ABSTRACT
Capsule endoscopy (CE) enables noninvasive visualization of the small bowel in Crohn’s disease (CD), but should not be conducted in patients with bowel obstruction. Patency capsule (PC) can be ingested before conducting the CE examination to ensure patency of the gastrointestinal (GI) tract. This study aimed to evaluate the clinical significance of GI patency which the PC demonstrated. A retrospective review of the medical records was conducted with 99 consecutive patients with CD who underwent PC and CE at Nagoya University Hospital from January 2010 to May 2015. By using the Cox proportional hazards model, the association between the GI patency evaluated using the PC and the outcome in terms of the rate of patients who needed admission or surgery during the 2-year follow-up was examined. Of all 99 patients who ingested the PC, 84 (84.8%) were diagnosed as not having bowel obstruction, and therefore were eligible for CE (P group). Of the 15 patients in whom bowel obstruction was suspected (NP group), 12 patients underwent either the balloon-assisted endoscopy (n=10) or enteroclysis (n=2), and 11 were confirmed to have small bowel stricture. Non-admission rates of the P and NP groups during the 2-year observation period were 74/84 (88.0%) and 8/15 (53.3%), respectively (P<0.001). Non-operation rates of the P and NP groups during the 2-year observation period were 80/84 (95.2%) and 9/15 (60.0%), respectively (P<0.001). In conclusion, GI patency as diagnosed using the PC was associated with a significantly lower incidence of admission or surgical intervention.
Key Words: capsule endoscopy, patency, Crohn’s disease, small bowel
INTRODUCTION
Mucosal healing (MH) has emerged as a major therapeutic goal in Crohn’s disease (CD) in clinical practice. MH is strongly associated with lower relapse rates, lower hospitalization rates, and reduced need for surgery and1) can also predict sustained clinical remission in patients with early-stage CD.2) Information on how to evaluate and monitor MH in patients with CD during the remission period is very important, and several devices for this purpose are currently available. Capsule endoscopy (CE) is a noninvasive form of endoscopic examination suitable for observation of the small bowel and is clinically indicated for the evaluation of CD.3-6) CE is known to be superior to other modalities, particularly, in diagnosing CD.7) In addition, small bowel MH assessment by using CE is reported to be useful for predicting long-term clinical remission in CD.8)
One of the important issues for safely conducting CE is to prevent retention of the capsule which may necessitate surgery for its retrieval. PillCam patency capsule (PC, FUJIFILM Holdings Corporation, Tokyo, Japan, and Medtronic Ltd, Ireland) is a partner product of PillCam SB, the same company that manufactured CE, and has been designed to be ingested prior to using the CE to confirm patency of the gastrointestinal tract (GI) in a noninvasive manner. The PC consists of a small identification tag (RFID), detectable by radiofrequency, which is surrounded by an absorbable material with lactose and a small amount of barium, all this covered by an external cover. The PC has the same dimensions (11.4 mm × 26.4 mm) and the same shape as the regular CE and can be caught in the GI in case of strictures. If such retention occurs, timer plug erodes after 40 h, allowing penetration of body fluids into the capsule and subsequent dissolution of the body. The remaining RFID fragments of the capsule can then pass through even tight strictures, thus avoiding untoward consequences.9) Although the PC was introduced into clinical practice earlier and prospective studies were designed to assess the clinical usefulness and safety of the PC in patients with intestinal strictures suspected from clinical and/or radiological data, clinical experience with the PC remains scarce at this time, and the gold standard for confirming the patency of the GI tract has not been established. However, if the PC is not administered to patients who have a risk of CE retention, such as long-term users of nonsteroidal anti-inflammatory drugs and patients suspected to have CD, CE retention is expected to occur at certain percentages. Our team started to evaluate the PC in 2010 as a preliminary preclinical study and utilized the device more extensively in clinical practice after its approval by the Japanese Ministry of Health, Labor, and Welfare in July 2012. In the current study, data of patients with CD who were administered the PC were retrospectively analyzed, and the information regarding the association between GI patency achieved using the PC and the 2-year clinical outcome was evaluated.
PATIENTS AND METHODS
Patients
Consecutive patients with CD who were administered the PC from January 2010 to May 2015 in Nagoya University Hospital were enrolled in this study, and medical records of these patients were retrospectively reviewed. Only patients with CD of the ileal or ileocolic type whose colonic lesions were in a remission status were eligible.
Protocol for the examination with the PC
The PC was ingested either in the morning or just before going to bed. The timing depended on the physician’s choice. The patients were free to have any food before and after the PC ingestion, but were not administered any drug to enhance bowel movement for the passage of the PC. The diagnosis of GI patency is defined as the passage of the PC within 33 h of ingestion. When this was not the case, the PC was searched for either through radiography or computed tomography (CT). Once GI patency was confirmed, CE was scheduled within a week.3)
Analyses
Analysis 1: Diagnoses regarding stenotic lesions were reviewed among the patients who were not found to have GI patency according to the PC test. Analysis 2: The clinical risk factors associated with GI patency were analyzed with age, sex, disease duration, history of surgery, abdominal pain, Crohn’s Disease Activity Index (CDAI), and laboratory data as the variables. Analysis 3: The clinical outcome in terms of the need for hospital admission and surgery was compared between the patients in whom the GI patency was proven using the PC (P group) and those who were diagnosed to have bowel stenosis by using the PC (NP group). This retrospective study was approved by the clinical ethics committee of the Nagoya University Hospital (2015-0485).
Statistical analysis
The factors associated with GI patency evaluated using the PC were analyzed using the Fisher’s exact probability test, Mann-Whitney U test, and Student’s t-test. The relation between GI patency and the clinical prognosis was analyzed with comparison to the P and NP groups using the Kaplan-Meier method. The Statistical Package for the Social Sciences (SPSS) for Windows (IBM Corp., Armonk, NY) was used to analyze the data. In all analyses, a P value of <0.05 was considered statistically significant.
RESULTS
Table 1 shows the demographic data of the patients at the first examination by using the PC. CD activity of this population based on the CDAI was relatively low, although 71 patients (71.7%) had the history of abdominal surgery at the time of examination. Figure 1 shows the examination flowchart. Of all 99 patients who ingested the PC during the study period, GI patency was suggested in 84 patients (84.8%) and was confirmed using CE in 83 patients. In one other patient, the subsequent CE resulted in retention of the CE, and the patient underwent both surgical removal and segmental resection of the small bowel. Of the 15 patients in whom the bowel stenosis was suggested, 12 patients (80%) underwent either balloon-assisted endoscopy (n=10) or enteroclysis (n=2), and 11 were confirmed to have small bowel stenosis which included the luminal dilatation at the oral side of the lesion. The sensitivity, specificity, positive predictive value, and negative predictive value of the PC test for detecting small bowel stenosis were 91.7%, 98.8%, 98.9%, and 91.7%, respectively (Table 2).
Table 1.
Demographic data of the patients at the first examinations
| Male / Female | 68 / 31 |
| Age (mean, years old) | 37.1 ± 13.4 |
| Duration, months (range) | 87 (6–413) |
| Small bowel / small and large bowel type | 54 / 45 |
| History of abdominal surgery | 71 (71.7%) |
| CDAI | 82 (6–375) |
Fig. 1.
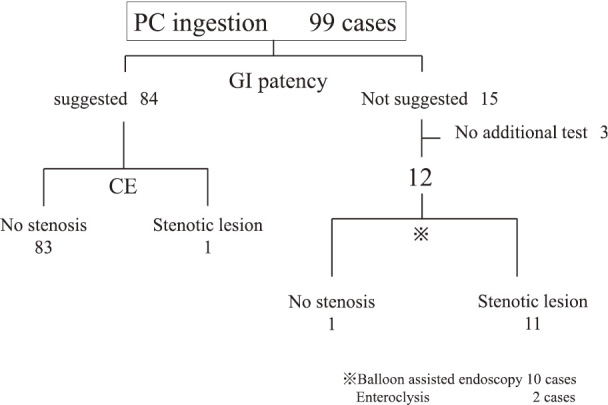
Flowchart for the clinical course according to PC and CE
Table 2.
Accuracy of the evaluation of small bowel stenosis by PC
| Small bowel stenosis on imaging | ||
|---|---|---|
| confirmed | not confirmed | |
| GI patency diagnosed by PC | ||
| Suggested | 1 | 83 |
| Not suggested | 11 | 1 |
PC, Pillcam patency capsule; GI, gastrointestinal.
The univariate analysis identified the age, post endoscopic balloon dilation (EBD) status, and abdominal pain after meal as the important factors associated with the results of the PC examination (Table 3). Multivariate analysis showed that the post-EBD status and abdominal pain after meal were the independent factors associated with the lack of GI patency (Table 4).
Table 3.
Comparisons of clinical backgrounds between patency (P) and non-patency (NP) groups
| Factors | P group | NP group | P value |
|---|---|---|---|
| N | 84 | 15 | |
| Gender (Male/Female) | 52 / 25 | 9 / 6 | 0.547a |
| Age±SD | 35.9±13.5 | 43.8±13.7 | 0.035b |
| Duration, months (range) | 81.5 (0–413) | 170 (0–396) | 0.074b |
| Post surgery, positive / negative | 59 / 25 | 12 / 3 | 0.546a |
| Post Endoscopic balloon dilation,
positive / negative |
2 / 82 | 4 / 11 | 0.005a |
| Abdominal pain after meal,
positive / negative |
9 / 75 | 8 / 7 | 0.0005a |
| CDAI (range) | 79 (6–375) | 95 (9–279) | 0.223b |
| CRP, Median (range) | 0.100 (0.01–3.13) | 0.185 (0.02–1.63) | 0.369b |
| Albumin level, Mean | 4.09±0.53 | 3.79±0.57 | 0.058c |
aFisher’s exact probability test.
bMann-Whitney U test.
cStudent’s t-test.
Table 4.
Factors associated with non-patency status
| Factors | Odds ratio | 95%CI | P value |
|---|---|---|---|
| Age | 0.97 | 0.920–1.01 | 0.164 |
| EBD | 0.15 | 0.037–0.52 | 0.024 |
| Abdominal pain | 0.14 | 0.030–0.78 | 0.004 |
EBD, post endoscopic balloon dilation.
The nonadmission rates of the P and NP groups during the 2-year observation period were 74/84 (88.0%) and 8/15 (53.3%), respectively (P<0.001, Figure 2). The non-operation rates of P and NP groups during the 2-year observation period were 80/84 (95.2%) and 9/15 (60.0%), respectively (P<0.001, Figure 3).
Fig. 2.
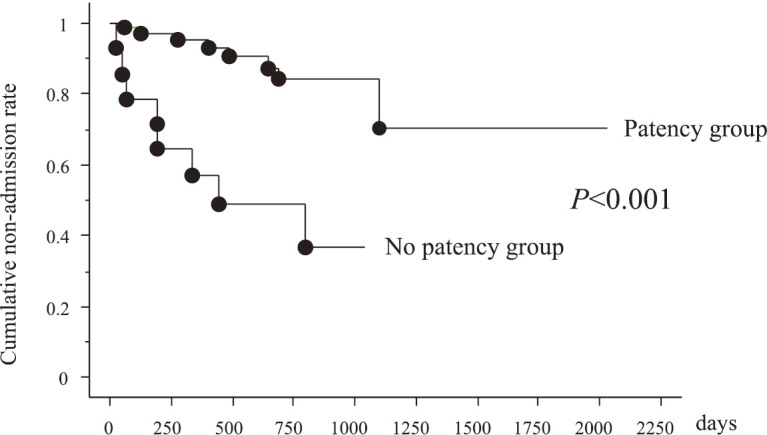
Cumulative non-admission rate between patency and non-patency groups
Fig. 3.
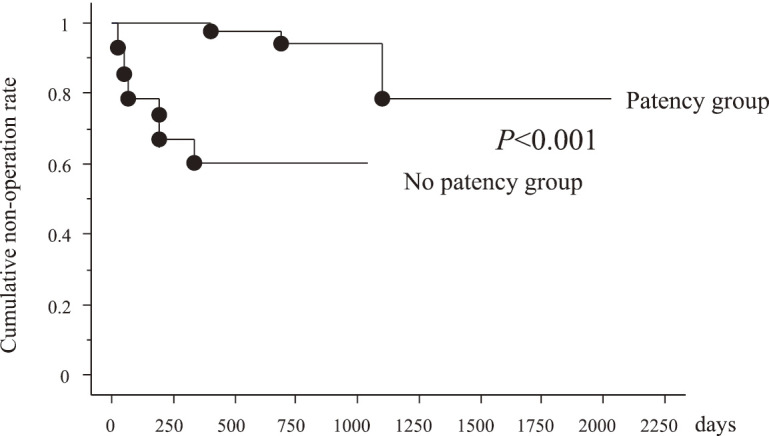
Cumulative non-operation rate between patency and non-patency groups
DISCUSSION
In the current study, the clinical impact of the PC for CD patients with and without GI patency was evaluated. GI patency was suggested in 84 of the 99 patients (84.8%). CE was safely conducted in 83 of the 84 patients, although CE retention was observed in one remaining patient.
The major reasons for the lack of GI patency according to the PC test was either a severe GI stenosis that resulted in dilatation of the lumen in the oral side of the stenosis, or poor intestinal mobility that resulted in a delayed PC passage. In the present study, almost all patients whose GI patency could not be established by the PC test had small bowel stenosis (Table 2). Thus, the lack of GI patency should be considered as the consequence of GI stenosis in case of CD, even if the patient had not developed any symptoms. CT scan or small bowel radiology can miss minor bowel stenosis, especially lower ileal lesion and narrow segmental lesion. MR enterography is considered to be more useful for detecting the small bowel lesions10), but short-segment stenosis could still be undetectable.
Significant risk factors affecting the GI patency with CD patients were analyzed. Abdominal pain after meal and post-EBD status were listed up as the independent factors. Meals would temporarily become burden for the prestenostic lumen till the food passes through the stricture. In such a case, food will be staying there for some time and stimulate the small bowel mucosa as a kind of antigen. The relevance of postprandial abdominal pain as a sign of severe stricture should be kept in mind. The effectiveness of EBD for fibrotic stricture was proved by several original articles, but the effect may be temporary.11-13) If medical treatment post-EBD is not satisfactory, the stricture will be complicated with active ulcerations and local edema, and the stenotic symptoms will recur. Thus, several reasons exist as to why these two factors should be identified as independent significant risk factors for the stenosis.
According to Figures 2 and 3, GI patency affects the clinical prognosis of CD. However, not all patients with CD are eligible for the PC because of the risk of capsule retention due to bowel strictures and the induction of the stenotic symptom.14,15) Alternative examinations by using double-balloon enteroscopy (DBE) and cross-sectional imaging should be performed instead of CE whenever GI patency cannot be confirmed using the PC examination and in case of clinically apparent bowel obstruction. DBE will provide detailed information of the stenotic area, although the procedure has a risk for small bowel bleeding and perforation.16) In addition, DBE has therapeutic ability for EBD alongside having a role in retrieval of the retained capsule endoscope.17,18) Thus, CE and DBE are major and complementary methods for small bowel examination in the 21st century. CE seems to be superior as the first examination, and DBE is useful for detailed examination and endoscopic therapy, but further clinical studies to explore effective diagnostic and therapeutic strategies by using these two modalities to cope for unknown or unsolved small bowel disorders are required. For established CD, CE was the most useful for patients in clinical remission with positive CRP and without stenosis, whereas DBE was useful for patients with symptoms of stenosis16). Once GI patency is confirmed, CE will be a very promising tool in the management of CD, and annual monitoring of CD activity can be accomplished by CE.19,20)
The PC may give false-positive results. Sawada et al. demonstrated that constipation was significantly related to false positivity.21) Patients with CD are not likely to have constipation and excrete the PC earlier than in other types of bowel disease. As shown in Figure 1, of the 12 cases whose GI patency was not suggested by the PC, 11 (91.6%) had some form of strictures in the small bowel. We could predict that the patients without GI patency have some active lesions influencing the clinical prognosis or real stenotic lesion in the small bowel and should have alterations in the treatment strategy.
This study has some limitations due to the inherent nature of a single-institution retrospective study. In addition, not all 15 patients for whom the GI patency was not proven using the PC underwent alternative examination in search of the true nature of the stenosis.
In conclusion, this study demonstrated that GI patency evaluated using the PC has a clinical impact for the management of CD.
ACKNOWLEDGMENT
This work was supported by a JSPS KAKENHI Grant Number 16K09406.
CONFLICTS OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
- 1).Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, et al. Scientific Committee of the European Crohn’s and Colitis Organization. Results from the 2nd Scientific Workshop of the ECCO. I: impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis, 2011; 5: 477–483. [DOI] [PubMed]
- 2).Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol, 2010; 7: 15–29. [DOI] [PubMed]
- 3).Nakamura M, Hirooka Y, Yamamura T, Miyahara R, Watanabe O, Ando T, et al. Clinical usefulness of novel tag-less agile patency capsule prior to capsule endoscopy for patients with suspected small bowel stenosis. Dig Endosc, 2015; 27: 61–66. [DOI] [PubMed]
- 4).Nakano M, Oka S, Tanaka S, Kunihara S, Igawa A, Aoyama T, et al. Clinical usefulness of transabdominal ultrasonography prior to patency capsule for suspected small-bowel strictures. Scand J Gastroenterol, 2016; 51: 281–287. [DOI] [PubMed]
- 5).Shiotani A, Hata J, Manabe N, Imamura H, Ishii M, Fujita M, et al. Clinical relevance of patency capsule combined with abdominal ultrasonography to detect small bowel strictures. Eur J Gastroenterol Hepatol, 2014; 26: 1434–1438. [DOI] [PubMed]
- 6).Omori T, Nakamura S, Shiratori K. Localization of the patency capsule by abdominal tomosynthesis. Digestion, 2015; 91: 318–325. [DOI] [PubMed]
- 7).Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol, 2011; 9: 124–129. [DOI] [PubMed]
- 8).Niv Y. Small-bowel mucosal healing assessment by capsule endoscopy as a predictor of long-term clinical remission in patients with Crohn’s disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol, 2017; 29: 844–848. [DOI] [PubMed]
- 9).Herrerias JM, Leighton JA, Costamagna G, Infantolino A, Eliakim R, Fischer D, et al. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc, 2008; 67: 902–909. [DOI] [PubMed]
- 10).Takenaka K, Ohtsuka K, Kitazume Y, Nagahori M, Fujii T, Saito E, et al. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn’s disease. Gastroenterology, 2014; 147: 334–342. [DOI] [PubMed]
- 11).Hirai F, Beppu T, Takatsu N, Yano Y, Ninomiya K, Ono Y, et al. Long-term outcome of endoscopic balloon dilation for small bowel strictures in patients with Crohn’s disease. Dig Endosc, 2014; 26: 545–551. [DOI] [PubMed]
- 12).Ohmiya N, Arakawa D, Nakamura M, Honda W, Shirai O, Taguchi A, et al. Small-bowel obstruction: diagnostic comparison between double-balloon endoscopy and fluoroscopic enteroclysis, and the outcome of enteroscopic treatment. Gastrointest Endosc, 2009; 69: 84–93. [DOI] [PubMed]
- 13).Sunada K, Shinozaki S, Nagayama M, Yano T, Takezawa T, Ino Y, et al. Long-term outcomes in patients with small intestinal strictures secondary to Crohn’s disease after bouble-balloon endoscopy-assisted balloon dilation. Inflamm Bowel Dis, 2016; 22: 380–386. [DOI] [PubMed]
- 14).Kato S, Osada H, Yakabi K. Rare case of temporary intestinal obstruction induced by novel tag-less Agile patency capsule in a patient with Crohn’s disease. Dig Endosc, 2016; 28: 481. [DOI] [PubMed]
- 15).Saito K, Nakagawa T, Koseki H, Taida T, Sakurai T, Yoshihama S, et al. Retention of the cellophane wall of a patency capsule by intestinal stenosis: a report of three cases. Clin J Gastroenterol, 2016; 9: 365–368. [DOI] [PubMed]
- 16).Nakamura M, Hirooka Y, Watanabe O, Yamamura T, Funasaka K, Ohno E, et al. A retrospective evaluation of the utility of capsule endoscopy and double-balloon endoscopy in Crohn’s disease. Gastroenterol Res Pract, 2016; 2016: 1085027. [DOI] [PMC free article] [PubMed]
- 17).Nakamura M, Hirooka Y, Watanabe O, Yamamura T, Nagura A, Yoshimura T, et al. Minimally invasive extraction of a foreign body from the small intestine using double-balloon endoscopy. Nagoya J Med Sci, 2015; 77: 189–194. [PMC free article] [PubMed]
- 18).Mitsui K, Fujimori S, Tanaka S, Ehara A, Omori J, Akimoto N, et al. Retrieval of retained capsule endoscopy at small bowel stricture by double-balloon endoscopy significantly decreases surgical treatment. J Clin Gastroenterol, 2016; 50: 141–146. [DOI] [PubMed]
- 19).Papay P, Ignjatovic A, Karmiris K, Amarante H, Milheller P, Feagan B, et al. Optimising monitoring in the management of Crohn’s disease: a physician’s perspective. J Crohns Colitis, 2013; 7: 653–669. [DOI] [PubMed]
- 20).Kopylov U, Ben-Horin S, Seidman EG, Eliakim R. Video capsule endoscopy of the small bowel for monitoring of Crohn’s disease. Inflamm Bowel Dis, 2015; 21: 2726–2735. [DOI] [PubMed]
- 21).Sawada T, Nakamura M, Watanabe O, Yamamura T, Ishikawa T, Furukawa K, et al. Clinical factors related to false positive rates of patency capsule examination. Therap Adv Gastroenterol, 2017; 10: 589–598. [DOI] [PMC free article] [PubMed]


