Abstract
Repetitive thinking styles such as rumination are considered to be a key factor in the development and maintenance of mental disorders. Different situational triggers (e.g., social stressors) have been shown to elicit rumination in subjects exhibiting such habitual thinking styles. At the same time, the process of rumination influences the adaption to stressful situations. The study at hand aims to investigate the effect of trait rumination on neuronal activation patterns during the Trier Social Stress Test (TSST) as well as the physiological and affective adaptation to this high-stress situation.
Methods
A sample of 23 high and 22 low ruminators underwent the TSST and two control conditions while their cortical hemodynamic reactions were measured with functional near-infrared spectroscopy (fNIRS). Additional behavioral, physiological and endocrinological measures of the stress response were assessed.
Results
Subjects showed a linear increase from non-stressful control conditions to the TSST in cortical activity of the cognitive control network (CCN) and dorsal attention network (DAN), comprising the bilateral dorsolateral prefrontal cortex (dlPFC), inferior frontal gyrus (IFG) and superior parietal cortex/somatosensory association cortex (SAC). During stress, high ruminators showed attenuated cortical activity in the right IFG, whereby deficits in IFG activation mediated group differences in post-stress state rumination and negative affect.
Conclusions
Aberrant activation of the CCN and DAN during social stress likely reflects deficits in inhibition and attention with corresponding negative emotional and cognitive consequences. The results shed light on possible neuronal underpinnings by which high trait rumination may act as a risk factor for the development of clinical syndromes.
Keywords: Trier Social Stress Test (TSST), Functional near-infrared spectroscopy (fNIRS), Inferior frontal gyrus (IFG), Functional connectivity, Rumination, Cognitive control network (CCN)
Highlights
-
•
This is the first study that assessed cortical activity during the Trier Social Stress Test (TSST) in low and high ruminators
-
•
High trait ruminators were more strongly affected by the TSST on negative affect, state-rumination and cortical activation
-
•
During the TSST, a significant increase of cortical activity was observed in parts of the cognitive control network
-
•
High ruminators showed impairments in the activation of the right inferior frontal gyrus (IFG) during stress
-
•
IFG reactivity mediated effects of group membership on post-stress negative affect and state rumination
1. Introduction
Rumination is an enduring self-referential pessimistic repetitive thinking style about problems with little or no goal and change-orientation (Teismann, 2012). The process is considered to be an important factor in the development and maintenance of major depression since it is related to the onset, severity and treatment stability of the disorder (Smith and Alloy, 2009). Ruminative tendencies elevate the risk for depression even in the absence of other acute symptoms in healthy individuals (Eshun, 2000; Ito et al., 2006; Koval et al., 2012; Michalak et al., 2011; Smith and Alloy, 2009; Teismann et al., 2008). However, also other mental disorders – such as anxiety disorders – and physical health – such as immune system and fitness – are affected by high levels of rumination (Mellings and Alden, 2000; Thomsen et al., 2004a, Thomsen et al., 2004b).
On a neuronal level, rumination is associated with aberrant functional activity within several brain areas. Studies showed that activity in the subgenual prefrontal cortex is associated with higher levels of rumination (Bratman et al., 2015), and that activity in this area and parts of the default mode network (DMN) (e.g., posterior cingulate) and cognitive control network (CCN) (e.g., dorsolateral prefrontal cortex (dlPFC)) can be elicited by a rumination induction (Cooney et al., 2010). However, in comparison to task positive network activity, relative DMN dominance has been associated with rumination (Hamilton et al., 2011). Also, in depressed subjects – a sample that is known to show elevated levels of rumination – meta-analytic data showed decreased activity within the frontal parts of the CCN (Zhong et al., 2016). Moreover, stimulation of the right prefrontal cortex with transcranial direct current stimulation (tDCS) led to higher state rumination after an anger induction (Kelley et al., 2013). In this framework, the midline structures of the cortex – mostly belonging to the DMN – are thought to play an important role in self-referential processing, while the lateral parts of the cortex – mostly corresponding to the CCN and attention network – are involved in cognitive control and attention processes (Nejad et al., 2013).
Usually, rumination is directly induced in experimental designs by instructing participants to think in a certain way, or by using autobiographical paradigms (Berman et al., 2014; Ottaviani et al., 2016). Since rumination is thought to be elicited by stressful life events (Smith and Alloy, 2009), stress induction methods (Skoluda et al., 2015) have also been used to induce rumination. While some did not find effects of stress on the induction of rumination (Young and Nolen-Hoeksema, 2001), others found that state rumination can be elicited by stress (Gianferante et al., 2014; Hilt et al., 2015; Shull et al., 2016). However, the stress response itself is also affected by rumination as indicated by a reduced decline of cortisol in high ruminators (Capobianco et al., 2018; Denson et al., 2009; Hilt et al., 2015; LeMoult and Joormann, 2014; Shull et al., 2016). Indeed, meta-analytic data suggests that rumination is associated with higher heart rate, systolic and diastolic blood pressure and cortisol levels in experimental designs (Ottaviani et al., 2016). Yet, the neural links between rumination, cortical activation and the stress response are still unclear.
In the following work, we sought to investigate how far rumination can be induced through social stress in low and high trait ruminators. Further, we aimed to assess the neural underpinnings of the stress response in these individuals by using functional near-infrared spectroscopy (fNIRS), an optical imaging method that has proven to be compatible with the standard procedure of the Trier Social Stress Test (TSST) (Rosenbaum et al., 2018). We hypothesized that stress-induced increases in state rumination would be stronger in high trait-ruminating individuals. Further, we predicted that the stress response in terms of heart rate, cortisol reactivity and subjective stress would be higher in high trait ruminators and would correlate with the increases in state rumination. On a neural level, we hypothesized that high trait-ruminators would show lower hemodynamic responses in parts of the CCN in comparison to low trait-ruminators during the TSST.
2. Materials and methods
2.1. Participants
This study was approved by the ethics committee at the University Hospital and University of Tübingen. All participants gave their written informed consent. A total of 45 subjects were recruited at the University of Tübingen according to their total Rumination Response Scale (RRS) (Nolen-Hoeksema, 1991) score out of a sample of 400 subjects that completed the online assessment. To maximize differences in trait rumination, only subjects with high (PR > 65) and low (PR < 27) RRS scores were recruited. RRS score means for high (n = 23) ruminators were m = 2.59 (SD = 0.17, range: 2.36–3.04) and for low (n = 22) ruminators m = 1.53 (SD = 0.21 range: 1.09–1.86). The average age was 22 (SD = 3 years) and 83% of the sample were female. Low and high ruminators did not differ in terms of these variables (see Table 1). High ruminators had a mean Beck Depression Inventory (BDI) score of 8.5 (SD = 5.79, range: 0–23) and low ruminators of 1.9 (SD = 2.2, range: 0–9) (Beck et al., 1994). No participant fulfilled full criteria for clinical depression. As expected, high ruminators reported to spend more time per day ruminating than low ruminators (t(43) = −2.105, p < .05, d = 0.63). All subjects were right-handed, none took medication (except for contraceptive medication) and no subjects had medical conditions that influence the stress response. High and low ruminators did not differ on their general intelligence as assessed with the Mehrfachwahl-Wortschatz-Intelligenztest (t(43) = −0.5, p > .1) (Lehrl, 2005).
Table 1.
Demographic, clinical and performance variables of the high and low ruminators. BDI = Beck Depression Inventory, RRS = Rumination Response Scale, TSST = Trier Social Stress Test.
| Variable | Low-ruminators (n = 22) |
High-ruminators (n = 23) |
t/χ2 | p | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (years) | 22.3 | 3.88 | 21.69 | 2.68 | t(43) < 1 | p > .1 |
| Percent of female participants | 86% | 79% | χ2(1) = 0.5 | p > .1 | ||
| BDI | 1.9 | 2.25 | 8.5 | 5.80 | t(43) = 4.99 | p < .001 |
| RRS | 1.5 | 0.21 | 2.6 | 0.17 | t(43) = 19.32 | p < .001 |
| Time spent ruminating per day (hours) | 0.25 | 0.38 | 0.55 | 0.55 | t(43) = −2.105 | p < .05 |
| Mean errors (control task) | 0.6 | 0.27 | 0.6 | 0.41 | t(43) < 1 | p > .1 |
| Mean calculations (control task) | 8.0 | 2.88 | 8.5 | 3.00 | t(43) < 1 | p > .1 |
| Mean errors TSST | 1.5 | 0.64 | 1.5 | 0.61 | t(43) < 1 | p > .1 |
| Mean calculations TSST | 9.6 | 3.80 | 9.7 | 3.50 | t(43) < 1 | p > .1 |
2.2. Procedures
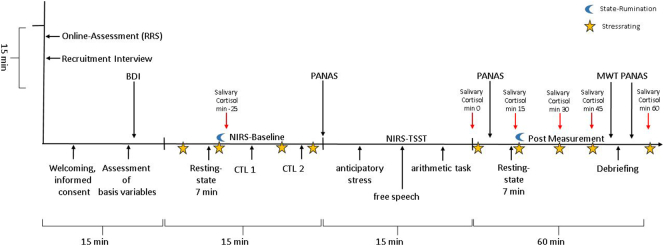
Subjects were screened via online assessment of the RRS score. After inclusion into the study, subjects completed the baseline assessment including demographic variables, the Beck Depression Inventory and a 10-minute interview about rumination symptoms. Afterwards, a 7-minute, eyes-open resting-state measurement was conducted using fNIRS. After the resting-state measurement, state rumination was assessed (see supplementary material). Two control tasks were completed afterwards including a number reading task (CTL1) and an arithmetic task (CTL2) without social stress, i.e., without judges or videotaping. Both tasks consisted of 6 blocks with 40 s task performance and 20 s pausing. During CTL1, subjects had to read decreasing numbers from 1023 in steps of 13 (i.e., 1023, 1010, 997 and so on). During CTL2, subjects had to subtract the number 13 from 6 different starting points between 1026 and 1014. For the control tasks, subjects were instructed by a friendly study nurse. If errors occurred, the study nurse said: “Ok, please go on from …” and gave the correct answer. Afterwards, the TSST was performed. The TSST committee – comprising a female and male judge – entered the laboratory and sat down in front of the participants. According to the TSST standard protocol, subjects had a 5 min preparation phase before performing a 5 min free speech about their personal strengths and qualifications during which they stood in front of the TSST committee and were videotaped. Then a 6 min arithmetic stress challenge followed. Again, subjects had to subtract the number 13 from different starting points between 1026 and 1014 in 6 task blocks. If subjects made an error, one committee member interrupted them saying: “Stop! Please start again from…”. Different starting points were chosen for CTL2 and the arithmetic stress condition. The TSST committee was non-verbally neutral and emotionally non-responsive throughout the TSST. After the completion of the TSST, the committee left the room without any comment. Directly after the TSST, subjects completed a second resting-state measurement. During all experimental conditions, subjects gave subjective stress ratings and heart rate was measured. Cortisol samples were taken after the first resting-state measure, after the TSST and in 15 minute steps up to 60 min following the completion of the TSST. After the resting-state measurements, state rumination was assessed. Further, positive and negative affect was measured with the Positive and Negative Affect Schedule (PANAS) following the control conditions, the TSST and before the last salivary sample was taken (Watson et al., 1988) (see Fig. 1 and Supplementary material).
Fig. 1.
Design and measurements of the experiment.
2.3. Cortisol sampling and assays
Saliva was collected in salivettes (Sarstedt AG & Co., REF 51.1534.500) and was further stored at −20 °C. For analysis of cortisol levels, salivettes were thawed and centrifuged for 2 min at 1000g to collect saliva. Further analysis was performed with enzyme immunoassay (IBL International, Cortisol ELISA, REF RE52611) according to the manufacturer's instructions. Average cortisol levels were taken from duplicate runs if intra-assay variation was below 10%. Finally, daytime was regressed out of cortisol coefficients to account for circadian rhythm fluctuations that are not related to the TSST and values were log-transformed. Participants were instructed not to drink alcohol the day before the measurement, to sleep as long as they usually do and to perform no physical activities at the day of the measurement. Also subjects were told not to drink or eat 30 min before the measurement started.
2.4. Heart rate
The heart rate was recorded with a one channel electro cardiogram (ECG). For ECG recordings, two standard Ag/AgCl EEG ring electrodes of 8 mm diameter were attached to the abraded skin above the left and right collar bone. FPz according to the 10/20 system was taken as a reference. Signal recordings were done with a BrainAmp ExG amplifier and Brain Vision recorder software (Brain Products, Munich, Germany) at 1000 Hz sampling rate. Data was further preprocessed and analyzed using MATLAB R2017a routines (MathWorks Inc., Natick, USA). Preprocessing steps were as follows: Band-pass filtering (0.25–50 Hz) and (for one subject) 50 Hz notch filtering. Afterwards intervals between R complexes and the average beats per minute were calculated.
2.5. fNIRS
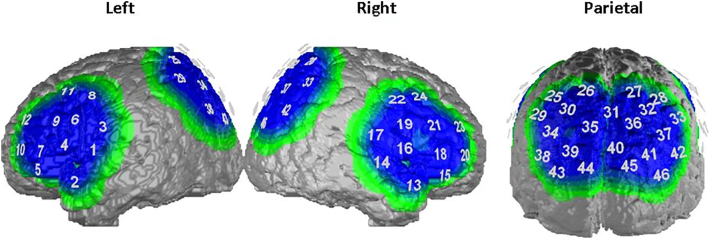
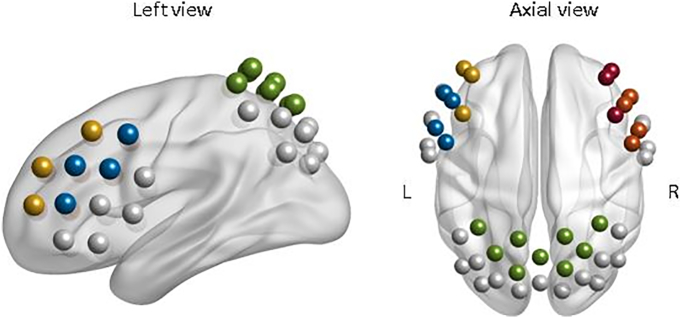
Cortical activation was measured with a continuous wave, multichannel NIRS system (ETG-4000 Optical Topography System; Hitachi Medical Co., Japan) with a temporal resolution of 10 Hz. The measurement array consisted of two frontal and one parietal probeset (see Table 2). Optodes were positioned on a combined electrode Easycap with sponge rings for additional fixation. The system consisted of three probesets, two frontal probesets (reference points F3 and F4 according to the international 10–20 System (Jasper, 1958)) with 9 optodes each and one parietal probeset (reference point Pz) with 15 optodes, resulting in a total of 46 channels (see Table 1, Supplementary Figs. S1 and S2). The combined electrode caps were positioned at reference point Cz according to the international 10–20-system on each participants head. Corresponding brain areas of each channel were extrapolated from reference points based on the Colin 27 template (Cutini et al., 2011; Tsuzuki and Dan, 2014).
Table 2.
Channels of the used fNIRS probeset and corresponding brain areas.
| Brain area | Probeset A: (left frontal) |
Probeset B: (right frontal) |
|---|---|---|
| Retrosubicular area | 1 | 14, 16 |
| Dorsolateral prefrontal cortex | 5, 10, 11, 12 | 15, 20, 23, 24 |
| Temporopolar area | 2 | 13 |
| Subcentral area | 3 | 17 |
| Pre-motor and supplementary motor cortex | 8 | 22 |
| Pars opercularis | 6 | 19 |
| Pars triangularis | 4, 7, 9 | 18, 21 |
| Probeset C: (parietal) | ||
| Somatosensory association cortex | 25, 26, 27, 28, 30, 31, 32, 34, 35, 36, 37 | |
| V3 | 38, 39, 40, 41, 43, 44, 45, 46 | |
| Angular gyrus | 42 | |
| Supramarginal gyrus | 29, 33 | |
After the assessment, data was further analyzed using MATLAB R2017a (MathWorks Inc., Natick, USA). Data was first bandpass filtered (0.1–0.001 Hz) before the movement artefact reduction by the algorithm of Cui et al. (Brigadoi et al., 2014; Cui et al., 2010) was performed and a first interpolation of single artefact-loaded channels was done. As we used the correlation-based signal correction of Cui et al. (2010), we further only analyzed the data of the oxygenated signal (which was corrected for correlation with the deoxygenated signal). The oxygenated signal was further selected due to its higher signal-to-noise ratio, higher variability and excitability. Afterwards, an ICA based reduction of clenching artifacts was done and a second bandpass filtering (0.1–0.01 Hz) was performed before a global signal reduction was done with a spatial Gaussian kernel filter (Zhang et al., 2016) with a standard deviation of σ = 50. Finally, data was averaged over the 6 task blocks with a 5 s baseline correction for the total 40 s of task performance.
2.6. Data analysis
The different datasets – behavioral, physiological, endocrinological and cortical activation data – were analyzed with respect to the hypothesized group (low vs. high ruminators) by condition interaction. For all measures, repeated measurement ANOVAs were performed with IBM SPSS Statistics Version 24. We hypothesized that high ruminators would have higher stress-ratings, heart rates, state rumination, negative affect and cortisol levels in the post TSST phase than non-ruminators. Due to different path lengths of the near-infrared light, group (high ruminators vs. low ruminators) by condition (CTL1 vs. CTL2 vs. TSST) repeated measures ANOVAs were performed for five ROI (bilateral dlPFC, IFG and SAC) separately (see Supplementary Fig. S2). We hypothesized a linear relationship between blood oxygenation and stress-loading of the task (CTL1 < CTL2 < TSST) in the low ruminators and that this relationship would be disturbed in the high ruminators (Zhong et al., 2016). Finally, we tested in how far effects of group on behavioral measures were mediated by changes in cortical activation from CTL1 to the TSST by using regression analysis and Sobel's-Z-test for mediation (Sobel, 1982, Sobel, 1986). In the paper at hand, only the experimental effects on the hemodynamic response during the control conditions and the TSST are reported. Resting-state measurements were analyzed separately with respect to functional connectivity (FC) differences and will be reported elsewhere since both measures – FC and activity – have differential and independent informational content.
3. Results
3.1. Behavioral, endocrinological and sympathetic changes
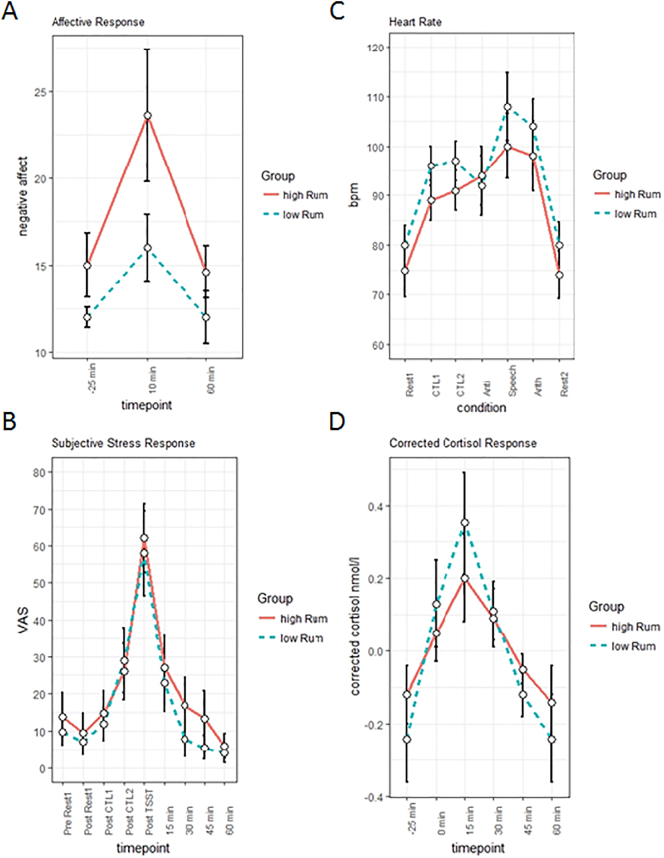
As indicated by repeated measurement ANOVA (group*condition), both the number of arithmetical computations (F(1, 43) = 37.051, p < .001, η2 = 0.46) and errors (F(1, 43) = 114.621, p < .001, η2 = 0.72) increased from CTL2 to TSST. However, no significant differences were found between high- and low-ruminators. Regarding negative (NA) and positive affect (PA), we found a significant group (high vs. low ruminators) by time (pre TSST vs. 5 min post TSST vs. 50 min post TSST) interaction for negative affect (F(2, 82) = 6.092, p < .01, η2 = 0.13). Results indicated a generally higher NA level for high ruminating subjects – reflected by a main effect of group (F(1, 42) = 11.649, p < .001, η2 = 0.22) – and higher negative affective reactivity in the high ruminators due to the stress-induction in terms of a quadratic significant interaction (F(1, 41) = 7.394, p < .01, η2 = 0.15) (see Fig. 2A). In the same way, we found a group (high vs. low ruminators) by time (pre vs. post TSST) interaction for state rumination (F(1, 43) = 4.49, p < .05, η2 = 0.095), reflecting higher overall state rumination (F(1,43) = 27.47, p < .001, η2 = 0.39) and higher increases in state rumination during the experiment for the high ruminators (t(43) = 2.12, p < .05, d = 0.64).
Fig. 2.
Responses in negative affect (A), subjective stress ratings (B), heart rate (C) and salivary cortisol (D). Timepoints are centered at post TSST (0 min).
Subjective stress ratings showed a significant main effect for time (F(1, 43) = 94.703, p < .001, η2 = 0.68). While there was no significant interaction between time and group, planned comparisons indicated that the subjective stress rating was significantly higher in the high ruminators at 30 min post TSST (t(43) = 2.12, pone-sided < .05, d = 0.63) and 45 min post TSST (t(43) = 1.93, pone-sided < .05, d = 0.57) (see Fig. 2B).
Regarding sympathetic activation, heart rate measurements indicated a significant variation over conditions (resting-state pre TSST vs. CTL1 vs. CTL2 vs. TSST anticipation vs. TSST free speech vs. TSST arithmetic task vs. resting-state post TSST; F(6, 252) = 90.610, p < .001, η2 = 0.68) and a marginally significant difference for the main effect of group (F(1, 42) = 3.9, p < .1, η2 = 0.086), showing a trend towards lower heart rates in the high ruminators. Heart rates increased in the whole group from the resting-state measure to CTL1 (t(43) = 12.75, p > .001, d = 1.9), from CTL1 to CTL2 (t(43) = 2.74, p > .01, d = 0.41) and decreased from CTL2 to the anticipation phase of the TSST (t(43) = 3.71, p > .001, d = 0.56). During the free speech, heart rates increased significantly (t(43) = 11.35, p > .001, d = 1.7) and decreased again during the post resting-state measurement (t(43) = 14.23, p > .001, d = 2.1). Importantly, heart rate was significantly elevated during the TSST arithmetic task in comparison to CTL1 (t(43) = 5.7, p > .001, d = 0.86) and CTL2 (t(43) = 5.4, p > .001, d = 0.81) (see Fig. 2C).
In line with this, cortisol levels showed a significant increase through the stress induction (F(1, 43) = 24.203, p < .001, η2 = 0.36; see Fig. 2D). However, no significant differences in cortisol levels were found between the groups.
3.2. Cortical activation
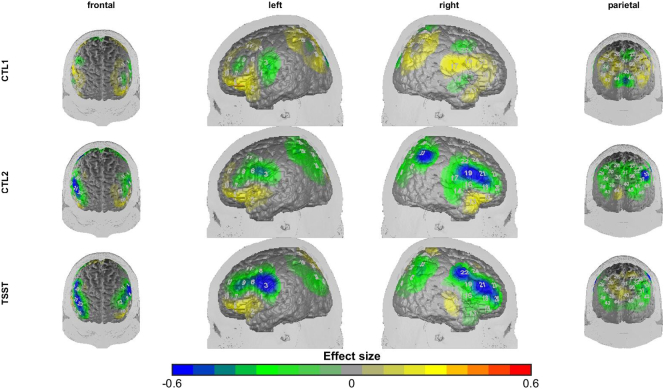
As indicated by repeated measurement ANOVA with the factors group (high vs. low ruminators) and condition (CTL1 vs. CTL2 vs. TSST arithmetic challenge), we found significant main effects for condition in the ROIs of the left dlPFC (F(2, 86) = 4.79, p < .05, η2 = 0.10), left IFG (F(2, 86) = 4.19, p < .05, η2 = 0.09), right dlPFC (F(2, 86) = 5.10, p < .01, η2 = 0.11) and SAC (F(2, 86) = 6.6, p < .01, η2 = 0.13). Post-hoc tests revealed a significant increase from CTL1 to CTL2 in all of these ROI (t(43) = 3.22 to 4.23, p < .001, d = 0.48 to 0.59). Increases from CTL2 to TSST were found in the left IFG (t(43) = 1.73, p < .05, d = 0.26) and SAC (t(43) = 1.89, p < .05, d = 0.28). Also, planned comparisons for the right dlPFC showed a significant linear group by condition contrast (F(1, 43) = 4.75, p < .05, η2 = 0.10) indicating a higher increase in cortical activation from the non-stressful to stressful conditions in the low ruminators than in the high ruminators (see Fig. 3).
Fig. 3.
Differences in cortical activation between high and low ruminators in the experimental conditions. Cold colors indicate higher activation in the low ruminators. (For interpretation of the references to color in this figure legend, the reader is referred to the online version of this chapter.)
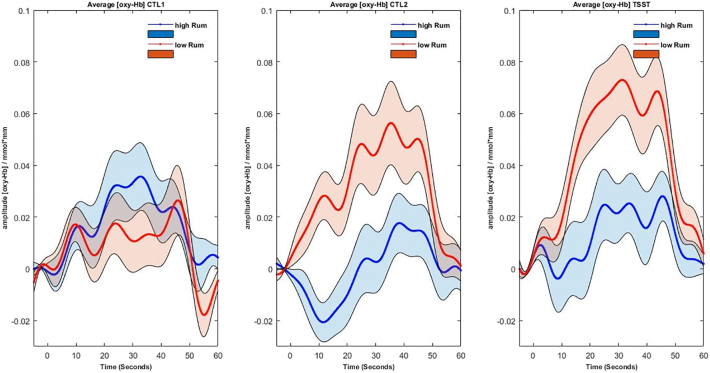
A significant group by condition interaction was found for the right IFG (F(2, 86) = 4.3, p < .05, η2 = 0.09). As for the right dlPFC, the linear contrast indicated a higher increase in cortical activation for the low ruminators from the control conditions to the TSST (F(1,43) = 7.19, p < .01, η2 = 0.14). Post-hoc tests revealed that low ruminators had higher activity within the right IFG during the CTL2 (t(43) = 2.87, p < .01, d = 0.85) and TSST (t(43) = 2.38, p < .05, d = 0.70) than high ruminators, but not during CTL1 (see supplementary Fig. S3). Further post-hoc comparisons revealed that a significant increase in IFG activity from CTL1 to TSST occurred only in low ruminators (t(21) = 3.6, p > .01, d = 0.77) (see Fig. 4).
Fig. 4.
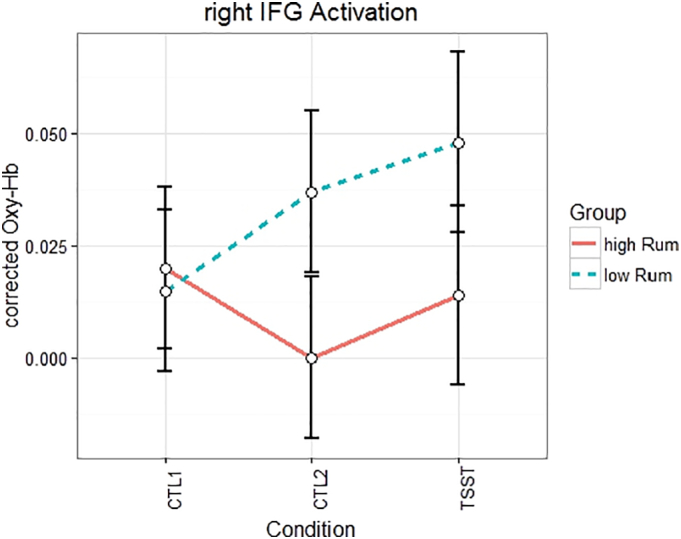
Interaction of condition by group-membership in the right IFG in cortical activation.
3.3. Mediation analysis
As indicated by Sobels Z-Test, we found a full mediation of the group effect on negative affect at the end of the experiment (B = 2.275 (1.104), t(42) = −2.18, p < .05, R2 = 0.10), by the increase of cortical activation from CTL1 to the TSST in the right IFG (B = −26.279 (9.85), t(42) = −2.66, p < .05, R2 = 0.145; Z = 2.697, p < .05). The mediation indicates that the high ruminators had a lower increase in right IFG activation that led to higher negative affect at the end of the experiment.
Further, the group effect on stress-induced changes in state rumination (B = 1.008 (0.245), t(42) = −4.12, p < .001, R2 = 0.28) was partially mediated by the increase in right IFG activation (B = −5.42 (0.295), t(42) = −2.03, p < .05, R2 = 0.09; Z = 3.25, p < .05). As for negative affect, our results indicate that the reduced IFG activation during the TSST in the high ruminators led to higher state rumination after the experiment. No such mediation effects were found for the effects on subjective stress.
4. Discussion
The aim of this study was to explore the effects of rumination on the stress response. We hypothesized that stress would induce ruminative processes (state rumination) and that this effect would be higher in high-trait ruminators. Further, we assumed that high ruminators would show a distinct pattern in subjective stress, sympathetic activity, the endocrinological stress response and cortical activation during and/or following the TSST.
Firstly, as expected, we found significant increases in behavioral, physiological and endocrinological stress indices during the stress induction of the TSST as compared to two control conditions. These were accompanied by elevated cortical activity in regions of cognitive and attentional control, namely the dorsolateral prefrontal cortex, inferior prefrontal cortex and superior parietal lobule/somatosensory association cortex. Additionally, the TSST condition led to further increases in activity of the left IFG and SAC in comparison to the CTL2. These main effects of within-subject comparisons reflect a successful induction of psychosocial stress and their cortical correlates.
With regards to our primary research hypothesis, our results showed that high ruminators showed a higher reactivity in negative affect and state rumination through the stress induction. No differences were found with regards to heart rate and cortisol responses. In line with our hypotheses, we found reduced cortical activity in the right IFG in this group. Finally, a mediation analysis showed that the group effects on negative affect and state rumination were mediated by cortical activation in the right IFG.
The found difference between high and low ruminators in the right IFG fits well with the present literature on the function of the IFG which has been reported to be central to inhibition during cognitive tasks and during physiological and psychological stress paradigms (Aron et al., 2004b; Depue et al., 2007; Kogler et al., 2015; Wang et al., 2005). For example, previous data suggest its involvement during response inhibition in Go-NoGo tasks (Garavan et al., 1999; Konishi et al., 1998; Rubia et al., 2003), task switching paradigms (Aron et al., 2004a), cold pressure tests and arithmetic stress challenges (Kogler et al., 2015). Also, rumination has been related to deficits in cognitive control and inhibition (Smith and Alloy, 2009). From our data, we would suggest that the lower activation of the right IFG during CTL2 and TSST conditions in high ruminators reflects such inhibitory deficits. Moreover, these inhibitory deficits during social stress situations led to higher negative affect and higher state rumination in the post TSST phase. These findings indicate – in terms of a more general interpretation – that inhibition deficits in high ruminators might lead to a reduced resilience to adverse events and impaired psychological (and physiological) health (Joormann, 2005, Joormann, 2006; Rosenbaum et al., 2017). Interestingly, also data of lesion studies suggests that IFG damage is associated with problems in “directed forgetting”, which means that subjects with IFG damage have problems to suppress or exclude material from memory retrieval (Conway and Fthenaki, 2003). This is in line with some characteristics of rumination, in which subjects can't stop ruminating after stressful events and have problems to stop thinking about their past failures. Herein lies a potential explanation for the found mediation of group membership effects on state rumination and negative affect by right IFG activation: The high ruminators were not able to sufficiently activate their right IFG during the stress tasks, which might reflect insufficient inhibition of stress-related emotional and cognitive responses during the TSST. In the aftermath, these inhibitory deficits resulted in elevated levels of state rumination and negative emotionality. In line with this suggestion, Herrmann et al. (2016) found reduced stress responses in a threat task after stimulation of the right IFG with transcranial direct current stimulation (Herrmann et al., 2016). However, with respect to our data it is unclear in how far the reduced IFG activation during the TSST may already be a correlate of intrusive negative thoughts while performing the arithmetic task.
Interestingly, differences between the high and low ruminators in right IFG activation were already found during the second control task. However, also subjective stress levels and heart rate measures were significantly increased during this control task, when compared to CTL1 and resting-state measurements. From this point, one could argue that the arithmetic control task (CTL2) already induced moderate levels of stress that were accompanied by reduced cortical activation in the right IFG in the high ruminators. Indeed, arithmetic tasks – even without explicit social stressors as in the TSST (camera and judges) – have been shown to elicit stress in individuals (Beilock, 2008; Noto et al., 2005).
Planned comparisons by a linear contrast showed a significant group by condition effect in the right dlPFC. The direction of this effect was in line with the reported results of the right IFG, showing attenuated cortical reactivity in the high ruminators. Both areas – IFG and dlPFC – are part of the CCN and have strong functional and structural connections. The adaption during the TSST demands several cognitive functions comprising – besides inhibitory control – also attentional processes, which is likely reflected by an increase in dlPFC activation. Indeed, inhibition and attentional control are both cognitive processes that are deeply entangled and sometimes even interchanged. It has been shown previously that depression and rumination are associated with deficits in tasks that require attention switching (Koster et al., 2013; Whitmer and Banich, 2007), cognitive and attentional control (Ottowitz et al., 2002) with attentional biases towards negative information (Koster et al., 2005). It is thus very likely that such deficits in high ruminators are also relevant in the TSST in which subjects have to refocus their attention after miscalculations or distractions by emotional non-reactivity of the reviewer board.
Although effects of rumination on heart rate and cortisol levels are reported on a meta-analytic level (Ottaviani et al., 2016), we did not find group differences in these variables, although they showed an expected reactivity pattern through the stress induction. One possible explanation may lie in the found meta-analytic effect sizes for heart rate (g = 0.20 to 0.28) and cortisol (g = 0.32 to 0.36), which are small to medium, and the power in our sample, which requires medium to high effect sizes.
Despite these conclusive findings, some limitations have to be noted. Firstly, through the fNIRS method's depth resolution, our results are restricted to the upper 2–3 cm of the cortical parts of the brain (Haeussinger et al., 2011). Potential effects in other areas of the brain could not be measured in the study at hand. Another limitation concerns the study sample. We used a non-clinical sample to prevent the influence of therapeutic interventions on the results. As previous studies have shown, the habit to ruminate is also a predictor for mental and physical health in non-clinical populations and might be considered a risk factor (Michalak et al., 2011; Teismann et al., 2008). Since the mental process per se is likely similar in clinical and non-clinical populations (and might only differ in the amount of time spent ruminating and its controllability), the results of this study should mostly be generalizable to clinical populations. In fact, the trait rumination – as measured with the RRS – of the high ruminators in this sample (m = 2.6, SD = 0.17) were comparable to those of depressed patients in our clinic (N = 24, m = 2.6, SD = 0.56). Nonetheless, in future studies, the reported effects should be replicated in clinical populations, with additional consideration of potential effects of medication status. Also, the results of the mediation analysis have to be interpreted with caution due to the relatively small sample size. In future studies, the reported results should be replicated in clinical samples with larger sample sizes. Further, a classification system of behavioral reactions of participants during the TSST that could be videotaped could give further insight into the specific processes that lead to cortical differences between subject groups.
In conclusion, we found reduced stress-related cortical activation in the right IFG in high ruminators, an effect that is likely related to inhibitory deficits and led to heightened negative affect and ruminative thinking following the stress task. The fNIRS method was shown to be usable in subclinical subjects in the original TSST setting, which might also be valuable for the investigation of depression and other stress-related clinical disorders. Overall, the present findings provide insight into possible mechanisms by which high trait rumination may act as a risk factor for the development of clinical syndromes and maladaptive stress responses.
The following are the supplementary data related to this article.
Fig. S1.
Channel positions of the probesets on the brain.
Fig. S2.
Definition of the five ROIs within the used probesets.
Fig. S3.
Waveforms of the hemodynamic response (oxy-Hb) averaged over right IFG ROIs for low ruminators (red) and high ruminators (blue) in the three conditions (left: CTL1, middle: CTL2, right: TSST arithmetic).
Financial disclosures
Ann-Christine Ehlis was partly supported by IZKF Tübingen (Junior Research Group 2115-0-0). David Rosenbaum was partly supported by STORZ MEDICAL AG. No author reported conflicts of interest.
Authors contributions
D.R., M.T. & P.H. contributed to the analysis and interpretation of the data for the work and did the primary drafting. A.J.F., H.C.N., V.N., F.G.M., F.B.H. & A.-C.E. contributed to the design and acquisition of the work and revised it critically for important intellectual content.
All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Ramona Taeglich, Betti Schopp and Larissa Metzler for their excellent work and their valuable support with the measurements.
References
- Aron A.R., Monsell S., Sahakian B.J., Robbins T.W. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain J. Neurol. 2004;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Hautzinger M. 1994. Beck-Depressions-Inventar: (BDI); Testhandbuch, 1. Aufl. ed. Huber, Bern. [Google Scholar]
- Beilock S.L. Math performance in stressful situations. Curr. Dir. Psychol. Sci. 2008;17:339–343. [Google Scholar]
- Berman M.G., Misic B., Buschkuehl M., Kross E., Deldin P.J., Peltier S., Churchill N.W., Jaeggi S.M., Vakorin V., McIntosh A.R., Jonides J. Does resting-state connectivity reflect depressive rumination? A tale of two analyses. NeuroImage. 2014;103:267–279. doi: 10.1016/j.neuroimage.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Bratman G.N., Hamilton J.P., Hahn K.S., Daily G.C., Gross J.J. Nature experience reduces rumination and subgenual prefrontal cortex activation. Proc. Natl. Acad. Sci. 2015;112:8567–8572. doi: 10.1073/pnas.1510459112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigadoi S., Ceccherini L., Cutini S., Scarpa F., Scatturin P., Selb J., Gagnon L., Boas D.A., Cooper R.J. Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data. NeuroImage. 2014;85:181–191. doi: 10.1016/j.neuroimage.2013.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianco L., Morris J.M., Wells A. Worry and rumination: do they prolong physiological and affective recovery from stress? Anxiety Stress Coping. 2018;12:1–13. doi: 10.1080/10615806.2018.1438723. [DOI] [PubMed] [Google Scholar]
- Conway M.A., Fthenaki A. Disruption of inhibitory control of memory following lesions to the frontal and temporal lobes. Cortex J. Devoted Study Nerv. Syst. Behav. 2003;39:667–686. doi: 10.1016/s0010-9452(08)70859-1. [DOI] [PubMed] [Google Scholar]
- Cooney R.E., Joormann J., Eugène F., Dennis E.L., Gotlib I.H. Neural correlates of rumination in depression. Cogn. Affect. Behav. Neurosci. 2010;10:470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Bray S., Reiss A.L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. NeuroImage. 2010;49:3039–3046. doi: 10.1016/j.neuroimage.2009.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutini S., Scatturin P., Zorzi M. A new method based on ICBM152 head surface for probe placement in multichannel fNIRS. NeuroImage. 2011;54:919–927. doi: 10.1016/j.neuroimage.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Denson T.F., Fabiansson E.C., Creswell J.D., Pedersen W.C. Experimental effects of rumination styles on salivary cortisol responses. Motiv. Emot. 2009;33:42–48. [Google Scholar]
- Depue B.E., Curran T., Banich M.T. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- Eshun S. Role of gender and rumination in suicide ideation: a comparison of college samples from Ghana and the United States. Cross-Cult. Res. 2000;34:250–263. [Google Scholar]
- Garavan H., Ross T.J., Stein E.A. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianferante D., Thoma M.V., Hanlin L., Chen X., Breines J., Zoccola P.M., Rohleder N. Post-stress rumination predicts HPA axis responses to repeated acute stress. Psychoneuroendocrinology. 2014;49:244–252. doi: 10.1016/j.psyneuen.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeussinger F.B., Heinzel S., Hahn T., Schecklmann M., Ehlis A.-C., Fallgatter A.J. Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol. Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M.J., Beier J.S., Simons B., Polak T. Transcranial direct current stimulation (tDCS) of the right inferior frontal Gyrus attenuates skin conductance responses to unpredictable threat conditions. Front. Hum. Neurosci. 2016;10 doi: 10.3389/fnhum.2016.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt L.M., Aldao A., Fischer K. Rumination and multi-modal emotional reactivity. Cognit. Emot. 2015;29:1486–1495. doi: 10.1080/02699931.2014.989816. [DOI] [PubMed] [Google Scholar]
- Ito T., Takenaka K., Tomita T., Agari I. Comparison of ruminative responses with negative rumination as a vulnerability factor for depression. Psychol. Rep. 2006;99:763–772. doi: 10.2466/PR0.99.3.763-772. [DOI] [PubMed] [Google Scholar]
- Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1958;10:370–375. [Google Scholar]
- Joormann J. Inhibition, rumination, and mood regulation in depression. In: Engle R.W., Sedek G., von Hecker U., McIntosh D.N., editors. Cognitive Limitations in Aging and Psychopathology. Cambridge University Press; Cambridge: 2005. pp. 275–312. [Google Scholar]
- Joormann J. Differential effects of rumination and dysphoria on the inhibition of irrelevant emotional material: evidence from a negative priming task. Cogn. Ther. Res. 2006;30:149–160. [Google Scholar]
- Kelley N.J., Hortensius R., Harmon-Jones E. When anger leads to rumination. Psychol. Sci. 2013;24:475–481. doi: 10.1177/0956797612457384. [DOI] [PubMed] [Google Scholar]
- Kogler L., Müller V.I., Chang A., Eickhoff S.B., Fox P.T., Gur R.C., Derntl B. Psychosocial versus physiological stress — meta-analyses on deactivations and activations of the neural correlates of stress reactions. NeuroImage. 2015;119:235–251. doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S., Nakajima K., Uchida I., Sekihara K., Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging: no-go dominant brain activity revealed by fMRI. Eur. J. Neurosci. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Koster E.H.W., De Raedt R., Goeleven E., Franck E., Crombez G. Mood-congruent attentional bias in dysphoria: maintained attention to and impaired disengagement from negative information. Emotion. 2005;5:446–455. doi: 10.1037/1528-3542.5.4.446. [DOI] [PubMed] [Google Scholar]
- Koster E.H.W., De Lissnyder E., De Raedt R. Rumination is characterized by valence-specific impairments in switching of attention. Acta Psychol. 2013;144:563–570. doi: 10.1016/j.actpsy.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Koval P., Kuppens P., Allen N.B., Sheeber L. Getting stuck in depression: the roles of rumination and emotional inertia. Cognit. Emot. 2012;26:1412–1427. doi: 10.1080/02699931.2012.667392. [DOI] [PubMed] [Google Scholar]
- Lehrl S. 2005. Manual zum MWT-B: Mehrfachwahl-Wortschatz-Intelligenztest, 5., unveränd. Aufl. ed. Spitta-Verl, Balingen. [Google Scholar]
- LeMoult J., Joormann J. Depressive rumination alters cortisol decline in major depressive disorder. Biol. Psychol. 2014;100:50–55. doi: 10.1016/j.biopsycho.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellings T.M., Alden L.E. Cognitive processes in social anxiety: the effects of self-focus, rumination and anticipatory processing. Behav. Res. Ther. 2000;38:243–257. doi: 10.1016/s0005-7967(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Michalak J., Hölz A., Teismann T. Rumination as a predictor of relapse in mindfulness-based cognitive therapy for depression: rumination as a predictor of relapse. Psychol. Psychother. Theory Res. Pract. 2011;84:230–236. doi: 10.1348/147608310X520166. [DOI] [PubMed] [Google Scholar]
- Nejad A.B., Fossati P., Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front. Hum. Neurosci. 2013;7(666) doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J. Abnorm. Psychol. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Noto Y., Sato T., Kudo M., Kurata K., Hirota K. The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesth. Analg. 2005:1873–1876. doi: 10.1213/01.ANE.0000184196.60838.8D. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Thayer J.F., Verkuil B., Lonigro A., Medea B., Couyoumdjian A., Brosschot J.F. Physiological concomitants of perseverative cognition: a systematic review and meta-analysis. Psychol. Bull. 2016;142:231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Ottowitz W.E., Dougherty D.D., Savage C.R. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harv. Rev. Psychiatry. 2002;10:86–99. doi: 10.1080/10673220216210. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D., Haipt A., Fuhr K., Haeussinger F.B., Metzger F.G., Nuerk H.-C., Fallgatter A.J., Batra A., Ehlis A.-C. Aberrant functional connectivity in depression as an index of state and trait rumination. Scientific Reports. 2017;7 doi: 10.1038/s41598-017-02277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum D., Hilsendegen P., Thomas M., Haeussinger F.B., Metzger F.G., Nuerk H.-C., Fallgatter A.J., Nieratschker V., Ehlis A.-C. Cortical hemodynamic changes during the Trier Social Stress Test: An fNIRS study. NeuroImage. 2018;171:107–115. doi: 10.1016/j.neuroimage.2017.12.061. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Shull A., Mayer S.E., McGinnis E., Geiss E., Vargas I., Lopez-Duran N.L. Trait and state rumination interact to prolong cortisol activation to psychosocial stress in females. Psychoneuroendocrinology. 2016;74:324–332. doi: 10.1016/j.psyneuen.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Skoluda N., Strahler J., Schlotz W., Niederberger L., Marques S., Fischer S., Thoma M.V., Spoerri C., Ehlert U., Nater U.M. Intra-individual psychological and physiological responses to acute laboratory stressors of different intensity. Psychoneuroendocrinology, this issue includes a special section on biomarkers in the military - new findings from prospective. Studies. 2015;51:227–236. doi: 10.1016/j.psyneuen.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Smith J.M., Alloy L.B. A roadmap to rumination: a review of the definition, assessment, and conceptualization of this multifaceted construct. Clin. Psychol. Rev. 2009;29:116–128. doi: 10.1016/j.cpr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel M.E. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol. Methodol. 1982;13:290–312. [Google Scholar]
- Sobel M.E. Some new results on indirect effects and their standard errors in covariance structure models. Sociol. Methodol. 1986;16:159–186. [Google Scholar]
- Teismann T. Springer; Berlin; New York: 2012. Kognitive Verhaltenstherapie depressiven Grübelns. [Google Scholar]
- Teismann T., Willutzki U., Michalak J., Schulte D. Bedeutung von Rumination und Ablenkung fuer den Therapieerfolg depressiver Patienten. Verhaltenstherapie. 2008;18:215–222. [Google Scholar]
- Thomsen D.K., Mehlsen M.Y., Hokland M., Viidik A., Olesen F., Avlund K., Munk K., Zachariae R. Negative thoughts and health: associations among rumination, immunity, and health care utilization in a young and elderly sample. Psychosom. Med. 2004;66 doi: 10.1097/01.psy.0000127688.44363.fb. [DOI] [PubMed] [Google Scholar]
- Thomsen D.K., Mehlsen M.Y., Olesen F., Hokland M., Viidik A., Avlund K., Zachariae R. Is there an association between rumination and self-reported physical health? A one-year follow-up in a young and an elderly sample. J. Behav. Med. 2004;27:215–231. doi: 10.1023/b:jobm.0000028496.41492.34. [DOI] [PubMed] [Google Scholar]
- Tsuzuki D., Dan I. NeuroImage, Celebrating 20 Years of Functional Near Infrared Spectroscopy (fNIRS) Vol. 85. 2014. Spatial registration for functional near-infrared spectroscopy: From channel position on the scalp to cortical location in individual and group analyses; pp. 92–103. [DOI] [PubMed] [Google Scholar]
- Wang J., Rao H., Wetmore G.S., Furlan P.M., Korczykowski M., Dinges D.F., Detre J.A. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc. Natl. Acad. Sci. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whitmer A.J., Banich M.T. Inhibition versus switching deficits in different forms of rumination. Psychol. Sci. 2007;18:546–553. doi: 10.1111/j.1467-9280.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- Young E.A., Nolen-Hoeksema S. Effect of ruminations on the saliva cortisol response to a social stressor. Psychoneuroendocrinology. 2001;26:319–329. doi: 10.1016/s0306-4530(00)00059-7. [DOI] [PubMed] [Google Scholar]
- Zhang X., Noah J.A., Hirsch J. Separation of the global and local components in functional near-infrared spectroscopy signals using principal component spatial filtering. Neurophotonics. 2016;3 doi: 10.1117/1.NPh.3.1.015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Pu W., Yao S. Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naïve patients with major depressive disorder: a meta-analysis of resting-state fMRI data. J. Affect. Disord. 2016;206:280–286. doi: 10.1016/j.jad.2016.09.005. [DOI] [PubMed] [Google Scholar]