Abstract
Background
Left ventricular assist devices (LVADs) have revolutionized and improved the care of the sickest heart failure (HF) patients, and it is imperative that they receive appropriate ventricular unloading. Assessing this critical parameter with current methodologies (labs, imaging) is usually suboptimal in this patient population. Hence it is imperative to elucidate the molecular underpinnings involved in ventricular unloading. We have previously identified the cytoskeletal protein βII spectrin as an essential nodal protein involved in post-translational targeting and βII spectrin protein levels are significantly altered in multiple forms of human and animal HF. We therefore hypothesized that the βII spectrin pathway would play a critical role in LVAD remodeling.
Methods
Human heart failure samples were obtained from patients undergoing heart transplantation. Wild type (WT) mice and our previously validated βII spectrin conditional knock out (βII cKO) mice were used for animal experiments. Transaortic constriction (TAC) was performed on WT mice. Protein expression was assessed via immunoblots, and protein interactions were assessed with co-immunoprecipitation. Transcriptome analysis was performed using isolated whole hearts from control adult WT mice (n = 3) compared to βII cKO spectrin mice (n = 3).
Results
We report that hearts from mice selectively lacking βII spectrin expression in cardiomyocytes displayed altered transcriptional regulation of cardiac ankyrin repeat protein (CARP). Notably, CARP protein expression is increased after TAC. Additionally, our findings illustrate that prior to LVAD support, CARP levels are elevated in HF patients compared to normal healthy controls. Further, for the first time in a LVAD population, we show that elevated CARP levels in HF patients return to normal following LVAD support.
Conclusion
Our findings illustrate that CARP is a dynamic molecule that responds to reduced afterload and stress, and has the potential to serve as a prognostic biomarker to assess for an adequate response to LVAD therapy.
Keywords: Medicine, Cardiology
1. Introduction
Heart Failure (HF) is a national epidemic [1], and the lifetime risk for the development of HF is 20% [2]. Cardiac transplantation is the preferred long-term treatment option with respect to survival for end-stage HF, but a severe shortage of donor organs is a significant limiting factor and many patients are poor candidates for transplantation due to age or co-morbidities [3, 4]. Left ventricular assist devices (LVADs) have revolutionized and improved the care of the sickest HF patients, and for New York Heart Association (NYHA) class IV patients who require LVAD support, overall quality of life and functional capacity is significantly improved with LVADs [5]. Survival with continuous-flow pumps exceeds 80% at one year, yet that short term survival benefit must be balanced against the increased risk of complications (>60% composite event rate at one year), reduced long-term survival compared to transplants, and exceedingly high one-year readmission rates which approach 80% [3, 6, 7].
For patients to receive optimal benefits from LVAD support, it is imperative that they receive appropriate ventricular unloading. Assessing this critical parameter is limited to either invasive hemodynamic measurements or crude estimates based on ventricular measurements via echocardiograms, which are usually suboptimal in this patient population. Hence it is imperative to not only identify optimal unloading and ways to measure this non-invasively, but to elucidate the molecular underpinnings involved in the unloading process. This is particularly challenging as many different molecular and cellular pathways are directly impacted by ventricular unloading induced by LVADs [8].
Given their critical roles in regulation of membrane integrity and cellular signaling, it is not surprising that cytoskeletal proteins are directly impacted by LVAD support. Investigators have previously demonstrated that following LVAD support, dystrophin, β-actin, α-tropomyosin, lamin, sarcomeric actin, troponins-C and -T, and titin show altered expression and may serve as markers of ventricular recovery/remodeling [9, 10, 11, 12, 13, 14]. We have previously identified the cytoskeletal protein βII spectrin as an essential nodal protein involved in post-translational targeting and localization of critical membrane and proteins in heart [15]. Moreover, βII spectrin protein levels are significantly altered in multiple forms of human cardiovascular disease as well as in large and small animal cardiovascular disease models following structural and electrical remodeling [15, 16]. While our initial findings support a critical role of the βII spectrin-based cytoskeletal infrastructure in heart, we lack a complete understanding of how this pathway is regulated in HF. As an initial investigation into the molecular underpinnings of not only βII spectrin in HF, but broader cytoskeletal pathways in LVAD-based unloading in human subjects, we analyzed common transcriptional and protein signatures. We report that hearts from mice selectively lacking βII spectrin expression in cardiomyocytes displayed altered transcriptional regulation of cardiac ankyrin repeat protein (CARP, encoded by ANKRD1). As CARP is well known to be modified by muscle strain it was an ideal target to assess in LVAD patients. Consistent with these data, CARP protein expression is increased in a heart murine model of pressure-overload HF. Most notably, our findings illustrate that prior to LVAD support, CARP levels are elevated in HF patients compared to normal healthy controls. Further, for the first time in a LVAD population we show that elevated CARP levels in HF patients return to normal following continuous ventricular unloading. In summary, our findings illustrate that in a critical patient population, CARP is a dynamic molecule that responds to reduced afterload and stress, and has the potential to serve as a prognostic biomarker to assess for an adequate response to LVAD therapy.
2. Methods
2.1. Human tissue
Approval for use of human subjects was obtained from the Institutional Review Board (IRB) of Ohio State University, and informed consent was obtained from all human participants according to our IRB approved protocol. Left ventricular tissue from healthy donor hearts not suitable for transplantation (subclinical atherosclerosis, age, no matching recipients) was obtained through Lifeline of Ohio and used as controls, and none of the nonfailing hearts had signs of coronary bypass surgery or prior myocardial infarctions, while wall thicknesses, heart weights, and heart weight-to-body weight ratios were all in the normal range (n = 6). As previously described by our group, explanted hearts were obtained in the operating room, flushed immediately after removal from living donors, and thereafter transferred to the laboratory in cold cardioplegic solution, and then immediately placed into a −80° freezer for long-term storage. Hearts were procured and treated with identical protocols and timing regardless of their source [17]. The investigation conforms to the principles outlined in the Declaration of Helsinki. Ischemic heart failures samples were defined as the presence of any epicardial coronary vessels with ≥75% stenosis or any history of myocardial infarction or coronary revascularization (either percutaneous transluminal coronary angioplasty or coronary artery bypass grafting) [18]. For human heart failure samples used in the LVAD experiments, left ventricular tissue was obtained from six explanted hearts (3 ischemic and 3 non-ischemic) of patients undergoing heart transplantation at The Ohio State University. An additional four non-failure human samples and four non-ischemic HF samples were used for microarray analysis. Baseline demographic was collected on each patient at the time of LVAD implantation.
2.2. Animals (mice)
All experiments were performed in age-matched (2–4 month) littermates. All animal procedures were approved and were in accordance with institutional guidelines (IACUC; Ohio State University) [19]. Wild type mice (C57BL/6J, Jackson laboratory) and our previously validated βII spectrin conditional knock out (cKO) mice were used. To create our cKO mice we used a cre-flox system with exon 3 of SPTBN1 flanked by LoxP sites and used a cre-recombinase driven by the α-MHC promoter to excise βII in heart [15].
2.3. Transverse aortic constriction (mice)
Transverse aortic constriction was performed as previously described [16, 20]. Wild type mice were anesthetized (ketamine, 100 mg/kg I.P. plus xylazine, 5 mg/kg), intubated, and placed on a respirator (120 breaths min−1, 0.1 mL tidal volume). Anesthesia was monitored by repeated hind limb response to pinch. Aorta was exposed via a midline sternotomy. A 6.0 Prolene suture was placed around the aorta distal to the brachiocephalic artery. The suture was tightened around a blunted 27-gauge needle placed next to the aorta. The needle was removed, and the chest was closed. Echocardiography was performed before surgery and at regular intervals for six weeks following surgery to assess cardiac function and to ensure a dilated heart failure phenotype (left ventricular ejection fraction <40%) using the Vevo 2100 (Visualsonics). The MS-400 transducer was used in the short axis M-mode to assess heart function and contractile features. Mice were sacrificed at 6 weeks post-surgery (TAC or sham). With our laboratory protocol, this protocol produces decreased ejection fraction and increased heart to body weight ratio when compared with Sham mice [20]. Age-matched littermates were used as sham operated controls. Hearts were harvested from sacrificed mice (2% Avertin, 20 μL/g, I.P.) via rapid thoracotomy.
2.4. Immunoblots
To create lysates, 30 μg of human left ventricular free wall tissue were placed into ice cold Homogenization Buffer (in mM: 50 Tris–HCl, 10 NaCl, 320 sucrose, 5 EDTA, 2.5 EGTA; supplemented with 1:500 protease inhibitor cocktail). Following BCA quantitation, 30 μg of tissue lysates were electrophoresed using the Mini-PROTEAN tetra cell (BioRad) and a 4–15% precast TGX gel (BioRad). Gels were transferred to a nitrocellulose membrane using the Mini-PROTEAN tetra cell (BioRad) and blocked for 1 h at room temperature. Primary antibody incubation was carried out overnight at 4 °C. Donkey anti-rabbit secondary HRP antibodies (Jackson ImmunoReseach; catalog number 711-035-152) were used for ANKRD1/CARP, β-II spectrin, and Vinculin. Donkey anti-Mouse HRP (Jackson ImmunoReseach; catalogue number 715-035-150) was used for GAPDH. Membranes were developed using Super Signal West Femto Maximum Sensitivity Substrate (Thermo Scientific; catalogue number 34095) and then imaged using ChemiDoc XRS+ with image lab software (BioRad; Universal Hood II). Densitometric analyses were performed using ImageLab software (BioRad). For all experiments, protein values were normalized against an internal loading control validated against the specific pathology. The following antibodies were used: ANKRD1 (ABCAM) and βII spectrin (Covance).
2.5. Co-immunoprecipitation
Beads were made using Pierce® Co-Immunoprecipitation Kit (thermos Fisher). Fresh mouse hearts were obtained and lysates were made in tissue homogenization buffer (0.025 M Tris–HCL, 0.15 M NaCl, 0.001 M EDTA, 1% NP-40, 5% Glycerol; supplemented with 1:500 protease inhibitor cocktail). Lysate was spun at 14,000 rpm for 18 min. Supernatant was then removed and protein concentration was determined using PierceTM BCA Protein Assay Kit (Thermo Scientific). 1 mg of protein was added to 80 μl of agarose resin control beads and spun for an hour. Then 0.5 mg of pre-cleared lysate was added to the IgG beads and βII-Spectrin beads. The Pierce® Co-Immunoprecipitation Kit instructions were then followed. Once eluted tissue lysates were electrophoresed using the Mini-PROTEAN tetra cell (BioRad) and a 4–15% precast TGX gel (BioRad). Gels were transferred to a nitrocellulose membrane using the Mini-PROTEAN tetra cell (BioRad) and blocked for 1 h at room temperature. Primary antibody incubation was carried out overnight at 4 °C. Densitometric analyses were performed using ImageLab software (BioRad). For all experiments, protein values were normalized against an internal loading control validated against the specific pathology.
2.6. Microarray experiments
Transcriptome analysis was performed using isolated whole hearts from control adult wild type mice (n = 3) compared to βII spectrin cardiac knockout mice (n = 3) and control non-failure human left ventricular samples to non-ischemic HF samples (n = 4 for both groups). For total RNA isolation 30 μg on heart tissue was homogenized. RNA was then isolated using miRNeasy mini kit (Qiagen). Sample integrity was assessed and samples with 260/280 and 260/230 ratios ≥2 were sent to the Ohio State University core microarray lab for additional processing and data analysis.
2.7. Statistics
Data are presented as mean ± SEM. For the comparison of 2 groups, we performed Wilcoxon-Mann–Whitney U tests. For the comparison of >2 groups, we applied a Kruskal-Wallis test. When we obtained a significant P-value, we continued with pair-wise comparisons using Wilcoxon-Mann–Whitney U tests according to the closed-testing principle. For our study, a value of P < 0.05 was considered statistically significant.
2.8. Statistical methods for transcriptional analyses
Signal intensities were analyzed by Affymetrix Expression Console software. Background correction and quantile normalization was performed to adjust technical bias, and gene expression levels were summarized by RMA method. A filtering method based on percentage of arrays above noise cutoff was applied to filter out low expression genes. Linear model was employed to detect differentially expressed genes. In order to improve the estimates of variability and statistical tests for differential expression, a variance smoothing method with fully moderated t-statistic was employed for this study. The significance level was adjusted by controlling the mean number of false positives. A heatmap was generated for visualizing expression of significant genes. Hierarchical clustering with average linkage was superimposed on the heatmap to show gene clustering. Statistical software SAS 9.2 and R was used for analysis.
3. Results
3.1. βII spectrin expression is reduced in human heart failure and levels remain reduced following LVAD support
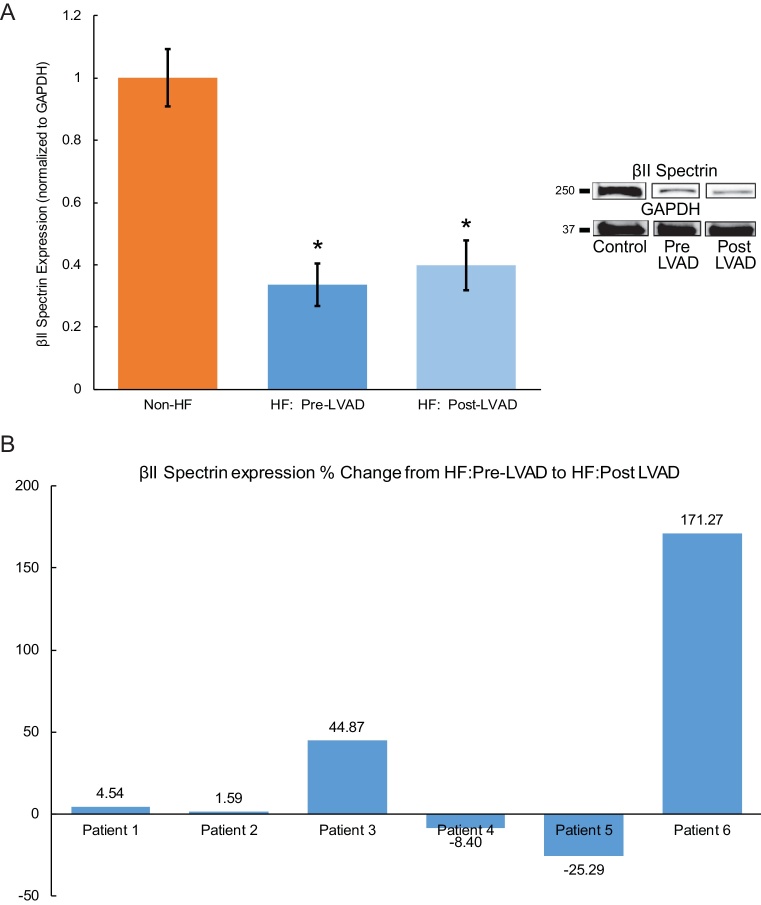
We previously reported that βII spectrin expression is reduced in human ischemic and non-ischemic HF [16]. Our current data comparing 6 non-failing left ventricular (LV) control samples with 6HF samples (3 ischemic and 3 non-ischemic) confirmed this relationship as there was a significant decrease of spectrin expression in failing hearts (Fig. 1A). Furthermore, after LVAD support (at the time of transplantation), βII spectrin expression remained significantly decreased compared to control non-failing samples (Fig. 1A), although when assessing the individual patient response, the expression was quite variable as 2/6 patients had a marked increase in βII spectrin expression after a period of LVAD support (Fig. 1B).
Fig. 1.
βII spectrin expression is reduced in human heart failure and levels remain reduced following LVAD support. Comparing six non-failing (Non-HF) left ventricular (LV) control samples with six HF samples (3 ischemic and 3 non-ischemic) demonstrated there was a significant decrease of spectrin expression in failing hearts (HF-Pre-LVAD, Fig. 1A and Supplemental Fig. 1). After LVAD support (at the time of transplantation), βII spectrin expression remained significantly decreased compared to control non-failing samples (HF: Post-LVAD, Fig. 1A). When assessing the individual patient response (percent change/%), the expression was quite variable as 2/6 patients had a marked increase in βII spectrin expression after a period of LVAD support (Fig. 1B).
3.2. CARP transcript levels are increased in βII spectrin cKO hearts
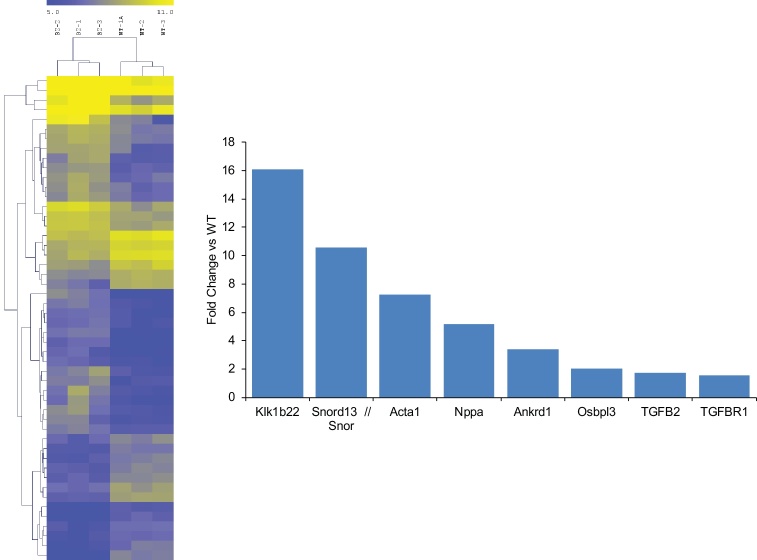
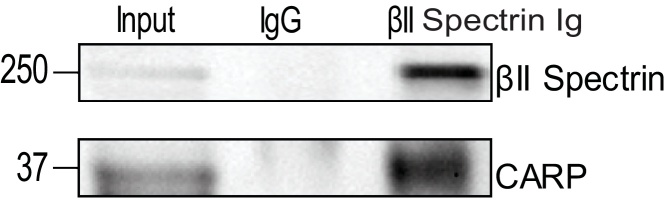
We previously demonstrated that βII spectrin is a critical cytoskeletal protein required to maintain normal cardiovascular structural and electrical physiology, and loss of βII spectrin in heart leads to lethal arrhythmias, aberrant electric and calcium handling phenotypes, and abnormal expression/localization of cardiac membrane proteins [15, 16]. To assess the pathways associated with reduced βII spectrin expression in heart, we isolated RNA from βII spectrin cKO mice (n = 3) and wildtype (WT) mice (n = 3) and performed microarray analysis. From ∼25,000 transcripts analyzed, surprisingly only a small subset of transcripts (79) met the strict criteria of having both a 1.5-fold change in expression and a p-value of <0.05 as assessed by ANOVA (Fig. 2). One transcript that was notable is the gene ANKRD1 that encodes CARP, and ANKRD1 expression was increased ∼three-fold (p < 0.001) in the hearts of βII spectrin cKO mice compared to WT mice (Fig. 2). CARP and βII spectrin share a common pathway involving transforming growth factor β (TGFβ). Notably TGFβR1 (1.58-fold increase, p < 0.001) and TGFβ2 (1.75-Fold increase, p < 0.001) transcript levels were both significantly upregulated in βII spectrin cKO mice compared to WT controls (Fig. 2). To further assess the relationship between spectrin and CARP we subsequently determined that βII spectrin Ig co-immunoprecipitates with CARP from detergent-soluble lysates from non-failing adult mouse heart (Fig. 3), supporting the microarray data. Of the various transcripts discovered we determined that CARP was the best target for further investigation in LVAD patients. LVAD loading conditions can be quite variable, and since CARP is modified by muscle strain it was reasonable to conclude that LVADs could directly impact this protein which serves as a transcriptional co-factor.
Fig. 2.
ANKRD1 transcription levels are increased in βII spectrin cKO hearts. In microarray analysis, ANKRD1 was upregulated ∼three-fold (p < 0.001) in the hearts of βII spectrin cKO mice compared to WT mice (n = 3 for control and βII-spectrin cKO).
Fig. 3.
CARP and βII Spectrin Are Molecular Partners in Heart. βII spectrin Ig co-immunoprecipitates with CARP from detergent-soluble lysates from non-failing adult mouse heart (Supplemental Fig. 2).
3.3. CARP protein expression is increased in murine and human heart failure
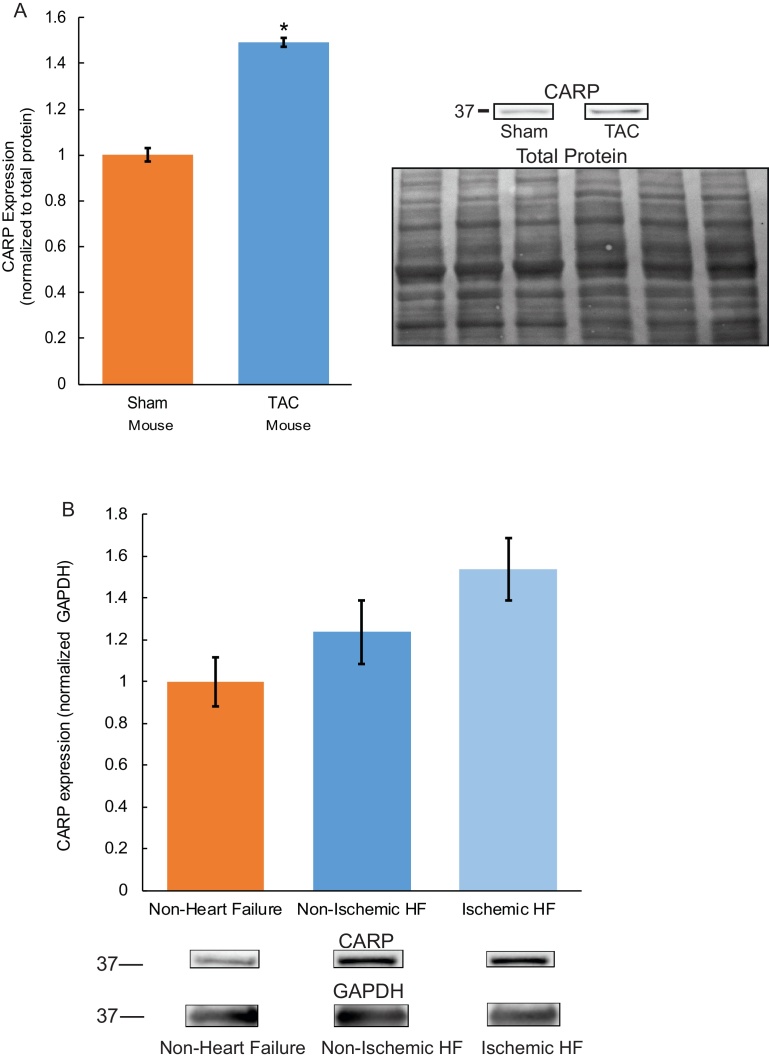
Based on previous data in humans [21], we hypothesized that CARP protein levels are significantly altered in non-ischemic murine heart failure. To directly examine this hypothesis, we tested if CARP protein levels were altered in the left ventricle of wild type (WT) mice subjected to six weeks of transaortic constriction (TAC; induces pressure-overload heart failure, ejection fraction reduced ∼50%) [20]. In support of our hypothesis, we observed a significant increase of CARP levels in the left ventricle of WT TAC mice versus sham control animals (Fig. 4A; n = 3/group, p < 0.05), consistent with previous studies in human HF [21, 22, 23]. As our goal is ultimately to define new molecular markers to define remodeling in human heart, we performed a transcriptional analysis of human non-ischemic left ventricular heart failure samples (n = 4) and non-failing control left ventricular samples (n = 4). In human ischemic and non-ischemic HF samples ANKRD1 transcription levels were increased by >20% compared to control samples, suggestive of a positive upward trend (Table 1, p = 0.07). Notably in both ischemic and non-ischemic human HF samples, CARP protein expression trended higher compared to control non-failure samples, although this did not reach statistical significance (Fig. 4B). In summary, our findings illustrate that CARP transcript and protein levels are increased in murine and human HF.
Fig. 4.
CARP protein expression is increased in murine and human heart failure. There is a significant increase of CARP levels in left ventricle of TAC mice versus sham control animals (n = 3/group, p < 0.05, normalized to total protein, Supplemental Fig. 3A) (A). In both ischemic and non-ischemic human HF samples, CARP protein expression trended higher compared to control non-failure samples, although this did not reach statistical significance (B, 3 ischemic HF and 3 non-ischemic HF samples/6 control non-HF samples, Supplemental Fig. 3B).
Table 1.
Expression Profile of Genes by Microarray in Mouse and Non-Ischemic Human Heart Tissue.
| Gene | Protein | Fold-change/p-value in βII cKO Mouse vs WT, n = 3 (each group) | Fold-change/p-value in HF human vs non-failing control, n = 4 (each group) | ||
|---|---|---|---|---|---|
| ANKRD1 | CARP | 3.4 | p < 0.001 | 1.24 | p = 0.07 |
| Baseline Clinical Characteristics of Non-Ischemic HF Patients Used in Microarray | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Age | Gender | Race | LVEFa | Cardiac output/Index | Peak VO2b | LVAD | Inotrope | Diabetes Mellitus |
| 1 | 69 | Female | White | 15% | 3.8/2.1 | 12.2 | No | Milrinone | Yes |
| 2 | 64 | Male | White | 20% | 4.0/2.0 | – | No | Milrinone | No |
| Dobutamine | |||||||||
| 3 | 64 | Male | White | 15% | 4.5/2.2 | – | No | Milrinone | Yes |
| 4 | 56 | Female | White | 10% | 2.2/1.2 | – | No | Dobutamine | Yes |
LVEF = Left Ventricular Ejection Fraction.
VO2 = maximal oxygen consumption (ml/kg/min).
3.4. CARP protein expression decreases with LVAD mechanical unloading
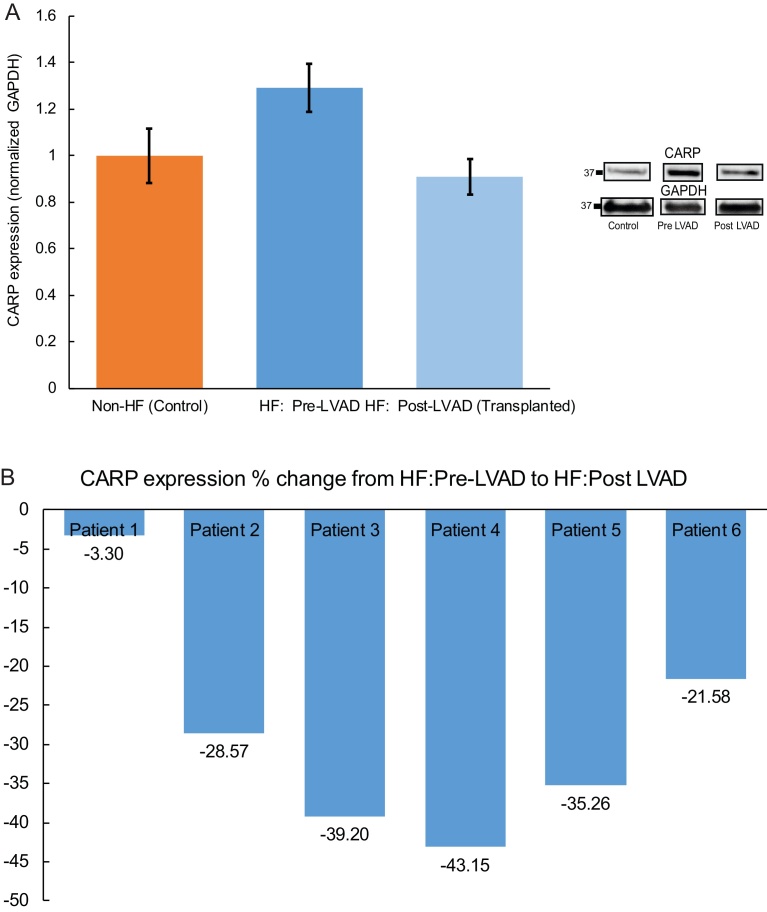
ANKRD1 mRNA levels are increased in human heart failure (Fig. 4B) [22, 23, 24]. However, the molecular mechanisms underlying/associated with heart disease may differ between human and animal disease models, as well as between various forms of HF (ischemic/non-ischemic). To date CARP expression has not been assessed in LVAD patients, a unique population of patients with end-stage HF who benefit from mechanical unloading. We therefore examined CARP protein expression in humans who received LVAD support for end-stage HF and subsequently survived to cardiac transplantation, at which time the LVAD was removed (Table 2). Notably all patients were bridged to transplant and were electively transplanted only, and post-LVAD right heart catheterization numbers demonstrated normal filling pressures suggestive of adequate ventricular unloading. LV samples were obtained from 6 patients (3 ischemic HF and 3 non-ischemic HF) at the time of LVAD implantation and compared to 6 non-HF LV samples. Contrary to our findings in human HF pre-LVAD support, there was decreased trend in CARP expression following LVAD support (at the time of cardiac transplantation) using LV samples from these same six HF patients (Fig. 5A, p = 0.0764), mirroring the expression levels in the non-failing controls. Notably all six patients demonstrated a decrease in CARP expression post-LVAD (Fig. 5B). In summary, these data suggest that while CARP protein expression is elevated in human HF, mechanical unloading returns these levels back to baseline.
Table 2.
End-Stage Heart Failure (HF) Patients Who Received LVADs and Survived to Cardiac Transplantation.
| Patient | Days From LVAD to Transplant | Type of HF | Body Mass Index (kg/m2) | Hypertension | Diabetes Mellitus | Myocardial Infarction | Atherosclerosis | Stroke | Inotrope | Left Ventricular Ejection Fraction (%) | Pulmonary artery systolic pressure (mmHg) | Mean pulmonary artery pressure (mmHg) | Pulmonary capillary wedge pressure (mmHg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 428 | Ischemic | 26 | Yes | Yes | No | No | No | No | <20% | 24 | 16 | 5 |
| 2 | 294 | Ischemic | 34.1 | Yes | Yes | Yes | No | No | Yes | <20% | 32 | 20 | 9 |
| 3 | 287 | Ischemic | 30.5 | No | Yes | Yes | Yes | No | No | 15% | 30 | 20 | 8 |
| 4 | 516 | Non-Ischemic | 23.2 | Yes | No | No | No | No | No | <20% | 30 | 17 | 3 |
| 5 | 325 | Non-Ischemic | 25.6 | No | No | No | No | No | No | 10% | 14 | 8 | 3 |
| 6 | 251 | Non-Ischemic | 30.7 | No | No | No | No | No | Yes | <20% | 28 | 21 | 14 |
Fig. 5.
CARP Protein Expression Decreases with LVAD Mechanical Unloading. There was a trend towards decreased CARP expression following LVAD support (at the time of cardiac transplantation) using LV samples from six HF patients (p = 0.076) (A, and Supplemental Fig. 4). All six HF patients demonstrated a decrease (percent change/%) in CARP expression post-LVAD support (B).
4. Discussion
βII spectrin levels are significantly altered in human cardiovascular disease as well as in large and small animal cardiovascular disease models, and the spectrin pathway is emerging as a new target to investigate the fundamental aspects of heart failure [16]. Notably CARP functions as a transcription co-factor in the setting of mechanical stress and pressure overload. Specifically, in response to stress, CARP translocates from the cytosol to the nucleus to regulate gene expression [22, 25, 26, 27]. Here, our goal was to assess the expression of βII spectrin and CARP in HF patients before and after LVAD support. Our new data demonstrate that CARP protein levels are consistently increased in human HF as well as small animal models of HF at the transcriptional and translational level, and that mechanical unloading with an LVAD leads to a marked reduction in CARP expression. For the first time, the role of CARP in LVAD patients has been investigated. Notably, after LVAD support CARP levels decreased and were akin to non-failure control levels, thus supporting our hypothesis that CARP is a dynamic protein whose expression changes based on loading conditions/stress. LVAD tissue samples are an ideal situation to examine the effects of LV unloading on the native heart. Thus, CARP has the potential to be a biomarker to assess hemodynamic loading conditions and overall response to LVAD therapy [26]. It is important to note that in patients 4 and 5 there was a lack of correlation regarding spectrin and CARP expression (Figs. 1 B and 5 B). While βII spectrin and CARP interact in a larger molecular complex as suggested by our co-immunoprecipitation data, they may operate independently regarding loading conditions and additional post-translational changes or other protein interactions may govern these changes, which warrant further investigation.
The totality of our data from mouse to human demonstrates that CARP expression is increased in HF/under stressed-conditions, and notably this is driven primarily by changes at the transcriptome level which directly impact ANKRD1. It is worth noting that since LVAD unloading and support alters ventricular loading conditions and strain patterns, alternate modifiers of CARP could play prominent roles. For instance it has been demonstrated that myofibrillar muscle ankyrin proteins are regulated by stretch, which is linked to the titin-N2A-based myofibrillar based pathway which regulates muscle gene expression [26, 28]. One of the fundamental roles of titan is to retain the cardiomyocyte in an optimal length, and this is critical in the mechanosensory pathway and is likely impacted by ventricular unloading.
4.1. The role of ANKRD1 in heart failure
Several investigators have demonstrated that ANKRD1 expression is increased in human HF. CARP is predominantly expressed in cardiac muscle, with lower expression levels in skeletal muscle and endothelial cells based on mRNA sequencing. It has a critical role in the Nkx2.5 transcriptional pathway that defines ventricular muscle gene expression in the developing heart and it is an early marker of cardiac muscle lineages in general [22]. Notably CARP is activated and plays a critical role in the embryonic gene program during cardiac hypertrophy prior to the development of HF, and CARP expression is down-regulated in the adult heart [23]. One of the seminal studies in the field showed that CARP mRNA and protein expression were significantly increased in failing left ventricle tissue specimens obtained explanted hearts from patients with end-stage HF [21]. Notably these investigators noticed that alterations in CARP expression were restricted to ventricular tissue and were not observed in atria, and CARP was predominantly in the nucleus [21].
4.2. Congenital heart failure and myopathies
ANKRD1 is involved in the pathogenesis of congenital HF as five heterozygous mutations in highly conserved ANKRD1-coding sequences leading to mononucleotide substitutions have been associated with dilated cardiomyopathies, likely by impairing CARP nuclear function [29]. Another group identified three separate missense mutations in ANKRD1 which could impair the stretch-induced gene expression required for normal CARP function in the presence of increased mechanical stretch and could contribute to the development of dilated cardiomyopathy [30]. Hypertrophic cardiomyopathy (HCM) has also been linked to ANKRD1 missense mutations which increased binding of CARP to both titin/connectin and myopalladin, and this increased binding of sarcomeric CARP to these proteins could lead to mislocalization of CARP to the nucleus and could play a role in the pathogenesis of HCM [31].
4.3. LVADs and ventricular unloading
For patients who receive LVADs, it is imperative to obtain adequate ventricular unloading to ensure they are receiving the benefits of the device, and to increase their chance of survival until cardiac transplantation. Ideally a combination of clinical/histological criteria and serum biomarkers could be used to enrich our ability to identify patients who are receiving adequate unloading. The utilization of biomarkers as a tool to assess for ventricular unloading has been assessed by many investigators and to date no specific candidate has been identified which consistently demonstrates the required sensitivity and specificity to be used clinically. Brain natriuretic peptide (BNP) has been considered a possible biomarker and indicator of reverse remodeling as unloading of the left ventricle with LVAD support has been shown to decrease BNP levels in the heart based on mRNA and protein expression [32]. A contemporary study suggests that at 2 months serum BNP levels ≤322 pg/ml are significantly associated with all-cause mortality [33]. Yet the challenge and limitation at this time is that mechanical unloading in LVAD recipients is not associated with a rapid reduction of standard plasma biomarkers [34]. Additional investigation is required to determine if CARP could serve as a biomarker of ventricular unloading and if the changes occur in a dynamic and rapid fashion in the majority of patients. Our data clearly demonstrated that 6/6 patients had a reduction in CARP levels over time (Fig. 5B), and this needs to be validated in larger cohorts, and will need to be assessed/replicated in serum and urine samples to have clinical utility. CARP could be used in conjunction with BNP to increase the overall specificity of left ventricular unloading. While previous data demonstrated an overall decrease in BNP levels after LVAD unloading (22/27), 5 patients actually had an increase in BNP levels. Notably in our study all LVAD patients (6/6) had a decrease in CARP expression after ventricular unloading. Hence the utilization of several biomarkers and clinical parameters in concert may provide the best non-invasive assessment of clinical unloading.
4.4. Limitations and future goals
The present study is not without limitations. We realize that our sample size is small, yet with this limited cohort and even with the heterogeneity naturally found in humans we were still able to observe an increased trend regarding CARP levels pre- and post-LVAD support. Ideally serum and plasma would have been collected on each patient when tissue was procured, and we would have collected serum at discrete time points to truly assess the rate of CARP response to unloading. Future studies will examine the relationship and expression of CARP in HF and LVAD serum samples, as that will provide better translation insight regarding its role as a potential biomarker of ventricular unloading.
4.5. Conclusion
We propose that the CARP could enhance our ability to assess the response to ventricular unloading and identify patients who will have a good response with chronic LVAD therapy as a bridge to cardiac transplant.
Declarations
Author contribution statement
Amber Kempton, Matt Cefalu, Cody Justice, Tesla Baich, Mohamed Derbala: Performed the experiments; Analyzed and interpreted the data.
Benjamin Canan, Paul Janssen, Peter J Mohler: Contributed reagents, materials, analysis tools or data.
Sakima A Smith: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by the Robert Wood Johnson Harold Amos Faculty Development Grant and National Institutes of HealthK08 HL135437 (SAS).
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
References
- 1.Writing Group Members, Mozaffarian D., Benjamin E.J. Executive summary: heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D.M., Larson M.G., Leip E.P. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 3.Yusen R.D., Edwards L.B., Dipchand A.I. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J. Heart Lung Transplant. 2016;35(10):1170–1184. doi: 10.1016/j.healun.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Abraham W.T., Smith S.A. Devices in the management of advanced, chronic heart failure. Nat. Rev. Cardiol. 2013;10(2):98–110. doi: 10.1038/nrcardio.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers J.G., Aaronson K.D., Boyle A.J. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J. Am. Coll. Cardiol. 2010;55(17):1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 6.Kirklin J.K., Naftel D.C., Pagani F.D. Seventh INTERMACS annual report: 15,000 patients and counting. J. Heart Lung Transplant. 2015;34(12):1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Estep J.D., Starling R.C., Horstmanshof D.A. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: results from the ROADMAP study. J. Am. Coll. Cardiol. 2015;66(16):1747–1761. doi: 10.1016/j.jacc.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 8.Birks E.J. Molecular changes after left ventricular assist device support for heart failure. Circ. Res. 2013;113(6):777–791. doi: 10.1161/CIRCRESAHA.113.301413. [DOI] [PubMed] [Google Scholar]
- 9.Vatta M., Stetson S.J., Perez-Verdia A. Molecular remodelling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet. 2002;359(9310):936–941. doi: 10.1016/S0140-6736(02)08026-1. [DOI] [PubMed] [Google Scholar]
- 10.Vatta M., Stetson S.J., Jimenez S. Molecular normalization of dystrophin in the failing left and right ventricle of patients treated with either pulsatile or continuous flow-type ventricular assist devices. J. Am. Coll. Cardiol. 2004;43(5):811–817. doi: 10.1016/j.jacc.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 11.Birks E.J., Hall J.L., Barton P.J. Gene profiling changes in cytoskeletal proteins during clinical recovery after left ventricular-assist device support. Circulation. 2005;112(9 Suppl):I57–64. doi: 10.1161/CIRCULATIONAHA.104.526137. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigue-Way A., Burkhoff D., Geesaman B.J. Sarcomeric genes involved in reverse remodeling of the heart during left ventricular assist device support. J. Heart Lung Transplant. 2005;24(1):73–80. doi: 10.1016/j.healun.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Latif N., Yacoub M.H., George R., Barton P.J., Birks E.J. Changes in sarcomeric and non-sarcomeric cytoskeletal proteins and focal adhesion molecules during clinical myocardial recovery after left ventricular assist device support. J. Heart Lung Transplant. 2007;26(3):230–235. doi: 10.1016/j.healun.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 14.de Jonge N., van Wichen D.F., Schipper M.E. Left ventricular assist device in end-stage heart failure: persistence of structural myocyte damage after unloading. An immunohistochemical analysis of the contractile myofilaments. J. Am. Coll. Cardiol. 2002;39(6):963–969. doi: 10.1016/s0735-1097(02)01713-8. [DOI] [PubMed] [Google Scholar]
- 15.Smith S.A., Sturm A.C., Curran J. Dysfunction in the betaII spectrin-dependent cytoskeleton underlies human arrhythmia. Circulation. 2015;131(8):695–708. doi: 10.1161/CIRCULATIONAHA.114.013708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith S.A., Hughes L.D., Kline C.F. Dysfunction of the beta2-spectrin-based pathway in human heart failure. Am. J. Physiol. Heart Circ. Physiol. 2016;310(11):H1583–H1591. doi: 10.1152/ajpheart.00875.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milani-Nejad N., Canan B.D., Elnakish M.T. The Frank-Starling mechanism involves deceleration of cross-bridge kinetics and is preserved in failing human right ventricular myocardium. Am. J. Physiol. Heart Circ. Physiol. 2015;309(12):H2077–H2086. doi: 10.1152/ajpheart.00685.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felker G.M., Shaw L.K., O'Connor C.M. A standardized definition of ischemic cardiomyopathy for use in clinical research. J. Am. Coll. Cardiol. 2002;39(2):210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 19.Pugach E.K., Richmond P.A., Azofeifa J.G., Dowell R.D., Leinwand L.A. Prolonged cre expression driven by the alpha-myosin heavy chain promoter can be cardiotoxic. J. Mol. Cell Cardiol. 2015;86:54–61. doi: 10.1016/j.yjmcc.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudmundsson H., Curran J., Kashef F. Differential regulation of EHD3 in human and mammalian heart failure. J. Mol. Cell Cardiol. 2012;52(5):1183–1190. doi: 10.1016/j.yjmcc.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zolk O., Frohme M., Maurer A. Cardiac ankyrin repeat protein, a negative regulator of cardiac gene expression, is augmented in human heart failure. Biochem. Biophys. Res. Commun. 2002;293(5):1377–1382. doi: 10.1016/S0006-291X(02)00387-X. [DOI] [PubMed] [Google Scholar]
- 22.Zou Y., Evans S., Chen J., Kuo H.C., Harvey R.P., Chien K.R. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development. 1997;124(4):793–804. doi: 10.1242/dev.124.4.793. PMID: 9043061, Online ISSN. [DOI] [PubMed] [Google Scholar]
- 23.Kuo H., Chen J., Ruiz-Lozano P., Zou Y., Nemer M., Chien K.R. Control of segmental expression of the cardiac-restricted ankyrin repeat protein gene by distinct regulatory pathways in murine cardiogenesis. Development. 1999;126(19):4223–4234. doi: 10.1242/dev.126.19.4223. PMID: 10477291, Online ISSN 1477-9129. [DOI] [PubMed] [Google Scholar]
- 24.Zolk O., Marx M., Jackel E., El-Armouche A., Eschenhagen T. Beta-adrenergic stimulation induces cardiac ankyrin repeat protein expression: involvement of protein kinase A and calmodulin-dependent kinase. Cardiovasc. Res. 2003;59(3):563–572. doi: 10.1016/s0008-6363(03)00476-0. [DOI] [PubMed] [Google Scholar]
- 25.Chu W., Burns D.K., Swerlick R.A., Presky D.H. Identification and characterization of a novel cytokine-inducible nuclear protein from human endothelial cells. J. Biol. Chem. 1995;270(17):10236–10245. doi: 10.1074/jbc.270.17.10236. [DOI] [PubMed] [Google Scholar]
- 26.Miller M.K., Bang M.L., Witt C.C. The muscle ankyrin repeat proteins: CARP, ankrd2/arpp and DARP as a family of titin filament-based stress response molecules. J. Mol. Biol. 2003;333(5):951–964. doi: 10.1016/j.jmb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Torrado M., Nespereira B., Bouzamayor Y., Centeno A., Lopez E., Mikhailov A.T. Differential atrial versus ventricular ANKRD1 gene expression is oppositely regulated at diastolic heart failure. FEBS Lett. 2006;580(17):4182–4187. doi: 10.1016/j.febslet.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 28.Buyandelger B., Ng K.E., Miocic S. Genetics of mechanosensation in the heart. J. Cardiovasc. Transl. Res. 2011;4(3):238–244. doi: 10.1007/s12265-011-9262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duboscq-Bidot L., Charron P., Ruppert V. Mutations in the ANKRD1 gene encoding CARP are responsible for human dilated cardiomyopathy. Eur. Heart J. 2009;30(17):2128–2136. doi: 10.1093/eurheartj/ehp225. [DOI] [PubMed] [Google Scholar]
- 30.Moulik M., Vatta M., Witt S.H. ANKRD1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J. Am. Coll. Cardiol. 2009;54(4):325–333. doi: 10.1016/j.jacc.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arimura T., Bos J.M., Sato A. Cardiac ankyrin repeat protein gene (ANKRD1) mutations in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2009;54(4):334–342. doi: 10.1016/j.jacc.2008.12.082. [DOI] [PubMed] [Google Scholar]
- 32.Bruggink A.H., de Jonge N., van Oosterhout M.F. Brain natriuretic peptide is produced both by cardiomyocytes and cells infiltrating the heart in patients with severe heart failure supported by a left ventricular assist device. J. Heart Lung Transplant. 2006;25(2):174–180. doi: 10.1016/j.healun.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Sato T., Seguchi O., Iwashima Y. Serum brain natriuretic peptide concentration 60 days after surgery as a predictor of long-term prognosis in patients implanted with a left ventricular assist device. ASAIO J. 2015;61(4):373–378. doi: 10.1097/MAT.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milting H., Ellinghaus P., Seewald M. Plasma biomarkers of myocardial fibrosis and remodeling in terminal heart failure patients supported by mechanical circulatory support devices. J. Heart Lung Transplant. 2008;27(6):589–596. doi: 10.1016/j.healun.2008.02.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.