Abstract
Objectives
To determine the contemporary effectiveness of exercise-based cardiac rehabilitation (CR) in terms of all-cause mortality, cardiovascular mortality and hospital admissions.
Data sources
Studies included in or meeting the entry criteria for the 2016 Cochrane review of exercise-based CR in patients with coronary artery disease.
Study eligibility criteria
Randomised controlled trials (RCTs) of exercise-based CR versus a no-exercise control whose participants were recruited after the year 2000.
Study appraisal and synthesis methods
Two separate reviewers independently screened the characteristics of studies. One reviewer quality appraised any new studies and assessed their risk of bias using the Cochrane Collaboration’s recommended risk of bias tool. Data were reported as the risk difference (95% CI).
Results
We included 22 studies with 4834 participants (mean age 59.5 years, 78.4% male). We found no differences in outcomes between exercise-based CR and a no-exercise control at their longest follow-up period for: all-cause mortality (19 studies; n=4194; risk difference 0.00, 95% CI −0.02 to 0.01, P=0.38) or cardiovascular mortality (9 studies; n=1182; risk difference −0.01, 95% CI −0.02 to 0.01, P=0.25). We found a small reduction in hospital admissions of borderline statistical significance (11 studies; n=1768; risk difference −0.05, 95% CI −0.10 to −0.00, P=0.05).
Conclusions and implications of key findings
Our analysis indicates conclusively that the current approach to exercise-based CR has no effect on all-cause mortality or cardiovascular mortality, when compared with a no-exercise control. There may be a small reduction in hospital admissions following exercise-based CR that is unlikely to be clinically important.
PROSPERO registration number
Keywords: coronary artery disease, exercise-based cardiac rehabilitation, all-cause mortality, cardiovascular mortality, hospital admissions.
Strengths and limitations of this study.
To our knowledge, this is the first systematic review of exercise-based cardiac rehabilitation (CR) that has pooled data relevant to the current medical management of patients diagnosed with coronary artery disease.
For analysis, we present the data as the risk difference (95% CI), which ensures all studies reporting data on the outcomes of interest were included.
This systematic review pools data from studies that deliver an intervention recognised as best practice in exercise-based CR, where multiple approaches, including educational/psychosocial components, as well as the exercise component were used.
We have not done a de novo quality assessment of 21/22 studies included in this review and instead rely on a previous Cochrane assessment.
We did not include health-related quality of life as an outcome measure as this is unsuitable for meta-analysis.
Background
Cardiovascular disease is the world’s biggest killer, accounting for 15 million deaths in 2015.1
Secondary prevention of coronary artery disease through exercise-based CR in those who have a diagnosis of coronary artery disease has the potential to reduce mortality, reduce hospital admissions and increase quality of life. Guidelines internationally endorse the use of exercise-based cardiac rehabilitation (CR) programmes.2–5
Typically, exercise-based CR aims to achieve 20–60 min of moderate intensity continuous exercise, 3–5 times a week, with muscular strength and endurance exercises prescribed in conjunction.6 Additionally, most programmes include supplementary education (coronary risk factors and cardiac misconceptions), advice on diet and access to psychological support.2 4 7 8 Typically, exercise-based CR is delivered in a supervised centre-based setting, although home-based programmes are used.9
A 2016 Cochrane review (63 studies, n=14 486 participants) found benefits of exercise-based CR for patients with coronary artery disease. Both cardiovascular mortality (27 studies, risk ratio (RR) 0.74, 95% CI 0.64 to 0.86) and hospital readmissions were reduced (15 studies, RR 0.82, 95% CI 0.70 to 0.96), when compared with a no-exercise control. However, in contrast to previous systematic reviews and meta-analyses, there was no significant reduction in risk of reinfarction (36 studies, RR 0.90, 95% CI 0.79 to 1.04) or all-cause mortality (47 studies, RR 0.96, 95% CI 0.88 to 1.04).10
Over recent decades, the medical management of coronary artery disease has been transformed. The introduction of primary percutaneous coronary intervention has reduced short-term major adverse cardiac events and increased long-term survival.11–14 Simultaneously, there have also been widespread advances in secondary preventative medical therapy. This includes the introduction of aspirin and beta-blockers in the 1980s,15 16 lipid-lowering statins and ACE inhibitors in the 1990s17 18 and, more recently, the introduction of clopidogrel, a secondary antiplatelet, in 2007.19 20 Age-adjusted mortality has decreased substantially in this population.21 Systematic reviews and meta-analyses that include data from older studies may not correctly assess the potential effect of exercise-based CR. We hypothesise that previous reviews have overestimated the benefit of exercise-based CR.
Objectives
To determine the contemporary effectiveness of exercise-based CR on all-cause mortality, cardiovascular mortality and hospital readmissions in patients with coronary artery disease.
Methods
We conducted and reported this meta-analysis in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses.22
Search strategy
To identify relevant studies, we started with the latest Cochrane review of exercise-based CR in patients with coronary artery disease.10 Studies identified as ‘awaiting assessment’ or ‘on-going’ in this review were revisited to establish whether publication had been reached. To identify any new studies published since the completion of the Cochrane review, an updated search was run on 28 February 2017. This search used the same search strategies as the latest Cochrane review.10 We searched Cochrane Central Register of Controlled Trials (online supplementary appendix 1), MEDLINE (Ovid), Embase (Ovid) and CINAHL (EBSCO) databases. This approach allowed us to efficiently identify all relevant studies. Where appropriate, we contacted original authors for clarification of any new included studies.
bmjopen-2017-019656supp001.pdf (83.7KB, pdf)
Two separate reviewers (RP and GM) independently screened the characteristics of studies in the latest Cochrane review, studies identified as ‘awaiting assessment’ or ‘on-going’ and studies identified in the updated search. Full-text publications were retrieved to allow for further examination and to verify study inclusion. Any discrepancies were resolved by a third reviewer (MU).
Criteria for considering studies
In 1996, The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology first recommended early (within 2 hours) primary percutaneous interventions in preference to thrombolytic therapy for acute myocardial infarction.23 Two years later, guidelines set by the Joint British recommendations on prevention of Coronary Heart Disease in Clinical Practice were published outlining the recommendations for best practice for secondary prevention medical therapies.24 Although there have been some changes, notably the introduction of a second antiplatelet agent in the early 2000s,19 20 the approach to secondary prevention medical therapies has not changed since then. Allowing time for implementation of these guidelines and recommendations, we identified and included studies whose participants were recruited after the year 2000 to represent a contemporary population engaging in exercise-based CR.
Where there was no indication of recruitment period, the diagnosis and the secondary preventative medical therapy received by participants included in the trial determined the inclusion or exclusion of the study in the analysis.
Types of studies
We included randomised controlled trials of exercise-based CR compared with a no-exercise control with a minimum follow-up period of 6 months. Data reported at the longest follow-up period were included in the analysis.
Types of participants
We used the same entry criterion as previous Cochrane reviews:
people who have had a myocardial infarction or who had undergone revascularisation (coronary artery bypass grafting or percutaneous coronary intervention) or who have angina pectoris or coronary artery disease defined by angiography.
on optimal secondary preventative medical therapy defined by the Joint British recommendations on prevention of Coronary Heart Disease in Clinical Practice.24
recruited to hospital-based, community-based or home-based CR programmes.
Types of intervention(s)
Randomised controlled trials consisted of supervised or non-supervised exercise-based CR. The intervention was exercise alone or exercise as part of a comprehensive CR programme (consisting of educational/psychosocial components). ‘No exercise control’ consisted of standard medical care, including optimal secondary preventative medical therapy, education and advice about diet and exercise, psychosocial support but with no formal exercise intervention.
Types of outcome measures
We extracted data on: all-cause mortality, cardiovascular mortality and hospital readmissions. We did not include health-related quality of life as the authors of the 2016 Cochrane review found this unsuitable for meta-analysis.
Data collection, statistical analysis and quality assessment
We pooled data using Review Manager V.5.3.25 Previous Cochrane reviews have presented the data as individual and pooled risk ratio (95% CI). Using risk ratios automatically removed studies with no events in either study arm from the analysis. Nine studies (n=936 participants) reporting on all-cause mortality, cardiovascular mortality or hospital readmissions were excluded from one or more meta-analyses in the 2016 Cochrane review for this reason. We therefore present the data as the risk difference (95% CI), which ensures all studies reporting data on the outcomes of interest were included.
We applied a random-effects model to all analyses given the clinical heterogeneity of individual studies. Heterogeneity of included studies were tested statistically using the χ2 test of heterogeneity and I2 statistic.26
We did not repeat quality assurance checks already completed by the authors of the Cochrane review. For separate study risk of bias breakdown for these studies, we refer the reader to the existing characteristics of studies.10 For studies identified as ‘awaiting assessment’ or ‘on-going’ in the latest Cochrane review or in the updated search, we quality appraised these studies and assessed their risk of bias using the Cochrane Collaboration’s recommended risk of bias tool.27
Assessment of risk of bias in additional included study
One reviewer (RP) assessed the risk of bias in any additional included studies (table 1). Assessment of three further quality domains as outlined in the latest Cochrane review was also conducted (groups balanced at baseline, intention-to-treat analysis and groups received comparable treatment (except exercise)). A breakdown of the criteria used for assessing these three domains can be found in the latest Cochrane review. Risk of bias assessments were checked by a second reviewer (GM) and any discrepancies were resolved by a third reviewer (MU).
Table 1.
Risk of bias assessment for additional study
| Santaularia et al 29 | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list in blocks of 10 was created by a computer random number generator. The randomisation list and the allocation of patients to each group were independently controlled by the Clinical Trials Unit. |
| Allocation concealment (selection bias) | Low risk | A randomisation list in blocks of 10 was created by a computer random number generator. The randomisation list and the allocation of patients to each group were independently controlled by the Clinical Trials Unit. |
| Blinding of outcome assessment (detection bias): all outcomes | Low risk | An independent committee that was blind to the patients’ treatment group assessed the main outcomes. This committee comprised a cardiologist, a rehabilitation cardiologist and a health information manager, all from different centres. |
| Incomplete outcome data (attrition bias): all outcomes | Low risk | There was no loss to follow-up. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results. Results regarding quality of life are presented in supplementary data but were not required for the current review. |
| Groups balanced at baseline | Low risk | No significant differences between groups were observed, with the exception of gender: 23% of the control group were women compared with 7% in the intervention group (P=0.049). |
| Intention-to-treat analysis conducted | High risk | No analysis was conducted. |
| Groups received same treatment (apart from the intervention) | Low risk | Patients assigned to the control group received standard care given at the hospital. In addition to standard care, patients randomised to the intervention group. |
Patient involvement
No patients were involved in setting the objectives or outcome measures of this review, nor were they involved in the design or implementation. No patients were involved in the analysis or interpretation of the results, nor the writing of any drafts. There are no plans to disseminate the results of the review to participants included in the studies of the review or any relevant patient networks.
Results
Studies retrieved
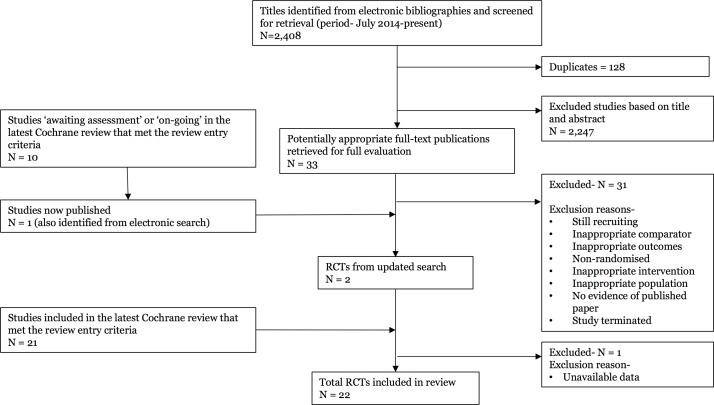
Of the 63 studies included in the Cochrane review, 21 studies met our entry criteria. We identified two additional relevant papers not included in the 2016 Cochrane review.28 29 One was excluded because data for our specific research question were not available in a useable format.28 In total, 22 studies (n=4834 participants) contributed to the analysis (figure 1). For the study identified from the updated search,29 there was a low risk of bias in all eight domains, apart from the intention-to-treat analysis, where there was no evidence of this analysis being conducted (table 1).
Figure 1.
Summary of study selection process. RCTs, randomised controlled trials.
Three studies (3/22; 14%) reported on all three outcomes of interest, 11 studies (11/22; 50%) reported on two outcomes of interest and 8 studies (8/22; 36%) reported on one outcome of interest.
Two studies for all-cause mortality30 31 and one study for cardiovascular mortality30 reported data at varying follow-up periods (6–12 months; >12–36 months; >3 years). Data from these studies were taken at their longest follow-up period. Mean maximum follow-up period was 24.7 months. Maximum follow-up period ranged from 24 weeks to 10 years (table 2).
Table 2.
Overview of participants, recruitment period, patient diagnosis and medical therapy
| Reference, country | N | Mean age (years) | Male participants (%) | Recruitment period (years) | Maximum follow-up period | Patient diagnosis | Medication |
| Aronov et al,39 Russia | 392 | 61.4 | 73.5 | None specified | 1 year | AMI, stable angina, unstable angina or myocardial revascularisation. | Standard medical therapy: β-blocker, acetylsalicyclic acid or other antithrombotic drug, nitrate, ACE inhibitor. Some patients on lipid-lowering drugs. |
| Belardinelli et al,32 Italy | 118 | 61 | 100 | None specified | 33 months | CAD including AMI. Successful PCI in one or two native epicardial coronary arteries only. | According to international accepted protocols: aspirin, ticlopidine, calcium antagonists and nitrates. |
| Briffa et al,40 Australia | 113 | 47.5 | 89.5 | None specified | 1 year | Uncomplicated AMI or recovery from unstable angina. PCI, CABG, thrombolytic therapy. | Aspirin, antiarrhytmic agent, β-blocker, ACE inhibitor, calcium antagonist, long-acting nitrate and diuretic. |
| Giallauria et al,33 Italy | 61 | 58.5 | 78.5 | None specified | 6 months | AMI and undergone primary or rescue PCI only. | Aspirin, β-blocker, ACE inhibitor, ARB and statin. |
| Hambrecht et al,30 Germany | 101 | 56 | 87.3 | 1997–2001 | 1 year | Stable CAD defined by angina pectoralis and amenable to PCI. AMI patients excluded. | β-receptor antagonists, β-HMG-CoA reductase inhibitors, ACE inhibitor and acetylsalicyclic acid. |
| Higgins et al,41 Australia | 105 | 60.8 | 81.3 | 1995–1997 | 51 weeks | Post-PCI patients only. No AMI 1-month preprocedure. | Reference to medical therapy, only breakdown for lipid-lowering medication. |
| Houle et al,42 Canada | 65 | 51.5 | 100 | 2007–2008 | 12 months | Patients hospitalised for an ACS (unstable angina, non-ST-elevation or ST elevation MI). PCI, CABG or no revascularisation procedure. | Reference to medication in usual care group but no breakdown. |
| Kovoor et al,43 Australia | 142 | 51.5 | 100 | None specified | 6 months | AMI only. Thrombolytic therapy, one patient in the exercise treatment group had primary angioplasty. | Aspirin, β-blocker, ACE inhibitor, calcium channel blockers, nitrates and cholesterol-lowering agents. |
| Maddison et al,44 New Zealand | 171 | 59 | 20 | 2010–2012 | 24 weeks | Diagnosis of IHD (angina, MI, revascularisation, including angioplasty, stent or CABG). | No description. |
| Maroto et al,34 Spain | 180 | 76.9 | 57.5 | (None specified) 2-year enrolment period | 10 years | AMI only. | Medication regimens employed in secondary prevention at discharge were clearly insufficient by standard criteria but currently meet Spanish and European guidelines. |
| Munk et al,35 Norway | 40 | 56.4 | 84.8 | None specified | 6 months | Stable angina and unstable angina, post-PCI only. AMI patients excluded. | Aspirin, β-blocker, ACE inhibitor, ARB, statin and acetylsalicyclic acid. |
| Mutwalli et al,45 Saudi Arabia | 49 | 69.7 | 100 | 2008–2010 | 6 months | Undergone CABG surgery. Unknown whether AMI patients included. | Participants received advice that focused on medications, no breakdown. |
| Oerkild et al,36 Denmark | 40 | 63.5 | 0 | 2007–2008 | 12 months (mortality data after 5.5 years) | Recent coronary event defined as AMI, PCI, CABG or without invasive procedure. | β-blocker, antithrombotics, calcium antagonists, lipid-lowering agents and diuretics. |
| Reid et al,46 Canada | 223 | 54.5 | 87.3 | 2004–2007 | 12 months | ACS including AMI, underwent successful PCI only. | Reference to a ‘descriptive summary in supplemental table’, no access. |
| Santaularia et al,29 Spain | 85 | 59.6 | 84.7 | None specified | 12 months | AMI only, no evidence of revascularisation procedure. | Reference to cardiac medication but no breakdown |
| Seki et al,47 Japan | 39 | 57.8 | 83.8 | None specified | NR | AMI, PCI or CABG. | Reference to ‘lipid-lowering drugs and other medications’, no breakdown. |
| Toobert et al,48 USA | 25 | 64.5 | 0 | None specified | 24 months | CAD defined as atherosclerosis, AMI, PCI or CABG. | Antihypertensive and hypolipidaemic medications. |
| Vestfold Heartcare Study Group,37 Norway | 197 | 64 | 75.8 | None specified | 2 years | AMI, unstable angina pectoris or after PCI or CABG. | Aspirin, β-blocker, statin, ACE inhibitor, calcium antagonist and warfarin. |
| Wang et al,49 China | 160 | 67 | 63.5 | 2005–2007 | 6 months | AMI only. | Antiplatelet, nitrate, β-blocker, ACE inhibitor, calcium antagonist and statin. |
| West et al,31 UK | 1813 | 51.9 | 93.9 | 1997–2000 | 7–9 years | AMI only. | Aspirin, β-blocker, ACE inhibitor, diuretic, long-acting nitrate/calcium channel blocker, statin and GTN. |
| Yu et al,50 China | 269 | 56 | 83.9 | None specified | 2 years | Recent AMI, after elective PCI or thrombolytic therapy. | Antiplatelet, β-blocker, calcium channel blocker, nitrate, statin, ACE inhibitor and diuretic. |
| Zwisler et al,38 Denmark | 446 | 55.5 | 72.1 | 2000–2003 | 1 year | AMI, angina pectoris or after PCI or CABG. | Antithrombotics, lipid-lowering drugs, β-blocker, calcium antagonists, ACE inhibitor, diuretic and long-acting nitrates. |
ACS, acute coronary syndrome; AMI, acute myocardial infarction; ARB, angiotensin receptor blockers; CABG, coronary artery bypass graft; CAD, coronary artery disease; GTN, glyceryl trinitrate; IHD, ischaemic heart disease; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Sample size, gender, age and study origin
Of our 22 studies, 10 studies were in Europe29–38 and 12 from outside of Europe.39–50 We included a total of 4834 participants (3788 (78.4%) males). Four studies included males only,30 34 45 47 and one study included women only.51 Participants mean age was 59.5 years. The mean age for individual studies ranged from 47.5 to 76.9 years (table 2).
Incomplete outcome data
The majority of trials (18/22; 82%) reported complete follow-up data, regardless of participants who were lost to follow-up or who dropped out. In four studies, outcome data were incomplete for 75 (75/4,834; 1.6%) participants with no description of withdrawal or dropout.41 47 48 50
Participant diagnosis of coronary artery disease and treatment received
The diagnosis of participants recruited to the studies was described in the majority of studies (21/22; 95%). Thirteen studies enrolled participants with mixed diagnoses, including angina pectoralis or coronary artery disease defined by angiography, myocardial infarction, percutaneous coronary interventions or coronary artery bypass grafts.32 36–42 44 46–48 50 Six studies enrolled participants following acute myocardial infarction only,29 31 33 34 43 49 and two studies enrolled participants diagnosed with angina pectoralis (unstable and stable angina) only.30 35 It was unclear from one study whether participants following myocardial infarction were included and instead the population was defined as ‘patients after coronary artery bypass graft surgery’45 (table 2).
Six studies recruited participants following percutaneous coronary intervention only30 32 33 35 41 46 and one study recruited participants following coronary artery bypass grafting only.45 Twelve studies included participants who had received thrombolysis, percutaneous coronary intervention, coronary artery bypass grafting and/or no revascularisation procedure.31 36–40 42–44 47 48 50 Three studies did not provide any breakdown of coronary intervention or surgical procedure received by participants prior to enrolment29 34 49 (table 2).
Medication
A full description and breakdown of the medication received by the participants, comparable with optimal secondary prevention medical therapy defined by the Joint British recommendations on prevention of Coronary Heart Disease in Clinical Practice set in 1998,24 was provided by 13/22 studies (59%).30–33 35–40 43 49 50 References to coexisting medical therapies were made in 7/22 (32%), but no breakdowns were provided.29 34 41 42 45–47 One study referred to the prescription of antihypertensive and hypolipidaemic medications without reference to other recommended medications.48 One study failed to provide any description or breakdown of coexisting medical therapies44 (table 2).
Clearly defined recruitment period
Seven studies (7/22; 32%) were explicit that they recruited participants after the year 2000.36 38 42 44–46 49 In three studies, participants were recruited either just before or during the year 2000.30 31 41 Due to participant diagnosis, treatment received and coexisting medical therapies, it was agreed by all reviewers to include these studies.
The remaining 12 studies failed to provide a recruitment period. Following further examination of the full papers, due to adequate description of patient diagnosis, treatment received and coexisting medical therapies, it was agreed by all reviewers to include these studies (table 2).
Content of the interventions
The content of the interventions tested was heterogeneous with multiple approaches being adopted. Sixteen studies (16/22; 73%) compared exercise in combination with other therapies (education and psychosocial management), while six studies compared exercise as a stand-alone intervention, against a no-exercise control. The exercise component alone varied considerably with respect to setting, training modality, duration, session length, frequency and intensity (table 3).
Table 3.
Overview of exercise interventions
| Reference, country | Exercise intervention | |||||
| Exercise | Modality | Study duration | Session duration/frequency/intensity | Additional | Control (comparator) |
|
| Aronov et al,39 Russia | Moderate intensity physical training (unknown setting). | Cycle ergometer. | 12 months | 45–60 min/three sessions per week/50%–60% of the performed capacity by bicycle ergometry. | None specified. | Standard medical therapy. |
| Belardinelli et al,32 Italy | Moderate intensity exercise (supervised in hospital gym). | Cycle ergometer. | 6 months | 53 min/three sessions per week/60% of peak oxygen uptake (VO2peak). | None specified. | Recommended to perform basic daily mild physical activities but to avoid any physical training. |
| Briffa et al,40 Australia | Aerobic circuit training (supervised in hospital). | Aerobic circuit training. | 6 weeks | 60–90 min/three sessions per week/not specified. | Education and psychosocial counselling. | Education, pharmacotherapy and lifestyle counselling. |
| Giallauria et al,33 Italy | Moderate intensity exercise (supervised in centre). | Cycle ergometer. | 6 months | 40 min/three sessions per week/60%–70% of peak oxygen uptake (VO2peak). | None specified. | Generic instructions on maintaining physical activity and a correct lifestyle. |
| Hambrecht et al,30 Germany | Moderate intensity exercise (supervised in hospital and unsupervised at home). | Cycle ergometer. | 12 months | 10 min, 42 sessions per week (hospital), 20 min, seven sessions per week (home) plus 60 min group training, one session per week/70% of symptom-limited max HR. | None specified. | Standard medical therapy. |
| Higgins et al,41 Australia | Moderate intensity walking programme (unsupervised at home). | Walking. | Not specified | Not specified/not specified/not specified. | Psychological plus education. | Psychological support, education, counselling and guidance. |
| Houle et al,42 Canada | Pedometer-based walking programme (unsupervised at home). | Walking. | 12 months | Not specified/not specified/not specified. | Education plus sociocognitive. | Sociocognitive support and advice regarding physical activity, diet and medication. |
| Kovoor et al,43 Australia | Standard cardiac rehabilitation programme (unknown setting). | Not specified. | 5 weeks | Not specified/2–4 sessions per week/not specified. | Education and counselling. | Encouraged to exercise at home and return to normal activities. |
| Maddison et al,44 New Zealand | Automated package of text messages to increase exercise behaviour (unsupervised at home). | Moderate to vigourous aerobic exercise, for example, walking and household chores. | 24 weeks | Minimum of 30 min/at least five sessions per week/not specified. | Optional access to other CR service or support. | Behaviour change therapy, encouragement to be physically active and advice to attend a cardiac club. |
| Maroto Montero et al,34 Spain | Individualised physical training (supervised in hospital gym). | Physiotherapy and aerobic training on mats or a cycle ergometer. | 3 months | 60 min/three sessions per week/75%–85% max HR. | Psychological support, education plus return to work counselling. | Psychological support, education plus return to work counselling. |
| Munk et al,35 Norway | Moderate/high intensity interval training (supervised in centre). | Cycle ergometer or running. | 6 months | 60 min/three sessions per week/60%–70% and 80%–90% max HR. | Spine and abdominal resistance training. | Usual care, including drug therapy. |
| Mutwalli et al,45 Saudi Arabia | Moderate intensity walking programme (unsupervised at home). | Walking. | 6 months | 30 min/seven sessions per week/not specified. | Education. | Education, standard hospital care |
| Oerkild et al,36 Denmark | Moderate intensity exercise (unsupervised at home). | Individualised. | 12 months | 30 min/six sessions per week/11–13 on the Borg Scale. | Risk factor management. | Usual care, no exercise education or dietary counselling. |
| Reid et al,46 Canada | Internet-based physical activity plan and motivational tool to increase physical activity (unsupervised at home). | Not specified. | 20 weeks | Not specified/not specified/not specified. | None specified. | Online education, physical activity guidance and an education booklet. |
| Santaularia et al,29 Spain | Outpatient exercise training programme (supervised in hospital). | Cycle ergometer. | 10 weeks | 60 min/three sessions per week/75%–90% max HR (RPE 11–15 on Borg Scale) | Resistance training, education and risk factor management. | Standard care, risk factor management, guidance on physical activity and adherence to medication. |
| Seki et al,47 Japan | Moderate intensity aerobic exercise (supervised in centre and unsupervised at home). | Walking, cycle ergometer and jogging. | 6 months | 50–110 min, one session per week (centre), ≥30 min, two sessions per week (home)/12–13 on the standard Borg Scale. | Education. | Education and outpatient follow-up with physician. |
| Toobert et al,48 USA | Walking or aerobics (supervised in centre and unsupervised at home). | Walking or aerobics. | 24 months | 60 min, seven sessions per week (centre), 60 min, three sessions per week (home)/individually prescribed. | Education and psychological support. | Cooking classes, stress management and education. |
| Vestfold Heartcare Study Group,37 Norway | Dynamic endurance physical activity (supervised, group sessions in centre). | Dynamic endurance training. | 15 weeks | 55 min/two sessions per week/RPE 11–13 on the Borg Scale, increased to 13–15 after 6 weeks. | Education and psychological support. | Education and psychological support. |
| Wang et al,49 China | Not specified. | Not specified. | Not specified | Not specified/not specified/not specified. | Education. | Education. |
| West et al,31 UK | Not specified, multicentre (supervised in centre). | Varied by centre (exercise equipment in physiotherapy gyms). | 6–8 weeks | Averaged 20 hours over 6–8 weeks/1–2 sessions per week/not specified. | Education plus psychological support. | Education plus psychological support. |
| Yu et al,50 China | Ambulatory and aerobic cardiovascular training (supervised in hospital and centre, unsupervised at home). | Walking, treadmill, cycle ergometry, rowing, stepper, arm ergometry and dumbbell | 8 1/2 months | 2 hours/two sessions per week (centre), not specified (home)/65%–85% of maximal aerobic capacity (VO2peak). | Resistance training and education. | Conventional medical therapy and education. |
| Zwisler et al,38 Denmark | Intensive CR programme (supervised in centre). | Not specified. | 6 weeks | Not specified/two sessions per week/not specified. | Education and psychosocial support. | Education and psychosocial support. |
CR, cardiac rehabilitation; HR, heart rate; RPE, rating of perceived exertion; VO2 Peak, peak oxygen uptake.
Overall effects of interventions
All-cause mortality
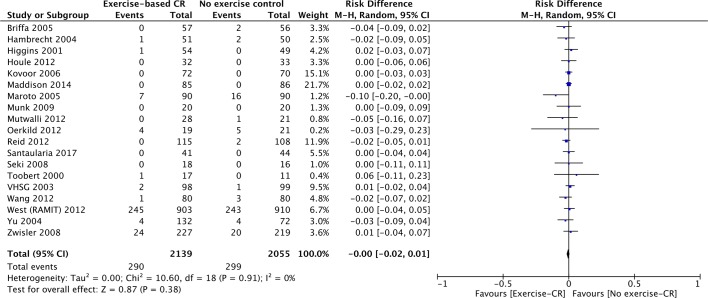
Nineteen studies (n=4194 participants) reported all-cause mortality (figure 2). There was no difference between groups at their longest follow-up (risk difference=0.00, 95% CI −0.02 to 0.01, P=0.38). There was no evidence of statistical heterogeneity across trials (P value=0.91, I2=0%).
Figure 2.
All-cause mortality for studies at their longest follow-up period. Filled squares represent the risk difference for individual studies at the longest reported follow-up. The boxes are proportional to the weight of each study, and the lines represent their 95% CI. The filled diamond represents the pooled risk difference. Weights are from random effects analysis. CR, cardiac rehabilitation.
Cardiovascular mortality
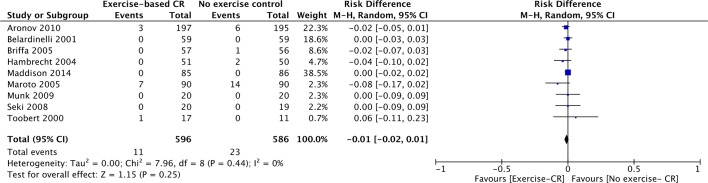
Nine studies (n=1182 participants) reported cardiovascular mortality (figure 3). There was no difference between groups at their longest follow-up (risk difference=−0.01, 95% CI −0.02 to 0.01, P=0.25). There was no evidence of statistical heterogeneity across trials (P value=0.44, I2=0%).
Figure 3.
Cardiovascular mortality for studies at their longest follow-up period. Filled squares represent the risk difference for individual studies at the longest reported follow-up. The boxes are proportional to the weight of each study and the lines represent their 95% CI. The filled diamond represents the pooled risk difference. Weights are from random effects analysis. CR, cardiac rehabilitation.
Hospital admissions
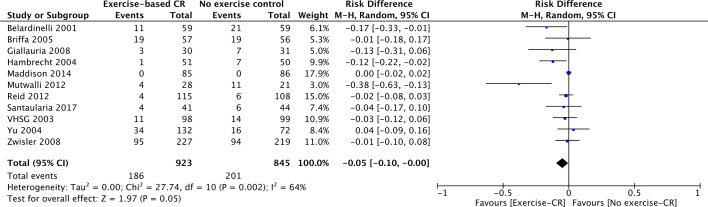
Eleven studies (n=1768 participants) reported on proportion with one or more hospital admissions (figure 4). There was a reduction of borderline statistical significance (risk difference=−0.05, 95% CI −0.10 to −0.00, P=0.05). There was evidence of statistical heterogeneity across trials (P value=0.002, I2=64%).
Figure 4.
Hospital admissions for studies at their longest follow-up period. Filled squares represent the risk difference for individual studies at the longest reported follow-up. The boxes are proportional to the weight of each study and the lines represent their 95% CI. The filled diamond represents the pooled risk difference. Weights are from random effects analysis. CR, cardiac rehabilitation.
Discussion
The effectiveness of exercise-based CR in patients with coronary artery disease has been determined by Cochrane systematic reviews and meta-analyses, providing clinicians and academics with the highest level of evidence over the last 17 years.10 52 53 The latest Cochrane review, conducted in 2016, found benefits of exercise-based CR in terms of reduced cardiovascular mortality and hospital admissions, but unlike previous Cochrane reviews, found no effect on all-cause mortality.10 We identified that data from studies included in this review dated back as far as 1975.54 By including such historical data, this Cochrane review may not be correctly assessing the potential effect of contemporary exercise-based CR.
The current review aimed to assess the effect of exercise-based CR in the era of improved reperfusion strategies and simultaneous advances in pharmacological management, by only including studies whose participants were recruited after the year 2000. The majority of interventions tested in the 22 included trials (table 3) delivered an intervention recognised as best practice in exercise-based CR, where multiple approaches, including educational/psychosocial components, as well as the exercise component were used.2 3 8 The interventions were tested against a no exercise control consisting of educational and psychosocial components alone (table 3).
The current analyses demonstrated no improvement in all-cause mortality from participation in exercise-based CR: the risk difference was 0.00 (95% CI −0.02 to 0.01). The largest trial included in our analysis, the UK-based Rehabilitation after myocardial infarction trial (RAMIT) trial, sought to show a 20% reduction in relative risk based on an 11% mortality, that is, a 2.2% risk difference.24 The limits of the 95% CI for the effect size in our analysis do not include the RAMIT trial’s prespecified clinically important difference. We therefore conclude that it is extremely unlikely that there is a worthwhile benefit from exercise-based CR on all-cause mortality. Furthermore, it is unlikely that future trials of similar interventions and populations will change this conclusion. This is supported by a recent meta-analysis that included participants with other forms of atherosclerotic cardiovascular disease, that is, peripheral artery disease, ischaemic cerebrovascular accidents, diabetes and hypertension. They too found a zero effect on all-cause mortality (relative risk 1.00, 95% CI 0.88 to 1.14).55 With the mean follow-up period for all studies included in our review being 24.7 months, it may be that any benefits on mortality will accrue over a longer follow-up. However, the absence of any kind of signal in this review means a substantial longer term benefit is unlikely.
The current analyses do not quite exclude a worthwhile benefit of exercise-based CR on hospital admissions. While a risk difference of −0.05 (95% CI −0.10 to −0.00) is of borderline statistical significance, it is probably clinically unimportant in the context of no change in all-cause mortality.
From the studies included in this review, we do not know if there is a worthwhile benefit on quality of life, as a meta-analysis was not conducted. However, the authors of the 2016 Cochrane review reported that in 4 of the 22 studies included in this review, there was a significantly higher quality of life in at least half or more of the subscales.32 45 46 49
Based on the present data, we are also unable to comment on whether exercise-based CR might be cost-effective. Five of the studies in this review included a within-trial health economic evaluation.30 40 43 44 50 Of these five papers, three studies showed no difference in healthcare costs between groups,40 43 50 one found healthcare costs to be lower for exercise-based CR30 and one failed to report a P value for cost difference.44 While a decrease, of borderline statistical significance, in hospital admissions may improve quality of life for patients, it is unclear if this confers any economic benefit, in the absence of robust cost-effectiveness analyses.
It may be that exercise-based CR has an effect on other outcomes, not specifically addressed in this review, such as cardiorespiratory fitness, lifestyle risk factor management, adherence to medication, diet, smoking cessation, psychosocial health and return to work.7 8 56 57 If the focus of future research is on measuring and improving these outcomes, attention will be needed to develop the best multicomponent intervention.
Strengths and limitations
To our knowledge, this is the first systematic review of exercise-based CR that has pooled data relevant to contemporary medical management of patients diagnosed with coronary artery disease. Although we have not done a de novo quality assessment of 21/22 studies included in this review and instead are relying on a previous Cochrane assessment, it is unlikely that we would have drawn different conclusions from such an assessment.10
The current review does not provide information on participant baseline characteristics. In the majority of studies (20/22; 91%), however, baseline characteristics were comparable between the intervention and control groups.10 29
While there was no evidence of statistical heterogeneity across trials for all outcome measures (P value <0.01, I2 >30%), except for hospital admissions, there was substantial context and interventional heterogeneity. The studies came from a wide range of clinical environments and countries, and the interventions delivered ranged greatly in quality. When compared with both the BACPR ‘minimum standards and core components’8 and Association of Chartered Physiotherapists in Cardiac Rehabilitation (ACPICR) guidelines,6 there was considerable variation in the exercise interventions delivered (table 3). Critics have questioned the exercise component reported in the largest included study—the RAMIT trial (n=1813).31 They argued that underdosage of exercise intensity and duration may have led to the inconclusive result.58 Several other studies included in this review also fail to report on the intensity, modality and/or duration of the exercise intervention. Exercise and physical activity has a ‘dose–response’ relationship with cardiovascular disease risk.59 Moreover, a higher exercise capacity (VO2 peak) is associated with an improvement in mortality risk.60 61 If patients engaging in exercise-based CR do not achieve the correct dose of exercise, a physiological benefit is unlikely. It is a legitimate concern that participants in many included trials may not have received an adequate dose of exercise. In the era of contemporary medical management, higher intensity exercise protocols might be appropriate and effective.62
One major concern is the reporting of adherence to, and fidelity of, exercise interventions.10 While the majority of studies included in this review report the intended prescription exercise dose29 30 32–37 39 40 47 50 (table 3), it is not possible to determine adherence and fidelity. Without basic reporting of these parameters, the actual exercise dose received cannot be quantified. This may have a significant bearing on intervention efficacy and the results of this meta-analysis. Moving forward, the introduction of checklists and reporting standards of interventional studies should improve reporting quality and trial interpretation.63
Conclusion
Based on the outcomes of all-cause mortality and cardiovascular mortality, our analysis indicates conclusively that the current approach to exercise-based CR has no effect when compared with a no-exercise control. There may be a small reduction in hospital admissions following exercise-based CR that is unlikely to be clinically important.
The continued delivery of exercise-based CR needs to be supported by new research to show its impact on health-related quality of life and whether it is a cost-effective intervention.
Supplementary Material
Footnotes
Contributors: RP and MU were principally responsible for the study concept and design. RP and GM were responsible for study selection, data extraction and risk of bias assessment. With the assistance of University Hospital Coventry & Warwickshire library services, RP updated and ran the searches. RP, MU and PKK were responsible for statistical analysis and interpretation of data. GM and SE provided clinical advice. RP and MU wrote the first draft of the review, and all coauthors contributed to review and editing of drafts of the report. All authors approved the final manuscript. RP is the study guarantor and had full access to all trial level data in the review, takes responsibility for the integrity of the data, and accuracy of the data analysis, and had final responsibility to submit for publication.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: This review was not funded, and hence no role was played by funders in the conception, data synthesis, analysis, interpretation or in the drafting of the manuscript.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1.World Health Organisation. Top 10 causes of death worldwide- Fact Sheet. 2017. http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed 29 May 2017).
- 2.NICE. Myocardial Infarction: cardiac rehabilitation and prevention of further cardiovascular disease. 2013. https://www.nice.org.uk/guidance/cg172/resources/myocardial-infarction-cardiac-rehabilitation-and-prevention-of-further-cardiovascular-disease-pdf-35109748874437 (accessed 31 May 2017).
- 3.BACPR. Cardiovascular disease prevention and rehabilitation. 2012. http://www.bacpr.com/resources/46C_BACPR_Standards_and_Core_Components_2012.pdf (accessed 31 May 2017).
- 4.Piepoli MF, Corrà U, Adamopoulos S, et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European association for cardiovascular prevention & rehabilitation. Endorsed by the committee for practice guidelines of the European society of cardiology. Eur J Prev Cardiol 2014;21:664–81. 10.1177/2047487312449597 [DOI] [PubMed] [Google Scholar]
- 5.Balady GJ, Williams MA, Ades PA, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American heart association exercise, cardiac rehabilitation, and prevention committee, the council on clinical cardiology; the councils on cardiovascular nursing, epidemiology and prevention, and nutrition, physical activity, and metabolism; and the American association of cardiovascular and pulmonary rehabilitation. J Cardiopulm Rehabil Prev 2007;27:121–9. 10.1097/01.HCR.0000270696.01635.aa [DOI] [PubMed] [Google Scholar]
- 6.ACPICR. Standards for physical activity and exercise in the cardiovascular population. 2015. http://acpicr.com/sites/default/files/ACPICR Standards 2015.pdf (accessed 31 May 2017).
- 7.Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. BMJ 2015;351:h5000 10.1136/bmj.h5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley JP, Furze G, Doherty P, et al. BACPR scientific statement: British standards and core components for cardiovascular disease prevention and rehabilitation. Heart 2013;99:1069–71. 10.1136/heartjnl-2012-303460 [DOI] [PubMed] [Google Scholar]
- 9.Dalal HM, Zawada A, Jolly K, et al. Home based versus centre based cardiac rehabilitation: cochrane systematic review and meta-analysis. BMJ 2010;340:b5631 10.1136/bmj.b5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016;67:1–12. 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 11.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20. 10.1016/S0140-6736(03)12113-7 [DOI] [PubMed] [Google Scholar]
- 12.D’Souza SP, Mamas MA, Fraser DG, et al. Routine early coronary angioplasty versus ischaemia-guided angioplasty after thrombolysis in acute ST-elevation myocardial infarction: a meta-analysis. Eur Heart J 2011;32:972–82. 10.1093/eurheartj/ehq398 [DOI] [PubMed] [Google Scholar]
- 13.Van de Werf F, Ardissino D, Betriu A, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The task force on the management of acute myocardial infarction of the european society of cardiology. Eur Heart J 2003;24:28–66. [DOI] [PubMed] [Google Scholar]
- 14.West RM, Cattle BA, Bouyssie M, et al. Impact of hospital proportion and volume on primary percutaneous coronary intervention performance in England and Wales. Eur Heart J 2011;32:706–11. 10.1093/eurheartj/ehq476 [DOI] [PubMed] [Google Scholar]
- 15.Anon. Reduction in mortality after myocardial infarction with long-term beta-adrenoceptor blockade. Multicentre international study: supplementary report. Br Med J 1977;2:419–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elwood PC, Cochrane AL, Burr ML, et al. A randomized controlled trial of acetyl salicylic acid in the secondary prevention of mortality from myocardial infarction. Br Med J 1974;1:436–40. 10.1136/bmj.1.5905.436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anon. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the scandinavian simvastatin survival study (4S). Lancet 1994;344:1383–9. [PubMed] [Google Scholar]
- 18.Anon. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials. ACE inhibitor myocardial infarction collaborative group. Circulation 1998;97:2202–12. [DOI] [PubMed] [Google Scholar]
- 19.Skinner JS, Minhas R. Commentary on NICE guidance for secondary prevention for patients following a myocardial infarction. Heart 2007;93:864–6. 10.1136/hrt.2007.124305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisen A, Bhatt DL. Optimal duration of dual antiplatelet therapy after acute coronary syndromes and coronary stenting. Heart 2017;103 10.1136/heartjnl-2015-309022 [DOI] [PubMed] [Google Scholar]
- 21.Gaziano TA, Bitton A, Anand S, et al. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol 2010;35:72–115. 10.1016/j.cpcardiol.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med 2009;3:e123–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Anon. Acute myocardial infarction: pre-hospital and in-hospital management. The task force on the management of acute myocardial infarction of the European society of cardiology. Eur Heart J 1996;17:43–63. [DOI] [PubMed] [Google Scholar]
- 24.Wood DA DP, Poutler N. Joint British recommendations on prevention of coronary heart disease in clinical practice. British cardiac society, British hyperlipidaemia association, British hypertension society, endorsed by the British diabetic association. Heart 1998;80(Suppl 2):S1–29. [PMC free article] [PubMed] [Google Scholar]
- 25.RevMan. The Nordic Cochrane Centre. The cochrane collaboration, version 5.3 (Review Manager), 2014. [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadda O, Kotanidou A, Manginas A, et al. Lifestyle intervention and one-year prognosis of patients following open heart surgery: a randomised clinical trial. J Clin Nurs 2015;24:1611–21. 10.1111/jocn.12762 [DOI] [PubMed] [Google Scholar]
- 29.Santaularia N, Caminal J, Arnau A, et al. The efficacy of a supervised exercise training programme on readmission rates in patients with myocardial ischemia: results from a randomised controlled trial. Eur J Cardiovasc Nurs 2017;16:201–12. 10.1177/1474515116648801 [DOI] [PubMed] [Google Scholar]
- 30.Hambrecht R, Walther C, Möbius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 2004;109:1371–8. 10.1161/01.CIR.0000121360.31954.1F [DOI] [PubMed] [Google Scholar]
- 31.West RR, Jones DA, Henderson AH. Rehabilitation after myocardial infarction trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart 2012;98:637–44. 10.1136/heartjnl-2011-300302 [DOI] [PubMed] [Google Scholar]
- 32.Belardinelli R, Paolini I, Cianci G, et al. Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol 2001;37:1891–900. 10.1016/S0735-1097(01)01236-0 [DOI] [PubMed] [Google Scholar]
- 33.Giallauria F, Cirillo P, Lucci R, et al. Left ventricular remodelling in patients with moderate systolic dysfunction after myocardial infarction: favourable effects of exercise training and predictive role of N-terminal pro-brain natriuretic peptide. Eur J Cardiovasc Prev Rehabil 2008;15:113–8. 10.1097/HJR.0b013e3282f00990 [DOI] [PubMed] [Google Scholar]
- 34.Maroto Montero JM, Artigao Ramírez R, Morales Durán MD, et al. [Cardiac rehabilitation in patients with myocardial infarction: a 10-year follow-up study]. Rev Esp Cardiol 2005;58:1181–7. 10.1016/S1885-5857(06)60397-6 [DOI] [PubMed] [Google Scholar]
- 35.Munk PS, Staal EM, Butt N, et al. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation A randomized controlled trial evaluating the relationship to endothelial function and inflammation. Am Heart J 2009;158:734–41. 10.1016/j.ahj.2009.08.021 [DOI] [PubMed] [Google Scholar]
- 36.Oerkild B, Frederiksen M, Hansen JF, et al. Home-based cardiac rehabilitation is an attractive alternative to no cardiac rehabilitation for elderly patients with coronary heart disease: results from a randomised clinical trial. BMJ Open 2012;2:e001820 10.1136/bmjopen-2012-001820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vestfold Heartcare Study Group. Influence on lifestyle measures and five-year coronary risk by a comprehensive lifestyle intervention programme in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil 2003;10:429–37. 10.1097/01.hjr.0000107024.38316.6a [DOI] [PubMed] [Google Scholar]
- 38.Zwisler AD, Soja AM, Rasmussen S, et al. Hospital-based comprehensive cardiac rehabilitation versus usual care among patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease: 12-month results of a randomized clinical trial. Am Heart J 2008;155:1106–13. 10.1016/j.ahj.2007.12.033 [DOI] [PubMed] [Google Scholar]
- 39.Aronov DM, Krasnitskiĭ VB, Bubnova MG, et al. [Physical training at ambulatory-polyclinical stage in complex rehabilitation and secondary prevention of patients with ischemic heart disease after acute incidents. Effect on physical working capacity, hemodynamics, blood lipids, clinical course and prognosis (Russian cooperative study)]. Kardiologiia 2009;49:49–56. [PubMed] [Google Scholar]
- 40.Briffa TG, Eckermann SD, Griffiths AD, et al. Cost-effectiveness of rehabilitation after an acute coronary event: a randomised controlled trial. Med J Aust 2005;183:450–5. [DOI] [PubMed] [Google Scholar]
- 41.Higgins HC, Hayes RL, McKenna KT. Rehabilitation outcomes following percutaneous coronary interventions (PCI). Patient Educ Couns 2001;43:219–30. 10.1016/S0738-3991(00)00164-6 [DOI] [PubMed] [Google Scholar]
- 42.Houle J, Doyon O, Vadeboncoeur N, et al. Effectiveness of a pedometer-based program using a socio-cognitive intervention on physical activity and quality of life in a setting of cardiac rehabilitation. Can J Cardiol 2012;28:27–32. 10.1016/j.cjca.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 43.Kovoor P, Lee AK, Carrozzi F, et al. Return to full normal activities including work at two weeks after acute myocardial infarction. Am J Cardiol 2006;97:952–8. 10.1016/j.amjcard.2005.10.040 [DOI] [PubMed] [Google Scholar]
- 44.Maddison R, Pfaeffli L, Whittaker R, et al. A mobile phone intervention increases physical activity in people with cardiovascular disease: results from the HEART randomized controlled trial. Eur J Prev Cardiol 2015;22:701–9. 10.1177/2047487314535076 [DOI] [PubMed] [Google Scholar]
- 45.Mutwalli HA, Fallows SJ, Arnous AA, et al. Randomized controlled evaluation shows the effectiveness of a home-based cardiac rehabilitation program. Saudi Med J 2012;33:152–9. [PubMed] [Google Scholar]
- 46.Reid RD, Morrin LI, Beaton LJ, et al. Randomized trial of an internet-based computer-tailored expert system for physical activity in patients with heart disease. Eur J Prev Cardiol 2012;19:1357–64. 10.1177/1741826711422988 [DOI] [PubMed] [Google Scholar]
- 47.Seki E, Watanabe Y, Shimada K, et al. Effects of a phase III cardiac rehabilitation program on physical status and lipid profiles in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP). Circ J 2008;72:1230–4. [DOI] [PubMed] [Google Scholar]
- 48.Toobert DJ, Glasgow RE, Radcliffe JL. Physiologic and related behavioral outcomes from the Women’s Lifestyle Heart Trial. Ann Behav Med 2000;22:1–9. 10.1007/BF02895162 [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Chair SY, Thompson DR, et al. Effects of home-based rehabilitation on health-related quality of life and psychological status in Chinese patients recovering from acute myocardial infarction. Heart Lung 2012;41:15–25. 10.1016/j.hrtlng.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 50.Yu CM, Lau CP, Chau J, et al. A short course of cardiac rehabilitation program is highly cost effective in improving long-term quality of life in patients with recent myocardial infarction or percutaneous coronary intervention. Arch Phys Med Rehabil 2004;85:1915–22. [DOI] [PubMed] [Google Scholar]
- 51.Toobert DJ, Strycker LA, Glasgow RE. Lifestyle change in women with coronary heart disease: what do we know? J Womens Health 1998;7:685–99. 10.1089/jwh.1998.7.685 [DOI] [PubMed] [Google Scholar]
- 52.Jolliffe JA, Rees K, Taylor RS, et al. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2001;1:CD001800 10.1002/14651858.CD001800 [DOI] [PubMed] [Google Scholar]
- 53.Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2011:CD001800 10.1002/14651858.CD001800.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilhelmsen L, Sanne H, Elmfeldt D, et al. A controlled trial of physical training after myocardial infarction. Effects on risk factors, nonfatal reinfarction, and death. Prev Med 1975;4:491–508. [DOI] [PubMed] [Google Scholar]
- 55.van Halewijn G, Deckers J, Tay HY, et al. Lessons from contemporary trials of cardiovascular prevention and rehabilitation: a systematic review and meta-analysis. Int J Cardiol 2017;232:294–303. 10.1016/j.ijcard.2016.12.125 [DOI] [PubMed] [Google Scholar]
- 56.Valkeinen H, Aaltonen S, Kujala UM. Effects of exercise training on oxygen uptake in coronary heart disease: a systematic review and meta-analysis. Scand J Med Sci Sports 2010;20:545–55. 10.1111/j.1600-0838.2010.01133.x [DOI] [PubMed] [Google Scholar]
- 57.Yohannes AM, Doherty P, Bundy C, et al. The long-term benefits of cardiac rehabilitation on depression, anxiety, physical activity and quality of life. J Clin Nurs 2010;19:2806–13. 10.1111/j.1365-2702.2010.03313.x [DOI] [PubMed] [Google Scholar]
- 58.Conraads VM, Denollet J, De Maeyer C, et al. Exercise training as an essential component of cardiac rehabilitation. Heart 2012;98:674–5. author reply 5 10.1136/heartjnl-2012-301912 [DOI] [PubMed] [Google Scholar]
- 59.Sattelmair J, Pertman J, Ding EL, et al. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 2011;124:789–95. 10.1161/CIRCULATIONAHA.110.010710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 2009;301:2024–35. 10.1001/jama.2009.681 [DOI] [PubMed] [Google Scholar]
- 61.Garber CE, Blissmer B, Deschenes MR, et al. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–59. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 62.McGregor G, Nichols S, Hamborg T, et al. High-intensity interval training versus moderate-intensity steady-state training in UK cardiac rehabilitation programmes (HIIT or MISS UK): study protocol for a multicentre randomised controlled trial and economic evaluation. BMJ Open 2016;6:e012843 10.1136/bmjopen-2016-012843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slade SC, Dionne CE, Underwood M, et al. Consensus on Exercise Reporting Template (CERT): modified delphi study. Phys Ther 2016;96:1514–24. 10.2522/ptj.20150668 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-019656supp001.pdf (83.7KB, pdf)