Abstract
The present study was aimed to investigate the protective effect of Pterocarpus marsupium bark extracts against cataract in streptozotocin-induced diabetic male albino rats. Aldose reductase is a key enzyme in the intracellular polyol pathway, which plays a major role in the development of diabetic cataract. Rats were divided into five groups as normal control, diabetic control, and diabetic control treated with different concentrations of Pterocarpus marsupium bark extracts. Presence of major constituents in Pterocarpus marsupium bark extract was performed by qualitative analysis. Body weight changes, blood glucose, blood insulin, and reduced glutathione (GSH) and aldose reductase mRNA and protein expression were determined. Rat body weight gain was noted following treatment with bark extracts. The blood glucose was reduced up to 36% following treatment with bark extracts. The blood insulin and tissue GSH contents were substantially increased more than 100% in diabetic rats following treatment with extracts. Aldose reductase activity was reduced up to 79.3% in diabetic rats following treatment with extracts. Vmax, Km, and Ki of aldose reductase were reduced in the lens tissue homogenate compared to the diabetic control. Aldose reductase mRNA and protein expression were reduced more than 50% following treatment with extracts. Treatment with Pterocarpus marsupium bark was able to normalize these levels. Taking all these data together, it is concluded that the use of Pterocarpus marsupium bark extracts could be the potential therapeutic approach for the reduction of aldose reductase against diabetic cataract.
Keywords: Diabetic cataract, Aldose reductase, Pterocarpus marsupium, Blood insulin, Glucose
Introduction
Diabetes is a well-known metabolic disorder affecting millions of people worldwide due to defective insulin secretion or insulin resistance (Muthuraman and Srikumar 2009; Muthuraman et al. 2009; Muthuraman and Kim 2016). Thylefors (1990) has reported that the diabetic cataract is the main reason for most of the blind population. Klein et al. (1992) have reported that the diabetic cataract occurs in younger age populations in developing countries. Aldose reductase is a well-known enzyme in the intracellular polyol pathway, which plays a vital role in the development of cataract. Aldose reductase plays a very important in role in hyperglycemia than in normoglycemia (Gabbay 1973; Ghahary et al. 1989). Aldose reductase converts glucose into sorbitol which accumulates in the lens of the eye and further leads to swelling of tissues due to increased osmotic pressure (Corso et al. 2008). Hence, the inhibition of aldose reductase in lens epithelial tissues is the possible therapeutic approach for diabetic cataract. Sorbinil, fidarestat, zenarestat, epalrestat, torestat, and ranirestat are well-known inhibitors of aldose reductase (Zhu 2013). Even though several aldose reductase inhibitors are identified, there are a lot of unacceptable adverse effects and low pharmacokinetics observed (Schemmel et al. 2010). Therefore, development of potent aldose reductase inhibitors with little side effects is mandatory for the prevention and management of diabetic cataract.
The identification of natural products for the treatment of diabetic cataract is highly required (Hung et al. 2012; Narayana and Dobriyal 2000). Grover et al. (2002) have reported that the antidiabetic potential of Pterocarpus marsupium. It is a deciduous tree called as Malabar kino or vijayasar which grows up to 30 m. The plant Pterocarpus marsupium is widely abundant in India, Sri Lanka, and Nepal (Hariharan et al. 2005). Warrier (1995) has reported that the Pterocarpus marsupium bark has been used for the treatment of toothache and diarrhea, and utilized as anti-diarrheal and astringent. Therefore, the present study investigates the effect of Pterocarpus marsupium bark extracts on the inhibition of aldose reductase in diabetic rats.
Materials and methods
Experimental animals
Male Wistar rats (170–200 g) were purchased from the animal house of The Second Affiliated Hospital of Da Lian Medical University. Rats were allowed to free access to food and water, with standard relative humidity (60 ± 5%) and room temperature was maintained. Conventional photoperiod of 12 h/day was kept in rat polypropylene cages. Animal experiments were approved by the ethical committee of The Second Affiliated Hospital of Da Lian Medical University. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Preparation of Pterocarpus marsupium bark extracts
The plant Pterocarpus marsupium bark (500 g) was collected from Theerthalli region (Karnataka, India). The bark was finely ground into a fine powder with use of an electric grinder and dissolved in water, and continuously stirred for 12 h. The prepared concentrated extract was lyophilized to yield a fine powder of 50 g/kg of bark paste (Vats et al. 2004), and preserved at 4 °C for further experiments. Qualitative analysis of Pterocarpus marsupium bark extract was performed to identify the presence of key constituents (Harborne 1998).
Experimental diabetes induction and treatment
Experimental diabetes was induced by the intraperitoneal administration of streptozotocin (60 mg/kg of body weight, 0.1 M citrate buffer, pH 4.5), and serum glucose level was increased threefold compared to the normal level at the end of the 15th day (Muthuraman et al. 2009). Extracts were dissolved in carboxymethyl cellulose (1%), and rats were administered extracts with use of an oral gauge. The experimental groups were designated as follows. Group I normal rats were received carboxy-methyl cellulose and served as normal control. Group II diabetic rats were received carboxy-methyl cellulose and served as diabetic control, whereas group III–V received extracts 2, 4 and 8 g/kg of body weight, respectively. The experimental treatment was continued for 60 consecutive days.
Preparation of lens tissue homogenate
At the end of the 60 days, eyeballs were collected from rats immediately the following sacrifice by cervical dislocation. The surgically removed lenses were adequately washed, and weight was recorded. Lens tissue homogenate (10%) was prepared in phosphate buffered saline (PBS) and centrifuged at 10,000g for 5 min. The supernatant was taken for further experiments (Kumar et al. 2011).
Determination of body weight changes, serum glucose, and plasma insulin content
The body weight changes in the rat were recorded at all time periods. Serum blood glucose was determined by glucose oxidase method. Glucose oxidase converts glucose into gluconic acid H2O2. The resultant pink colored product was measured at 540 nm (Wang et al. 2010). Plasma insulin content was measured by radioimmunoassay method (RIA, Sigma-Aldrich) (Koksal 2015).
Determination of protein and glutathione level
The proteins in the lens tissue homogenate react with Folin–Ciocalteau reagent and form a blue colored complex. This color formation was due to a reaction between aromatic amino acids present in the protein and of the alkaline copper, and due to the reduction of phosphomolybdate in the reaction mixture by tyrosine and tryptophan present in the protein. The resultant colored product was measured at 680 nm (Lowry et al. 1951). Reduced glutathione (GSH) in the lens tissue homogenate was determined according to Ellman’s reaction. The 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) reacts with the sulfhydryl group of GSH. The resultant absorbance was measured at 412 nm (Schwartz and Durham 1979; Lou and Dickerson 1992).
Determination of aldose reductase activity and kinetics
Aldose reductase activity was measured in the homogenate according to Patel and Mishra (2009). Quercetin was used as a standard for the calculation of the percentage of enzyme inhibition. Also, the kinetic properties of aldose reductase were determined. The Cheng–Prusoff equation was used to determine Vmax, Km, and Ki (Daniellou et al. 2006).
RT-PCR
Total RNA was isolated from lens homogenate, and RNA was transcribed into cDNA using oligo (dt) primers. Aldose reductase mRNA expression was quantified by qPCR with primer specific (forward: 5′-GGACCTCTACCTTATTCACTG-3′, reverse: 5′-TTGGCCCAGGGCCTGTCAG-3′). GAPDH (forward: 5′-GGTCACCAGGGCTGCTTTT-3′, reverse: 5′-ATCTCGCTCCTGGAAGATGGT-3′) was used as housekeeping gene. The 2−∆∆CT method was used to calculated relative ratios of aldose reductase mRNA expression (Masatoshi et al. 2001).
Western blot analysis
Proteins in the lens tissue homogenate were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to membranes. Membranes were probed with an aldose reductase primary antibody (MABS18, Sigma-Aldrich) for overnight. The horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG was incubated with the membrane for 1 h. Aldose reductase protein expression was determined using enhanced chemiluminescence (ECL) method (Massfelder et al. 2004).
Immunohistochemistry
Dissected lens sections were fixed in formalin and paraffin embedded. Then, sections were deparaffinized, rehydrated with xylene and graded alcohol. Endogenous peroxide activity was inhibited by 0.3% H2O2. Then, sections were incubated with 2% bovine serum albumin (BSA) for 45 min to block non-specific binding sites. Sections were incubated with the aldose reductase primary antibody (1:300 in 5% goat serum, MABS18, Sigma-Aldrich) for overnight at 4 °C. Then, the section was incubated with FITC conjugated secondary antibody (1:300) for 1 h (Balic et al. 2011). Relative staining intensity was measured in control and treated lens sections.
Statistical analysis
Values are given as mean with standard error of the mean (SEM). The difference between treated and control group was analyzed using analysis of variance (ANOVA) and Student’s t test. Statistically, significance was considered when P < 0.05.
Results
The protective effect of Pterocarpus marsupium bark extracts against cataract is through the inhibition of aldose reductase activity in streptozotocin-induced diabetic male albino rats. The presence of phenol, flavones, flavonoid, tannin, terpenoids, alkaloids and cardiac glycoside was identified in the phytoscreening analysis of bark extracts (Table 1). Supplementation of extracts improved rat body weight gain than control. The body weight gain was 22.3, 40.5 and 50% in group III, IV and V, respectively (Table 2, P < 0.05). Supplementation of bark extracts reduced blood glucose 12.1, 19.7 and 36% in group III, IV and V, respectively (Table 2, P < 0.05). Blood insulin content was increased 38.8, 60.8 and 125.5% in group III, IV and V, respectively (Table 2, P < 0.05).
Table 1.
Qualitative phytochemical analysis of Pterocarpus marsupium bark extracts
| S. no. | Compounds | Inference |
|---|---|---|
| 1 | Phenol | + |
| 2 | Flavones | + |
| 3 | Flavonoid | + |
| 4 | Tannin | + |
| 5 | Terpenoids | + |
| 6 | Alkaloids | + |
| 7 | Cardiac glycoside | + |
Table 2.
Effect of Pterocarpus marsupium bark extracts on body weight, blood glucose and insulin
| Experimental groups | Body weight (g) | Blood glucose (mmol/L) | Blood insulin (microunit/mL) | |||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | |
| Group I | 178 ± 3.8 | 310 ± 7.1 | 5.4 ± 0.3 | 5.2 ± 0.4 | 15.4 ± 0.4 | 15.9 ± 0.3 |
| Group II | 172 ± 4.1 | 209 ± 3.2* | 22.6 ± 1.1* | 24.8 ± 1.1* | 4.8 ± 0.2* | 4.4 ± 0.2* |
| Group III | 184 ± 5.1 | 225 ± 4.9a | 23.1 ± 1.2 | 20.3 ± 0.9a | 4.5 ± 0.2 | 6.8 ± 0.3a |
| Group IV | 180 ± 3.8 | 253 ± 5.4a | 23.3 ± 1.0 | 18.7 ± 1.2a | 5.1 ± 0.2 | 8.2 ± 0.3a |
| Group V | 178 ± 4.3 | 267 ± 6.2a | 22.5 ± 1.2 | 14.4 ± 1.1a | 4.7 ± 0.1 | 10.6 ± 0.3a |
*P < 0.5 (group I vs group II) and aP < 0.05 (group II vs group III, IV and V)
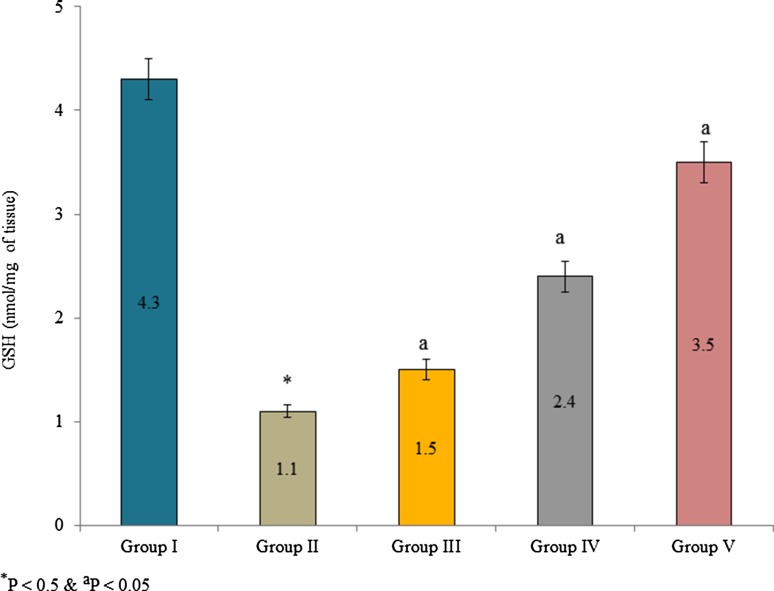
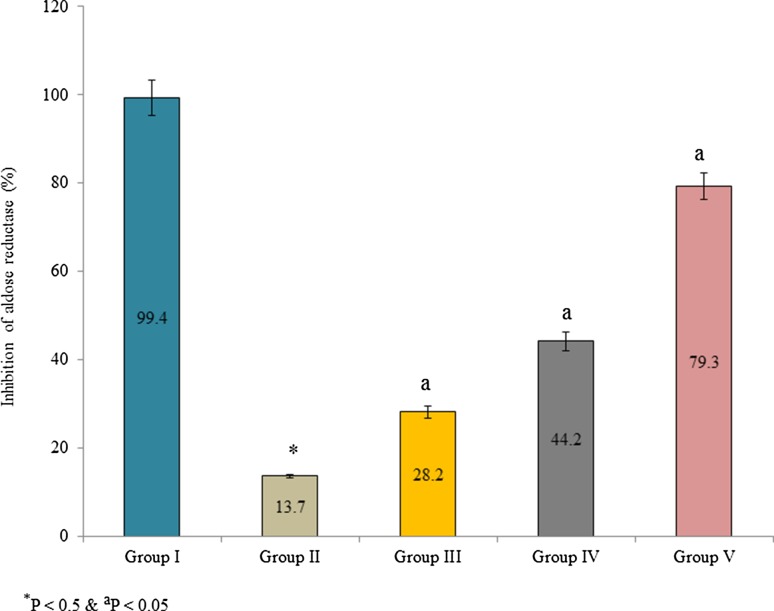
Supplementation of Pterocarpus marsupium bark extracts significantly improved GSH content in the lens tissue than control. GSH content was substantially increased 36.4, 118.2 and 218.1% in the group III, IV and V, respectively (Fig. 1, P < 0.05). Aldose reductase activity was expressed as a percentage of inhibition. Supplementation of bark extracts inhibited aldose reductase than control. The percentage of enzyme inhibition was 99.4, 13.7, 28.2, 44.2 and 79.3% in group I, II, III, IV, and V, respectively (Fig. 2, P < 0.05). Kinetic properties of aldose reductase such as Vmax, Km, and Ki were also determined. Vmax, Km, and Ki of aldose reductase were substantially reduced in the lens tissue homogenate than control. The Vmax of aldose reductase was 0.25612, 0.22832 and 0.16736 in the group III, IV and V, respectively (Table 3, P < 0.05). The Km of aldose reductase was 2.88451, 1.94238 and 0.94523 × 10−3 mM in the group III, IV and V, respectively (Table 3, P < 0.05). The Ki of aldose reductase was 1.73941, 1.16248 and 0.84521 in the group III, IV and V, respectively (Table 3, P < 0.05).
Fig. 1.
Effect of Pterocarpus marsupium bark extracts on GSH content in lens tissue homogenate of male albino rats. Values are expressed as nmol/mg of tissue. *P < 0.5 (group I vs group II) and aP < 0.05 (group II vs group III, IV and V)
Fig. 2.
Effect of Pterocarpus marsupium bark extracts on aldose reductase enzyme activity in lens tissue homogenate of male albino rats. Values are expressed as a percentage of inhibition. *P < 0.5 (group I vs group II) and aP < 0.05 (group II vs group III, IV and V)
Table 3.
Effect of Pterocarpus marsupium bark extracts on kinetic parameters of aldose reductase
| Experimental groups | V max | Km × 10−3 mM | K i |
|---|---|---|---|
| Group I | 0.15274 ± 0.0002 | 0.57243 ± 0.0031 | 0.00000 ± 0.0000 |
| Group II | 0.41841 ± 0.0004* | 3.15352 ± 0.0241* | 2.14322 ± 0.0217* |
| Group III | 0.25612 ± 0.0010a | 2.88451 ± 0.0315a | 1.73941 ± 0.0152a |
| Group IV | 0.22832 ± 0.0010a | 1.94238 ± 0.0411a | 1.16248 ± 0.0214a |
| Group V | 0.16736 ± 0.0040a | 0.94523 ± 0.0013a | 0.84521 ± 0.0032a |
*P < 0.5 (group I vs group II) and aP < 0.05 (group II vs group III, IV and V)
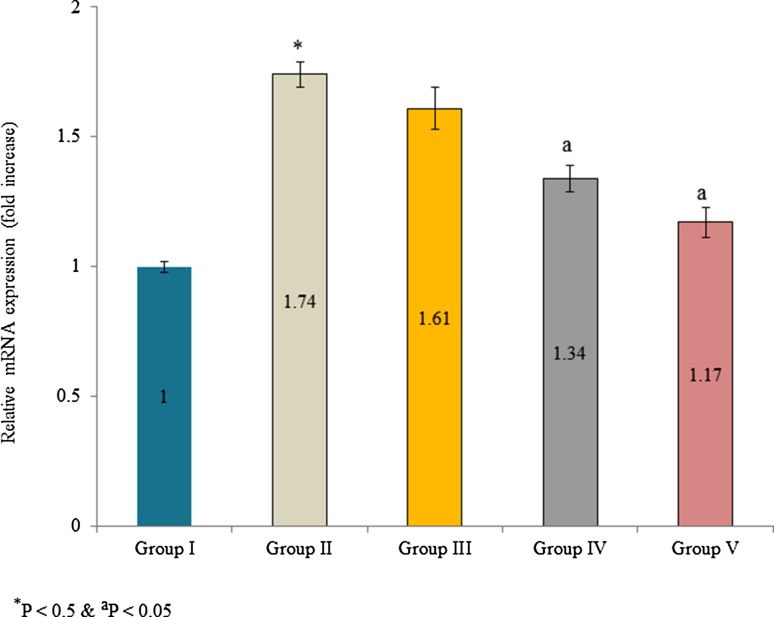
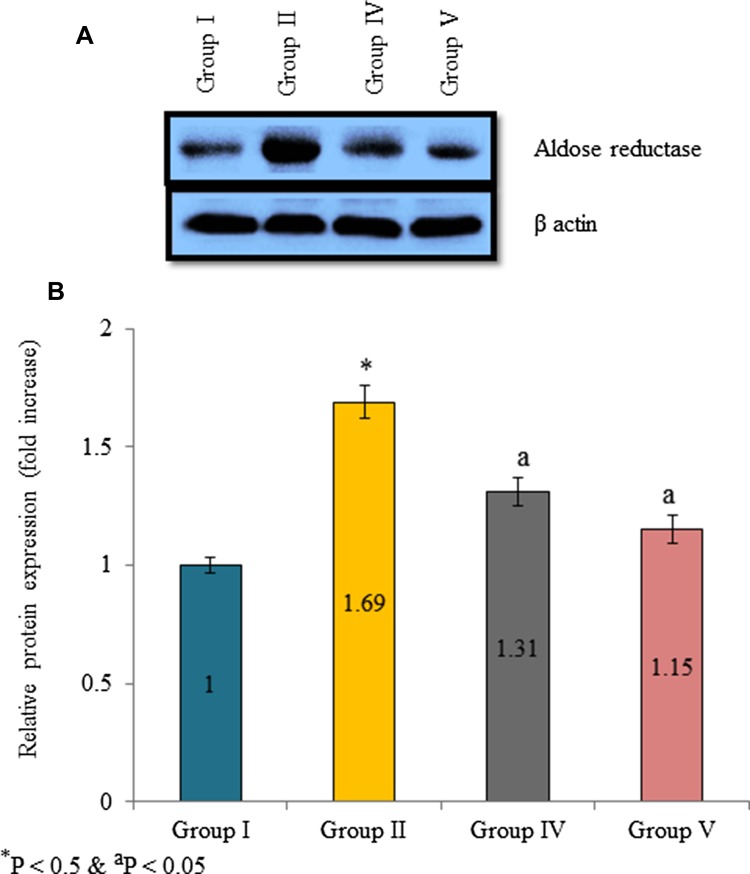
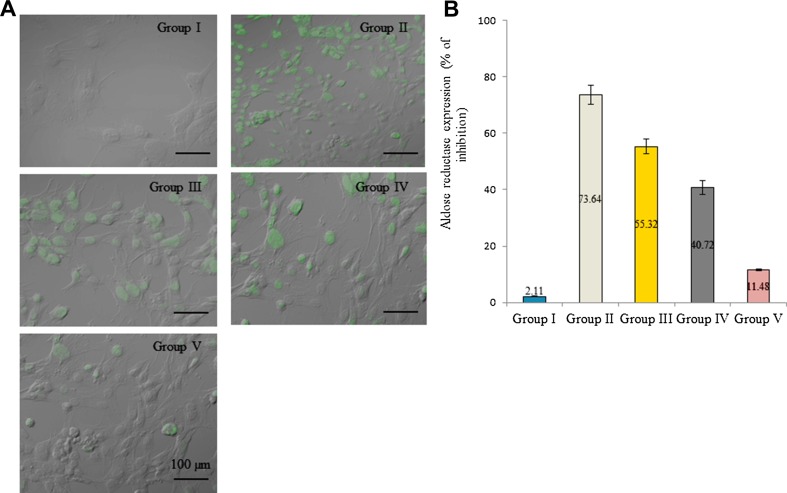
Aldose reductase mRNA expression was increased in diabetic rats. The mRNA expression was increased 0.74-fold in diabetic control than normal control. However, supplementation of extracts significantly reduced aldose reductase mRNA expression. The mRNA expression was reduced 17.6, 54.1 and 77% in the group III, IV and V, respectively (Fig. 3, P < 0.05). Protein expression was determined by Western blot analysis. Aldose reductase protein expression was increased 0.69-fold in diabetic control. Extracts supplementation substantially reduced aldose reductase protein expression in the lens tissue homogenate. The protein expression was reduced 55.1 and 74.3% in the group IV and V, respectively (Fig. 4b, P < 0.05). Further, immunofluorescence analysis was used investigate aldose reductase expression and expressed as a percentage of inhibition. Aldose reductase inhibition was 2.11, 73.64, 55.32, 40.72 and 11.48% in group I, II, III, IV, and V, respectively (Fig. 5b, P < 0.05).
Fig. 3.
Effect of Pterocarpus marsupium bark extracts on aldose reductase mRNA expression in lens tissue homogenate of male albino rats. Values are expressed as fold changes. *P < 0.5 (group I vs group II) and aP < 0.05 (group II vs group III, IV and V)
Fig. 4.
Effect of Pterocarpus marsupium bark extracts on aldose reductase protein expression in lens tissue homogenate of male albino rats. a Representative Western blot band images of aldose reductase. b Densitometry analysis of aldose reductase Western blot bands with reference to control. *P < 0.5 (group I vs group II) and aP < 0.05 (group II vs group III, IV and V)
Fig. 5.
Effect of Pterocarpus marsupium bark extracts on aldose reductase expression in lens tissue homogenate of male albino rats. a Representative immunofluorescent images of aldose reductase. b Percentage of aldose reductase enzyme expression with reference to control. *P < 0.5 (group I vs group II) and aP < 0.05 (group II vs group III, IV and V)
Discussion
The present study investigated the protective effect of Pterocarpus marsupium bark extracts against cataract through the inhibition of aldose reductase activity in streptozotocin-induced diabetic male albino rats. Researchers have reported that the diabetes mellitus and cataract have a positive relationship, and diabetic cataract is the primary cause of blindness. Lens removal from the eye is only approved surgical treatment for diabetic cataract. Srivastava et al. (1988) have reported that oral antidiabetic agents and insulin are regular treatments for diabetes mellitus. Secondary complications of diabetes mellitus are still uncontrollable. Therefore, identification of novel antidiabetic agent which control the occurrence of secondary complications is vital.
Aldose reductase is an essential key enzyme which converts aldose into polyol in polyol pathway. Aldose reductase was increased in order to convert excess of glucose into sorbitol under diabetic condition (Jung et al. 2009). Several researchers have reported that the aldose reductase activity act as a central biochemical link in the pathogenesis of several diabetic complications such as diabetic cataract. Retinopathy, neuropathy, corneal epitheliopathy and microangiopathy are well-known complications of diabetes mellitus. These complications have been prevented through the inhibition of aldose reductase (Reddy et al. 2008; Scotti et al. 2011). Our present study on the Pterocarpus marsupium bark extracts showed a significant reduction in aldose reductase activity in the lens of diabetic rats.
Qualitative phytoscreening analysis of Pterocarpus marsupium bark extracts showed the presence of phenols, flavonoids, and tannin (Harborne 1998). Polyphenols, flavonoids, and sugar derivatives are regarded as effective inhibitors of aldose reductase (Lee 2002). Oxidative stress can be induced by hyperglycemia through several mechanisms such as activation of polyol pathway and glucose auto-oxidation. Further, it leads to the sorbitol accumulation in epithelial cells and onset of various ocular lesions and peripheral neuropathy (Pastene et al. 2007). Harding (1992) has reported that the use of sorbitol-lowering drugs reduced diabetic cataract development in clinical experiments. In this study, reduction in blood glucose indicates the delay in development of cataract. Diabetic cataract was not entirely cured following supplementation of rats with Pterocarpus marsupium bark extracts. However, the intensity of cataract formation was lowered.
Conclusion
In summary, the Pterocarpus marsupium bark extracts supplementation was most useful for the prevention and management of cataract in streptozotocin-induced diabetic rats. The extracts offered the excellent inhibitory potential on aldose reductase. However, further clinical experiments are required to evaluate the therapeutic effect of Pterocarpus marsupium bark extracts.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
References
- Balic M, Rapp N, Stanzer S, Lin H, Strutz J, Szkandera J, Daidone MG, Samonigg H, Cote RJ, Dandachi N. Novel immunofluorescence protocol for multimarker assessment of putative disseminating breast cancer stem cells. Appl Immunohistochem Mol Morphol. 2011;19:33–40. doi: 10.1097/PAI.0b013e3181ebf4e8. [DOI] [PubMed] [Google Scholar]
- Corso AD, Cappiello M, Mura U. From a dull enzyme to something else: facts and perspectives regarding aldose reductase. Curr Med Chem. 2008;15:1452–1461. doi: 10.2174/092986708784638870. [DOI] [PubMed] [Google Scholar]
- Daniellou R, Zheng H, Palmer DRJ. Kinetics of the reaction catalyzed by inositol dehydrogenase from Bacillus subtilis and inhibition by fluorinated substrate analogs. Can J Chem. 2006;84:522–527. doi: 10.1139/v06-033. [DOI] [Google Scholar]
- Gabbay KH. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973;288:831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- Ghahary A, Luo JM, Gong YW, Chakrabarti S, Sima AA, Murphy LJ. Increased renal aldose reductase activity: immunoreactivity and mRNA in streptozotocin-induced diabetic rats. Diabetes. 1989;38:1067–1071. doi: 10.2337/diab.38.8.1067. [DOI] [PubMed] [Google Scholar]
- Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/S0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. 3. London: Chapman & Hall Pub; 1998. [Google Scholar]
- Harding JJ. Pharmacological treatment strategies in age-related cataracts. Drugs Aging. 1992;2(4):287–300. doi: 10.2165/00002512-199202040-00003. [DOI] [PubMed] [Google Scholar]
- Hariharan RS, Venkataraman S, Sunitha P, Rajalakshmi S, Samal KC, Routray BM, Jayakumar RV, Baiju K, Satyavati GV, Muthuswamy V, Gupta AK. Efficacy of vijayasar (Pterocarpus marsupium) in the treatment of newly diagnosed patients with type 2 diabetes mellitus: a flexible-dose double-blind, multicenter randomized controlled trial. Diabetologia Croatica. 2005;34:13–20. [Google Scholar]
- Hung HY, Qian K, Morris Natschke SL, Hsu CS, Lee KH. The Recent discovery of plant-derived anti-diabetic natural products. Nat Prod Rep. 2012;29:580–606. doi: 10.1039/c2np00074a. [DOI] [PubMed] [Google Scholar]
- Jung HA, Kim YS, Choi JS. Quantitative HPLC analysis of two key flavonoids and inhibitory activities against aldose reductase from different parts of the Korean thistle, Cirsium maackii. Food Chem Toxicol. 2009;47(11):2790–2797. doi: 10.1016/j.fct.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE. Epidemiology of proliferative diabetic retinopathy. Diabetic Care. 1992;15:1875–1891. doi: 10.2337/diacare.15.12.1875. [DOI] [PubMed] [Google Scholar]
- Koksal B. Effect of streptozotocin on plasma insulin levels of rats and mice: a meta-analysis study. Open Access Maced J Med Sci. 2015;3:380–383. doi: 10.3889/oamjms.2015.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Patel DK, Laloo D, Sairam K, Hemalatha S. Inhibitory effect of two Indian medicinal plants on aldose reductase of rat lens in vitro. Asian Pac J Trop Med. 2011;4:694–697. doi: 10.1016/S1995-7645(11)60176-4. [DOI] [PubMed] [Google Scholar]
- Lee H. Inhibitory activity of Cinnamomum cassia bark-derived component against rat lens aldose reductase. J Pharm Pharmaceut Sci. 2002;5(3):226–230. [PubMed] [Google Scholar]
- Lou MF, Dickerson JE., Jr Protein-thiol mixed disulfides in the human lens. Exp Eye Res. 1992;55:889–896. doi: 10.1016/0014-4835(92)90015-K. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Masatoshi M, Nobuo O, Shinichi S, Shahabuddin A, Jen-Yue T, Peter FK, Sanai S. The role of aldose reductase in sugar cataract formation: aldose reductase plays a key role in lens epithelial cell death (apoptosis) Chem Biol Interact. 2001;130–132:617–625. doi: 10.1016/s0009-2797(00)00289-1. [DOI] [PubMed] [Google Scholar]
- Massfelder T, Lang H, Schordan E, Lindner V, Rothhut S, Welsch S, Simon-Assmann P, Barthelmebs M, Jacqmin D, Helwig JJ. Parathyroid hormone-related protein is an essential growth factor for human clear cell renal carcinoma and a target for the von Hippel-Lindau tumor suppressor gene. Cancer Res. 2004;64:180–188. doi: 10.1158/0008-5472.CAN-03-1968. [DOI] [PubMed] [Google Scholar]
- Muthuraman P, Kim DH. Therapeutic potential of cyanobacteria against streptozotocin-induced diabetic rats. 3 Biotech. 2016;6(1):94. doi: 10.1007/s13205-016-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuraman P, Srikumar K. A comparative study on the effect of homobrassinolide and gibberellic acid on lipid peroxidation and antioxidant status in normal and diabetic rats. J Enzyme Inhib Med Chem. 2009;24:1122–1127. doi: 10.1080/14756360802667563. [DOI] [PubMed] [Google Scholar]
- Muthuraman P, Senthilkumar R, Srikumar K. Alterations in beta-islets of Langerhans in alloxan-induced diabetic rats by marine Spirulina platensis. J Enzyme Inhib Med Chem. 2009;24:1253–1256. doi: 10.3109/14756360902827240. [DOI] [PubMed] [Google Scholar]
- Narayana DB, Dobriyal RM. Complimentary medicines and 21st-century therapeutics: challenges for pharmacologist. In: Gupta SK, editor. Pharmacology and therapeutics in the new millennium. New Delhi: Narosa Publishing House; 2000. pp. 326–335. [Google Scholar]
- Pastene E, Avello M, Letelier MM, Sanzana E, Vega M, González M. Preliminary studies on antioxidant and anti-cataract activities of Cheilanthes glauca (Cav.) Mett. through various in vitro models. Electron J Food Plants Chem. 2007;2(1):1–8. [Google Scholar]
- Patel MB, Mishra SM. Aldose reductase inhibitory activity of a C-glycosidic flavonoid derived from Enicostemma hyssopifolium. J Altern Complement Med. 2009;6:1–17. doi: 10.1625/jcam.6.1. [DOI] [Google Scholar]
- Reddy GB, Satyanarayana A, Balakrishna N, Ayyagari R, Padma M, Viswanath K, et al. Erythrocyte aldose reductase activity and sorbitol levels in diabetic retinopathy. Mol Vis. 2008;14:593–601. [PMC free article] [PubMed] [Google Scholar]
- Schemmel K, Padiyara R, D’souza J. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complicat. 2010;24:354–360. doi: 10.1016/j.jdiacomp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Schwartz JE, Durham BC. Evaluation of three methods of protein analysis for serum and heart homogenates. Ann Clin Lab Sci. 1979;9:139–143. [PubMed] [Google Scholar]
- Scotti L, Fernandes MB, Muramatsu E, Pasqualoto KFM, Emereciano VP, Tavares LC, et al. Self-organizing maps and volsurf approach to predict aldose reductase inhibition by flavonoid compounds. Rev Bras Phcog. 2011;21(1):170–180. doi: 10.1590/S0102-695X2011005000028. [DOI] [Google Scholar]
- Srivastava Y, Venkatakrishna-Bhatt H, Verma Y. Effect of Momordica charantia Linn. pomous aqueous extract on cataractogenesis in murrin alloxan diabetics. Pharmacol Res Commun. 1988;20:201–209. doi: 10.1016/S0031-6989(88)80041-9. [DOI] [PubMed] [Google Scholar]
- Thylefors B. The World Health Organization’s program for the prevention of blindness. Int J Ophthalmol. 1990;14:211–219. doi: 10.1007/BF00158321. [DOI] [PubMed] [Google Scholar]
- Vats V, Yadav SP, Biswas NR, Grover JK. Anti-cataract activity of Pterocarpus marsupium bark and Trigonella foenum-graecum seeds extract in alloxan diabetic rats. J Ethnopharmacol. 2004;93:289–294. doi: 10.1016/j.jep.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yang Y, Xiang X, Zhu Y, Men J, He M. Estimation of the normal range of blood glucose in rats. Wei Sheng Yan Jiu. 2010;39:133–137. [PubMed] [Google Scholar]
- Warrier PK. Ocimum basilicum Linn. In: Warrier PK, Nambiar VPK, Ramankutty C, editors. Indian medicinal plants. Madras: Orient Longman Limited; 1995. pp. 160–163. [Google Scholar]
- Zhu C. Aldose reductase inhibitors as potential therapeutic drugs of diabetic complications. In: Oguntibeju OO, editor. Diabetes mellitus-insights and perspectives. Crotia: InTech; 2013. pp. 17–46. [Google Scholar]