. https://doi.org/10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Hardy JK, Evans DR, Greenberg RN. Vulnerability, distress, and immune response to vaccination in older adults. Brain Behav Immun, Aging, Brain, Behavior, and Immunity. 2012;26:747–753. doi: 10.1016/j.bbi.2011.10.009. https://doi.org/10.1016/j.bbi.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Herman CM, Bolger N. The costs and benefits of practical and emotional support on adjustment: A daily diary study of couples experiencing acute stress. Pers Relatsh. 2006;13:115–134. https://doi.org/10.1111/j.1475-6811.2006.00108.x. [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. http://dx.doi.org/10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci. 2010;107:14817–14822. doi: 10.1073/pnas.1009164107. https://doi.org/10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellar JE, John-Henderson N, Anderson CL, Gordon AM, McNeil GD, Keltner D. Positive affect and markers of inflammation: Discrete positive emotions predict lower levels of inflammatory cytokines. Emotion. 2015;15:129–133. doi: 10.1037/emo0000033. https://doi.org/10.1037/emo0000033. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. https://doi.org/10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, O’Donnell K, Badrick E, Kumari M, Marmot M. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: The Whitehall II study. Am J Epidemiol. 2008;167:96–102. doi: 10.1093/aje/kwm252. https://doi.org/10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- Strike PC, Wardle J, Steptoe A. Mild acute inflammatory stimulation induces transient negative mood. J Psychosom Res. 2004;57:189–194. doi: 10.1016/S0022-3999(03)00569-5. https://doi.org/10.1016/S0022-3999(03)00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Ji Y, Kersten S, Qi L. Mechanisms of Inflammatory Responses in Obese Adipose Tissue. Annu Rev Nutr. 2012;32:261–286. doi: 10.1146/annurev-nutr-071811-150623. https://doi.org/10.1146/annurev-nutr-071811-150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Hanson NQ, Straka RJ, Hoke TR, Ordovas JM, Peacock JM, Arends VL, Arnett DK. Effect of influenza vaccine on markers of inflammation and lipid profile. J Lab Clin Med. 2005;145:323–327. doi: 10.1016/j.lab.2005.03.009. https://doi.org/10.1016/j.lab.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. The PANAS-X: Manual for the positive and negative affect schedule-expanded form 1999 [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. https://doi.org/10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferon- α-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res. 2007;62:207–214. doi: 10.1016/j.jpsychores.2006.09.007. https://doi.org/10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2015;8:447. doi: 10.3389/fnins.2014.00447. https://doi.org/10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: Mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. https://doi.org/10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. https://doi.org/10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]