Abstract
Tyrosine kinases are expressed in many tissues, particularly in the central nervous system, and regulate various cellular functions. We report here that a src family tyrosine kinase-specific inhibitor, PP2, enhances neurotransmitter release from PC12 cells and primary cultured neurons. PP2 enhances only Ca2+-dependent release; it does not affect basal release. These effects result from an enhancement of vesicular exocytosis and not from the reuptake or refilling of neurotransmitters because Ca2+-dependent secretion of an exogenously expressed reporter protein, the human growth hormone (hGH), is also enhanced by PP2. Overexpression of constitutive active v-src, but not of a kinase-inactive mutant, suppressed Ca2+-dependent release. In PP2-treated cells, Pyk2, paxillin, and some other proteins showed a decrease in tyrosine phosphorylation, and the enhancement of tyrosine phosphorylation of these proteins in response to Ca2+ influx was also reduced. Electron and fluorescence microscopy showed that PP2 treatment induced morphological change and decreased phalloidin reactivity at the filopodium-like structures on the processes of PC12 cells. Interestingly, inhibition of actin polymerization with cytochalasin D and latrunculin A enhanced Ca2+-dependent, but not basal, release. It is possible that a src family tyrosine kinase, through the regulation of actin dynamics, has an inhibitory function to regulate neurotransmitter release.
Neurotransmitters are accumulated in synaptic vesicles in presynaptic nerve terminals. Ca2+ influx through voltage-gated Ca2+ channels triggers the release of neurotransmitters into the synaptic cleft by means of an exocytosis of synaptic vesicles. The modulation of neurotransmitter release is one of the cellular mechanisms for the regulation of synaptic transmission, and the involvement of various kinases has been suggested (1). Tyrosine kinases are classified as receptor types or nonreceptor types, both of which are abundant in the central nervous system. The receptor-type tyrosine kinase generally acts as a receptor for growth and trophic factors and plays an important role in the regulation of synaptic transmission through the up-regulation of neurotransmitter release from synapses of developing and mature neurons (2). The nonreceptor-type tyrosine kinase is well documented to have postsynaptic functions and is shown to phosphorylate neurotransmitter receptors, including nicotinic acetylcholine receptor (nACh-R), N-methyl-d-aspartate receptor (NMDA-R), and γ-aminobutyric acid type A receptor (GABAA-R) (3). src kinase, a nonreceptor tyrosine kinase expressed in the central nervous system, is accumulated in synaptic vesicles and accounts for the majority of synaptic-vesicle tyrosine kinase activity (4, 5). Ca2+ influx, which triggers neurotransmitter release, activates src kinase and induces tyrosine phosphorylation of several proteins, including synaptophysin, a synaptic vesicle protein (6–8). On synaptic vesicles, c-src interacts with synapsin, a perimembrane protein on these vesicles, through its SH3 domain (9, 10). These results suggest that src family tyrosine kinases are involved in the regulation of neurotransmitter release; however, no direct evidence to support this has been obtained. In this study, we analyzed the functional roles of tyrosine kinase in the regulation of neurotransmitter release by using clonal rat pheochromocytoma PC12 cells and cultured rat cerebellar granule cells. We found that src family tyrosine kinase plays an inhibitory role in the regulation of neurotransmitter release, possibly through a rearrangement of actin cytoskeleton.
Experimental Procedures
Materials.
The materials and their sources are genistein (Research Biochemicals, Natick, MA); genistin (Fujicco, Kobe, Japan); PP2, PP3, and cytochalasin D (Calbiochem); monoclonal anti-Pyk2 and anti-paxillin antibodies, (Transduction Laboratories, Lexington, KY); polyclonal anti-Pyk2 antibody (Santa Cruz Biotechnology); monoclonal anti-phosphotyrosine antibody (4G10) (Upstate Biotechnology, Lake Placid, NY); latrunculin A (Wako Biochemicals, Osaka); peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch); protein G-Sepharose (Amersham Pharmacia).
Cell Culture.
PC12 cells were plated on polyethylenimine-coated, 35-mm plates (Becton Dickinson) at a density of 2.5 × 105 cells per cm2 and maintained in DMEM (Life Technologies, Rockville, MD) with 5% heat-inactivated FBS (Life Technologies) and 5% horse serum (Life Technologies) at 37°C in a humidified atmosphere with 10% CO2. Cerebellar granule cells were isolated from Wistar rats according to the method of Levi et al. (11) with some modifications. Briefly, cerebella were dissected from 8- to 10-day-old rats and incubated in 0.2% trypsin and 0.02% DNase I for 15 min at 37°C. The trypsinized tissue was triturated with a Pasteur pipette until no tissue aggregates were seen. Then the cells were washed with DMEM containing 10% heat-inactivated FBS and plated on polyethylenimine-coated, 4-well plates (Nalge Nunc International, Rochester, NY) at a density of 5 × 105 cells per cm2 and maintained in culture medium [DMEM supplemented with 26 mM KCl, 1 μM cytosine arabinonucleoside (Sigma), 50 units per ml penicillin (Sigma), and 100 μg/ml streptomycin (Sigma)] containing 10% heat-inactivated FBS at 37°C in a humidified 10% CO2 atmosphere. Experiments were carried out after 19–20 days of culture.
Plasmids.
A DNA fragment corresponding to the human growth hormone (hGH) gene was isolated from the pXGH5 vector and subcloned into pcDNA3 (Invitrogen). The resulting vector was named pcDNA3/hGH. The v-src cDNA and kinase-inactive mutant v-srcK295M cDNA were generously provided by M. Iwashima (Medical College of Georgia, Augusta) and Y. Hakak (Univ. of California, Berkeley), respectively. They were subcloned into pcDNA3 to construct the mammalian expression vectors pcDNA3/v-src and pcDNA3/K295M, respectively. The DNA sequences of pcDNA3/v-src and pcDNA3/K295M are identical except for one nucleotide, which introduces an amino acid substitution at Lys-295 to Met.
Transfection.
PC12 cells were plated in polyethylenimine-coated, 35-mm culture dishes at a density of 1.5 × 105 cells per cm2. After 18–24 h, cells were transfected with various vectors by using Lipofectamine 2000 (Life Technologies), in accordance with the instruction manual, in the presence of serum. The pcDNA3/hGH vector was used for hGH release assay. In the cotransfection experiments, 2.2 μg of pcDNA3/v-src, pcDNA3/K295M, or empty pcDNA3 vector (as a control) was added with pcDNA3/hGH. The cells were subsequently maintained for 24–48 h and used for hGH release assay.
Release Assay.
All of the release assays were carried out at 37°C. Cells were washed five times with low-K+ solution (140 mM NaCl/4.7 mM KCl/1.2 mM KH2PO4/2.5 mM CaCl2/1.2 mM MgSO4/11 mM glucose/15 mM Hepes-NaOH, pH 7.4). After the wash, cells were incubated with low-K+ solution for 2 min and then the buffer was immediately changed every 2 min with ionomycin-containing low-K+ solution to stimulate Ca2+ influx. In some experiments, a 1-min interval was used instead of 2 min as indicated in the figure legends. Sample buffer solutions were collected into tubes on ice. Secreted dopamine was assayed by HPLC with an electrochemical detector system (12). For glutamate measurement, samples were mixed with o-phthalaldehyde and allowed to stand for 5 min at room temperature to make derivatives of amino acids detectable by fluorescence monitoring. Samples were then subjected to HPLC and analyzed by using a fluorescence monitor (13). hGH was measured by using a radioimmune assay kit (Nichols Institute, San Juan Capistrano, CA) or hGH ELISA kit (Roche Molecular Biochemicals) (14). For dopamine or hGH, secretion was evaluated as a percentage of total cellular content. Student's t test was used for statistical analysis.
Immunoprecipitation and Immunoblotting Analysis.
Whole-cell extracts were prepared by detergent solubilization in lysis buffer (50 mM Tris⋅HCl, pH 7.5/150 mM NaCl/1% Nonidet P-40/10 mM NaF/1 mM Na3VO4/1 mM EDTA/500 units per ml aprotinin/10 μg/ml leupeptin/1 mM phenylmethylsulfonyl fluoride) for 1 h at 4°C. The solubilized materials were centrifuged, and the supernatants were incubated with polyclonal anti-Pyk2, or monoclonal anti-paxillin antibodies for 0.5–1 h at 4°C. These solutions were incubated with protein G-Sepharose for 2–3 h at 4°C. The immunoprecipitates were washed several times with lysis buffer and then subjected to SDS/PAGE. Proteins were immobilized on poly(vinylidene difluoride) (PVDF) membranes after separation by SDS/PAGE, subjected to immunoblotting, and visualized with the enhanced chemiluminescence detection system (Amersham Pharmacia). Anti-phosphotyrosine antibody was stripped from PVDF membranes according to the instruction manual, and membranes were reprobed with anti-Pyk2 or anti-paxillin monoclonal antibody. Data collection and processing were performed with luminescent image analyzer LAS-1000 plus and Science Lab 99 image gauge software (Fujifilm).
Fluorescence Microscopy.
PC12 cells were plated on Lab-Tek chamber slides (Nalge Nunc International) and cultured for 18 h. They were then washed with PBS and fixed with 4% paraformaldehyde in PBS for 15 min. After another wash with PBS, cells were permeabilized and blocked with buffer G (5% goat serum/0.1% Triton-X100 in PBS) for 30 min at room temperature. They were then incubated with tetramethylrhodamine B isothiocyanate-labeled phalloidin (Sigma) in buffer G for 2 h at 4°C, washed several times with PBS, and mounted with ProLong antifade reagent (Molecular Probes). Phalloidin-stained cells were observed with a fluorescence microscope, the Olympus AX-80.
Scanning Electron Microscopy.
For scanning electron microscopy, the cells were fixed with 2.0% glutaraldehyde and 0.5% tannic acid in PBS. The samples were then treated with tannic acid in PBS, postfixed with 1% OsO4 in PBS, dehydrated in ethanol, coated with platinum-palladium, and viewed in a Hitachi S-4500 scanning electron microscope.
Results
Enhancement of Dopamine Release from PC12 Cells by Tyrosine Kinase Inhibitors.
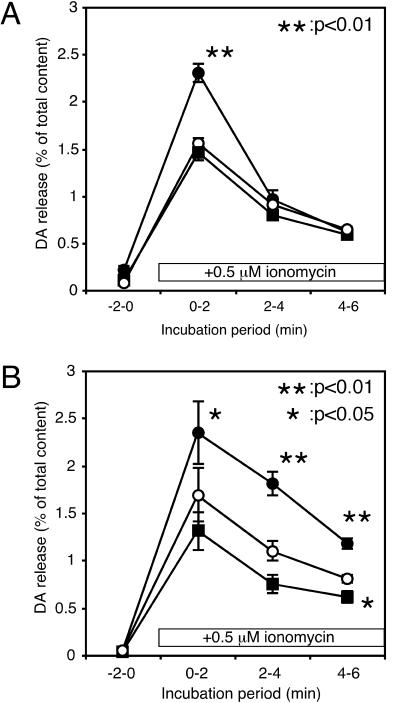
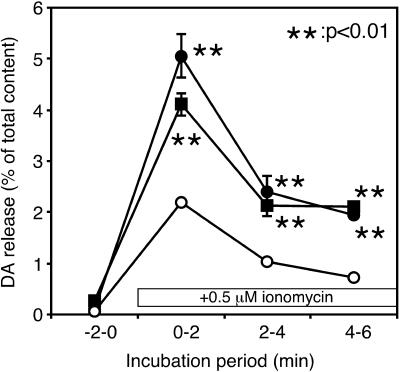
To investigate the role of tyrosine phosphorylation in neurotransmitter release, first we tested the effect of genistein, a nonselective tyrosine kinase inhibitor, on Ca2+-dependent dopamine release from clonal rat pheochromocytoma PC12 cells. We induced Ca2+ channel-independent Ca2+ influx with a Ca2+ ionophore, ionomycin, to evoke Ca2+-dependent dopamine release because some kinase inhibitors were reported to affect ion channel activities (15–17). Pretreatment of the cells with 50 μM genistein for 20 min caused a marked enhancement of dopamine release evoked by ionomycin from PC12 cells (Fig. 1A). This effect seemed to be specific because genistin, an inactive analog of genistein, did not significantly enhance the dopamine release. Enhancement of ionomycin-induced dopamine release was also observed with PP2, a potent and selective inhibitor of src family tyrosine kinases (18), in a concentration-dependent manner. Marked enhancement was observed by PP2 at 20 μM (Fig. 1B), but not at 1 μM (data not shown). PP3, an inactive analog of PP2, had no effect on dopamine release under these conditions. The total content of dopamine did not change after the pretreatment with genistein (control, 0.97 ± 0.02; 50 μM genistein, 0.89 ± 0.03; 50 μM genistin, 1.05 ± 0.01 nmol per 35-mm dish; the values are the mean ± SEM from four representative experiments in Fig. 1A) or PP2 (control, 1.62 ± 0.21; 1 μM PP2, 1.66 ± 0.21; 20 μM PP2, 1.60 ± 0.19; 20 μM PP3, 1.71 ± 0.21 nmol per 35-mm dish; the values are the mean ± SEM from six representative experiments in Fig. 1B.). Thus, tyrosine kinase inhibitors did not affect dopamine synthesis. Moreover, neither genistein nor PP2 affected the basal dopamine release observed in the absence of ionomycin. All of these findings suggest that src family protein tyrosine kinase has an inhibitory role in Ca2+-dependent neurotransmitter release from PC12 cells.
Figure 1.
Effects of tyrosine kinase inhibitors on Ca2+-dependent dopamine (DA) release from PC12 cells. PC12 cells were incubated for 20 min in the presence (●) or absence (○) of the tyrosine kinase inhibitor genistein (50 μM) (A) or PP2 (20 μM) (B) in low-K+ solution. An inactive genistein analogue (genistin, 50 μM) or an inactive PP2 analogue (PP3, 20 μM) was used as a control (■) to estimate the side effects of these inhibitors. After treatment, the cells were washed three times with low-K+ solution, then the extracellular buffer was changed four times every 2 min. Dopamine release was evoked with 0.5 μM ionomycin during the period indicated. The amount of dopamine released in the medium was expressed as percentages of the total cellular content. The values are the mean ± SEM from four (A) or six (B) representative experiments.
Overexpression of v-src-Inhibited Ca2+-Dependent Release from PC12 Cells.
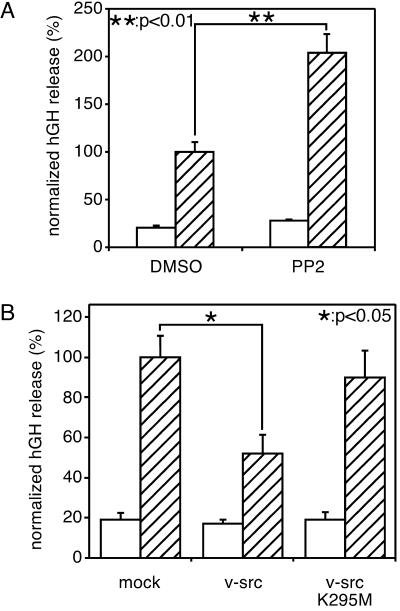
To further evaluate the role of src family tyrosine kinases in the regulation of neurotransmitter release, next we examined the effect of transient transfection of constitutively active avian v-src into PC12 cells by using a hGH-cotransfection system developed by Holz and his colleagues (19). As in the case of dopamine release, Ca2+-dependent hGH secretion was markedly enhanced by PP2 (Fig. 2A) and by genistein (data not shown). By contrast, overexpression of constitutive active v-src suppressed hGH release remarkably; however, the suppression was not observed on expression of the kinase inactive mutant, v-srcK295M (Fig. 2B). These results clearly indicate that the activation of src tyrosine kinase resulted in an inhibition of Ca2+-induced neurotransmitter release from PC12 cells and that the inhibitory mechanism depends on the kinase activity.
Figure 2.
Transfected hGH release from PC12 cells. (A) Effect of PP2 on release of transfected hGH from PC12 cells. PC12 cells were transfected with a plasmid encoding hGH (pcDNA3/hGH). After 2 days of culture, cells were washed five times with low-K+ solution and then incubated for 20 min in low-K+ solution with or without 20 μM PP2. After that, cells were washed three times and incubated for 2 min each in low-K+ solution (open bars) and low-K+ solution with 1 μM ionomycin (hatched bars) sequentially. (B) Suppression of Ca2+-dependent release of hGH by exogenously expressed v-src. PC12 cells were transfected with a plasmid encoding hGH (pcDNA3/hGH) with pcDNA3/v-src or pcDNA3/v-srcK295M. After two days of culture, cells were washed five times with low-K+ solution and then incubated for 2 min each in low-K+ solution (open bars) and low-K+ solution with 1 μM ionomycin (hatched bars) sequentially. To standardize results with the same plasmids from repeated experiments, the averaged hGH release, determined as a percentage of the total cellular contents in the control transfection, was set at 100% for all experiments. The values are the mean ± SEM from four representative experiments.
Identification of PP2-Sensitive Tyrosine-Phosphorylated Proteins in PC12 Cells.
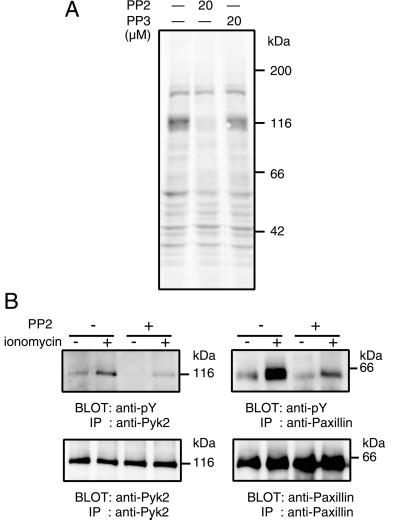
Because the inhibition of tyrosine phosphorylation appears to enhance neurotransmitter release, next we tried to identify the PP2-sensitive tyrosine-phosphorylated proteins. We examined the cellular proteins by Western blotting using an anti-phosphotyrosine antibody. As shown in Fig. 3A, several tyrosine-phosphorylated proteins were identified in the cellular homogenate of PC12 cells. PP2, but not PP3, treatment decreased the levels of tyrosine phosphorylation of several proteins (noticeable at 116–120 kDa, 110–90 kDa, and 60 kDa). Among tyrosine-phosphorylated proteins, Pyk2, a nonreceptor-type tyrosine kinase related to focal adhesion kinase, and paxillin, a cytoskeletal protein and focal adhesion adapter molecule, are known to be endogenous substrates of src family tyrosine kinases, the phosphorylation of which is regulated by Ca2+ influx into cells. Because the molecular masses of the tyrosine-phosphorylated proteins were similar to those of Pyk2 and paxillin, we examined whether Pyk2 and paxillin were phosphorylated in PC12 cells by an endogenous src family tyrosine kinase in a PP2-sensitive manner. Pyk2 and paxillin were immunoprecipitated from PC12 cell lysates and then immunoblotted with an anti-phosphotyrosine antibody (Fig. 3B). Ionomycin treatment, which induced Ca2+ influx into the cells, increased the amounts of tyrosine-phosphorylated Pyk2 and paxillin as reported previously (20, 21). Both the basal and ionomycin-induced tyrosine phosphorylation of Pyk2 and paxillin were decreased remarkably after the PP2 treatment. These results indicated that Pyk2 and paxillin were among the tyrosine-phosphorylated proteins sensitive to PP2. Synaptophysin is another well-known protein substrate of src kinase localized in synaptic vesicles. However, when tyrosine phosphorylation levels for synaptophysin in PC12 cells were estimated by immunoprecipitation and Western blotting, only faint bands were detected with anti-phosphotyrosine antibody, and they did not change in intensity under any conditions (with or without PP2 or membrane depolarization with high concentration of KCl), suggesting that synaptophysin was not a good substrate for src family kinases in PC12 cells, at least in the present condition (data not shown).
Figure 3.
Identification of PP2-sensitive tyrosine-phosphorylated proteins in PC12. (A) PC12 cells were incubated for 20 min at 37°C in low-K+ solution with or without 20 μM PP2 or PP3. After that, cells were lysed with lysis buffer and proteins from total cell lysates were subjected to Western blotting using a monoclonal anti-phosphotyrosine antibody. (B) PC12 cells were treated with or without 20 μM PP2 for 20 min at 37°C, and then cells were incubated in low-K+ solution with or without 1 μM ionomycin for 5 min at 37°C. Cells were lysed with lysis buffer, and the lysates were subjected to immunoprecipitation with anti-Pyk2 polyclonal antibody and anti-paxillin monoclonal antibody. The precipitated proteins were then subjected to Western blotting using a monoclonal anti-phosphotyrosine antibody (anti-pY). Blot sheets were reprobed with anti-Pyk2 and anti-paxillin monoclonal antibody after the stripping of anti-phosphotyrosine antibody. Positions of molecular mass markers are indicated on the right. IP, immunoprecipitation.
PP2 Treatment Altered Cell Shape and Decreased Phalloidin Reactivity.
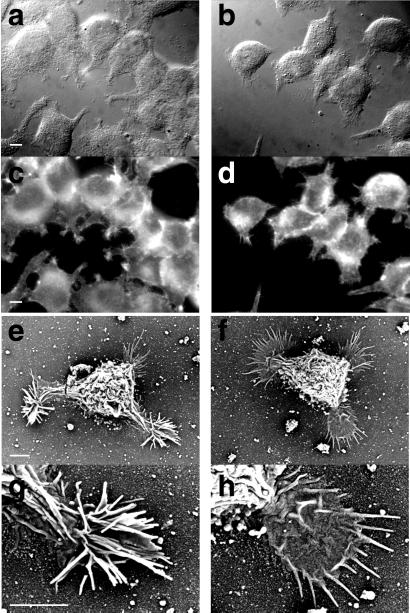
In focal adhesion plaques of various kinds of cells, Pyk2 and paxillin are localized as a complex with src kinase and involved in the regulation of cytoskeleton (22, 23). Therefore, we next examined the effect of PP2 on the dynamics of the F-actin cytoskeleton in PC12 cells by using rhodamine-phalloidin (Fig. 4 a–d). In the absence of PP2, PC12 cells had several processes attached to the substrate, and a very intensive staining of phalloidin was obvious at the tip of these processes. PP2 treatment, at a concentration high enough to abolish the tyrosine phosphorylation of Pyk2 and paxillin, diminished the accumulation of F-actin at the tip of the processes. PP2-induced morphological changes were observed more clearly by scanning electron microscopy (Fig. 4 e–h). In the absence of PP2, PC12 cells possessed many filopodium-like structures near the site of attachment to the culture substrate, and these structures were positioned three-dimensionally. PP2 treatment caused a marked change in the morphology: the filopodium-like structures disappeared and the extrusions attached to the substrate became flattened. These results suggested that a PP2-sensitive src family tyrosine kinase was involved in the formation of actin cytoskeleton in PC12 cells.
Figure 4.
Morphological change of PC12 cells to PP2 treatment. (a and b) Differential interference contrast microscopy. (c and d) Fluorescence microscopy (phalloidin staining). (e–h) Scanning electron microscopy. PC12 cells were incubated in low-K+ solution with (b, d, f and h) or without (a, c, e, and g) 20 μM PP2 for 20 min at 37°C. [a–d ×1000 (bar, 10 μm); e and f ×2000 (bar, 15 μm); g and h ×5000 (bar, 5 μm)].
Inhibition of Actin Dynamics Enhances Ca2+-Dependent Dopamine Release from PC12.
Because PP2 induced a change in actin dynamics, it is possible that the regulation of the actin cytoskeleton takes part in the enhancement of neurotransmitter release. To assess this possibility, we next examined the effect of membrane-permeant inhibitors of actin polymerization, cytochalasin D and latrunculin A, on the Ca2+-dependent dopamine release from PC12 cells. As shown in Fig. 5, both of these drugs enhanced Ca2+-induced dopamine release remarkably. The basal release in the absence of ionomycin was not affected by these treatments. These results indicate that actin dynamics have some inhibitory effect on the Ca2+-dependent exocytosis of secretory vesicles, and thus it is likely that src family tyrosine kinases take part in that regulation.
Figure 5.
Enhancement of dopamine (DA) release by actin filament disruption. PC12 cells were incubated for 2 h in the presence or absence (○) of 3 μM cytochalasin D (●) or 1 μM latrunculin A (■) under culture conditions. After the incubation, the cells were washed three times with low-K+ solution, and then the extracellular buffer was changed four times every 2 min. Dopamine release was evoked with 0.5 μM ionomycin. The amount of dopamine released into the medium was determined and expressed as a percentage of the total cellular content. The values are the mean ± SEM from four representative experiments.
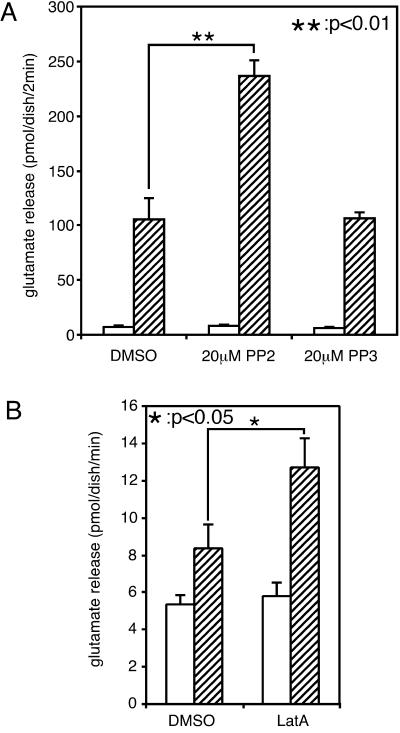
PP2 and Latrunculin A Enhance Ca2+-Dependent Glutamate Release from Cultured Cerebellar Granule Cells.
It is important to ascertain whether the inhibitory role of src family kinases in neurotransmitter release was also observed in neurons of the central nervous system. To this end, we examined the effect of PP2 on the release of glutamate, an excitatory neurotransmitter, from cultured cerebellar granule cells. Ionomycin-induced glutamate release was markedly enhanced by treatment with PP2 but not PP3 (Fig. 6A). Additionally, as observed in PC12 cells, inhibition of actin polymerization with latrunculin A also enhanced glutamate release from granule cells (Fig. 6B). These findings indicate that src family tyrosine kinases and the actin cytoskeleton play inhibitory roles even in synaptic transmission of the central nervous system as in PC12 cells.
Figure 6.
Enhancement of glutamate release from cultured cerebellar granule cells. (A) Cultured rat cerebellar granule cells were incubated for 20 min in the presence or absence of 20 μM PP2 (or PP3) in low-K+ solution in a water bath at 37°C. After treatment, cells were washed three times and incubated for 2 min each in low-K+ solution (open bars) and low-K+ solution with 5 μM ionomycin (hatched bars) sequentially to evoke neurotransmitter release. The amount of glutamate released into the medium was determined. The values are the mean ± SEM from four representative experiments. (B) Cultured rat cerebellar granule cells were incubated for 5 min in the presence or absence of 20 μM latrunculin A in low-K+ solution in a water bath at 37°C. After treatment, cells were washed three times and incubated for 1 min each in low-K+ solution (open bars) and low-K+ solution with 3 μM ionomycin (hatched bars) sequentially to evoke neurotransmitter release. The amount of glutamate released into the medium was determined. The values are the mean ± SEM from eight representative experiments.
Discussion
In the present study, we found (i) that tyrosine kinase inhibitors enhanced Ca2+-dependent neurotransmitter release from PC12 cells and cerebellar granules cells, and (ii) that a transient transfection of constitutive active v-src resulted in an enhancement of release from PC12 cells. We also found that the involvement of the actin cytoskeleton in the regulation of neurotransmitter release and its dynamics is regulated by src family tyrosine kinase. These results suggest that the src family of tyrosine kinases plays an inhibitory role in neurotransmitter release from neuronal cells, possibly through the regulation of actin cytoskeleton dynamics.
Several src family tyrosine kinases, such as src, fyn, and yes (24, 25), are expressed in the brain. Among them, c-src, which is abundant in synaptic vesicles, accounts for most of the tyrosine kinase activity in these vesicles (4, 5) and phosphorylates synaptic vesicle proteins, including synaptophysin (6). In the present study, we showed that neurotransmitter release from PC12 cells was suppressed by an overexpression of constitutive active v-src, but not by the inactive mutant v-srcK295M. All of these results suggest that c-src is involved in the suppression of neurotransmitter release from neuronal cells, but further study is necessary to specify the src kinase actually involved in the regulation of neurotransmitter release from PC12 cells and neurons.
Neurotransmitter release is a complex phenomenon and occurs in several steps, all of which could be modified by protein tyrosine phosphorylation. The tyrosine kinase inhibitors and v-src modified neurotransmitter release induced not only by high-K+ depolarization, but also by Ca2+/ionomycin, indicating that some steps after Ca2+ entry are regulated by tyrosine phosphorylation. It is possible that the tyrosine kinase inhibitors enhance dopamine release by affecting dopamine synthesis; however, this is unlikely because the dopamine content of PC12 cells was not changed by the treatment. Alternatively, it is possible that the src kinase apparently decreases dopamine release by stimulating dopamine reuptake by the dopamine transporter in the cell membrane. However, this possibility is also unlikely because we found that the tyrosine kinase inhibitors also enhanced the release of exogenously transfected hGH. Exogenously expressed hGH is coreleased with dopamine by an exocytosis of large dense-cored vesicles from PC12 cells. The released dopamine is reaccumulated into the cells by the dopamine transporter, but there is no reuptake system for hGH. Thus, it is quite likely that src kinase suppressed neurotransmitter release by modulating some exocytotic steps.
What is the protein substrate involved in the src-mediated inhibition of neurotransmitter release? Synaptophysin has been identified as an endogenous substrate of src kinase, but we did not detect significant tyrosine phosphorylation of synaptophysin in our experiment. By immunoblotting with an anti-phosphotyrosine antibody, we detected many tyrosine-phosphorylated proteins in the PC12 cell lysate. Among them, 116–120-kDa and 60-kDa proteins showed a reduction in tyrosine phosphorylation with PP2 treatment. In molecular size, these proteins were very similar to Pyk2 and paxillin, respectively, which are known to be phosphorylated by c-src in many cells (22, 26). Together with these findings, our specific immunoprecipitation assays indicated that Pyk2 and paxillin are major substrates of the src family kinases in PC12 cells. Paxillin is a cytoskeletal protein and functions as a scaffold for the recruitment of signaling molecules during cell adhesion and cell morphogenesis (22). In neuronal cells, paxillin is implicated in the regulation of cytoskeletal reorganization and/or cell adhesion changes during neuronal differentiation (27). Additionally, in association with Pyk2, paxillin regulates cytoskeletal reorganization during NGF-induced PC12 cell differentiation (28–30). Moreover, disruption of the actin cytoskeleton inhibits Pyk2 activation, suggesting that cytoskeletal integrity is required for Pyk2 activation (23). Several studies show that actin cytoskeleton has an important role in the fine regulation of neurotransmitter release (31–33). In the present study, we found that the src-family kinase inhibitor, PP2, exerted acute effects on the dynamics of the actin cytoskeleton and that inhibitors of actin polymerization enhanced neurotransmitter release from PC12 cells and cerebellar granule cells. These results suggest that the actin cytoskeleton has inhibitory roles in neurotransmitter release in these cells. Morales et al. (34) also suggested an inhibitory role in cultured hippocampal neurons. In their study, they showed that latrunculin A but not cytochalasin D increased neurotransmission. In fact, we also observed that cytochalasin D did not affect glutamate release from cerebellar granule neurons (data not shown). It may be possible that there are several kinds of cytoskeletons having different degrees of sensitivity to actin-depolymerizing reagents. It will be quite important to evaluate functional differences of them in synaptic functions. Although the signal-transduction mechanisms that regulate the actin cytoskeleton have yet to be elucidated, src family kinases are leading candidates for the key proteins regulating cytoskeleton in presynaptic nerve terminals.
In addition to Pyk2 and paxillin, we observed several other proteins that were tyrosine phosphorylated in a PP2-dependent manner (Fig. 3A). It is quite interesting and important to characterize these proteins for the further understanding of src family tyrosine kinase-mediated inhibition of neurotransmitter release.
In synaptic terminals, the excitatory response of neurons induces Ca2+ influx through voltage-dependent channels, and Ca2+ influx triggers the release of neurotransmitters. Ca2+ influx is reported to increase c-src kinase activity and to induce a redistribution of c-src from the cytosol to the plasma membrane (8). Indeed, we detected an increase in the tyrosine phosphorylation of src substrates, Pyk2 and paxillin, in response to Ca2+ influx and an inhibition by PP2 treatment. It is an attractive hypothesis that Ca2+ influx, first evoking exocytosis, acts as a negative-feedback signal on exocytosis through the src-family kinase pathway. In conclusion, we demonstrated that the src family of tyrosine kinases regulates exocytosis in neuronal cells. Various kinases, including protein kinase C, protein kinase A, and mitogen-activated protein kinase, stimulate neurotransmitter release from several neuronal preparations (1); however, reports on protein kinases that are involved in negative regulation as we reported in this paper are scarce. A next step is to examine the functional signaling pathway of tyrosine kinases regulating neurotransmitter release and synaptic plasticity.
Acknowledgments
We thank Dr. M. Iwashima and Dr. Y. Hakak for generously donating v-src and v-src K295 M cDNA, respectively, and Dr. M. J. Seagar for critical reading of this manuscript. This work was supported by Core Research for Evolutional Science and Technology (CREST) of Japan Science and Technology Corporation (JST).
Abbreviation
- hGH
human growth hormone
References
- 1.Turner K M, Burgoyne R D, Morgan A. Trends Neurosci. 1999;22:459–464. doi: 10.1016/s0166-2236(99)01436-8. [DOI] [PubMed] [Google Scholar]
- 2.Jovanovic J N, Czernik A J, Fienberg A A, Greengard P, Sihra T S. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- 3.Gurd J W. Neurochem Int. 1997;31:635–649. doi: 10.1016/s0197-0186(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 4.Pang D T, Wang J K T, Valtorta F, Benfenati F, Greengard P. Proc Natl Acad Sci USA. 1988;85:762–766. doi: 10.1073/pnas.85.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linstedt A D, Vesicle M L, Bishop J M, Kelly R B. J Cell Biol. 1992;117:1077–1084. doi: 10.1083/jcb.117.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnekow A, Jahn R, Schartl M. Oncogene. 1990;5:1019–1024. [PubMed] [Google Scholar]
- 7.Okumura N, Okada M, Nakagawa H. J Biochem (Tokyo) 1994;116:346–350. doi: 10.1093/oxfordjournals.jbchem.a124530. [DOI] [PubMed] [Google Scholar]
- 8.Rusanescu G, Qi H, Thomas S M, Brugge J S, Halegoua S. Neuron. 1995;15:1415–1425. doi: 10.1016/0896-6273(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 9.Onofri F, Giobedí S, Vaccaro P, Czernik A J, Valtorta F, Camilli P D, Greengard P, Benfenati F. Proc Natl Acad Sci USA. 1997;94:12168–12173. doi: 10.1073/pnas.94.22.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster-Barber A, Bishop J M. Proc Natl Acad Sci USA. 1998;95:4673–4677. doi: 10.1073/pnas.95.8.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levi G, Aloisi F, Ciotti M T, Thangnipon W, Kingsbury A, Balazs R. In: Dissection and Tissue Culture Manual of the Nervous System. Shahar A, de Vellis J, Vernadakis A, Haber B, editors. New York: Liss; 1989. pp. 211–214. [Google Scholar]
- 12.Nishiki T, Shoji-Kasai Y, Sekiguchi M, Iwasaki S, Kumakura K, Takahashi M. Biochem Biophys Res Commun. 1997;239:57–62. doi: 10.1006/bbrc.1997.7427. [DOI] [PubMed] [Google Scholar]
- 13.Takei N, Sasaoka K, Inoue K, Takahashi M, Endo Y, Hatanaka H. J Neurochem. 1997;68:370–375. doi: 10.1046/j.1471-4159.1997.68010370.x. [DOI] [PubMed] [Google Scholar]
- 14.Itakura M, Misawa H, Sekiguchi M, Takahashi S, Takahashi M. Biochem Biophys Res Commun. 1999;265:691–696. doi: 10.1006/bbrc.1999.1756. [DOI] [PubMed] [Google Scholar]
- 15.Wijetunge S, Hughes A D. Biochem Biophys Res Commun. 1995;217:1039–1044. doi: 10.1006/bbrc.1995.2874. [DOI] [PubMed] [Google Scholar]
- 16.Cataldi M, Taglialatela M, Guerriero S, Amoroso S, Lombardi G, Di Renzo G, Annunziato L. J Biol Chem. 1996;271:9441–9446. doi: 10.1074/jbc.271.16.9441. [DOI] [PubMed] [Google Scholar]
- 17.Paillart C, Carlier E, Guedin D, Dargent B, Couraud F. J Pharmacol Exp Ther. 1997;280:521–526. [PubMed] [Google Scholar]
- 18.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 19.Wick P F, Senter R A, Parsels L A, Uhler M D, Holz R W. J Biol Chem. 1993;268:10983–10989. [PubMed] [Google Scholar]
- 20.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 21.Khan M A, Okumura N, Okada M. Eur J Biochem. 1996;235:579–584. doi: 10.1111/j.1432-1033.1996.00579.x. [DOI] [PubMed] [Google Scholar]
- 22.Turner C E. Int J Biochem Cell Biol. 1998;30:955–959. doi: 10.1016/s1357-2725(98)00062-4. [DOI] [PubMed] [Google Scholar]
- 23.Avraham H, Park S-Y, Schinkmann K, Avraham S. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 24.Brown M T, Cooper J A. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 25.Thomas S M, Brugge J S. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 26.Girault J-A, Costa A, Derkinderen P, Studler J-M, Toutant M. Trends Neurosci. 1999;22:257–263. doi: 10.1016/s0166-2236(98)01358-7. [DOI] [PubMed] [Google Scholar]
- 27.Leventhal P S, Feldman E L. J Biol Chem. 1996;271:5957–5960. doi: 10.1074/jbc.271.11.5957. [DOI] [PubMed] [Google Scholar]
- 28.Alemá S, Casalbore P, Agostini E, Tató F. Nature (London) 1985;316:557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- 29.Haefner B, Frame M C. Biochem J. 1997;328:649–655. doi: 10.1042/bj3280649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S-Y, Avraham H, Avraham S. J Biol Chem. 2000;275:19768–19777. doi: 10.1074/jbc.M909932199. [DOI] [PubMed] [Google Scholar]
- 31.Matter K, Dreyer F, Aktories K. J Neurochem. 1988;52:370–376. doi: 10.1111/j.1471-4159.1989.tb09131.x. [DOI] [PubMed] [Google Scholar]
- 32.Doussau F, Augustine G J. Biochimie. 2000;82:353–363. doi: 10.1016/s0300-9084(00)00217-0. [DOI] [PubMed] [Google Scholar]
- 33.Trifaró J-M, Rosé S D, Lejen T, Elzagallaai A. Biochimie. 2000;82:339–352. doi: 10.1016/s0300-9084(00)00193-0. [DOI] [PubMed] [Google Scholar]
- 34.Morales M, Colicos M A, Goda Y. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]