Abstract
Background
The pathophysiology that drives the subacute hypercoagulable state commonly seen after traumatic brain injury (TBI) is not well understood. Alterations caused by TBI in platelet and microparticle (MP) numbers and function have been suggested as possible causes; however, the contributions of platelets and MPs are currently unknown.
Materials and methods
A weight-drop technique of TBI using a murine model of moderate head injury was used. Blood was collected at intervals after injury. MP enumeration and characterization were performed using Nanoparticle Tracking Analysis, and platelet counts and coagulation parameters were determined using thromboelastometry. A MP procoagulant assay was used to compare activity between injured and sham mice.
Results
At 24 h after injury, there were no changes in circulating platelet numbers. However, there was a decrease in platelet contribution to clot formation. In contrast, there was a decline in circulating total MP numbers. When MPs from sham mice were added to the blood from head-injured animals, there was a normalization of platelet contribution to clot formation. Conversely, when MPs from TBI mice were added to sham blood, there was a significant decrease in platelet contribution to clot formation. Notably, there was an increase in MP procoagulant activity in head-injured mice.
Conclusions
MPs generated after TBI likely contribute to altered coagulation after head injury and may play a key role in the development of a posttraumatic hypercoagulable state in TBI patients.
Keywords: Platelet function, Microparticles, TBI, Hypercoagulability, Thromboelastometry
1. Introduction
Traumatic brain injury (TBI) results in significant disability and mortality among trauma populations. Mortality rates have remained relatively stable over the past 10 years suggesting a need for better understanding and treatment of this disease process [1]. One of the major pathophysiologic disturbances shown to be predictive of increased morbidity and mortality after TBI is acute traumatic coagulopathy, with reported incidences ranges from 10%–97.5% [2–5]. After this immediate hypocoagulable state, a subacute hypercoagulable state develops with the incidence of deep vein thrombosis ranging from 1.8%–44% in major trauma patients [6–8]. More specifically, the presence of head injury is predictive of an increased risk for thrombotic events [9]. Although the mechanisms behind these changes are not fully understood [10–12], the commonly proposed causes include consumptive coagulopathy [13], tissue factor release [14], and plateletdysfunction [4,15–17].
More recent investigations have begun to explore the role of microparticles (MPs) in this process, as they have been identified as markers of and possible contributors to the hypercoagulable states seen in venous thromboembolism [18], cardiovascular disease [19], cancers [20], prothrombotic pregnancy complications [21], and posttraumatic states [22,23]. MPs are defined as 0.1–1 μm vesicles that bleb off cell membranes of activated or apoptotic cells and function in cell–cell communication [24]. Procoagulant MPs are often identified as those expressing tissue factor or phosphatidylserine, and previous studies have found them to be increased in the cerebrospinal fluid and blood of patients after intracranial hemorrhage [25] and head trauma [26–28]. However, the role of these particles in altering coagulation, and more specifically platelet function, has not been explored.
In the present study, we used a murine model to characterize alterations in coagulation after TBI to determine whether MPs contribute to these changes. Our hypotheses were that (1) TBI would result in a subacute hypercoagulable state, (2) increased circulating MPs would be seen after TBI, and (3) the changes in coagulation would be due to the altered MP populations.
2. Materials and methods
2.1. Materials
Male C57BL/6 mice between ages 6 and 8 wk and 20–28 g obtained from Taconic Labs (Hudson, NY) were used for all experiments. Only male mice were used as previous research has demonstrated that higher estrogen levels can impact immune status after trauma [29]. All murine experiments were performed between 8 AM and 10 AM and were performed under protocols approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
2.2. Traumatic brain injury
Mice were anesthetized with 2% inhaled isoflurane, and a traumatic closed-head injury was induced using an established weight-drop model [30]. In short, after anesthesia, mice were placed prone on a platform, and the posterior coronal suture of the mice was aligned below the weight. A 400 g weight was then dropped 1 cm to induce head injury. Sham mice were anesthetized and laid on the platform without head injury. Righting reflex time was measured immediately after TBI to ensure an injury of moderate severity.
2.3. Thromboelastometry
Rotational thromboelastometry (ROTEM; TEM Systems Inc, Durham, NC) analyses were performed to determine alterations in coagulation per manufacturer instructions. Whole blood collected via cardiac puncture from sham and TBI mice was anticoagulated with 10% citrate. All analyses were initiated within 10min ofwholeblood collection. Overall coagulationwas determined using NATEM, extrinsic pathway coagulation using EXTEM, and fibrin contribution to clot using the FIBTEM analysis. For EXTEM and FIBTEM analysis, 20 μL of thromboplastin and cytochalasin D were added to 300 μL of citrated blood. Clotting time (CT), clot formation time (CFT), clot lysis (LI30), α-angle (AA), and maximum clot firmness (MCF) were determined for each test. Treated samples had 30 μL of saline or MP preparations added. MPs were isolated as described in section 2.5. Percent of platelet contribution (%MCF-Platelet) was calculated by the equation: (EXTEMMCF – FIBTEMMCF)/EXTEMMCF, similar to the methods used by Kornblith et al. [31]. For NATEM analysis, sham mice (n = 9) and TBI mice at 10 min (n = 7), 24 h (n = 10), and 72 h (n = 11) were used. For EXTEM and FIBTEM analyses, the same treatment groups were used with 8–10 mice per group in both the untreated and MP-treated samples.
2.4. Platelet count determination
Whole blood collected via cardiac puncture from injured mice was anticoagulated with 10% citrate. Coulter AcT 10 Hematology Analyzer (Beckman Coulter, Brea, CA) was used to determine platelet counts.
2.5. MP isolation, enumeration, and characterization
The MP isolation protocol used was adapted from those previously published from our laboratory [32]. In short, whole blood collected via cardiac puncture from injured mice was anticoagulated with heparin. It was then centrifuged at 450g for 10 min; the supernatant was collected and centrifuged at 10,000g for 10 min to remove platelets. The platelet-free supernatant containing the MPs was then diluted at 1:1000 with Roswell Park Memorial Institute media and stained with 10 μL per sample CD41 antibody (Clone MWReg30; BD Pharmingen, San Jose, CA). Nanoparticle Tracking Analysis (NanoSight; Malvern Instruments Ltd, Worcestershire, United Kingdom) was then used to enumerate total and CD41+ MP concentrations. MP populations were characterized in sham mice (n = 26) and injured mice at 30 min (n = 13), 3 h (n = 9), 24 h (n = 21), and 72 h (n = 25) after TBI. For MPs used in ROTEM analyses, platelet-free plasma was collected per previously mentioned protocols and centrifuged at 25,000g for 30 min to pellet the MPs. MPs were then resuspended in sterile saline to a concentration of 3.0 × 108 MPs/mL and added to anticoagulated blood as stated in section 2.3. MPs isolated 24 h after injury from sham mouse blood will be referred to as sham MPs and those isolated from TBI mouse blood will be referred to as TBI MPs.
2.6. MP procoagulant activity
MP procoagulant activity was determined using a Zymuphen MP-Activity functional assay (Aniara, West Chester, OH). This assay first exposed the phospholipids on the surface of the MPs and then measured the activation of prothrombin into thrombin by these exposed phospholipids. Whole blood was collected via cardiac puncture from sham (n = 8) and TBI mice (n = 7) 24 h after injury and anticoagulated with 10% citrate. MPs were isolated per protocol in section 2.5, diluted at 1:20, and the assay was performed per manufacturer protocol.
2.7. Statistical analysis
A power calculation was performed to determine samples sizes required for all experiments. With a power of 0.8, an alpha value of 0.05 and an estimated difference between treatment groups of 25%, a sample size of six was required. Student t-tests were used when comparisons were made between two treatment groups. One-way analysis of variance with Tukey post hoc test was used to compare multiple populations. Prism 6 (GraphPad Software, La Jolla, CA) was used for all statistical analysis. Experiments containing multiple data points were used to calculate means and standard errors of the mean. A P value of ≤0.05 was considered significant.
3. Results
3.1. TBI does not alter overall clot strength
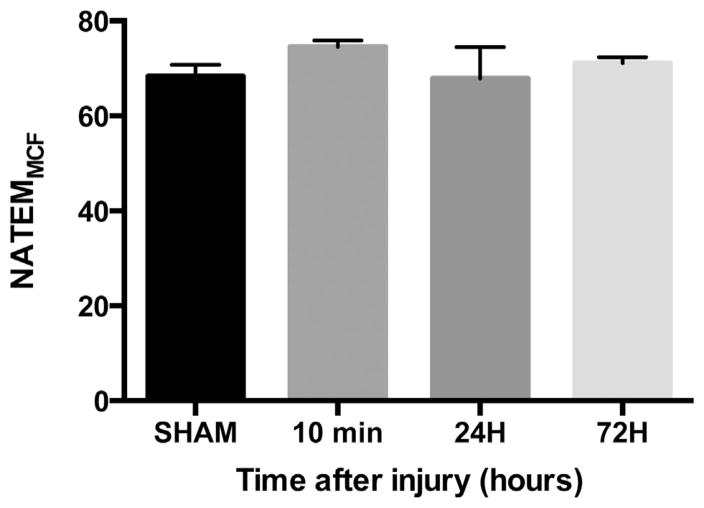
We analyzed whole blood from sham and injured mice at 10 min, 24 h, and 72 h after head trauma using the NATEM test on the ROTEM. We did not observe any differences in MCF at any time point after TBI compared with sham (Fig. 1). In the subacute phase of injury at 24 h after TBI, there were also no differences in CT, CFT, AA, or LI30. From these data, we conclude that in our TBI model, overall clot firmness is unchanged and overall coagulation is unchanged at 24 h after head injury.
Fig. 1.
Clot strength is unchanged after TBI. Whole blood NATEMMCF analysis for sham (n = 9) and TBI blood 10 min (n =7), 24 h (n =10), and 72 h (n =11) after injury. Data are expressed as the mean ± standard error of the mean. No statistically significant changes on one-way analysis of variance with Tukey post hoc test compared with sham.
3.2. Decreased platelet contribution to clot formation is seen after TBI
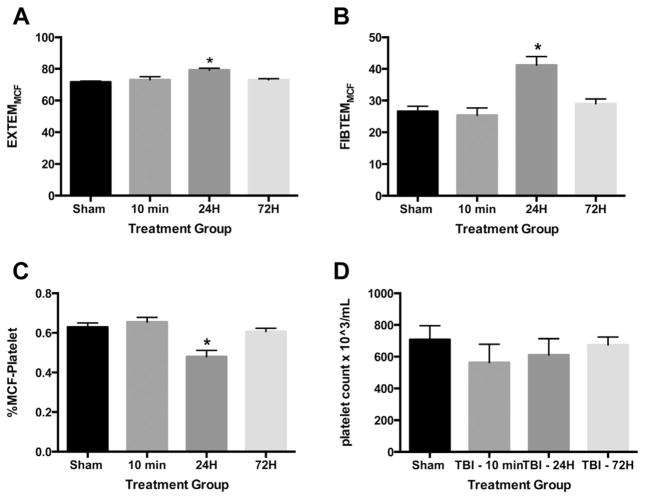
Evaluation of the extrinsic pathway after TBI demonstrated increased EXTEMMCF at 24 h after injury compared with sham with no differences seen at 10 min and 72 h after TBI (Fig. 2A). CT, CFT, LI30, and AA were not different at any time point on the EXTEM analysis (data not shown). Evaluation of fibrin function using FIBTEM analysis with platelet inhibition demonstrated increased FIBTEMMCF at 24 h after TBI compared with sham with no differences seen at the other injury time points compared with sham (Fig. 2B). CT, CFT, LI30, and AA were not different at any time point on the FIBTEM analysis (data not shown). The calculated %MCF-Platelet was found to be significantly lower 24 h after TBI compared with that of sham but unchanged 10 min and 72 h after injury (Fig. 2C). There were no significant changes in whole blood platelet counts after injury (Fig. 2D). Altogether, these data suggest that although overall clot formation remains unchanged after injury, 24 h after TBI there is a reduced platelet contribution to clot formation that is not the result of the altered platelet counts. Thus, there is another mediator that is replacing the role of platelets in clot formation after TBI.
Fig. 2.
Thromboelastometry measurements after TBI demonstrate decreased platelet contribution to clot formation. Whole blood from sham and TBI mice was collected 10 min, 24 h, and 72 h after injury and was analyzed using thromboelastometry. (A) EXTEMMCF, (B) FIBTEMMCF, (C) platelet contribution to clot formation, and (D) whole blood platelet counts. Samples sizes are 8–10 for each group. Data are expressed as the mean ± standard error of the mean. *P < 0.05 based on one-way analysis of variance with Tukey post hoc test compared with sham.
3.3. Circulating MP populations are altered after TBI
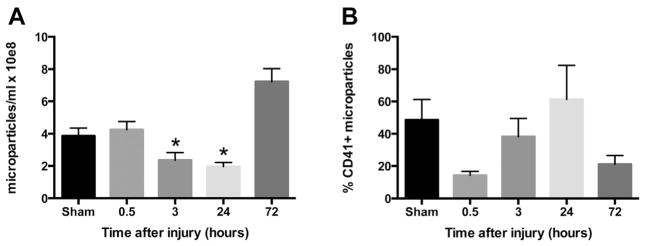
Overall MP populations were unchanged 30 min after injury, significantly decreased 3 h and 24 h after injury, and rebounded to sham levels by 72 h after injury (Fig. 3A). Platelet-derived MP populations were not significantly changed after injury (data not shown); however, the proportion of total MPs that are PMPs were altered over time with a trend toward increased PMP contribution at 24 h after injury compared with 30 min and 72 h after injury (Fig. 3B). These findings demonstrate that MP populations are altered after injury, with decreased overall circulating MPs 24 h after injury and a significantly increased proportion of PMPs at this time point compared with immediately after injury and 3 d after injury.
Fig. 3.
Circulating MP populations are altered after TBI. MPs were isolated from sham (n = 26) and TBI blood 30 min (n = 13), 3 h (n = 9), 24 h (n = 21), and 72 h (n = 24) after injury. (A) Total MP concentrations and (B) proportion of total MPs that are PMPs. Data are expressed as the mean ± standard error of the mean. *P < 0.01 based on one-way analysis of variance with Tukey post hoc test compared with sham.
3.4. MPs isolated from sham and TBI mice contribute to alterations seen in coagulation after head injury
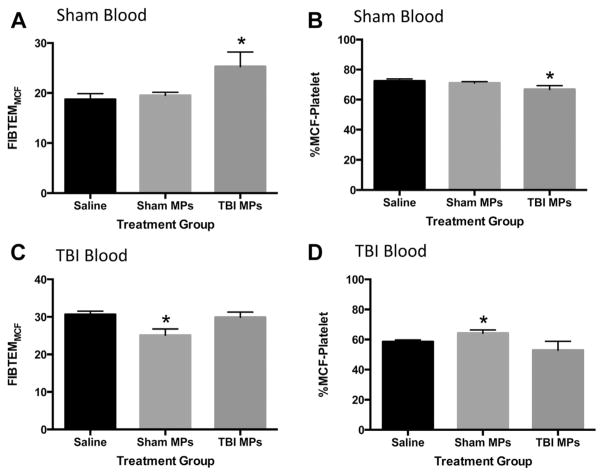
MPs were isolated from sham and TBI mice at 24 h after injury, concentrations normalized, and MP suspensions added back to sham and TBI whole blood 24 h after injury. Saline was used as a control to ensure any changes in coagulation parameters were not due to dilution of whole blood samples. ROTEM analysis using EXTEM and FIBTEM tests were performed. When sham MPs or TBI MPs were added to sham whole blood, there were no changes in the extrinsic pathway compared with saline (EXTEM test, data not shown). However, when platelet function was blocked on the FIBTEM test, there was a significant increase in FIBTEMMCF when TBI MPs were added to sham whole blood compared with saline and no change seen with addition of sham MPs to sham blood (Fig. 4A). This resulted in a significantly lower %MCF-Platelet (Fig. 4B). There were no changes in CT, CFT, LI30, or AA on EXTEM or FIBTEM tests (data not shown). Sham MPs and TBI MPs were then added to whole blood from head-injured mice 24 h after TBI. No changes were seen in any EXTEM test parameters (data not shown). When sham MPs were added to whole blood from mice 24 h after TBI, there was a significant decrease in FIB-TEMMCF compared with saline and no change seen when TBI MPs were added (Fig. 4C). This resulted in a significant increase in %MCF-Platelet in the sham MP-treated group compared with saline (Fig. 4D). There were no changes in CT, CFT, LI30, or AA with the addition of either population of MPs compared with saline (data not shown). These data suggest that addition of sham MPs or TBI MPs to blood from TBI or sham mouse blood, respectively, results in alterations to the platelet contribution to clot formation consistent with that seen in the MP donor. In summary, TBI MPs caused a significant decrease in platelet contribution to clot formation in sham blood, similar to that seen in TBI mice, and sham MPs caused a significant increase in platelet contribution to clot formation in TBI blood, consistent with coagulation profile seen in sham mice.
Fig. 4.
Serum MPs isolated from sham and TBI mice alter coagulation. MPs were isolated from whole blood from sham and TBI mice 24 h after injury. MP concentrations were normalized to 3.0 × 108 MPs/mL in 30 μL sterile saline were then added to whole blood from sham and TBI mice and thromboelastometry performed. (A) FIBTEMMCF and (B) platelet contribution to clot formation after saline, sham MPs, and TBI MPs are added to sham whole blood. (C) FIBTEMMCF and (D) platelet contribution to clot formation after saline, sham MPs, and TBI MPs are added to TBI whole blood. Data are expressed as the mean ± standard error of the mean. Samples sizes are 8–10 for each group. *P <0.01 based on one-way analysis of variance with Tukey post hoc test compared with sham.
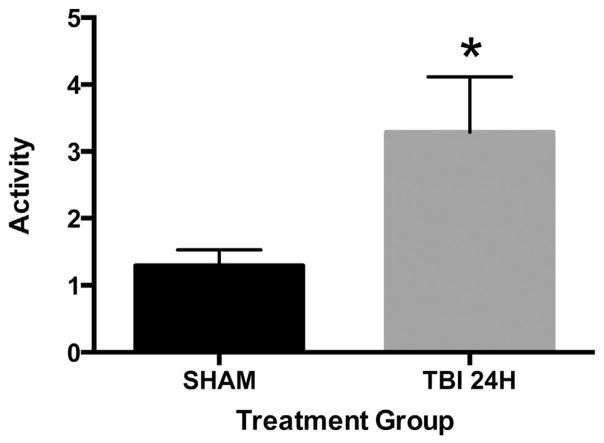
3.5. Circulating MPs in TBI mice have increased procoagulant activity 24 h after injury
To further elucidate the mechanism for MP-induced changes to clot formation, we next performed a MP procoagulant assay. At 24 h after injury, MP procoagulant activity was significantly increased compared with that of sham MPs (Fig. 5). From these data, we conclude that MPs isolated from TBI mice have an intrinsically higher procoagulant activity than those isolated from sham mice.
Fig. 5.
MP procoagulant activity is increased in TBI mice. Procoagulant activity of MPs isolated from sham (n = 8) and TBI (n = 7) blood was determined. Data are expressed as the mean ± standard error of the mean. *P = 0.04 based on Student t-test compared with sham.
4. Discussion
This study used a murine model to characterize the subacute hypercoagulable state after TBI and to determine whether MPs contribute to these changes. We demonstrated that in a murine model of TBI, there is no change in overall coagulation 24 h after injury (Fig. 1). Despite the lack of change in overall coagulation, we detected a significant decrease in platelet contribution to clot formation at 24 h after injury (Fig. 2). In addition to the altered platelet contribution 24 h after injury, there was an overall decline in circulating MP populations with a trend toward an increase in the proportion of PMPs (Fig. 3). When TBI MPs were added to sham blood, a similar decline in platelet contribution was seen, with a contrasting normalization of platelet contribution when sham MPs were added back to TBI blood 24 h after injury (Fig. 4). Furthermore, procoagulant MP activity was also increased in injured mice at this time (Fig. 5). All together, these data suggest that MPs are affecting the coagulation changes seen in these animals.
Traumatic injury, and more specifically TBI, is known to result in alterations in coagulation with an early coagulopathy and later development of a subacute hypercoagulable state. Viscoelastic coagulation testing, including thromboelastometry, is now more commonly used to characterize these postinjury states as its sensitivity for detecting the balance between thrombosis and LI30 [33]. Using this technology, human studies have shown that these tests are able to detect hypercoagulable states missed by common coagulation parameters such as plasma prothrombin time or activated partial thromboplastin time [34], and furthermore, that increased clot firmness on admission thromboelastography predicts increased rates of pulmonary embolism [35], highlighting the clinical importance of these tests. In addition, similar to our murine study, other human studies have shown that platelet contribution to clot strength steadily decreases over the 72 h after trauma, with a resulting increase in fibrinogen contribution [31]. Animal models have investigated altered platelet function after head injury [36]; however, our study is the first to demonstrate similar temporal changes in coagulation, specifically the platelet contribution to clot strength, after TBI using thromboelastometry in a murine model. With these new data, we are closer to both understanding the mechanisms behind coagulation changes after TBI and also to identifying appropriate therapeutic targets to prevent thromboembolic complications. As anticoagulation is often contraindicated in TBI patients immediately after injury, identifying specific mediators that can be targeted to prevent unwanted clot formation maybe key to minimizing life-threatening thromboembolic events.
Previous studies have investigated the time course of circulating MPs in blood [27,28] and cerebrospinal fluid [27] in human subjects after TBI, but our study is the first to describe these changes in a murine model. Interestingly in our model, circulating MP levels were significantly decreased at 24 h after injury compared with those of controls, whereas human studies suggest increased populations are present [25–28]. Similar to Nekludov et al. [28], we demonstrated a relative increase in PMPs and a similar decline in MPs soon after injury followed by an increase in MP levels at 72 h. In comparison with human studies where functional prothrombinase assays [25,27] and flow cytometry [26,28] were used for MP enumeration, MP populations in our study were determined using Nanoparticle Tracking Analysis. The different techniques used to identify MP populations may explain the differences seen as prothrombinase assays do not determine overall MP populations, and flow cytometry can only detect MPs down to a size of 200 nm, whereas Nanoparticle Tracking Analysis can reliably detect particles down to 50 nm [37].
As MP release increases with endothelial injury, platelet activation, and increased inflammation [38], and they have been shown to be procoagulant [24], it is not unexpected that they play a role in coagulation changes after injury. Platelet-derived MPs have been shown to be more prothrombotic than platelets themselves [39], and lower circulating PMPs are seen in patients that suffer from hemorrhagic complications after trauma [40]. In parallel with these studies, we noted a relative increase in PMPs 24 h after TBI and an associated increase in clot firmness at this time. By adding sham MPs and TBI MPs to whole blood ex vivo, we were also able to demonstrate that MPs may be responsible for these changes on thromboelastometry. The MPs induced measureable changes in the FIBTEMMCF that were not affected by platelet inhibition with cytochalasin D. Although we did not isolate PMPs specifically in these experiments, we also demonstrated that circulating MPs from mice 24 h after injury have significantly increased procoagulant activity. Combined with the associated increase in PMPs at this time point, we believe that the PMPs are likely the cause of these changes.
Although our study establishes an important connection between altered coagulation and the role of MPs in this process, there are limitations to the study. First, as our study was only performed in head-injured mice, it may not be applicable to all injury models. We also focused on the subacute hypercoagulable changes seen after injury and did not focus this investigation on the early coagulopathic changes immediately after injury. In addition, although we were able to demonstrate that MPs isolated from injured and sham mice altered coagulation profiles, we have yet to identify the definitive cell derivation of the MPs responsible for this change, which we speculate are PMPs. We also did not perform analyses to determine whether coagulation factors intrinsic to MPs were present in the MP preparation, including tissue factor, phosphatidylserine, and other mediators such as fibrinogen, and this will be an area of future research. Furthermore, we have yet to perform in vivo studies to verify altered coagulation when MPs are injected into injured and sham mice. Finally, we were unable to elucidate on a molecular level how MPs may alter platelet function during coagulation. When these limitations are addressed, we speculate that the knowledge gained will robustly advance our knowledge of the coagulation process after TBI.
5. Conclusions
Platelet contribution to clot formation is decreased 1 d after TBI despite unchanged platelet counts. Circulating MP populations are also altered at this time point with a significant increase in procoagulant MP activity. This suggests that MPs likely contribute to the altered platelet role in coagulation and to the development of a post-TBI hypercoagulable state.
Acknowledgments
Authors’ contributions: E.F.M., J.W.K., A.T.M., C.C.C., and M.D.G. contributed to the study design. E.F.M., P.L.J., L.A.F., R.V., A.T.M., and M.D.G. did the acquisition of data. E.F.M., P.L.J., J.W.K., R.V., A.T.M., C.C.C., and M.D.G. did the analysis of data. E.F.M. and M.D.G. did the drafting of the article. E.F.M., P.L.J., J.W.K., C.C.C., and M.D.G. did the approval of the final version. P.L.J., J.W.K., and C.C.C. did the critical revisions approval of the final version.
Footnotes
Disclosure
None of the mentioned authors has any disclosures.
References
- 1.Injury prevention & control: traumatic brain injury. Centers for Disease Control and Prevention; [Google Scholar]
- 2.Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir. 2008;150:165. doi: 10.1007/s00701-007-1475-8. discussion 175. [DOI] [PubMed] [Google Scholar]
- 3.Van Beek JG, Mushkudiani NA, Steyerberg EW, et al. Prognostic value of admission laboratory parameters in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:315. doi: 10.1089/neu.2006.0034. [DOI] [PubMed] [Google Scholar]
- 4.Carrick MM, Tyroch AH, Youens CA, Handley T. Subsequent development of thrombocytopenia and coagulopathy in moderate and severe head injury: support for serial laboratory examination. J Trauma. 2005;58:725. doi: 10.1097/01.ta.0000159249.68363.78. discussion 729–730. [DOI] [PubMed] [Google Scholar]
- 5.Kushimoto S, Yamamoto Y, Shibata Y, Sato H, Koido Y. Implications of excessive fibrinolysis and alpha(2)-plasmin inhibitor deficiency in patients with severe head injury. Neurosurgery. 2001;49:1084. doi: 10.1097/00006123-200111000-00011. discussion 1089–1090. [DOI] [PubMed] [Google Scholar]
- 6.Geerts WH, Jay RM, Code KI, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. New Engl J Med. 1996;335:701. doi: 10.1056/NEJM199609053351003. [DOI] [PubMed] [Google Scholar]
- 7.Paffrath T, Wafaisade A, Lefering R, et al. Venous thromboembolism after severe trauma: incidence, risk factors and outcome. Injury. 2010;41:97. doi: 10.1016/j.injury.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Brakenridge SC, Henley SS, Kashner TM, et al. Comparing clinical predictors of deep venous thrombosis versus pulmonary embolus after severe injury: a new paradigm for posttraumatic venous thromboembolism? J Trauma Acute Care Surg. 2013;74:1231. doi: 10.1097/TA.0b013e31828cc9a0. discussion 1237–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haut ER, Chang DC, Pierce CA, et al. Predictors of posttraumatic deep vein thrombosis (DVT): hospital practice versus patient factors-an analysis of the National Trauma Data Bank (NTDB) J Trauma. 2009;66:994. doi: 10.1097/TA.0b013e3181991adc. discussion 999–1001. [DOI] [PubMed] [Google Scholar]
- 10.Epstein DS, Mitra B, Cameron PA, Fitzgerald M, Rosenfeld JV. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: definition, incidence and outcomes. Br J Neurosurg. 2015;29:118. doi: 10.3109/02688697.2014.950632. [DOI] [PubMed] [Google Scholar]
- 11.Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT. Coagulopathy after traumatic brain injury. Neurosurgery. 2012;70:1334. doi: 10.1227/NEU.0b013e31824d179b. [DOI] [PubMed] [Google Scholar]
- 12.Kumar MA. Coagulopathy associated with traumatic brain injury. Curr Neurol Neurosci Rep. 2013;13:391. doi: 10.1007/s11910-013-0391-y. [DOI] [PubMed] [Google Scholar]
- 13.Vecht CJ, Sibinga CT, Minderhoud JM. Disseminated intravascular coagulation and head injury. J Neurol Neurosurg Psychiatry. 1975;38:567. doi: 10.1136/jnnp.38.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nekludov M, Antovic J, Bredbacka S, Blomback M. Coagulation abnormalities associated with severe isolated traumatic brain injury: cerebral arteriovenous differences in coagulation and inflammatory markers. J Neurotrauma. 2007;24:174. doi: 10.1089/neu.2006.0173. [DOI] [PubMed] [Google Scholar]
- 15.Nekludov M, Bellander BM, Blomback M, Wallen HN. Platelet dysfunction in patients with severe traumatic brain injury. J Neurotrauma. 2007;24:1699. doi: 10.1089/neu.2007.0322. [DOI] [PubMed] [Google Scholar]
- 16.Schnuriger B, Inaba K, Abdelsayed GA, et al. The impact of platelets on the progression of traumatic intracranial hemorrhage. J Trauma. 2010;68:881. doi: 10.1097/TA.0b013e3181d3cc58. [DOI] [PubMed] [Google Scholar]
- 17.Davis PK, Musunuru H, Walsh M, et al. Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit Care. 2013;18:201. doi: 10.1007/s12028-012-9745-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Qi XL, Xu MX, Mao Y, Liu ML, Song HM. Microparticles: new light shed on the understanding of venous thromboembolism. Acta Pharmacol Sin. 2014;35:1103. doi: 10.1038/aps.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiro A, Wilkinson FL, Weston R, Smyth JV, Serracino-Inglott F, Alexander MY. Endothelial microparticles as conveyors of information in atherosclerotic disease. Atherosclerosis. 2014;234:295. doi: 10.1016/j.atherosclerosis.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaptan K, Beyan C, Ifran A, Pekel A. Platelet-derived microparticle levels in women with recurrent spontaneous abortion. Int J Gynaecol Obstet. 2008;102:271. doi: 10.1016/j.ijgo.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Park MS, Owen BA, Ballinger BA, et al. Quantification of hypercoagulable state after blunt trauma: microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery. 2012;151:831. doi: 10.1016/j.surg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matijevic N, Wang YW, Wade CE, et al. Cellular microparticle and thrombogram phenotypes in the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study: correlation with coagulopathy. Thromb Res. 2014;134:652. doi: 10.1016/j.thromres.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacroix R, Dignat-George F. Microparticles as a circulating source of procoagulant and fibrinolytic activities in the circulation. Thromb Res. 2012;129(Suppl 2):S27. doi: 10.1016/j.thromres.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Huang M, Hu YY, Dong XQ. High concentrations of procoagulant microparticles in the cerebrospinal fluid and peripheral blood of patients with acute basal ganglia hemorrhage are associated with poor outcome. Surg Neurol. 2009;72:481. doi: 10.1016/j.surneu.2008.12.016. discussion 489. [DOI] [PubMed] [Google Scholar]
- 26.Patz S, Trattnig C, Grunbacher G, et al. More than cell dust: microparticles isolated from cerebrospinal fluid of brain injured patients are messengers carrying mRNAs, miRNAs, and proteins. J Neurotrauma. 2013;30:1232. doi: 10.1089/neu.2012.2596. [DOI] [PubMed] [Google Scholar]
- 27.Morel N, Morel O, Petit L, et al. Generation of procoagulant microparticles in cerebrospinal fluid and peripheral blood after traumatic brain injury. J Trauma. 2008;64:698. doi: 10.1097/TA.0b013e31816493ad. [DOI] [PubMed] [Google Scholar]
- 28.Nekludov M, Mobarrez F, Gryth D, Bellander BM, Wallen H. Formation of microparticles in the injured brain of patients with severe isolated traumatic brain injury. J Neurotrauma. 2014;31:1927. doi: 10.1089/neu.2013.3168. [DOI] [PubMed] [Google Scholar]
- 29.Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response—effect of gender differences. Injury. 2007;38:1382. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang SH, Gustafson J, Gangidine M, et al. A murine model of mild traumatic brain injury exhibiting cognitive and motor deficits. J Surg Res. 2013;184:981. doi: 10.1016/j.jss.2013.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornblith LZ, Kutcher ME, Redick BJ, Calfee CS, Vilardi RF, Cohen MJ. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76:255. doi: 10.1097/TA.0000000000000108. discussion 262–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash PS, Caldwell CC, Lentsch AB, Pritts TA, Robinson BR. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J Trauma acute Care Surg. 2012;73:401. doi: 10.1097/TA.0b013e31825a776d. discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251:604. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 34.Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266. doi: 10.1097/TA.0b013e3181ae6f1c. discussion 275–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cotton BA, Minei KM, Radwan ZA, et al. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma acute Care Surg. 2012;72:1470. doi: 10.1097/TA.0b013e31824d56ad. discussion 1475–1477. [DOI] [PubMed] [Google Scholar]
- 36.Ploplis VA, Donahue DL, Sandoval-Cooper MJ, et al. Systemic platelet dysfunction is the result of local dysregulated coagulation and platelet activation in the brain in a rat model of isolated traumatic brain injury. J Neurotrauma. 2014;31:1672. doi: 10.1089/neu.2013.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Sinauridze EI, Kireev DA, Popenko NY, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425. [PubMed] [Google Scholar]
- 40.Windeløv NA, Johansson PI, Sørensen AM, et al. Low level of procoagulant platelet microparticles is associated with impaired coagulation and transfusion requirements in trauma patients. J Trauma Acute Care Surg. 2014;77:692. doi: 10.1097/TA.0000000000000437. [DOI] [PubMed] [Google Scholar]