ABSTRACT
A 66-year-old male with advanced non-small-cell lung cancer (NSCLC) who was previously treated with carboplatin, pemetrexed, and bevacizumab consequently suffered from severe coughing during deglutition. Chest computed tomography (CT) revealed a tracheoesophageal fistula (TEF) between the left main bronchus and esophagus through a subcarinal metastatic lymph node. Given the extreme swelling of the lymph node due to metastatic cancer, it was determined that the walls of the bronchus and esophagus had been injured simultaneously. Delayed and dysfunctional wound healing due to bevacizumab resulted in necrosis of the contact region leading to fistula formation. This case suggests that using bevacizumab for NSCLC in patients with bulky subcarinal lymphadenopathy may increase the risk for TEF.
Key Words: bevacizumab, non-small-cell lung cancer, tracheoesophageal fistula
INTRODUCTION
A tracheoesophageal fistula (TEF) is a rare disorder that is either congenital or acquired. Acquired TEF due to esophageal or lung cancer, otherwise known as malignant TEF, is a life-threatening complication. The incidence of malignant TEF has been reported to be 0.16% to 0.3% for lung cancer and 4.9% for esophageal cancer1,2), with a median survival time (MST) from diagnosis of only 6 weeks in the absence of appropriate treatment1). Even after appropriate treatment, a MST of 2.8 months had been reported in a study of 264 patients3). Recently, a number of reports have been published regarding TEF and its relation to treatable radiation therapy (TRT) and bevacizumab4-6), though little is available on the causes of TEF without TRT in lung cancer.
Herein, we describe a case of TEF in a patient with non-squamous non-small-cell lung cancer (NSCLC) treated with bevacizumab without TRT.
CASE REPORT
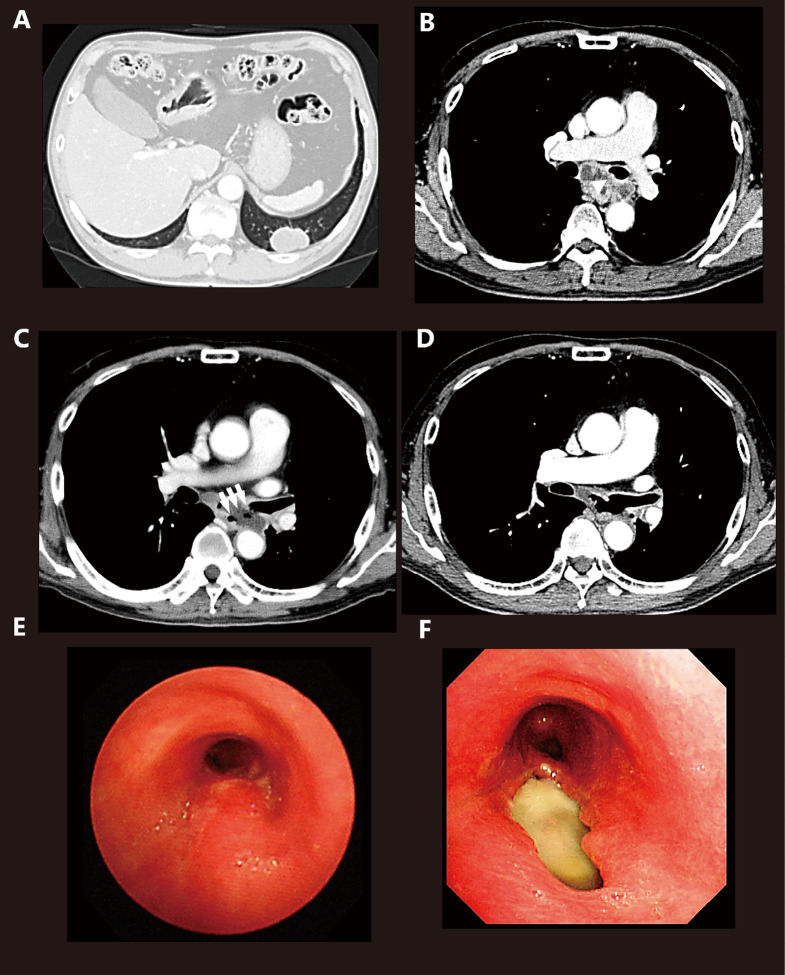
A 66-year-old male complaining of cough and back pain visited our clinic. He had a smoking history of 20 cigarettes per day for 40 years and no history of hemosputum, hemoptysis, and gastrointestinal disease. Although no significant abnormalities were found upon physical examination and blood work, a chest X-ray revealed a mass in the left lower lung. A chest CT confirmed the presence of a tumor in the left S9 and subcarinal lymphadenopathy (Fig. 1A, B). Bronchoscopy revealed an elevated membranous portion in the left main bronchus and no dilation of blood vessels. Transbronchial biopsy of the primary region was subsequently performed, after which a pathologic diagnosis of non-squamous NSCLC was established. The patient was negative for epidermal growth factor receptor mutation and echinoderm microtubule-associated protein-like 4 ALK. Systemic screening using fluourodeoxyglucose positron emission tomography and magnetic resonance imaging revealed stage IV disease (cT2aN3M1b, OSS). Bone metastasis was identified at the 10th and 12th thoracic vertebra, which prompted the initiation of palliative radiotherapy (30 Gy in 10 fractions). In addition, systemic chemotherapy with carboplatin (AUC5), pemetrexed (500 mg/m2), and bevacizumab (15 mg/kg) was administered one day after the start of palliative radiation therapy. However, 11 days after chemotherapy initiation, the patient complained of severe cough and fever, as well as the inability to swallow tablets without regurgitating them. Chest CT indicated aspiration pneumonia and a fistula between the left main bronchus and the middle part of the esophagus (Fig. 1C). Chemotherapy appreciably decreased the primary region from 40.4 mm to 33.8 mm and remarkably diminished subcarinal lymphadenopathy (Fig. 1D). The patient received no food by mouth and was placed on antibiotic therapy. Upper gastrointestinal endoscopy and bronchoscopy confirmed the presence of a TEF (Common Terminology Criteria for Adverse Events Grade 4) with a major and minor axis of 2.5 and 1.0 cm under direct visualization, respectively. After the placement of a covered esophageal stent (Niti-S stent, 100 mm), the patient’s symptoms improved. However, 3 months thereafter, the patient died due to primary disease.
Fig. 1.
Computed tomography (CT) and bronchoscopy
(A) Chest CT showing the primary region in the left S9.
(B) Chest CT showing the subcarinal lymph node compressing the esophagus before treatment and the subcarinal lymph node exerting pressure on the left main bronchus (arrow head).
(C) A tracheoesophageal fistula was observed between the left main bronchus and esophagus through the subcarinal lymph node (arrows).
(D) Chest CT performed 2 weeks after diagnosis of TEF showing a remarkably diminished subcarinal lymph node enlargement after chemotherapy.
(E) An elevated membranous portion of the left main bronchus observed by bronchoscopy before treatment.
(F) The fistula (2.5 cm × 1.0 cm) was observed in the left main bronchus.
DISCUSSION
The present case provides important clinical perspectives on the relationship between subcarinal lymphadenopathy and TEF formation. We tabulated previously reported cases that showed an associated between TEF and bevacizumab (Table 1)4-8). The identified cases showed that bevacizumab led to TEF formation when administered in the presence of additional risk factors. TRT had been suggested to be the most significant risk factor for bevacizumab-related TEF. Moreover, out of eight cases determined to have received TRT with bevacizumab, two cases developed TEF within the duration of bevacizumab administration after TRT. Meanwhile, six cases developed TEF 3 months or more after radiation therapy. In addition, disorders of the esophageal mucosa, such as esophagitis and esophagus dilatation, were observed in five cases. Damage to the esophagus was also determined as a risk factor for TEF. In contrast, Schreiber et al. reported that TEF occurred after chemotherapy, which included bevacizumab without TRT7). The aforementioned case involved an intratracheal tumor and a wall that was presumably destroyed before treatment. This suggested that the tracheal tumor directly injured the esophagus and resulted in TEF development without TRT. In the present case, the patient did not receive TRT, and the therapeutic radiologist confirmed that the site of the TEF was not within the irradiation field of palliative radiation therapy. Moreover, bronchoscopy revealed that the tumor was not on the surface of the bronchus, indicating that no intratracheal invasion had taken place. The patient had no symptoms of esophagitis until TEF development. The enlarged metastatic subcarinal lymph node exerted pressure on the bronchus and esophageal walls, which resulted in partial injury. CT and bronchoscopy showed an upward displacement of the mucosal wall in the left main bronchus before treatment, while CT showed remarkably diminished subcarinal lymph node enlargement after chemotherapy. The walls that may have been invaded by the tumor had rapidly necrotized, leading to their collapse and subsequent fistula formation.
Table 1.
Previously reported cases on the association between TEF and bevacizumab
| Article | Age,
Sex |
Cancer type | Treatment | Risk factors of Bev related TEF | Development TEF | Outcome |
|---|---|---|---|---|---|---|
| Goodgame 2008 | 28,
M |
NSCLC | 1st line:
CDDP+ETP+TRT 2nd line: PTX+CBDCA+Bev → Bev |
TRT | During second line | NA |
| Gore 2009 | 48,
M |
NSCLC | 1st line: PTX+CBDCA+TRT 2nd line: GEM+CBDCA+Bev → Bev |
TRT | 21 months after radiation therapy | NA |
| Spigel 2010 | 62,
F |
NSCLC | PEM+CBDCA+Bev+TRT | Esophageal ulcers/Esophageal dilation for esophageal stricture/TRT | 34 weeks after therapy | More than 10 months alive |
| 55,
M |
NSCLC | PEM+CBDCA+Bev+TRT | Esophageal esophagitis, ulcers/Esophageal dilation for esophageal stricture/TRT | 40 weeks after therapy | More than 10 months alive | |
| 57,
F |
SCLC | CPT-11+CBDCA+Bev+TRT | Esophagitis during chemotherapy/Esophageal dilation for esophageal stricture/TRT | 5 months after Bev maintenance initiation | NA | |
| 57,
F |
SCLC | CPT-11+CBDCA+Bev+TRT | Esophagitis during chemotherapy/TRT | 3 months after chemoradiation therapy | Dead due to TEF | |
| 67,
M |
SCLC | CPT-11+CBDCA+Bev+TRT | Barret’s esophagus/
Esophagitis/TRT |
6 weeks after chemo therapy | NA | |
| Socinski 2012 | NA | NSCLC | PTX+CBDCA+Bev→ PTX+Bev+TRT | TRT | 3.5 month after combined modality therapy | NA |
| Schereiber 2012 | 40, M |
NSCLC | PTX+CBDCA+Bev | Right main stem bronchus tumor/Barrett mucosa | 3 weeks after first cycle | Dead due to hemoptysis |
NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; TEF, tracheoesophageal fistula; TRT, thoracic radiation therapy; CDDP, Cisplatin; CBDCA, Carboplatin; ETP, Etoposide; PTX, Paclitaxel; Bev, Bevacizumab; CPT-11, Irinotecan; PEM, Pemetrexed; GEM, Gemcitabine; NA, No available
During the treatment of the current patient, no data was available on the association between TEF and bevacizumab, except during chemoradiation. We speculate that the large subcarinal lymph node was one of the risk factors for TEF formation. The concurrent destruction of both bronchial and adjacent esophageal walls is the essential element in the development of a TEF, for example, simultaneous injury to both walls due to TRT. The pressure exerted by the subcarinal lymph node on walls of the bronchus and esophagus was able to damage both walls simultaneously, leading to the fistula formation.
A few reports have described cases of TEF through a subcarinal lymph node9,10). Takeuchi et al. reported a case of TEF treated by concurrent chemoradiotherapy without bevacizumab in which bulky subcarinal lymphadenopathy was diagnosed after chest CT10). The fistula was identified through the subcarinal lymph node but did not directly connect the bronchus to the esophagus. Nishinari et al. reported a case in which bevacizumab administration to a patient with metastatic tumor of the hilar lymph node resulted in bronchopleural fistula and empyema. They suggested that the metastatic tumor’s contact with the trachea and thoracic cavity contributed to fistula formation. The importance of tumor location was also identified as a risk factor for fistula formation11).
This is the first case to provide perspective on the role of subcarinal lymphadenopathy on TEF formation. Subcarinal lymphadenopathy was suggested to have contributed to TEF formation. Moreover, the pressure exerted by the lymphadenopathy on the structurally vulnerable membranous portion of the bronchus could have resulted in injury to the wall. The high incidence of subcarinal lymph node metastasis among lung cancer patients increases the importance of this risk factor in clinical practice. We believe that the presented risk factor for TEF, i.e., mediastinal lymph node metastasis, is clearly different from the risk for hemoptysis due to large vessel invasion or tumor outcrops in the trachea. We may be able to determine the risk of wall invasion using several diagnostic techniques, such as endobronchial ultrasound, for physicians who plan on using bevacizumab in cases where the tumor or metastatic lymph node compresses the airway. Further studies are necessary to clarify the cause of TEF formation. In conclusion, the present case suggests that using bevacizumab in patients with large subcarinal lymph nodes increases the risk for TEF formation.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGMENT
The authors would like to thank Dr. Yuden Droma for her valuable advice and for kindly checking the English language usage in our manuscript.
REFERENCE
- 1). Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am, 2003; 13: 271–89. [DOI] [PubMed]
- 2). Martini N, Goodner JT, D’Angio GJ, Beattie EJ, Jr. Tracheoesophageal fistula due to cancer. J Thorac Cardiovasc Surg, 1970; 59: 319–24. [PubMed]
- 3). Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin. Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg, 2008; 34: 1103–7. [DOI] [PubMed]
- 4). Spigel DR, Hainsworth JD, Yardley DA, Raefsky E, Patton J, Peacock N, Farley C, Burris HA, 3rd, Greco FA. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol, 2010; 28: 43–8. [DOI] [PubMed]
- 5). Goodgame B, Veeramachaneni N, Patterson A, Govindan R. Tracheo-esophageal fistula with bevacizumab after mediastinal radiation. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer, 2008; 3: 1080–1. [DOI] [PubMed]
- 6). Gore E, Currey A, Choong N. Tracheoesophageal fistula associated with bevacizumab 21 months after completion of radiation therapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer, 2009; 4: 1590–1. [DOI] [PubMed]
- 7). Schreiber J, Waldburg N. Bronchoesophageal fistula and fatal hemoptysis after bevacizumab-containing chemotherapy without radiation in lung cancer. J Clin Oncol, 2012; 30: e324. [DOI] [PubMed]
- 8). Socinski MA, Stinchcombe TE, Moore DT, Gettinger SN, Decker RH, Petty WJ, Blackstock AW, Schwartz G, Lankford S, Khandani A, Morris DE. Incorporating bevacizumab and erlotinib in the combined-modality treatment of stage III non-small-cell lung cancer: results of a phase I/II trial. J Clin Oncol, 2012; 30: 3953–9. [DOI] [PubMed]
- 9). N Shirone, T Tamamoto, H Yoshimura. A case of esophago-bronchial fistula in patient with advanced lung cancer during chemoradiotherap. Jpn J Cancer Clin, 2002; 48: 419–423.
- 10). Takeuchi. H, Yonezawa. K, Kawaguchi. Y, Tokuyama. J, Osumi. K, Kishi. S, Wada. N, Hojo. T, Kim. S, Shimada. A, Oishi. T, Isobe. Y, Ikeuchi. S, Kubochi. K, Matsumoto. S. Self-expandable metallic stent for bronchoesophageal fistula due to lung cancer : report of a case. Progress of Digestive Endoscopy, 2006; 68: 90–91.
- 11). Nishinari Y, Kashiwaba M, Umemura A, Komatsu H, Sasaki A, Wakabayashi G. Pulmonary hilar lymph node metastasis of breast cancer induced bronchopleural fistula and superior vena cava syndrome. Am J Case Rep, 2014; 15: 492–5. [DOI] [PMC free article] [PubMed]