Abstract
Invasive pulmonary mucormycosis and aspergillosis are rare, life-threatening fungal infections. Most documented cases have been reported in non-cirrhotic patients with diabetes mellitus, neutropenia, or treatment with corticosteroids. The prevalence of each infection is low among patients with hepatic cirrhosis. We report the first likely case of combined invasive pulmonary mucormycosis and aspergillosis in a male with decompensated hepatic cirrhosis. This report also highlights the first non-diabetic case of invasive pulmonary mucormycosis with decompensated hepatic cirrhosis.
Keywords: Invasive, Pulmonary, Mucormycosis, Aspergillosis, Hepatic cirrhosis
1. Introduction
Mucormycosis and Aspergillosis are rare, life-threatening fungal infections with mortality rates reported to be over 50% despite surgical debridement and antifungal therapy [1], [2]. Rhizopus arrhizus, which is responsible for 70% of all cases of mucormycosis, is associated with a number of clinical diseases (rhino-orbital-cerebral, cutaneous, gastric, and pulmonary mucormycosis) in adults [1]. This fungal infection is particularly recognized worldwide by the healthcare community due to its highly pathogenic nature, which is characterized by rapid tissue destruction and invasion across tissue planes [3]. The major risk factors for mucormycosis include uncontrolled diabetes mellitus with ketoacidosis, hematological malignancy, stem cell and solid organ transplantations, iron chelation therapy with deferoxamine, and corticosteroid usage [3]. The diagnosis of mucormycosis is usually made by the identification of causative fungal organisms by histopathological analysis of tissue specimens from patients with suggestive signs and symptoms [3]. Cultures are only occasionally positive [3]. Initial treatment of mucormycosis typically requires early aggressive surgical debridement of infected tissues, combined with administration of amphotericin B deoxycholate (Amb) or liposomal amphotericin B (L-AmB) [3].
Aspergillus fumigatus is responsible for over 70% of all cases of invasive aspergillosis [4]. Invasive aspergillosis usually occurs in immunosuppressed patients such as those receiving chronic corticosteroid treatment or with prolonged neutropenia from malignancy [2], [4]. Other risk factors for infection include chronic obstructive pulmonary disease and hepatic cirrhosis [2]. The diagnosis of proven, probable, or possible invasive aspergillosis is made by the combination of host status, imaging and mycological findings [5]. The optimal treatment of aspergillosis is administration of voriconazole or isavuconazole, which must be used with caution in patients with hepatic cirrhosis [2], [4].
Here, we report a patient with decompensated hepatic cirrhosis who had combined invasive pulmonary mucormycosis and aspergillosis. To our knowledge, this is the first report of invasive pulmonary mucormycosis in a decompensated cirrhotic patient who did not have concomitant diabetes mellitus.
2. Case
A 58-year old homeless male was found unresponsive in a parking lot and was taken by ambulance to our emergency department (ED). The patient was minimally responsive and able to state only his name; no additional history could be obtained. Initial vital signs in the ED demonstrated a temperature of 37.1 °C, heart rate of 157 beats per minute, respiratory rate of 35 per minute, oxygen saturation of 78% while breathing 15 l of oxygen per minute via face mask, and blood pressure of 80/49 mmHg. On examination, he was ill appearing, icteric, and in respiratory distress. The patient had tachycardia, but no cardiac murmurs. Rales were heard at both lung bases. The entire right lower extremity was erythematous and there was extensive necrosis of the soft tissues extending from the hindfoot to the proximal thigh.
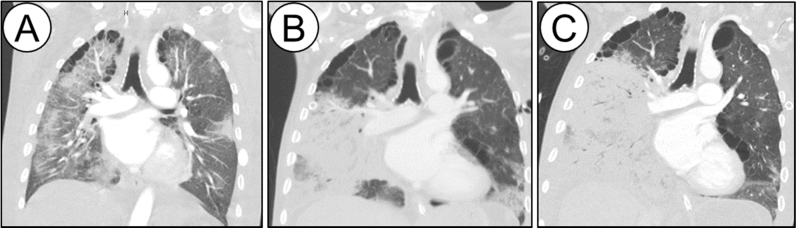
Pertinent laboratory studies on initial presentation revealed a white blood cell count of 19.2 × 103 cells/µm3, platelets of 19 × 103 cells/µm3, bicarbonate of 21 mEq/L, blood urea nitrogen of 67 mg/dL, creatinine of 1.23 g/dL, random glucose of 120 mg/dL, total bilirubin of 10.3 mg/dL, direct bilirubin of 6.1 mg/dL, alanine transaminase of 66 U/L, aspartate transaminase of 116 U/L, albumin of 1.4 g/dL, prothrombin time of 34, international normalized ratio of 3.3, partial thromboplastin time of 47 U/L, creatine phosphokinase of 851 U/L, and lactate of 3.3 mg/dL. A portable chest radiograph (CXR) showed patchy bibasilar infiltrates. Computed tomography (CT) of the chest revealed bilateral ground-glass opacities and infiltrates that were more extensive in the right lung (Fig. 1A).
Fig. 1.
Coronal images (slices) from CT of chest scans on days 1 (A), 16 (B), and 23 (C) of hospitalization demonstrating the progression of the infiltrates in the right lung.
The patient was treated with aggressive intravenous (IV) fluid hydration and vasopressors (norepinephrine, vasopressin, and epinephrine) for septic shock. He underwent tracheal intubation for acute hypoxic respiratory failure. The patient received empiric antibiotic treatment with IV vancomycin, piperacillin-tazobactam, and clindamycin. The patient was taken to the operating room where a right above-the-knee amputation was performed for his necrotizing soft tissue infection on day 0.
The patient's postoperative course was complicated by refractory septic shock requiring multiple vasopressors, acute anuric renal failure necessitating hemodialysis, bilateral chest tube drainage of pleural effusions, worsening liver failure, and progressive hypoxia requiring mechanical ventilation. On day+ 2, the patient underwent surgical disarticulation of his right hip to remove residual necrotic infected tissue. The intraoperative wound cultures grew methicillin-sensitive Staphylococcus aureus and Proteus penneri. On day+ 4, 1/4 bottles of the blood cultures grew Aerococcus species and Staphylococcus epidermidis. All four organisms were sensitive to vancomycin and/or piperacillin-tazobactam. No fungal organisms were seen on histopathological analysis of infected tissue and none were isolated from the cultured material.
The patient continued to deteriorate after surgery. On day+ 10, given the continued refractory septic shock and bibasilar patchy infiltrates seen on CXR, a bronchoalveolar lavage (BAL) was performed, and thick frothy respiratory secretions with pus were seen. Gram stain and bacterial culture of the BAL specimen were negative, and Xpert® MTB/RIF (Cepheid, Sunnyvale, CA) PCR of the sputum was negative for tuberculosis. Cytopathology was not performed.
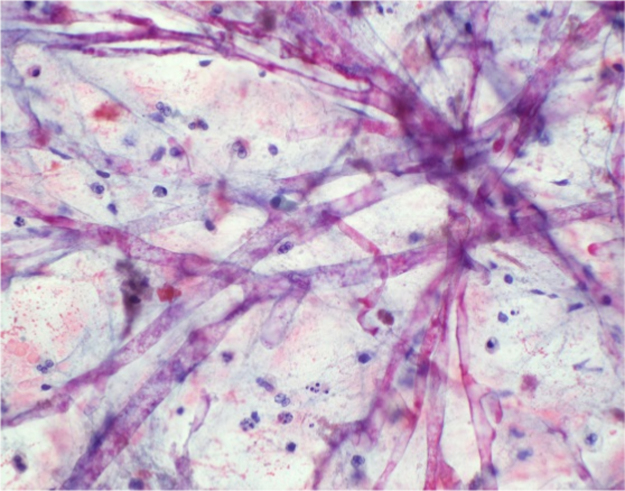
On day+ 12, an infectious diseases consultation was obtained. On initial evaluation, it was noted that the patient had a positive serum 1,3 β-D glucan (> 500, normal < 60). Empiric antifungal treatment with IV micafungin was initiated. Serum galactomannan, cryptococcal antigen, and coccidiomycosis complement fixation tests were negative, as was the urine histoplasma antigen test. On day+ 16, a repeat CT scan of the chest revealed worsening of right lung patchy infiltrates (Fig. 1B). A repeat BAL was performed on day+ 18, and thick frothy respiratory secretions were again seen. A bronchoscopic biopsy was not performed because of the patient's coagulopathy and thrombocytopenia. Nonseptate hyphae were seen on cytologic analysis of the repeat BAL specimen (Fig. 2). The Aspergillus galactomannan assay of the BAL fluid was also positive (6.78, normal < 0.5), and IV voriconazole was initiated on day+ 19. A rapidly growing mold grew in cultures of both the BAL sample and a swab from the right chest tube site; the specimens were sent to an outside reference laboratory for identification.
Fig. 2.
Nonseptate hyphae from BAL cytology specimen (original magnification X 400).
The patient continued to deteriorate. On day+ 21, when the total bilirubin increased to 23.5 mg/dL, the micafungin and voriconazole were discontinued and IV L-AmB (5 mg/kg daily) was initiated. On day+ 23, a CT scan of the chest showed worsening of the infiltrates in the right lung (Fig. 1C). In addition, a second mold grew in BAL fungal culture. This mold had septate hyphae and conidia, but was not sent for identification. The patient expired on day+ 25. Four days later, the rapidly growing mold from the BAL and right chest tube insertion site was identified as R. arrhizus, indicating the presence of invasive pulmonary mucormycosis.
3. Discussion
The data indicate that this patient had both invasive pulmonary mucormycosis and aspergillosis. The diagnosis of mucormycosis was made by the isolation of R. arrhizus from cultures of the BAL fluid and chest tube insertion site, and the finding of broad non-septate hyphae on the BAL cytology specimen. The patient also met the diagnostic criteria for probable invasive pulmonary aspergillosis based on his clinical presentation, the growth from the BAL culture of a second fungus with septate hyphae and conidia that resembled Aspergillus based on colony and microscopic morphologies, and positive BAL galactomannan and serum 1,3 B-D glucan tests [5]. Of note, neither test should be positive in patients with mucormycosis [6]. However, because no formal identification of the second mold from BAL fungal culture was performed, we cannot classify this case as proven aspergillosis [5]. The patient most likely acquired both organisms from the natural environment given his homelessness.
This is the first report of a case of combined invasive pulmonary mucormycosis and aspergillosis in a patient with decompensated cirrhosis. The patient did not have any of the traditional risk factors for both infections [2], [3], [4]. There was no evidence of diabetes mellitus given the patient's normal random glucose levels upon initial presentation and during hospitalization. The patient was never neutropenic, had only transient lactic acidosis, and did not receive prolonged corticosteroids.
Liver cirrhosis was the most likely contributing risk factor for both infections in our patient. Invasive aspergillosis is well-described in patients with decompensated liver cirrhosis [2]. Immune dysfunction is a known complication of liver cirrhosis [6]. A decline in both humoral and cell-mediated immunity is a risk factor for bacterial and fungal infections in patients with liver disease, especially in Child-Pugh (CP) class B and C cirrhosis [7], [8], [9]. The patient was also at higher risk for pulmonary aspergillosis given his positive emphysematous image findings (Fig. 1A-C) suggestive of chronic obstructive pulmonary disease [2]. Furthermore, the patient was in an iatrogenic iron overload state after receiving 22 units of packed red blood cells for anemia during his early-to-mid hospitalization course. This iron overload state can enhance fungal growth and virulence, possibly contributing to his clinical deterioration [3]. Another potential explanation to consider in our case was the early usage of voriconazole for aspergillosis. Animal models have demonstrated that voriconazole exposure increases virulence of R. arrhizus strains and mortality rates in pulmonary disease [10], [11].
Ultimately, our patient died from his invasive pulmonary mucormycosis and probable aspergillosis complicated by refractory septic shock, multi-organ failure, and severe immune dysfunction from decompensated liver cirrhosis. He also did not receive timely administration of L-Amb, the recommended drug of choice, for invasive pulmonary mucormycosis as this was an unexpected diagnosis given the rarity of this infection in decompensated liver cirrhosis.
To our knowledge, this is also the first case report of invasive pulmonary mucormycosis in a decompensated cirrhotic patient who did not have concomitant diabetes mellitus. The presenting and pertinent demographic and clinical characteristics of prior published case reports of invasive mucormycosis in patients with liver cirrhosis are shown in Table 1. Overall, these cases depict life-threatening infections associated with CP class C cirrhosis (14), diabetes mellitus (12), viral hepatitis C infection (9), rhino-orbital (8) and rhino-orbital-cerebral (7) involvements, CP class B cirrhosis (6), and alcohol use (6). The most intriguing findings are the vast disproportionate number of pulmonary (2) to rhino-orbital/rhino-orbital-cerebral (15) infections, no reports of infected cases associated with CP class A cirrhosis, and all cases with CP class C cirrhosis died despite IV Amb and surgical treatments. Our case differed from the 2 prior published cases (3 & 22) with pulmonary involvement in that there were no other risk factors of diabetes mellitus and chronic steroid usage as well as no association with CP class B cirrhosis.
Table 1.
Demographics and clinical characteristics of reported cases of invasive mucormycosis in patients with liver cirrhosis.
| Case | Age- years | Gender | Cause of liver disease | Child Pugh class | Other risk factors | Location of infection | Treatment | Outcome | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | Female | NR | B | None | RO | None | Died | [12] |
| 2 | 63 | Female | NR | NR | DM | ROC | None | Died | [13] |
| 3 | 44 | Male | ETOH | NR | DM | Pulmonary | AmB/Surgery | Alive | [14] |
| 4 | 53 | Male | HCV | B | DM | ROC | None | Died | [12] |
| 5 | 58 | Female | NR | C | DM | ROC | AmB | Died | [12] |
| 6 | 39 | Male | HCV | B | DM | ROC | AmB/Surgery | Died | [12] |
| 7 | 57 | Male | HCV | C | None | RO | AmB | Died | [12] |
| 8 | 55 | Male | HBV | C | None | RO | None | Died | [12] |
| 9 | 15 | Female | AIH | C | DM Steroids | RO | None | Died | [12] |
| 10 | 53 | Male | HCV | C | DM | ROC | AmB/Surgery | Died | [12] |
| 11 | 35 | Male | HCV | C | DM | RO | AmB | Died | [12] |
| 12 | 38 | Female | ETOH | C | Steroids | Cutaneous | Surgery | Died | [12] |
| 13 | 63 | Female | HCV | B | None | RO | AmB/Surgery | Alive | [12] |
| 14 | 42 | Male | HBV | C | None | RO | AmB | Died | [12] |
| 15 | 59 | Female | ETOH | C | None | ROC | None | Died | [12] |
| 16 | 65 | Male | HCV | B | DM | RO | AmB/Surgery | Alive | [12] |
| 17 | 47 | Male | HCV | C | DM | Gastric | AmB | Died | [12] |
| 18 | 48 | Female | ETOH | NR | None | Cutaneous | Surgery | Died | [12] |
| 19 | 25 | Female | AIH | C | Steroids | Cutaneous | AmB/Surgery | Died | [12] |
| 20 | 55 | Male | ETOH | NR | None | Gastric | AmB/Surgery | Alive | [15] |
| 21 | 55 | Female | AIH | C | DM Steroids | Gastric | AmB | Died | [16] |
| 22 | 68 | Woman | HCV | B | DM | Pulmonary | AmB/Surgery | Died | [17] |
| 23 | 28 | Male | ETOH | C | None | ROC | AmB | Died | [18] |
| 24 | 58 | Male | NR | C | None | Pulmonary | AmB | Died | This case |
Abbreviations: NR, not reported; RO, rhino-orbital; ROC, rhino-orbital-cerebral; DM, diabetes mellitus; ETOH, alcohol; AmB, amphotericin B; HCV, Hepatitis C Virus; HBV, Hepatitis B Virus; AIH, autoimmune hepatitis.
In conclusion, this case report highlights the need for healthcare professionals to be aware that decompensated liver cirrhosis is considered a risk factor for both invasive pulmonary mucormycosis and aspergillosis.
Acknowledgements
None.
Acknowledgments
Conflict of interest
There are none.
Ethical form
This study received no funding, and there are no potential conflicts of interests with respect to the research, authorship, and/or publication of this article. We were not able to obtain written and signed consent to publish the case report from the patient, and he had no known family members or legally authorized representatives.
References
- 1.Ibrahim A., Spellberg B., Walsh T., Kontoyiannis D. Pathogenesis of Mucormycosis. Clin. Infect. Dis. 2012;54(S1):S16–S22. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falcone M., Massetti A., Russo A., Vullo V., Venditti M. Invasive aspergillosis in patients with liver disease. Med. Mycol. 2011;49:406–413. doi: 10.3109/13693786.2010.535030. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim A., Kontoyiannis D. Update on mucormycosis pathogenesis. Curr. Opin. Infect. Dis. 2013;26(6):508–515. doi: 10.1097/QCO.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cicek N., Yildiz N., Kadayifci E., Gokce I., Alpay H. Invasive aspergillosis in a patient with end stage renal disease. Med. Mycol. Case Rep. 2017;18:12–14. doi: 10.1016/j.mmcr.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascioglu S., Rex J., De Pauw B., Bennett J., Bille J., Crokaert F. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 2002;34(1):7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 6.Bennett J., Dolin R., Mandell Blaser M. 8th ed. Elsevier; Philadelphia: 2015. Douglas, and Bennett's Principles and Practice of Infectious Diseases. [Google Scholar]
- 7.Cheruvattath R., Balan V. Infections in patients with end-stage liver disease. J. Clin. Gastroenterol. 2007;41:403–411. doi: 10.1097/01.mcg.0000248018.08515.f9. [DOI] [PubMed] [Google Scholar]
- 8.Fiuza C., Salcedo M., Clemente G., Tellado J. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J. Infect. Dis. 2000;182:526–533. doi: 10.1086/315742. [DOI] [PubMed] [Google Scholar]
- 9.Lombardo L., Capaldi A., Poccardi G., Vineis P. Peripheral blood CD3 and CD4 T-lymphocyte reduction correlates with severity of liver cirrhosis. Int. J. Clin. Lab. Res. 1995;25:153–156. doi: 10.1007/BF02592558. [DOI] [PubMed] [Google Scholar]
- 10.Lewis R., Liao G., Wang W., Prince R., Kontoyiannis D. Voriconazole pre-exposure selects for breakthrough mucormycosis in a mixed model of Aspergillus fumigatus-Rhizopus oryzae pulmonary infection. Virulence. 2011;2(4):348–355. doi: 10.4161/viru.2.4.17074. [DOI] [PubMed] [Google Scholar]
- 11.Lamaris G., Ben-Ami R., Lewis R., Chamilos G., Samonis G., Kontoyiannis D. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J. Infect. Dis. 2009;199(9):1399–1406. doi: 10.1086/597615. [DOI] [PubMed] [Google Scholar]
- 12.Elsiesy H., Saad M., Shorman M., Amr S., Abaalkhail F., Hashim A. Invasive mucormycosis in a patient with liver cirrhosis: case report and review of the literature. Hepat. Mon. 2013;13(8):e10858. doi: 10.5812/hepatmon.10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokuda T., Ono Y., Nishiya H., Aoki M., Yamanouchi S., Kunii O. An autopsy case of fungal (Mucor) cerebral aneurysm. Kansenshogaku Zasshi. 1995;69(4):438–443. doi: 10.11150/kansenshogakuzasshi1970.69.438. [DOI] [PubMed] [Google Scholar]
- 14.Tojima H., Tokudome T., Otsuka T. Chronic pulmonary mucormycosis that developed in preexisting cavities caused by tuberculosis in a patient with diabetes mellitus and liver cirrhosis. Nihon Kyobu Shikkan Gakkai Zasshi. 1997;35(1):100–105. [PubMed] [Google Scholar]
- 15.Lee S.H., Son Y.G., Sohn S.S., Ryu S.W. Successful treatment of invasive gastric mucormycosis in a patient with alcoholic liver cirrhosis: a case report. Exp. Ther. Med. 2014;8:401–404. doi: 10.3892/etm.2014.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corey K., Gupta N., Agarwal S., Xiao H. Case 32-2013: a 55-year-old woman with autoimmune hepatitis, cirrhosis, anorexia, and abdominal pain. New Engl. J. Med. 2013;369(16):1545–1553. doi: 10.1056/NEJMcpc1208153. [DOI] [PubMed] [Google Scholar]
- 17.Ayadi-Kaddour A., Braham E., Marghli A., Ismail O., Helal L., Mlika M. Fatal pulmonary mycosis in a diabetic and cirrhotic patient. Tunis Med. 2015;93(4):259–262. [PubMed] [Google Scholar]
- 18.Avelar Rodriquez D., Ochoa Virgen G., Miranda Ackerman R.C. A tip from the nose: rhinocerebral mucormycosis in a patient with alcoholic liver cirrhosis and cocaine abuse, an uncommon association. BMJ Case Rep. 2017 doi: 10.1136/bcr-2017-220730. [DOI] [PMC free article] [PubMed] [Google Scholar]