Abstract
Background
The “dysconnectivity hypothesis” was proposed 20 years ago. It characterized schizophrenia as a disorder with dysfunctional connectivity across a large range of distributed brain areas. Resting-state functional magnetic resonance imaging (rsfMRI) data have supported this theory. Previous studies revealed that the amygdala might be responsible for the emotion regulation-related symptoms of schizophrenia. However, conventional methods oversimplified brain activities by assuming that it remained static throughout the entire scan duration, which may explain why inconsistent results have been reported for the same brain region.
Methods
An emerging technique is sliding time window analysis, which is used to describe functional connectivity based on the temporal variability of regions of interest (e.g., amygdala) in patients with schizophrenia. Conventional analysis of the static functional connectivity between the amygdala and whole brain was also conducted.
Results
Static functional connectivity between the amygdala and orbitofrontal region was impaired in patients with schizophrenia. The variability of connectivity between the amygdala and medial prefrontal cortex was enhanced (i.e., greater dynamics) in patients with schizophrenia. A negative relationship was found between the variability of connectivity and information processing efficiency. A positive correlation was found between the variability of connectivity and symptom severity.
Conclusion
The findings suggest that schizophrenia was related to abnormal patterns of fluctuating communication among brain areas that are involved in emotion regulations. Unveiling the temporal properties of functional connectivity could disentangle the inconsistent results of previous functional connectivity studies.
Keywords: Dynamic functional connectivity, Temporal variability, Amygdala, Sliding-window correlation analysis, Schizophrenia
Highlights
-
•
FC between the amygdala and orbitofrontal region is impaired in patients with SZ.
-
•
The variability of FC between amygdala and MPFC was enhanced in patients with SZ.
-
•
Positive correlation was found between the variability of FC and symptom severity.
1. Introduction
Schizophrenia is a complex and heterogeneous mental disorder that was recognized approximately 100 years ago. Revealing the underlying neurobiological mechanisms associated with schizophrenia is crucial for effective diagnosis and treatment (McGlashan, 2011). The “dysconnectivity hypothesis” proposes that abnormal communication occurs across distributed brain areas and is crucial for the development of schizophrenia (Vogeley and Falkai, 1998; Bullmore et al., 1997; Zhou et al., 2015). Magnetic resonance image (MRI) studies provided preliminary evidence of alterations in functional and anatomical brain connectivity in patients with schizophrenia, particularly in the fronto-temporal system (Leroux et al., 2014). The amygdala and prefrontal cortex (PFC) play critical roles in the fronto-temporal system. Both regions are involved in affect perception and emotional and cognitive processing (Whitford et al., 2011; Shi et al., 2012; Samartzis et al., 2014; Voineskos, 2014). These functions are impaired in schizophrenia, underscoring the importance of examining the functional integrity of the amygdala and PFC.
Several neuroimaging studies have consistently reported smaller amygdala volumes and alterations of amygdala activity in patients with schizophrenia and their relatives. Imaging studies that analyzed structural data examined brain structures in high-risk offspring of schizophrenia patients and found that the volume of the left-amygdala was smaller in high-risk individuals (Keshavan et al., 2002). Amygdala activation has also been assessed during emotion-related tasks. However, since the seminal study by Schneider and colleagues that reported under-recruitment of the amygdala following mood induction in schizophrenia, no consensus has yet been reached on this issue (Schneider et al., 1998; Anticevic et al., 2011). Meta-analyses have shown that schizophrenia patients exhibit modest, albeit statistically significant, and decrease in amygdala recruitment in responses to aversive emotional stimuli (Anticevic et al., 2012; Taylor et al., 2012). Other studies found that functional connectivity between the amygdala and frontal regions is disrupted in schizophrenia (Hoptman et al., 2010; Liu et al., 2014). The present study focused on functional connectivity between the amygdala and whole brain in patients with schizophrenia. Based on the emotion-regulation related symptoms and the specific role of amygdala, our hypothesis was that functional connectivity between the amygdala and PFC was disrupted in patients with schizophrenia.
Conventional approaches that are used to study functional connectivity have an important disadvantage, in which brain activity is assumed to be static throughout the entire duration of scanning. Considering the complexity of the human brain and the ever-changing environment, this assumption that brain activity remains static is an oversimplification, which may omit important information. Brain connectivity should be considered flexible in integrating and transformatting information, in which it varies over time when responding to an ever-changing environment. Emerging studies have attempted to capture the dynamic nature of functional connectivity (Hutchison et al., 2013a; Hutchison et al., 2013b). Several recent studies showed that the dynamics of connectivity can capture uncontrolled but relatively robust patterns of temporal features among networks (Allen et al., 2012; Allen et al., 2014; Sakoğlu et al., 2010), which cannot be detected with static functional connectivity analyses.
One other promising approach to investigate variations in functional connectivity is sliding-time window correlation analysis (Hutchison et al., 2013a; Shakil et al., 2016). In this strategy, a time window with a fixed length is selected and used to calculate the functional connectivity metric. The window then slides by a fixed length to the next time window, which results in many functional connectivity metrics that can elucidate the temporal features of functional connectivity over the entire duration of the scan. Studies of major depression disorder, schizophrenia, and autism have revealed abnormal temporal attributes of functional connectivity (Kaiser et al., 2015; Demirtaş et al., 2016; Damaraju et al., 2014; Nguyen et al., 2016; Mulvey et al., 2013). The results have demonstrated that dynamic functional connectivity that is captured by the sliding time window method can facilitate the interpretation of communication across neural systems. Considering this emerging method and the inconsistent results of functional connectivity analyses between the amygdala and frontal brain areas, the present study investigated variations of functional connectivity, in addition to investigating the static functional connectivity between the amygdala and whole brain. Because of the unstable emotion regulation that is associated with schizophrenia, another hypothesis of the present study was that functional connectivity between the amygdala and PFC would present more variation and less stability in patients with schizophrenia.
2. Methods
2.1. Participants
We assessed a total 67 subjects: 34 healthy controls and 33 schizophrenia patients. The groups were matched for age and sex (age: p = 0.303, t = 1.2031, df = 2; sex: p = 0.745, χ2 = 0.59, df = 2; Table 1). Diagnoses were based on detailed medical and psychiatric histories and the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders. The exclusion criteria were the following: (i) age < 18 or >45 years, (ii) left handedness, (iii) history of brain trauma with loss of consciousness, neurological diseases, or serious physical diseases (e.g., respiratory disorders, cardiovascular diseases, and so on), (iv) diagnosis of alcohol/substance abuse within 12 months before participation in the study, and (v) contraindications for MRI. Seven of 33 patients were free of antipsychotic medication (medication-naive: n = 4; off antipsychotic medications for at least 2 weeks: n = 3), and all of the other patients were on antipsychotic medications at the time of the scan (olanzapine, n = 8; risperidone, n = 9; aripiprazole, n = 2; blonanserin, n = 5; amisulpride, n = 1; haloperidol, n = 2, and one patient received both risperidone and blonanserin at the same time). The Ethics Committee of Beijing Hui-Long-Guan Hospital (Beijing, China) approved the study, and all of the participants provided written informed consents.
Table 1.
Demographic and clinical characteristics of the participants in each group.
| Characteristic | Schizophrenia group |
Healthy control group |
p |
|---|---|---|---|
|
n = 33 |
n = 34 |
||
| Mean (SD) | Mean (SD) | ||
| Age (years) | 30.60 (8.13) | 28.12 (6.5) | 0.171 |
| Sex (male/female) | 11/22 | 14/20 | 0.51 |
| Education (years) | 12.36 (2.68) | 12.74 (3.79) | 0.686 |
| Age at disease onset (years) | 26.21 (8.242) | NA | |
| Length of illness (years) | 4.74 (2.52) | NA | |
| PANSS score | |||
| Total | 78.36 (7.95) | NA | |
| Positive | 25.61 (3.41) | NA | |
| Negative | 17.15 (2.81) | NA | |
| General | 35.60 (4.15) | NA | |
| Digit coding task | 41 (13.1) | 64.9 (11.9) | 0.00 |
PANSS, Positive and Negative Symptom Scale.
2.2. Data acquisition and preprocessing
fMRI data were acquired using a 3.0 Tesla Magnetom Trio scanner. The resting-state functional scans were obtained using a gradient-recalled echo-planar imaging sequence that was sensitive to blood oxygen level-dependent contrast (repetition time, 2000 ms; echo time, 30 ms; flip angle, 90°). The slice thickness was 4 mm (no gap), with a matrix size of 64 × 64 and field of view of 220 × 220 mm2, resulting in a voxel size of 3.4 × 3.4 × 4.0 mm3. Each brain volume comprised 33 axial slices, and each functional run contained 240 image volumes. During data acquisition, the subjects were instructed to close their eyes, relax, and stay awake. All of the images were checked for artifacts, structural abnormalities, and pathologies by a qualified neuroradiologist.
Image preprocessing was performed using statistical parametric mapping (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). To allow for magnetization equilibrium, the first 20 volumes of the functional images were discarded. The preprocessing procedure included slice-timing correction and head-motion correction. Four patients with schizophrenia were excluded because of head motion (>2.5 mm). Each fMRI scan was intensity-scaled to yield a whole-brain mean value of 10,000. Temporal band-pass filtering (0.01 < f < 0.08 Hz) was then performed. This range was selected to remove high-frequency activity that is related to cardiac and respiratory activity and low-frequency activity with a period that exceeds the duration of sliding windows that are used in dynamic analyses (Cordes et al., 2001; Leonardi and Van De Ville, 2015). The time series in white matter and cerebrospinal fluid and six affine motion parameters were also regressed from the data. The removal of linear and quadratic trends was also implemented. To obtain results at the group level, single-subject images were nonlinearly normalized to Montreal Neurological Institute (MNI) space using DARTEL in SPM8 and resampled to 3 × 3 × 3 mm3 cubic voxels. Finally, the data were spatially smoothed with a 6 mm full width at half-maximum (FWHM) Gaussian kernel.
2.3. Definition of regions of interest
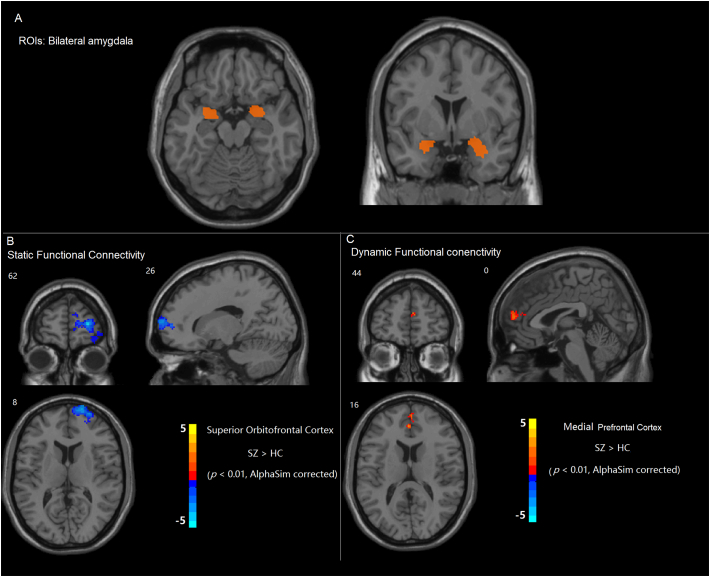
Bilateral amygdala areas were selected as the regions of interest (ROIs). Amygdala ROIs were determined using stereotaxic, probabilistic maps of cytoarchitectonic boundaries (Fig. 1).
Fig. 1.
(A) Regions of interests (ROIs): left and right amygdala. (B) Compared with the healthy control (HC) group, the schizophrenia (SZ) group exhibited a decrease in functional connectivity between the amygdala and regions of the orbitofrontal cortex. (C) Compared with the HC group, the SZ group exhibited greater temporal variability of functional connectivity between the left amygdala and medial prefrontal cortex.
2.4. Static and dynamic functional connectivity
Voxel-wise seed-based functional connectivity analyses were performed using the CONN toolbox (https://www.nitrc.org/projects/conn/ (Whitfield-Gabrieli and Nieto-Castanon, 2012)). Static functional connectivity was also performed to provide complementary information. Fisher's z-transformed Pearson's correlation coefficient was computed between the full time course of the amygdala and the time course of all of the other voxels.
For the dynamic analysis, the time course was segmented into 36-s windows (Kaiser et al., 2015; Leonardi and Van De Ville, 2015), sliding the onset of each window by 18 s, for a total of 19 windows. The duration of sliding windows was selected to optimize the balance between capturing rapidly shifting dynamic relationships (with shorter windows) and achieving reliable estimates of the correlated activity between regions (with longer windows) (Whitfield-Gabrieli and Nieto-Castanon, 2012). Fisher's z-transformed Pearson's correlation coefficient was then computed for each sliding window between the truncated time course of the seed and that of all of the other voxels, yielding a set of sliding-window beta maps for each participant. Dynamic connectivity was estimated by calculating the standard deviation (SD) in beta values at each voxel. Group-level dynamic analyses were conducted by performing group-level statistics on the SD in beta values at each voxel. The SD signals were then extracted from the prefrontal regions that showed differences in the dynamic analysis. Correlation analyses were then conducted between the SD signals and cognitive performance and symptom severity. The hypothesis of the present study is correlation between the functional connectivity and the symptoms and cognitive function in patients with schizophrenia, therefore, there are four correlations were attempted.
3. Results
3.1. Broken functional connectivity between the amygdala and orbitofrontal cortex in schizophrenia patients
Our findings indicated that, consistent with our predictions, the connectivity between the left amygdala and orbitofrontal regions decreased in patients with schizophrenia (p < 0.01, corrected for AlphaSim; Fig. 1B). However, no significant correlation was found between broken amygdala-prefrontal connectivity and symptom severity or cognition task performance.
3.2. Greater temporal variability of connectivity between the left amygdala and medial prefrontal cortex in patients with schizophrenia
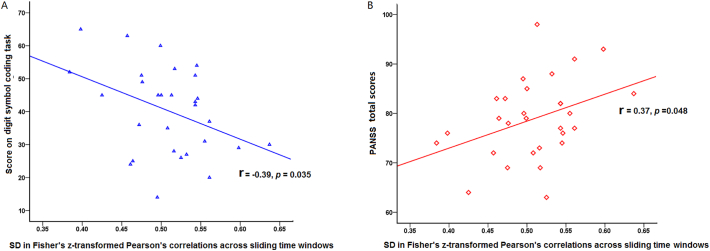
The dynamic functional connectivity analysis revealed greater variability of amygdala-PFC connectivity (p < 0.01, corrected for AlphaSim; Figs. 1C, 2: data from one healthy control and one patient). The subsequent correlation analyses revealed that the variability of connectivity negatively correlated with cognitive performance on the digit symbol coding task (r = −0.39, p = 0.035; Fig. 3A) and marginally positively correlated with symptom severity (r = 0.37, p = 0.048; Fig. 3B).
Fig. 2.
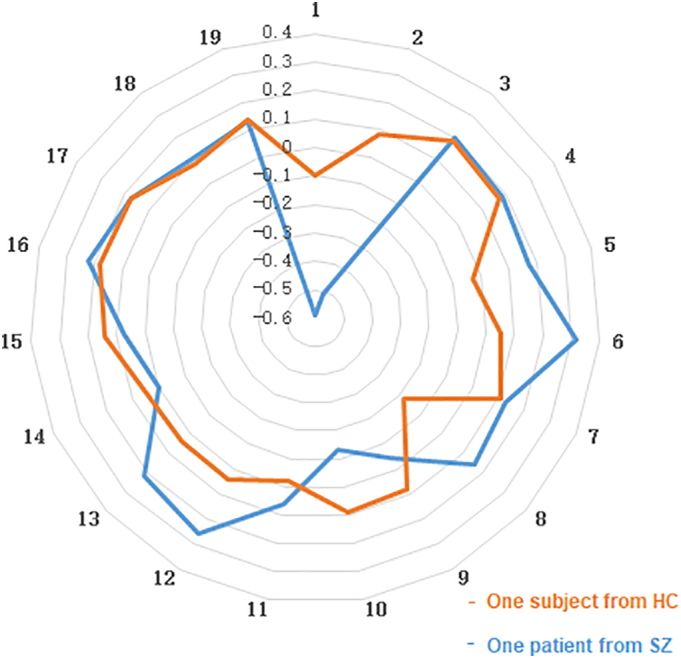
To illustrate time window-by-window patterns of functional connectivity, the figure shows amygdala-medial prefrontal cortex (mPFC) functional connectivity values (Fisher's z-transformed Pearson's correlations) for each of the 19 sliding time windows. And the data represented by orange line is from one healthy subject, and the data represented by orange line is from one patient with schizophrenia. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Relationships between temporal variability of the amygdala-medial prefrontal cortex (mPFC) functional connectivity and clinical severity and cognitive performance. (A) Negative correlation between temporal traits of amygdala-mPFC functional connectivity and information processing speed. (B) Positive correlation between temporal traits of amygdala-mPFC functional connectivity and total symptom severity.
4. Discussion
The present study found evidence of fluctuations of communication between the amygdala and prefrontal areas in patients with schizophrenia. The results suggested that static connectivity between the amygdala and prefrontal regions was broken in patients with schizophrenia. We also found greater temporal variability of connectivity between the amygdala and prefrontal regions in patients with schizophrenia. The increase in variability was negatively correlated with cognitive performance and positively correlated with symptom severity.
Static functional connectivity was also assessed in the present study. The results showed that connectivity between the amygdala and orbitofrontal cortex was disrupted in schizophrenia patients, which is consistent with previous studies. As a critical social skill, the emotion regulation is impaired in schizophrenia patients, especially the affective information processing ability. The role of the amygdala in emotion processing has also been well established. Abnormalities of brain circuits that involve the amygdala have been repeatedly reported in schizophrenia patients (Liu et al., 2014; Wright et al., 2000). Previous studies found increases in dopamine in the amygdala in the left hemisphere in patients with schizophrenia, and neurochemical evidences also indicated that schizophrenia was related to left hemisphere dysfunction (Reynolds, 1983; Reynolds, 1986). The disruption of functional connectivity between the orbitofrontal cortex and left amygdala is consistent with the supposition that dopamine abnormalities in the amygdala present lateral asymmetry in schizophrenia. A recent study suggested that functional connectivity between the amygdala and bilateral orbitofrontal cortices, bilateral precuneus, bilateral dorsolateral frontal cortices, and right insula is abnormal in schizophrenia patients (Lin Tian, 2011). Voxel-wise functional connectivity analyses revealed a decrease in connectivity between the amygdala and PFC, which is consistent with the static results of the present study. A significant negative linear relationship was found between symptoms and amygdala-orbitofrontal cortex connectivity across subjects (Mukherjee et al., 2016). However, in the present study, our correlation analysis did not detect a relationship between abnormal static amygdala-PFC connectivity and symptom severity (Anticevic et al., 2014). A structural diffusion tensor imaging study suggested that the loss of structural integrity of prefrontal pathways could lead to dysregulation in limbic regions in schizophrenia (Wagner et al., 2015). Such patterns of disruption have also been reported in animal models (Belujon et al., 2014). Structural analyses and resting-state functional connectivity studies also revealed that the strength of amygdala-PFC connectivity was negatively correlated with self-rated aggression (Hoptman et al., 2010). The impairments of amygdala-frontal functional connectivity that were found in the present study may reflect the disruption of top-down regulation. Patients with schizophrenia are emotionally responsive, particularly to stressful or negative stimuli, and such sensitivity may cause vulnerability to symptoms or to the disease itself. However, the present study did not detect any correlations between amygdala-OFC connectivity and symptom severity.
Quantifying fluctuations of functional connectivity metrics over time has been proposed to provide greater insights into the fundamental properties of brain networks. This measure of average connectivity through whole scan duration might not be sufficient to characterize dynamic changes in functional connectivity that are thought to be critical for integrating emotional and cognitive processing (Hutchison et al., 2013a; Cassidy et al., 2016). The present study found greater temporal variability of the amygdala-mPFC in patients with schizophrenia, which is consistent with a previous study that performed independent component analysis and found significantly more fluctuations between the frontoparietal, cerebellar, and temporal lobe regions in schizophrenia patients (Ma et al., 2014). Furthermore, the follow-up analysis revealed a negative correlation between temporal variability of amygdala-mPFC functional connectivity and cognitive performance on the digit symbol coding task. The digit symbol coding task reflects basic information processing ability, which has been reported to be impaired in patients with schizophrenia (Dickinson et al., 2007; Nazeri et al., 2013). The mPFC is critically related to higher cognitive functions, and abnormal amygdala-mPFC connectivity might lead to impairments of information integration. The present results may complement the results of static amygdala-mPFC connectivity. A positive correlation was found between the variability of functional connectivity and symptom severity, and such a correlation was not found in the static analysis. The decrease in amygdala-mPFC connectivity may lead to greater emotional responses and thus greater vulnerability to the disease. Additionally, the greater variability reduced the stability of amygdala-PFC connectivity.
5. Limitations
The present study has a few limitations. We employed a cross-sectional design rather than a longitudinal design, so the relationship between the course of the disease and functional connectivity could not be determined. We also did not examine possible correlations between antipsychotic medication dose/gender and the identified brain features, but the influence of medications on imaging measures has been previously reported (Szeszko et al., 2014).
6. Conclusions
The present study investigated dynamic attributes of functional connectivity in patients with schizophrenia, providing insights into the temporal stability of neural communication. Amygdala-frontal connectivity decreased in schizophrenia patients, with greater temporal variability, suggesting the unstable top-down regulation from prefrontal regions. Such impairments may be a pathological basis for the impairments in emotional evaluation and regulation. However, the ways in which impairments in these connections develop are still unclear. Further studies are needed to elucidate the relationship between changes in connectivity and the course of the illness.
Author contributions
Jing-Li Yue and Lin Lu designed the experiments. Peng Li, Jing-Li Yue, Le Shi, and Xiao Lin collected the data. Xiao Lin and Peng Li prepared the figures. Hong-Qiang Sun revised the manuscript and prepared the tables. Jing-Li Yue and Lin Lu discussed the results, advised on interpretation of the results, and contributed to the final draft of the manuscript. All of the authors contributed to and approved the final manuscript.
Competing financial interests
The authors declare no conflicts of interest.
Acknowledgements
This work was supported in part by the National Basic Research Program of China (no. 2015CB856400) and National Natural Science Foundation of China (no. 81501158, 81521063, 91432303, and 31230033).
Contributor Information
Xiao Lin, Email: LinXiaopku2015@pku.edu.cn.
Hong-Qiang Sun, Email: sunhq@bjmu.edu.cn.
Lin Lu, Email: linlu@bjmu.edu.cn.
References
- Allen E.A., Erhardt E.B., Wei Y. Capturing inter-subject variability with group independent component analysis of fMRI data: a simulation study. NeuroImage. 2012;59:4141–4159. doi: 10.1016/j.neuroimage.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Damaraju E., Plis S.M. Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Repovs G., Barch D.M. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr. Bull. 2011;38:967–980. doi: 10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Van Snellenberg J.X., Cohen R.E. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr. Bull. 2012;38:608–621. doi: 10.1093/schbul/sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A. Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophr. Bull. 2014;40:1105–1116. doi: 10.1093/schbul/sbt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P., Patton M.H., Grace A.A. Role of the prefrontal cortex in altered hippocampal-accumbens synaptic plasticity in a developmental animal model of schizophrenia. Cereb. Cortex. 2014;24:968–977. doi: 10.1093/cercor/bhs380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E.T., Frangou S., Murray R.M. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr. Res. 1997;28:143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Cassidy C.M. Dynamic connectivity between brain networks supports working memory: relationships to dopamine release and schizophrenia. J. Neurosci. 2016;36:4377–4388. doi: 10.1523/JNEUROSCI.3296-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am. J. Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Damaraju E., Allen E.A., Belger A. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtaş M., Tornador C., Falcón C. Dynamic functional connectivity reveals altered variability in functional connectivity among patients with major depressive disorder. Hum. Brain Mapp. 2016;37:2918–2930. doi: 10.1002/hbm.23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D., Ramsey M.E., Gold J.M. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch. Gen. Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Hoptman M.J., D'Angelo D., Catalano D. Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophr. Bull. 2010;36:1020–1028. doi: 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison R.M., Womelsdorf T., Allen E.A. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison R.M., Womelsdorf T., Gati J.S. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum. Brain Mapp. 2013;34:2154–2177. doi: 10.1002/hbm.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Whitfield-Gabrieli S., Dillon D.G. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology. 2015;41:1822–1830. doi: 10.1038/npp.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan M.S., Dick E., Mankowski L. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr. Res. 2002;58:173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- Leonardi N., Van De Ville D. On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage. 2015;104:430–436. doi: 10.1016/j.neuroimage.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Leroux E., Delcroix N., Dollfus S. Left fronto-temporal dysconnectivity within the language network in schizophrenia: an fMRI and DTI study. Psychiatry Res. 2014;223:261. doi: 10.1016/j.pscychresns.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Lin Tian C.M. Convergent evidence from multimodal imaging reveals amygdala abnormalities in schizophrenic patients and their first-degree relatives. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Tang Y., Womer F. Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophr. Bull. 2014;40:469–477. doi: 10.1093/schbul/sbt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Calhoun V.D., Phlypo R., Adalı T. Dynamic changes of spatial functional network connectivity in healthy individuals and schizophrenia patients using independent vector analysis. NeuroImage. 2014;90:196–206. doi: 10.1016/j.neuroimage.2013.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan T.H. Eugen Bleuler: centennial anniversary of his 1911 publication of dementia praecox or the group of schizophrenias. Schizophr. Bull. 2011;37:1101–1103. doi: 10.1093/schbul/sbr130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P. Disconnection between amygdala and medial prefrontal cortex in psychotic disorders. Schizophr. Bull. 2016;42:1056–1067. doi: 10.1093/schbul/sbw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M., Keown C.L., Handy M. Society for Neuroscience Annual Meeting. 2013. Temporal dynamics of resting state functional connectivity in autism: a sliding window fcMRI study. [Google Scholar]
- Nazeri A. Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology. 2013;38:1954–1962. doi: 10.1038/npp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.T., Kovacevic S., Dev S.I. Dynamic functional connectivity in bipolar disorder is associated with executive function and processing speed: a preliminary study. Neuropsychology. 2016;31:73–83. doi: 10.1037/neu0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G.P. Increased concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature. 1983;305:527–529. doi: 10.1038/305527a0. [DOI] [PubMed] [Google Scholar]
- Reynolds G.P. Dopaminergic Systems and their Regulation. Palgrave Macmillan UK; 1986. Amygdala dopamine asymmetry in schizophrenia: neurochemical evidence for a left temporal lobe dysfunction; pp. 285–291. [Google Scholar]
- Sakoğlu Ü., Pearlson G.D., Kiehl K.A. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. MAGMA. 2010;23:351–366. doi: 10.1007/s10334-010-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samartzis L., Dima D., Fusar-Poli P. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J. Neuroimaging. 2014;24:101–110. doi: 10.1111/j.1552-6569.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- Schneider F., Weiss U., Kessler C. Differential amygdala activation in schizophrenia during sadness. Schizophr. Res. 1998;34:133–142. doi: 10.1016/s0920-9964(98)00085-1. [DOI] [PubMed] [Google Scholar]
- Shakil S., Lee C.H., Keilholz S.D. Evaluation of sliding window correlation performance for characterizing dynamic functional connectivity and brain states. NeuroImage. 2016;133:111–128. doi: 10.1016/j.neuroimage.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Yap P.T., Gao W. Altered structural connectivity in neonates at genetic risk for schizophrenia: a combined study using morphological and white matter networks. NeuroImage. 2012;62:1622–1633. doi: 10.1016/j.neuroimage.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko P.R. White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacology. 2014;39:1324–1331. doi: 10.1038/npp.2013.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.F., Kang J., Brege I.S. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol. Psychiatry. 2012;71:136–145. doi: 10.1016/j.biopsych.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K., Falkai P. The dysconnectivity hypothesis of schizophrenia. Neurol. Psychiatry Brain Res. 1998;6:113–122. [Google Scholar]
- Voineskos A.N. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front. Hum. Neurosci. 2014;8:653. doi: 10.3389/fnhum.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. Structural and functional dysconnectivity of the fronto-thalamic system in schizophrenia: a DCM-DTI study. Cortex. 2015;66:35–45. doi: 10.1016/j.cortex.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Whitford T.J., Kubicki M., Shenton M.E. Diffusion tensor imaging, structural connectivity, and schizophrenia. Schizophr. Res. Treat. 2011;2011 doi: 10.1155/2011/709523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright I.C. Meta-analysis of regional brain volumes in schizophrenia. Am. J. Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Fan L., Qiu C. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci. Bull. 2015;31:207–219. doi: 10.1007/s12264-014-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]