Abstract
Historically, cancer treatment has emphasized measures for the “cure” regardless of the long-term consequences. Advances in cancer detection and treatment have resulted in improved outcomes bringing to the fore various quality of life considerations including future fertility. For many young cancer patients, fertility preservation is now an integral component of clinical decision-making and treatment design.
Optimal fertility-sparing options for young patients with gynecologic cancer are influenced by patient age, primary cancer, treatment regimens, and patient preferences. Possible approaches include embryo or oocyte cryopreservation, ovarian transposition, conservative surgery, and conservative medical treatment to delay radical surgery. These may be used alone or in combination to maximize fertility preservation.
Awareness of the various fertility-sparing options, eligibility criteria, and the central role of Magnetic Resonance Imaging (MRI) in the proper selection of patients will enable radiologists to produce complete clinically relevant imaging reports and serve as effective consultants to referring clinicians. Knowledge of the potential imaging pitfalls is essential to avoid misinterpretation and guide appropriate management.
Keywords: Oncofertility, Fertility preservation, Magnetic Resonance Imaging, Gynecologic cancer, Cancer
Introduction
Cancer survivorship has improved over the years and increasingly there are many young survivors including both children and women of reproductive age. It is estimated that in 2016, 10% of cancer survivors in the United States will be younger than 40 years (1). As such, quality of life issues including fertility preservation have become increasingly valuable to patients, families, and physicians. In the evaluation and treatment of cancer, there has been a paradigm shift leading to the emergence of oncofertility, a new field that recognizes the psychosocial impact of cancer-related infertility, bridges oncology with reproductive research, and assists patients to make informed decisions regarding cancer treatment and future fertility (2, 3).
Physicians need be familiar with the latest fertility-sparing approaches to effectively counsel women of reproductive age or parents/guardians of pediatric patients (4). Fertility-preserving options are influenced by the patient’s age, primary cancer, planned treatment, participating male partner or patient preferences regarding the use of donor sperm, and time interval to the initiation of therapy (4, 5). For appropriately selected patients with non-gynecologic or gynecologic cancer, fertility-sparing options include assisted reproductive technologies and ovarian transposition (5). For appropriately selected patients with gynecologic cancer, fertility-sparing surgeries such as conization or trachelectomy for cervical cancer, cystectomy or unilateral salpingo-oophorectomy (USO) for ovarian tumors, and conservative medical endocrine treatment for endometrial cancer are also possible (5).
Selection of the appropriate fertility-preserving option for the optimal benefit and safety of the patient depends on accurate cancer staging. MRI (magnetic resonance imaging) is particularly well suited to play an important role for assessing patient eligibility. MRI has unique utility for evaluation of gynecologic malignancies, particularly local staging of cervical and endometrial cancer (6) and characterization of sonographically indeterminate adnexal masses (7).
This review gives an overview of fertility-sparing options currently available to young patients with gynecologic cancers, illustrates the essential role of MRI in evaluating these options, and highlights the key imaging findings to include in the imaging report to address selection criteria.
General eligibility considerations in identifying suitable candidates for fertility preservation are summarized in Table 1.
Table 1.
General Eligibility Considerations in Identifying Suitable Candidates for Fertility Preservation (adapted from reference [8])
|
Part I: Fertility-Sparing Using Assisted Reproductive Technologies
All newly diagnosed cancer patients desiring future biological offspring require prompt consultation with a reproductive endocrinologist before the start of potentially fertility-impairing therapy (gonad-removing surgery, gonadotoxic chemotherapy, or radiation) (4).
Embryo and oocyte cryopreservation are the two most established artificial reproductive technologies utilized for fertility preservation (4). These approaches involve ovarian stimulation to induce development of multiple follicles, ultrasound-guided transvaginal oocyte retrieval, in-vitro fertilization (if embryo cryopreservation is chosen), and finally cooling to subzero temperatures for use at a later time (8).
While both embryo and oocyte cryopreservation are standard of care for reproductive age women and post pubertal girls, oocyte cryopreservation does not require a participating male partner or donor sperm, thereby permitting greater reproductive autonomy. Oocyte cryopreservation is therefore particularly well suited for single women, post-pubertal girls, or any patients with religious or ethical objections to embryo freezing (4, 8).
Two additional available methods include 1) ovarian tissue cryopreservation with the intent of future implantation, and 2) retrieval of immature oocytes, in-vitro maturation, and vitrification (rapid cooling to avoid ice formation) (4, 9). While still undergoing refinement, these are the only approaches available to pre-pubertal girls as they obviate the need for ovarian stimulation and sexual maturity (4, 9). The potential concern is reintroduction of cancer cells during implantation (4, 9). Thus, this approach is avoided in patients with an inherent predisposition to ovarian cancer or malignancies with moderate-to-high risk of ovarian metastases (e.g., leukemia, Ewing’s sarcoma, neuroblastoma, gastric, colon, and breast cancers) (9, 10).
Embryo and oocyte cryopreservation may be associated with ovarian hyperstimulation syndrome (OHSS), an exaggerated response to ovarian stimulation resulting in increased capillary permeability (11). Clinical signs/symptoms, imaging findings (ovarian size, presence of ascites, and pleural effusions), and biochemical parameters are combined to grade OHSS as mild, moderate, or severe (12). The mild form is common whereas severe OHSS accounts for only 0.1–2% of cases (12).
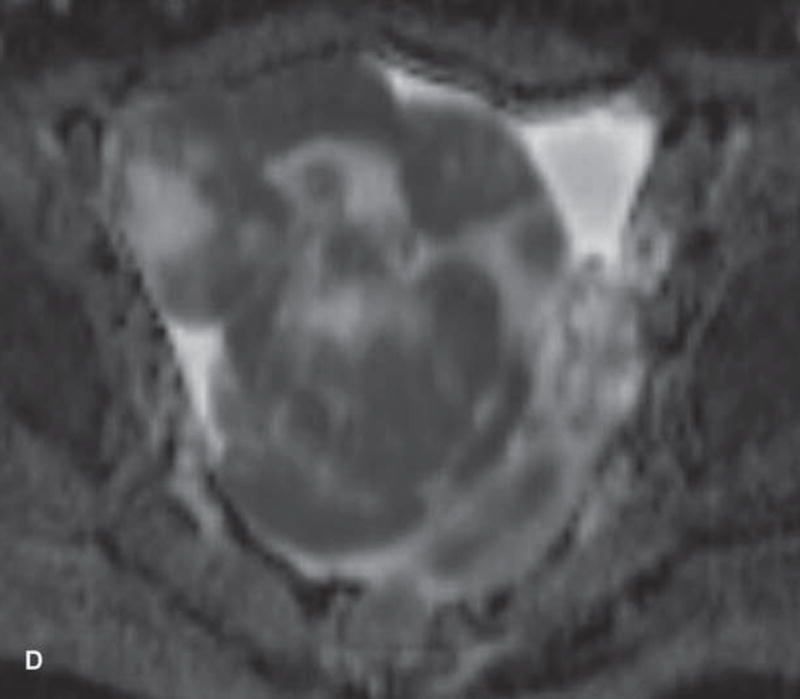
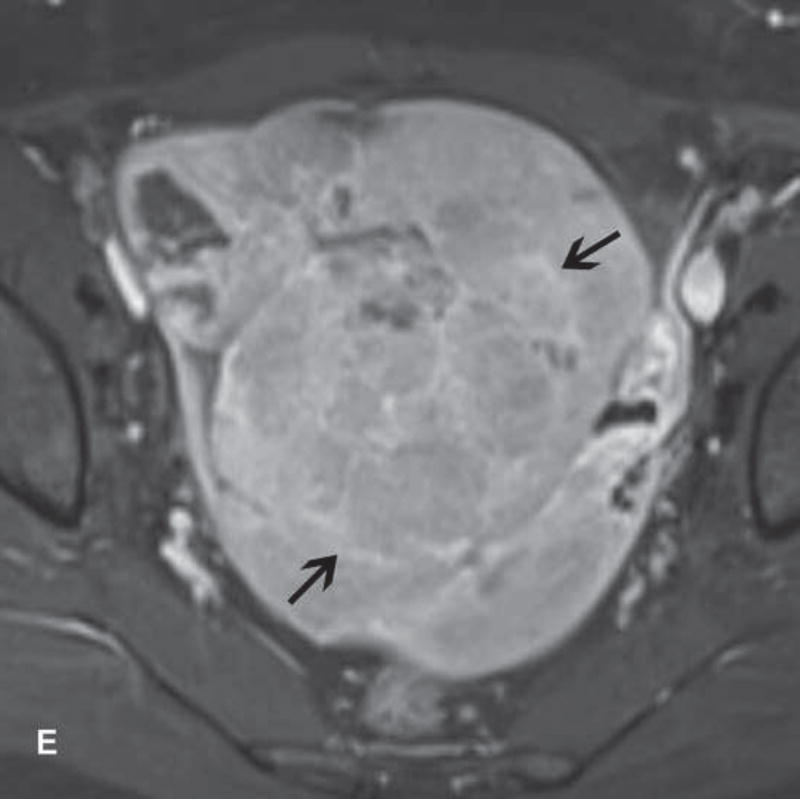
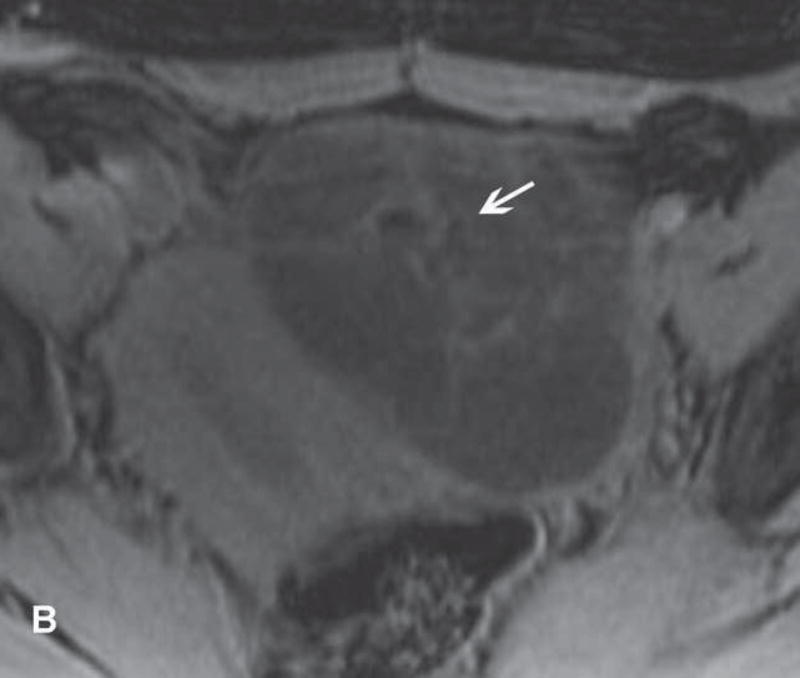
Imaging stigmata of OHSS include symmetric enlargement of both ovaries by multiple variable-sized follicular and corpus luteal cysts. Ovaries acquire a characteristic ‘spoke wheel’ appearance due to multiple peripheral ovarian cysts surrounding the central ovarian stroma (11, 13, 14) (Figure 1). Enlarged cystic ovaries should not be mistaken for cystic ovarian neoplasm (14). Presence of ascites indicates that OHSS is at least moderate in severity. Possible OHSS complications include thromboembolism and ovarian torsion (11, 15, 16).
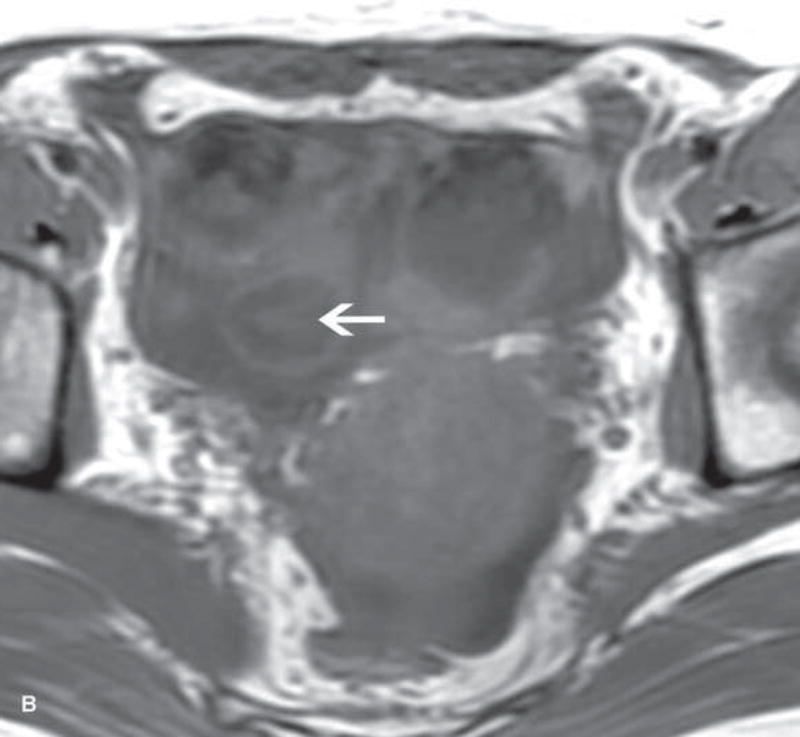
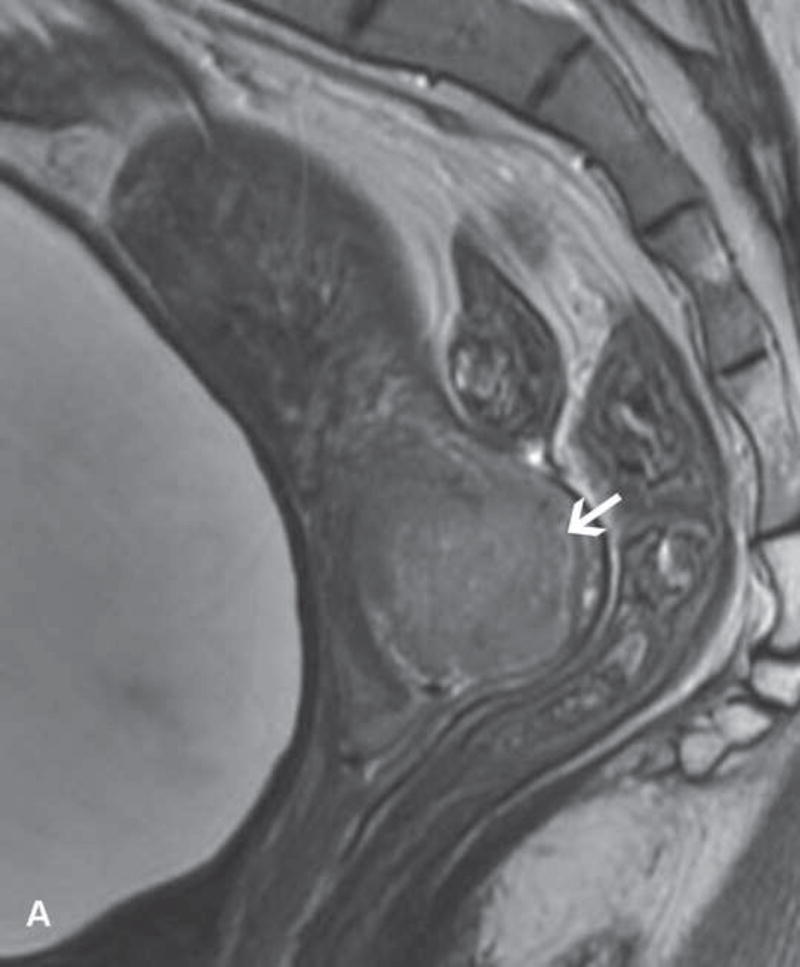
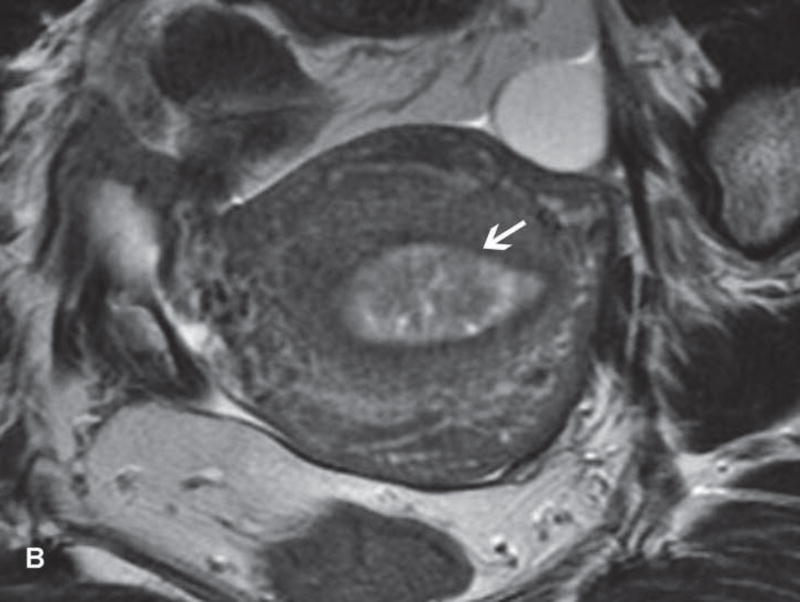
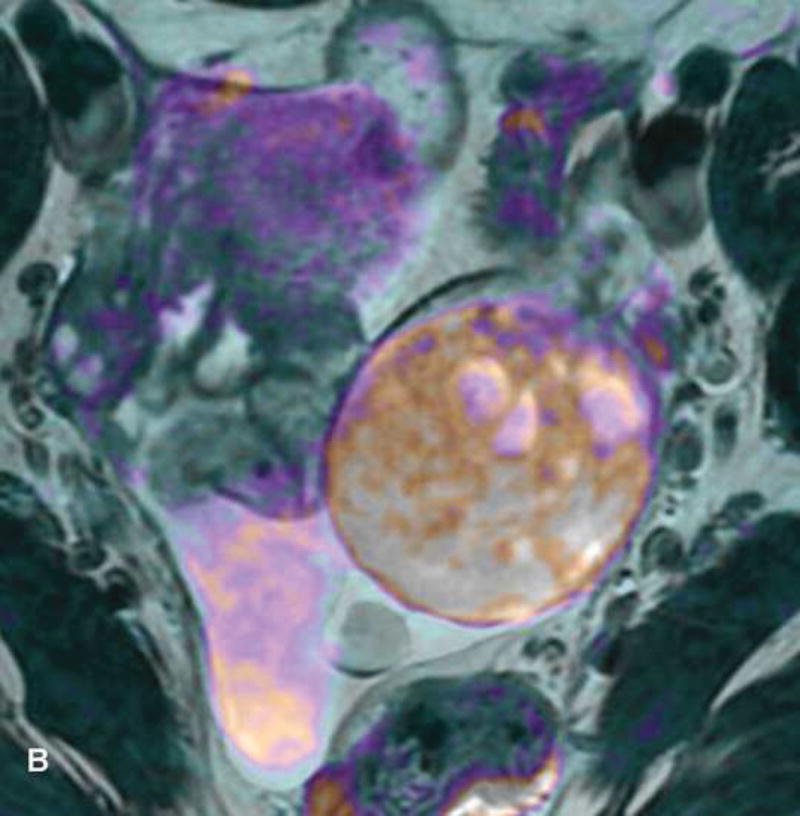
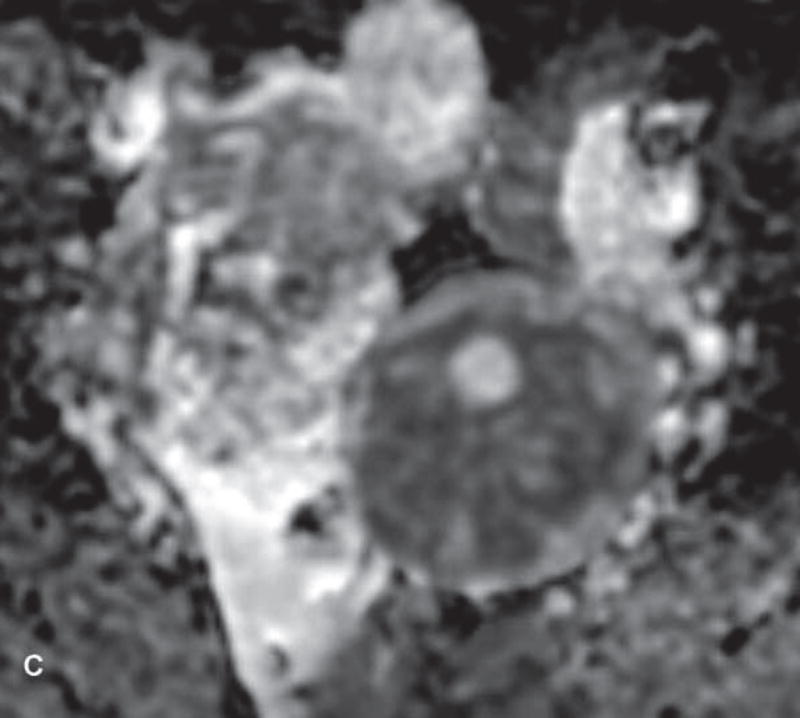
Figure 1.
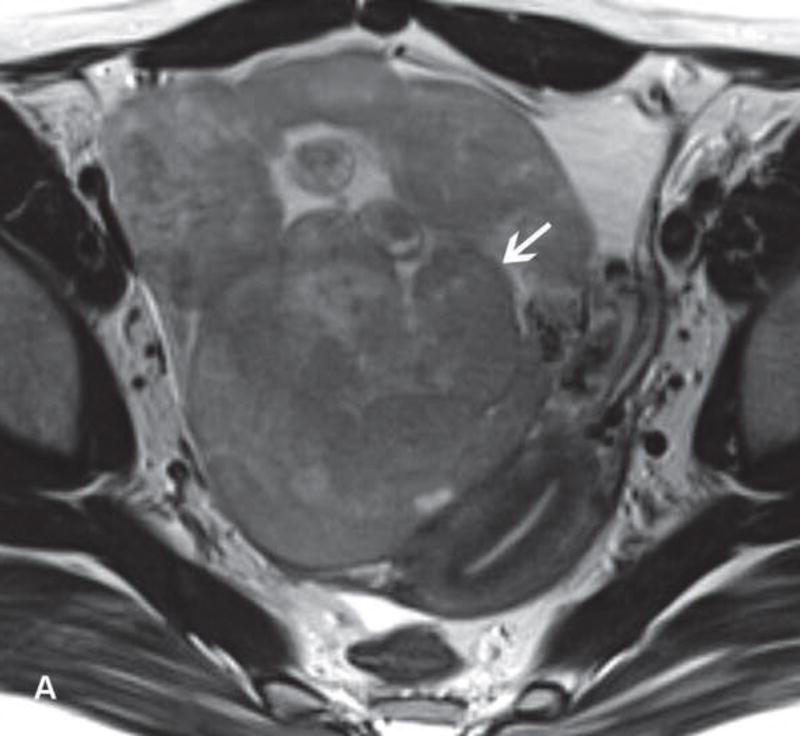
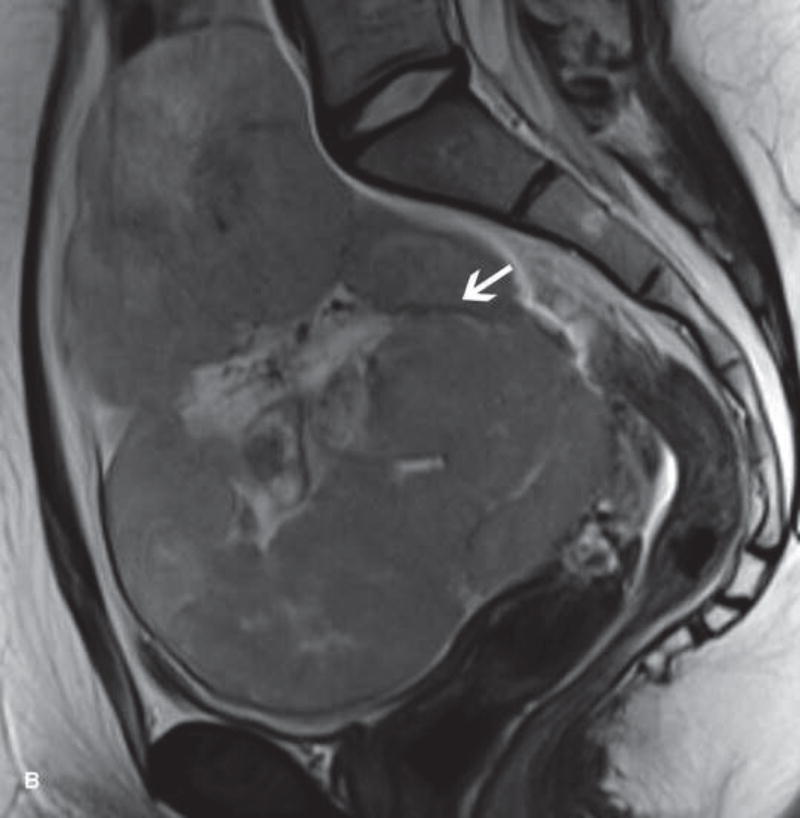
31-year-old woman with cervical cancer and ovarian hyperstimulation. A. A drawing shows the spoke-wheel appearance of hyperstimulated ovaries with multiple peripheral cysts centered on ovarian stroma. B. Axial T1WI shows symmetrically enlarged bilateral ovaries with low SI cysts; some cysts contain high SI blood products (white arrow). C. Axial oblique T2WI shows symmetrically enlarged bilateral ovaries with high SI cysts peripherally and edematous stroma centrally (black arrow); some cysts contain low SI foci due to blood products (white arrow). Note the small volume ascites (white arrowhead) and primary cervical tumor (black arrowhead). D. Axial oblique contrast-enhanced image shows symmetrically enlarged ovaries. Ovaries contain peripheral cysts (black arrow) with wall enhancement and enhancing central stroma. Note the absence of enhancing nodular septa or nodules. Primary cervical tumor is also seen (black arrowhead).
Part II: Fertility-Sparing Using Ovarian Transposition
Ovaries are extremely radiosensitive, with the effective sterilizing dose decreasing with age (17). The extent of the damage depends on patient age, cumulative dose to the ovaries, fractionation, and type of concurrent chemotherapy. Pelvic radiation also induces damage to a patient’s uterus consisting of changes to the muscles and vasculature, and hormone-resistant endometrial insufficiency, thereby leading to increased risk of infertility, miscarriage, and intrauterine growth retardation (18, 19). Women with a heavily irradiated uterus may require a surrogate (carrying of a pregnancy by a third party) (20).
Ovarian transposition (surgical placement of the ovaries outside the radiation field) is performed to preserve ovarian function in patients planned for lower abdominal and pelvic radiation (21). Recent evidence implicates fallopian tubes as a primary precursor of high-grade serous ovarian and peritoneal cancer.(22) Fallopian tubes are now often removed at the time of ovarian transposition to reduce the risk of ovarian cancer. The benefits of this approach remain to be proven.
Cervical cancer is the most common indication for ovarian transposition in reproductive age women, with vaginal and rectal cancers being less common indications (21). Similar to ovarian tissue cryopreservation and implantation, ovarian transposition is not recommended for patients with an inherited predisposition to ovarian cancer or malignancies at moderate-to-high risk of ovarian metastases (23, 24).
Ovarian transposition successfully preserves ovarian function in 65–94% of patients (23). Various factors such as patient age, radiation scatter, transposition locations, vascular pedicle status, types of radiation, and adjuvant chemotherapy impact the rate of success. To maximize fertility preservation, oocyte cryopreservation may be performed prior to ovarian transposition (8).
To confirm patient eligibility for ovarian transposition, MRI (and occasionally Positron Emission Tomography-Computed Tomography, PET/CT) is useful to delineate disease extent and exclude ovarian involvement.
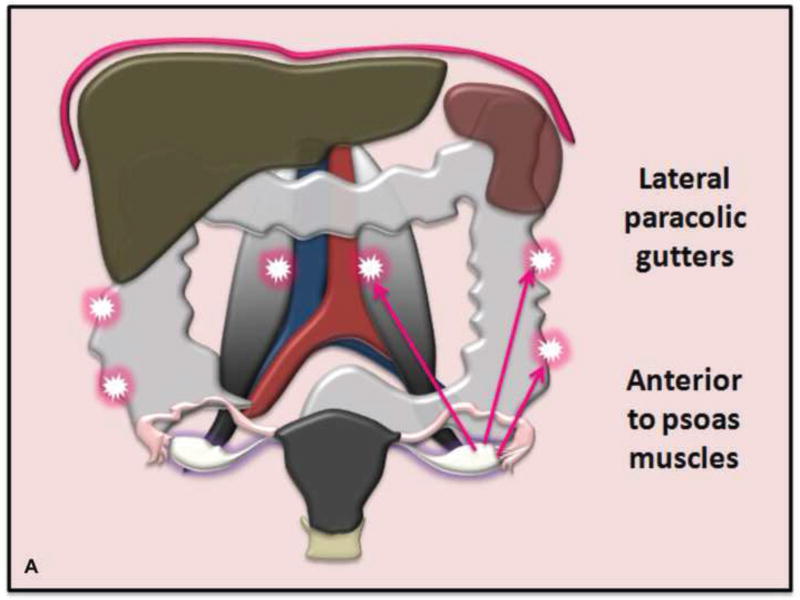
Various transposition locations can be considered depending on the size/location of radiation field (Figure 2). In patients with cervical cancer, ovaries are often placed into the paracolic gutters or anterior to the psoas muscles above the pelvic brim (21). Transposed ovaries are frequently marked with surgical clips to facilitate visualization during radiation planning.
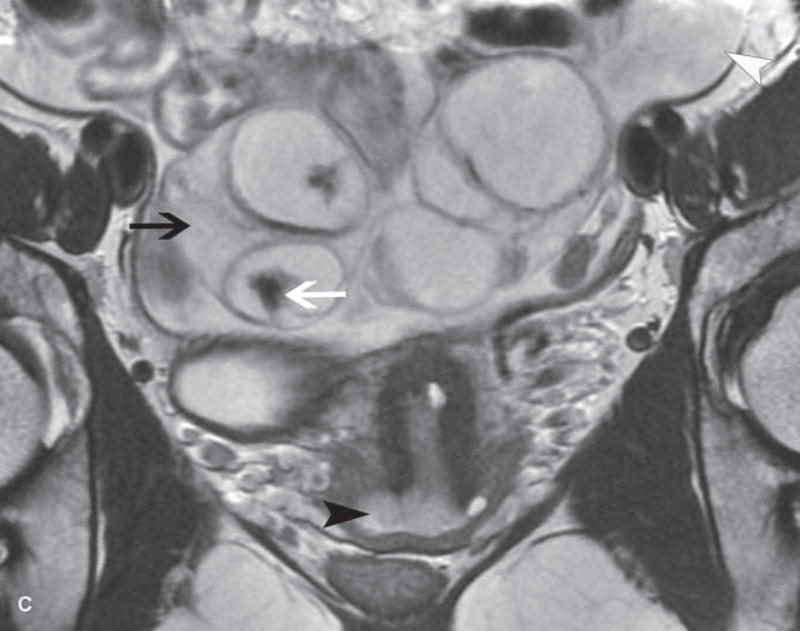
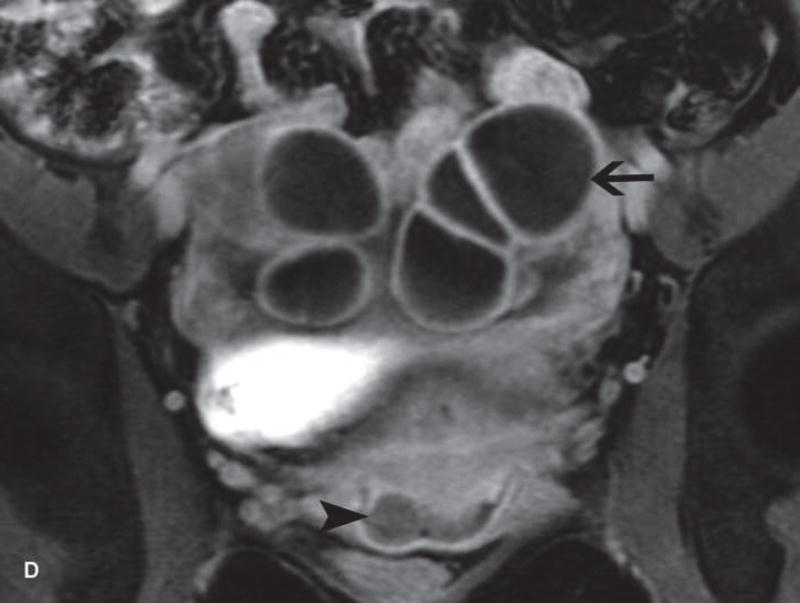
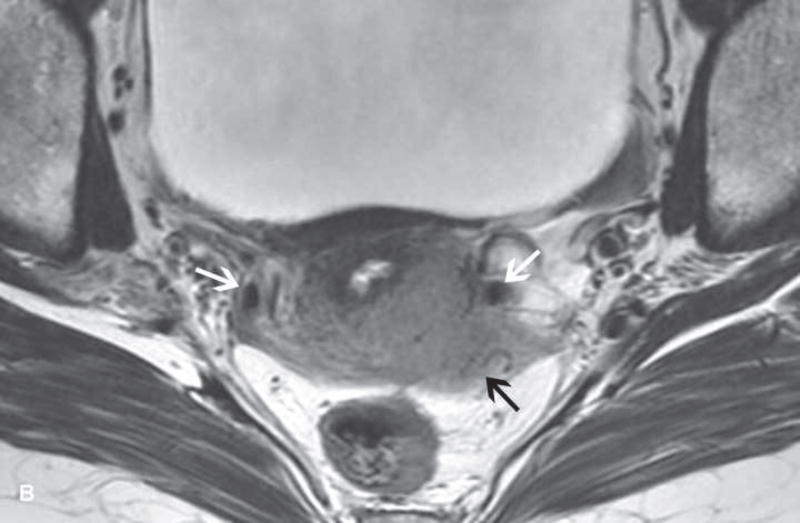
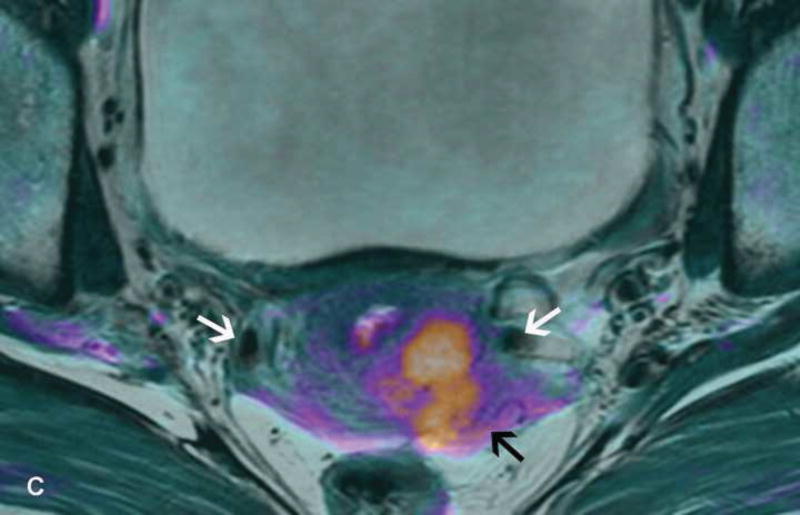
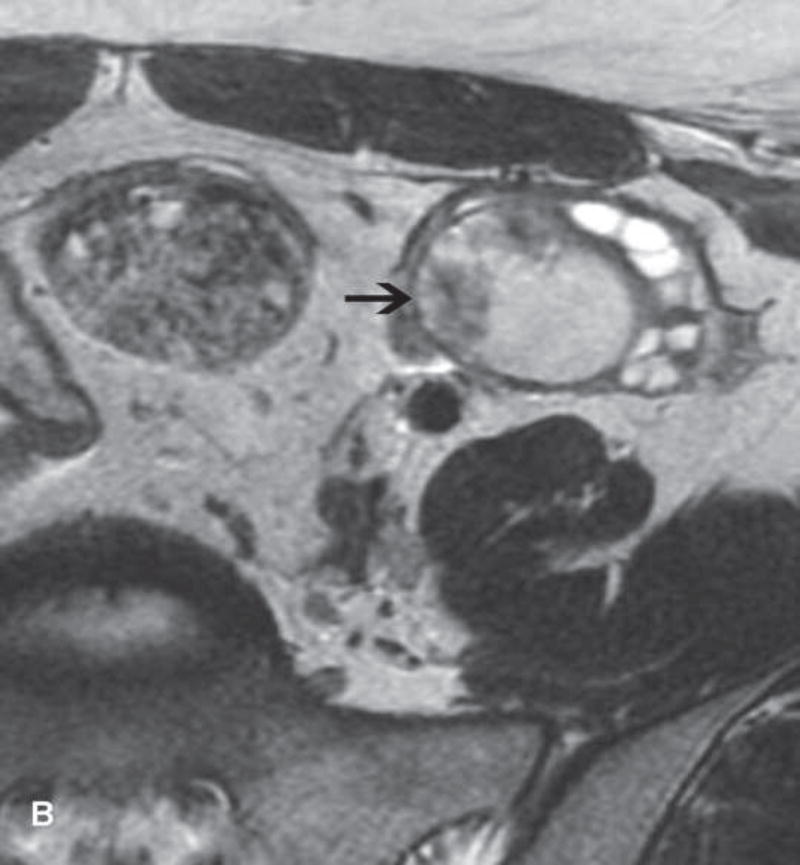
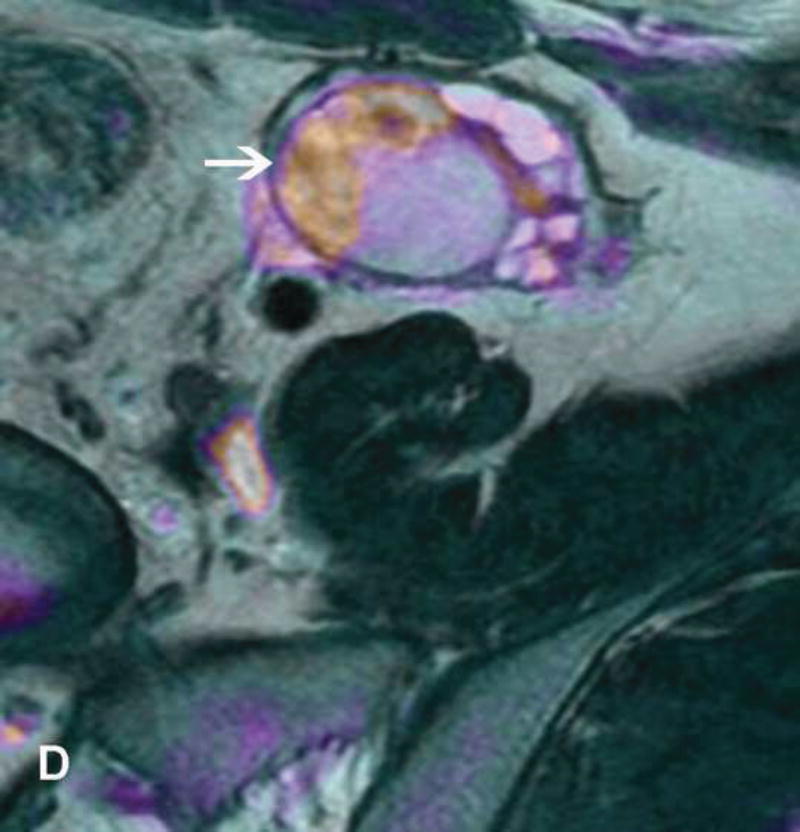
Figure 2.
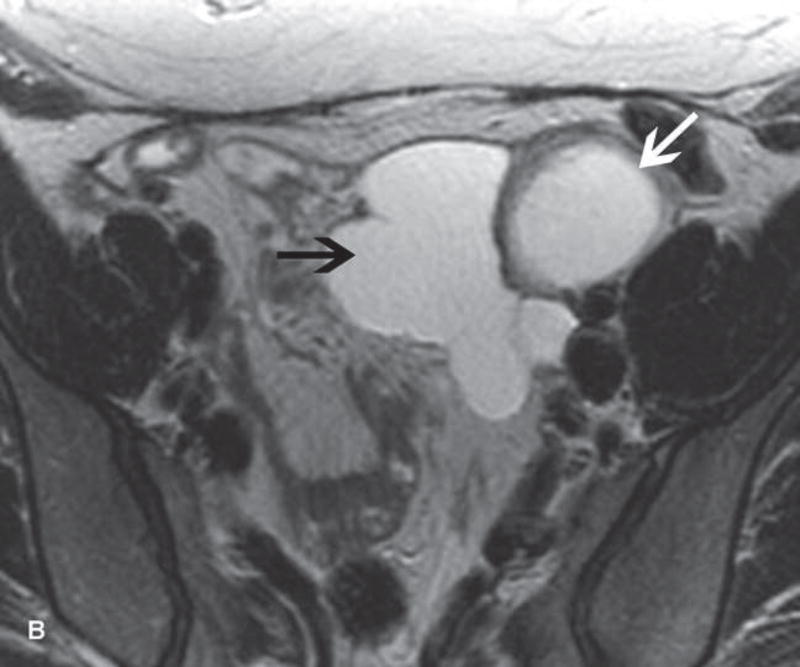
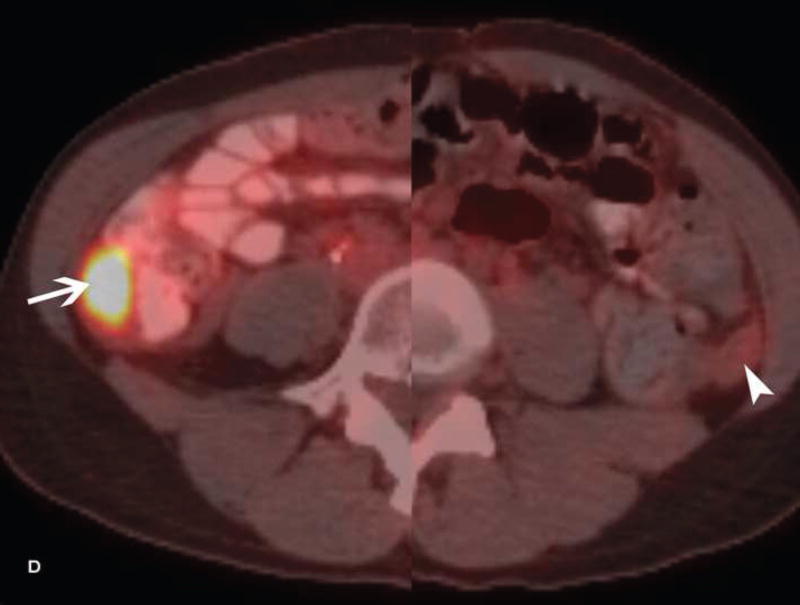
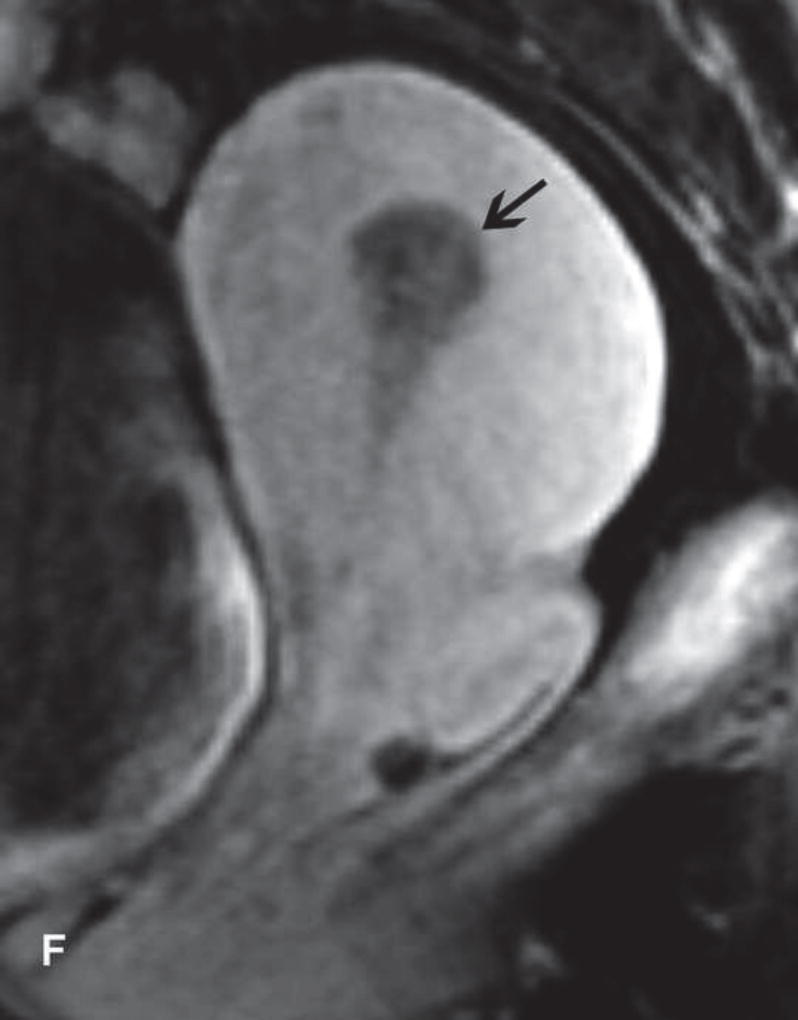
A. Drawing depicts common transposition locations for the ovaries. B. 48-year-old woman with cervical cancer who underwent ovarian transposition and definitive chemoradiation. Axial T2WI demonstrates transposed left ovary that re-migrated down into the pelvis (white arrow). It contains a dominant follicle, and is surrounded by a peritoneal inclusion cyst (black arrow). Transposed right ovary is not illustrated on this image. C and D. 32-year-old woman with cervical cancer and ovarian transposition prior to definitive chemoradiation. C. Coronal T2WI shows transposed ovaries in lower paracolic gutters (white arrows). D. Composite axial FDG-PET/CT image in the same patient demonstrates physiologically increased FDG uptake in the transposed right ovary (white arrow) and no increased FDG uptake in the transposed left ovary (white arrowhead).
On imaging, transposed ovaries appear as ovoid structures with follicles adjacent to surgical clips (25). Ovarian cysts and peritoneal inclusion cysts are common following ovarian transposition (Figure 2) (21, 25). Depending on the phase of ovulatory cycle, transposed ovaries may demonstrate physiologic fluorine 18-fluorodeoxyglucose (FDG) avidity on PET/CT (Figure 2). This should not be misinterpreted as metastatic disease (26). Metastases to transposed ovaries are extremely rare and have the same imaging features as metastases to non-transposed ovaries (23).
Part III: Suggested Tailored MRI Protocols for the Initial Work-Up of Gynecologic Cancers and Adnexal Lesions
MRI is the preferred imaging modality for the assessment of cervical and endometrial cancer (6). It is also the best problem-solving imaging technique for the characterization of adnexal masses indeterminate at ultrasonography, US (7, 27, 28). It is essential to tailor MR imaging protocols to the individual needs of the patient to provide accurate and complete information for optimal patient selection and initial treatment design.
Patient Preparation
Patients are encouraged to fast 4–6 hours prior to their MRI appointment and empty their bladder before the examination to reduce peristalsis-related motion artifacts. An anti-peristaltic agent is administered intramuscularly or intravenously at the start of the examination to further reduce peristalsis (6).
Patients are imaged in the supine position with ≥1.5-Tesla magnet and a multi-channel phase-array receiver coil. Endovaginal receiver coils are not in use routinely; however, they substantially increase the signal-to-noise ratio and can aid the evaluation of small cervical lesions including their detection, assessment of size, endocervical extent, and presence of parametrial invasion (29–32). The opacification of the vagina with gel is optional in women with cervical cancer (33).
Tailored MR Imaging Protocols (Table 2)
Table 2.
Tailored MR Imaging Protocols for the Initial Work-Up of Gynecologic Cancers and Adnexal Lesions
| Patient Preparation, Positioning, and Coil Selection | |||
|
| |||
| |||
|
| |||
| Tailored MR Imaging Protocols | |||
|
| |||
| ✓ Large FOV T1WI ±T2WI | • From the top of the kidneys to the iliac crests (Upper abdomen) | ||
| ✓ T1WI without and with fat saturation | • Pelvis, Axial plane | ||
|
|
|||
| Cervical cancer | Endometrial cancer | Adnexal mass | |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviations: IM = intramuscular, IV = intravenous, MR = magnetic resonance, FOV = field of view, T1WI = T1-weighted Images, T2WI = T2-weighted Images, DWI = Diffusion Weighted Imaging, ADC = Apparent Diffusion Coefficient, DCE-MRI = Dynamic Contrast-Enhanced MRI, CE = Contrast Enhancement
Large field-of-view T1-weighted (T1WI) ±T2-weighted (T2WI) images from the top of the kidneys to the iliac crests are acquired to exclude hydronephrosis, para-aortic lymphadenopathy, and osseous metastases (6, 33, 34). Axial T1WI of the pelvis without and with fat saturation are useful to distinguish blood from fat (28).
High-resolution small field-of-view T2WI in minimum two orthogonal planes is the cornerstone of local tumor staging. In patients with cervical cancer, these are usually obtained in the sagittal plane and axial oblique plane (perpendicular to the endocervical canal) (6, 33); the coronal oblique plane (parallel to the endocervical canal) may be optionally added (35). In patients with endometrial cancer, T2WI are obtained in the sagittal and axial oblique planes with the latter acquired perpendicular to the endometrial cavity (6, 34). In case of an adnexal mass, sagittal, axial, and axial oblique T2WI are obtained with axial oblique plane being parallel to the endometrial cavity, “ovarian axis” (27, 28). “Ovarian axis” images can help to confirm the ovarian origin of the mass by laying out the gonadal vessels leading to the ovaries.
Diffusion weighted imaging (DWI) is an integral part of the imaging protocol (6, 36). The standard DWI includes a minimum of two sets of images with b-values of 0 and ≥800 sec/mm2 to suppress high-SI (signal intensity) from freely diffusing water (using urinary bladder SI as a reference); apparent diffusion coefficient (ADC) maps are generated thereafter (6). In patients with endometrial and cervical cancer, sagittal, and axial oblique DWI are acquired in the same orientations as T2WI and are useful to detect myometrial invasion (endometrial cancer) and delineate tumor margins (cervical cancer). Coronal oblique DWI (parallel to the endocervical canal) may be included in the cervical protocol (35). Axial DWI is used for adnexal mass assessment.
DWI should always be interpreted in conjunction with other sequences such as T2WI and contrast-enhanced images. It is particularly important with adnexal masses because both benign (endometrioma, mature cystic teratoma) and malignant ovarian masses can show diffusion restriction (high SI on high b-value images and low SI on ADC maps).
Dynamic contrast-enhanced MRI (DCE-MRI) is also a part of the MR imaging protocol. While DCE-MRI is currently optional with newly diagnosed cervical cancer, it improves tumor-to-cervical stromal contrast in patients with small tumors (<2 cm), which is useful in patients being considered for fertility preservation (33, 37). DCE-MRI is essential for local staging of endometrial cancer and for characterization of adnexal lesions (27, 38). In case of endometrial cancer, DCE-MRI is acquired in the sagittal plane with delayed contrast-enhanced images being acquired in the axial oblique plane. In case of an adnexal mass, DCE-MRI is acquired axially using either dynamic multiphase approach or perfusion imaging. The latter is acquired for at least 3 minutes with temporal resolution under 15 seconds (39–42). Perfusion images are processed by placing regions of interest (ROIs) on the most avidly/rapidly enhancing component of the ovarian mass and on the outer myometrium resulting in one of three types of time-intensity curves (using the enhancement of the outer myometrium as a reference) (42). Type 1 curve, slow low level of enhancement without plateau, suggests benignity. Type 3 curve, rapid high level of enhancement (faster than that of the myometrium), indicates probable malignancy. An in-between Type 2 curve, moderate level of enhancement with a plateau, may point to a borderline ovarian tumor. The advantage of perfusion imaging over traditional dynamic multiphase acquisition is not yet proven.
Part IV: Fertility-Sparing Management of Cervical Cancer
Cervical cancer is the fourth most common cancer in women worldwide with the highest incidence in developing countries (43). It is one of the few common gynecologic malignancies with a predilection for women of reproductive age. In the United States, from 2009 to 2013, nearly 40% of new cases occurred in women younger than 45 years (44).
Traditionally, radical surgery was the only treatment option available to most women with early stage cervical cancer. The infrequent finding of parametrial invasion at radical hysterectomy in patients with cervix-confined tumors ≤ 2 cm led experts to suggest that patients with small tumors can be treated less aggressively (45). Fertility-sparing surgery has emerged as an option in appropriately selected young women desiring fertility and is endorsed by both the European Society of Medical Oncology (ESMO) and the National Cancer Care Network (NCCN) (46–48). Conservative surgery affords the opportunity for pregnancy and achieves long-term oncologic results similar to traditional radical surgery (49).
The International Federation of Gynecology and Obstetrics (FIGO) staging system for cervical cancer is based largely on clinical examination and suffers from the well-documented limitations pertaining to the accuracy of staging (50, 51). MRI is the best imaging modality to detect and demonstrate tumor size, endocervical extent, depth of cervical stromal invasion (CSI), and parametrial and nodal involvement; thus it overcomes FIGO limitations and is integral to the assessment of patient eligibility for conservative management of cervical cancer (47, 52–55).
Conservative Surgical Management
Fertility-sparing surgery starts with pelvic lymph node evaluation (pelvic lymphadenectomy or sentinel lymph node mapping), and, if no lymph node metastases are detected at frozen sections, continues with the resection of the cervix (cone resection, simple trachelectomy, or radical trachelectomy) (48, 56).
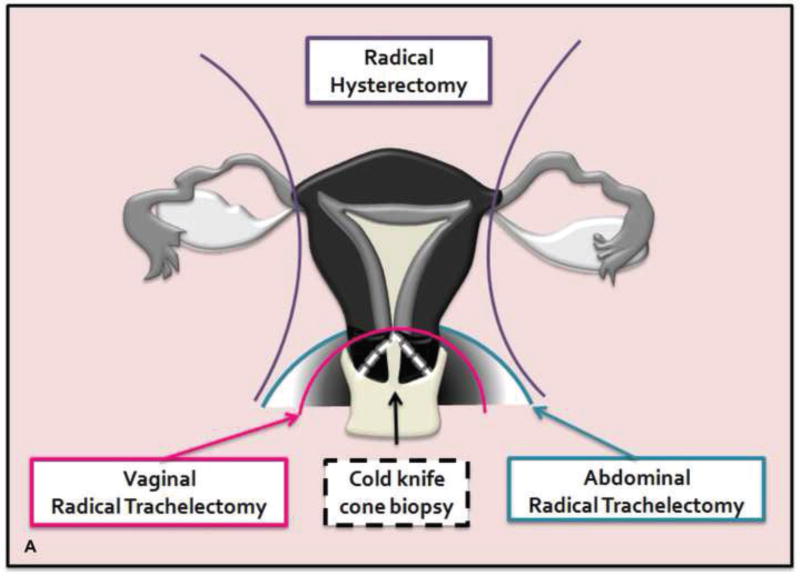
Cone resection or cone biopsy is an en bloc removal of the ectocervix and endocervical canal (47). Radical trachelectomy consists of cervical, vaginal cuff, and parametrial resection, followed by the creation of an isthmic vaginal anastomosis (Figure 3). Simple trachelectomy is similar to radical trachelectomy except parametrial resection is avoided. Intraoperative frozen sections are sent to confirm ≥ 1 cm tumor-free margin between the tumor and internal cervical os (minimum 0.5 cm for some centers) (56–58). Radical trachelectomy can be performed vaginally (Dargent’s procedure) or abdominally using open, laparoscopic, or robot-assisted approaches. Cervical cerclage is placed to prevent premature delivery from cervical incompetence (59).
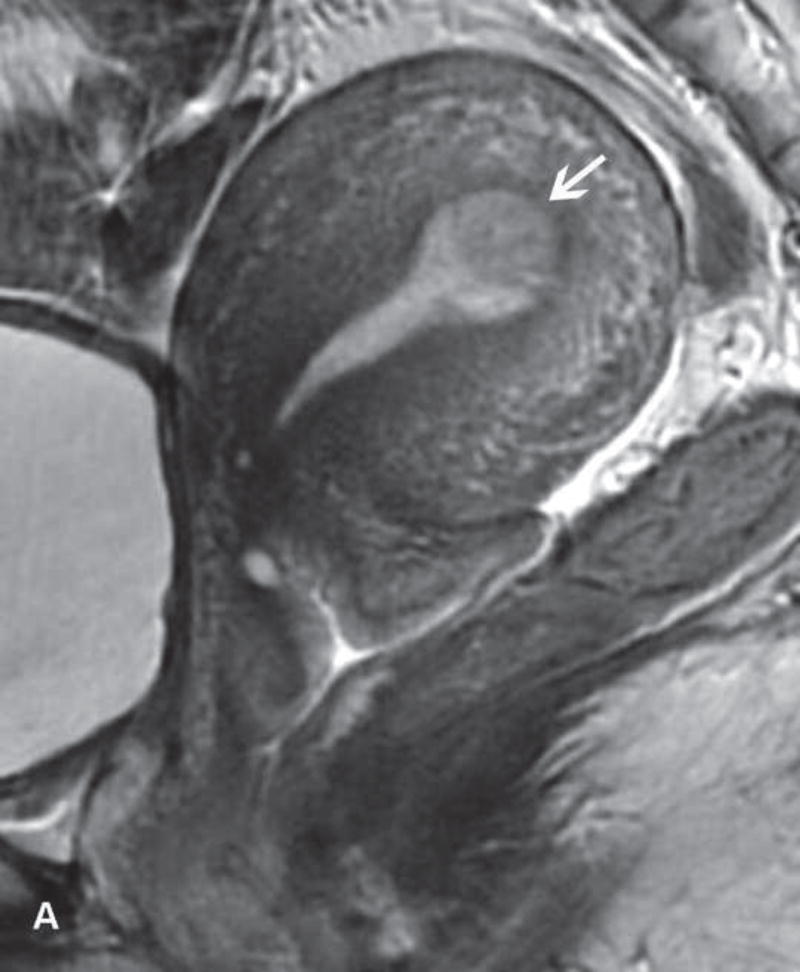
Figure 3.
A. Drawing portrays cone biopsy/resection (en bloc resection of ectocervix and endocervcial canal) and vaginal/abdominal radical trachelectomy (resection of cervix, adjacent parametria and a cuff of the upper vagina). B. The remaining uterus is anastomosed to the vagina with a resultant isthmic vaginal anastomosis.
Stage IA1 tumors without lymphovascular space invasion (LVSI) are managed with cone biopsy alone (with negative margins). Stage IA1 tumors with LVSI or Stage IA2 tumors are approached with pelvic lymph node assessment and cone resection or radical trachelectomy. Stage IB1 tumors ≤ 2cm are managed with pelvic lymph node assessment combined with cone resection or simple trachelectomy (if LVSI is absent) or radical trachelectomy (if LVSI is present) (48).
Fertility-sparing surgery is most validated for tumors ≤ 2cm (47). Larger stage IB1 lesions (2–4 cm) have a higher risk of extra-cervical spread and increased likelihood of recurrence after radical vaginal trachelectomy (Dargent’s procedure) (59). Therefore, these women are usually selected for abdominal radical trachelectomy or neoadjuvant chemotherapy plus conservative surgery (48, 60). Conservative management is considered unsafe for patients with Stage IB2 and Stage IIA1 disease (48).
Patient Eligibility Criteria and Role of MRI (Table 3 and Figure 4)
Table 3.
Cervical Cancer: Eligibility Criteria For Fertility-Sparing Management
| Tumor Characteristics |
|
|
|
|
|
| Criteria Evaluated at MRI |
|
|
|
|
|
|
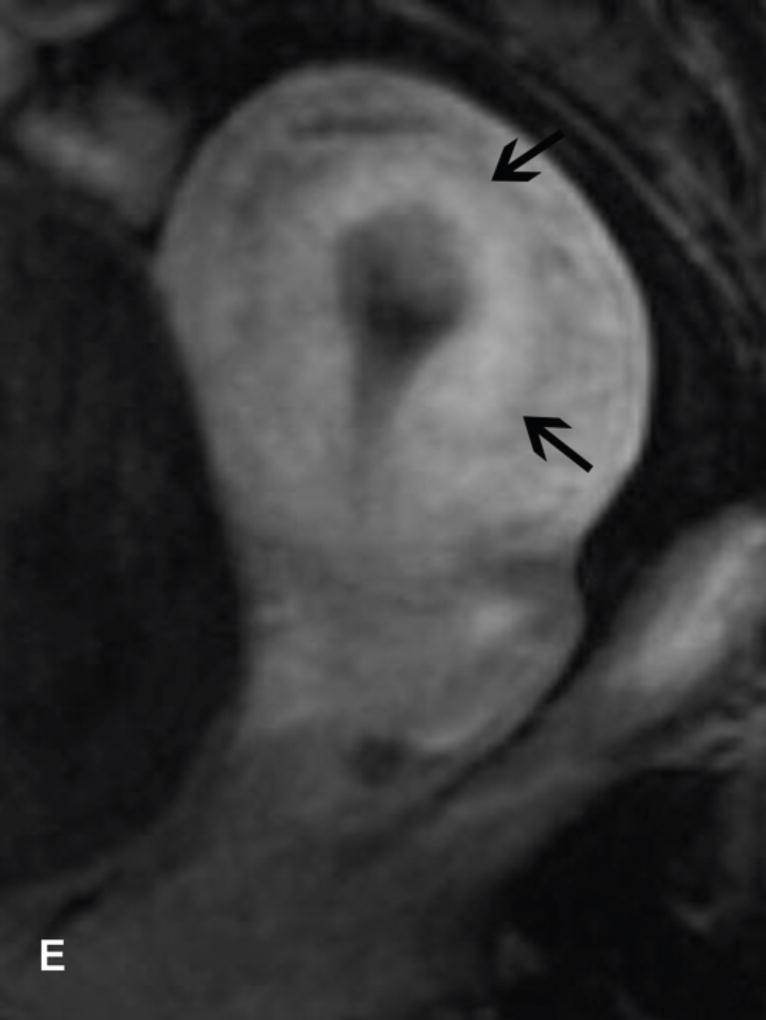
Figure 4.
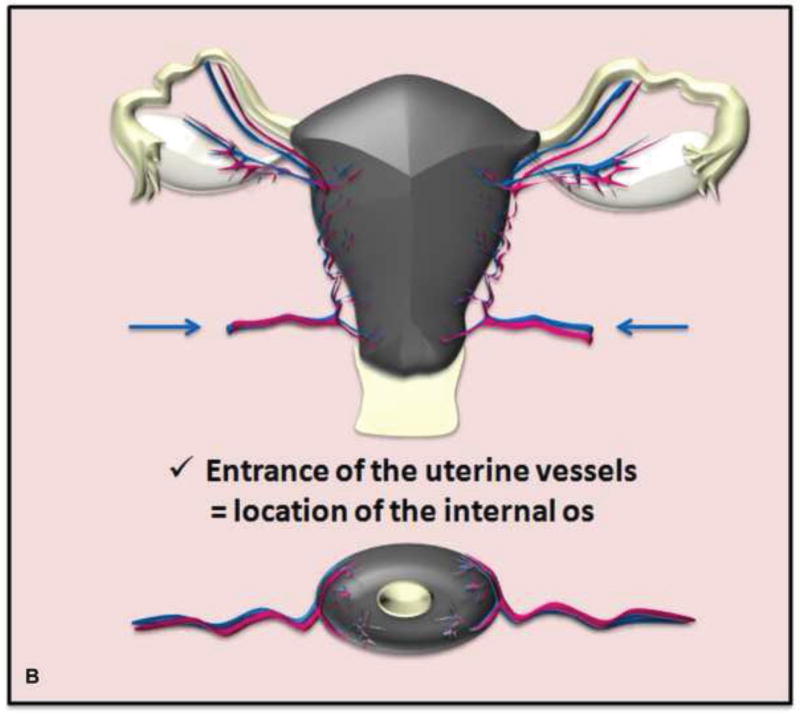
Drawings illustrate the location of the internal os seen as the waist of the uterine contour where low SI cervical stroma changes to intermediate SI myometrium (A) and as the entrance of uterine vessels (B).
1. Tumor Characteristics
Patients should have confirmed invasive cervical cancer of squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma histology (47). Patients with small cell neuroendocrine tumors (aggressive behavior) and gastric type minimum deviation adenocarcinoma or adenoma malignum (rare and challenging to diagnose) are ineligible (47).
2. Tumor size ≤ 2cm (some centers accept ≤ 4 cm, particularly if tumor is exophytic)
Tumor size directly affects patient eligibility. MR imaging can accurately measure tumor size including cranio-caudal dimension, which presents a challenge at clinical examination.
On T2WI, cervical cancer appears as an intermediate-SI lesion that may disrupt low-SI cervical stroma (Figures 5 and 6) (61). On DWI, the tumor has high SI on high-b-value images and low SI on ADC maps relative to the cervical stroma (62). While DCE-MRI is not required for initial staging, small tumors may enhance earlier than adjacent stroma with most tumors being iso or hypointense on delayed images (37, 63, 64).
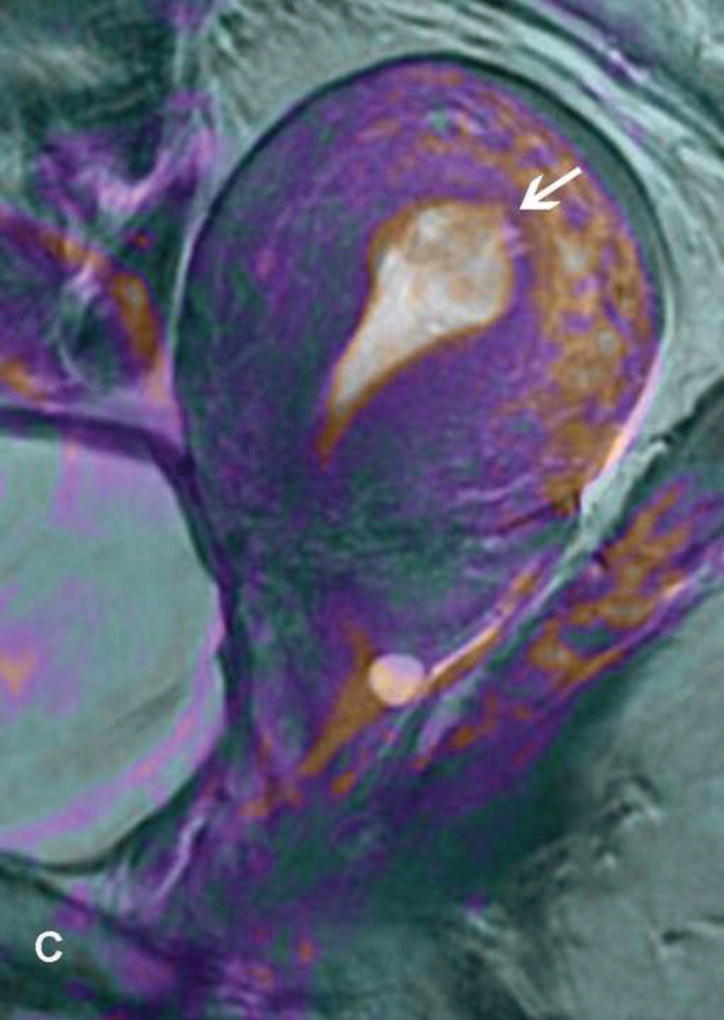
Figure 5.
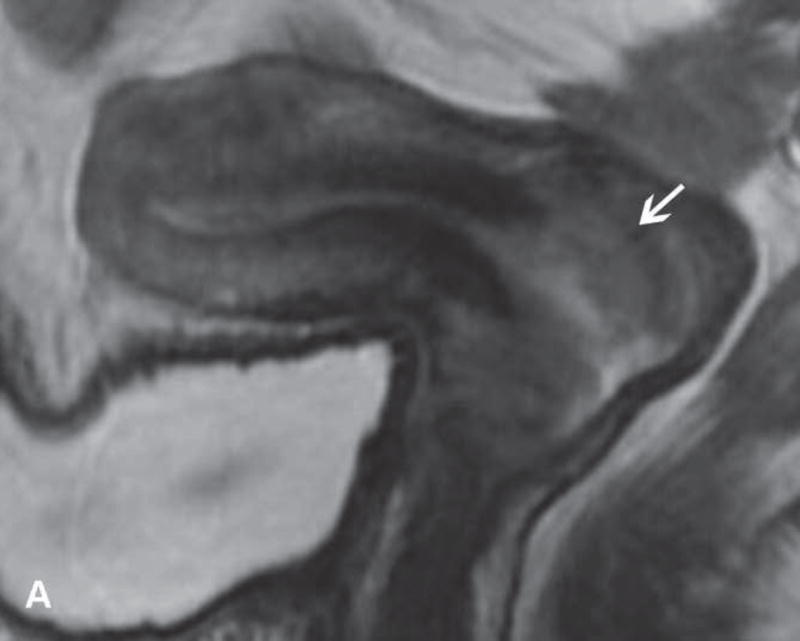
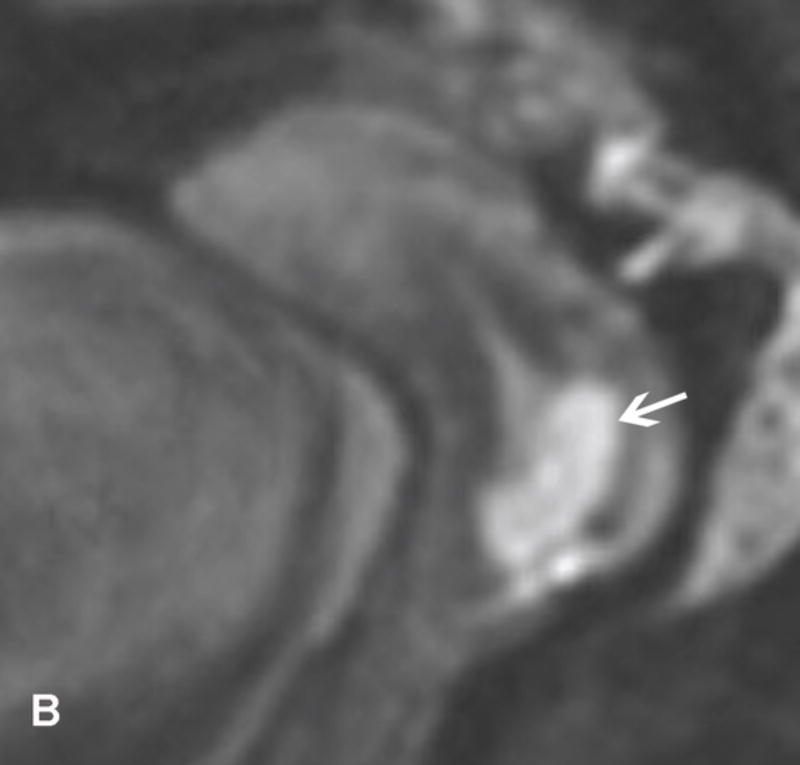
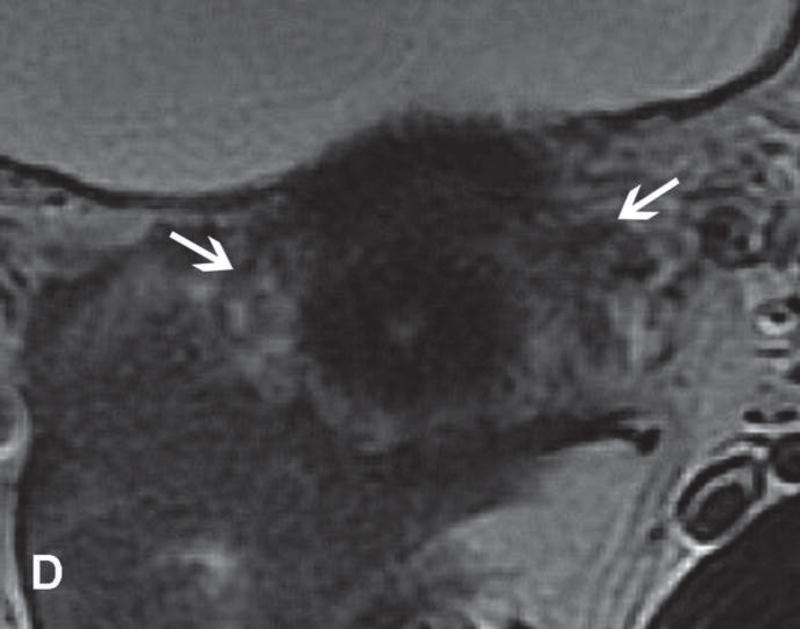
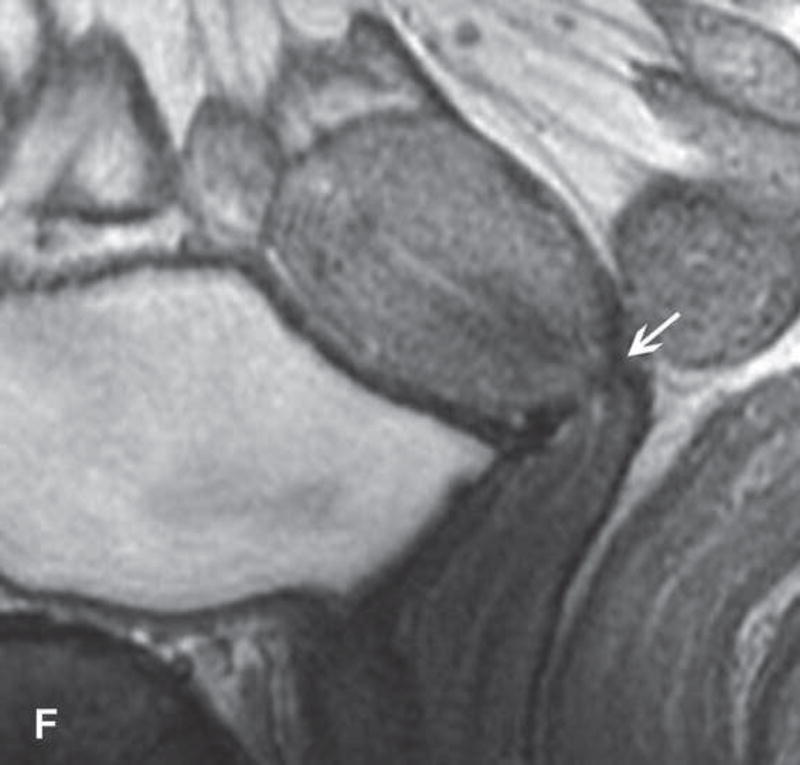
31-year-old woman with Stage IB1 cervical cancer. A. Sagittal T2WI demonstrates endophytic intermediate-SI tumor (white arrow). The tumor borders are obscured by post-cone resection changes making it challenging to accurately determine tumor size and endocervical extent. B and C. The tumor margins (white arrow) are more clearly defined on DWI and contrast-enhanced images, respectively, facilitating assessment of tumor size (2 cm in maximum dimension) and extent (1 cm caudal to internal os). D and E. Axial oblique and coronal oblique T2WI illustrate the location of internal os marked by the entrance of the uterine vessels (white arrows). F. Sagittal T2WI in the same patient after radical trachelectomy shows the normal appearing uterine remnant and isthmic vaginal anastomosis (white arrow).
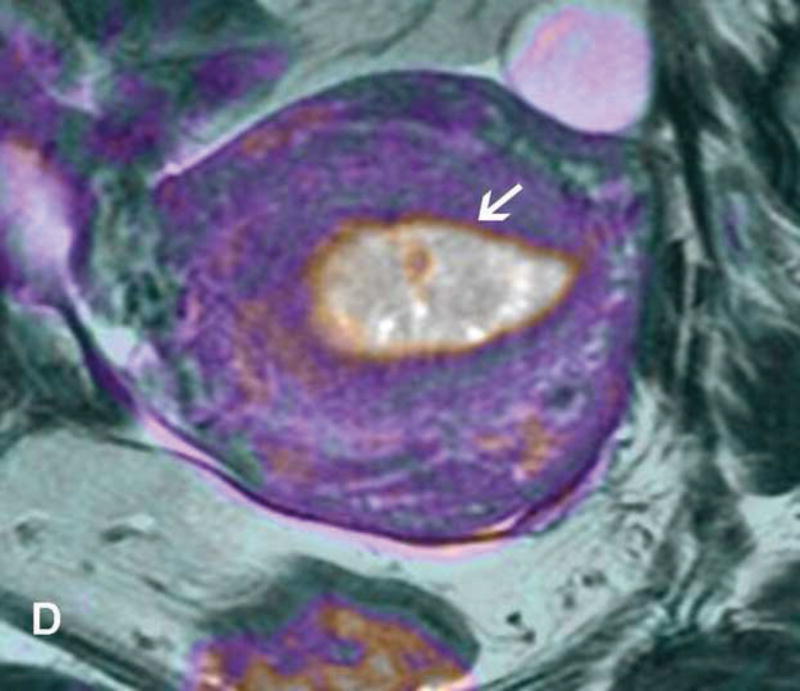
Figure 6.
37-year-old woman with Stage IB1 cervical cancer. A. Sagittal T2WI demonstrates 2.7 cm endophytic intermediate-SI tumor (white arrow). B and C. Axial oblique T2WI and fused (T2WI + DWI) images, respectively, show tumor extension to the internal os and left parametrial invasion (black arrows). The internal os is demarcated as the entrance of the uterine vessels (white arrows).
Tumor should be measured relative to the cervical axis with its size reported in three dimensions. On T2WI, the tumor size is reported to be within 0.5 cm of the surgical size in 70–90% of cases (65–67). Prior procedures can lead to overestimation secondary to edema, inflammation, and granulation tissue. The addition of DWI and DCE-MRI to T2WI may increase tumor conspicuity and aid the detection/size assessment of small tumors (32, 35, 37, 68).
Pattern of tumor growth may occasionally influence eligibility particularly in patients with larger tumors >2 cm (58). Therefore, the imaging report should indicate if the tumor is exophytic (grows outward and does not encroach on the endocervical canal), endophytic (grows inward along the endocervical canal), or a combination of both (61).
3. Tumor-to-internal os distance ≥ 1 cm (some centers accept ≥ 0.5 cm)
The ideal distance between the superior margin of the tumor and the internal os is ≥ 1 cm to allow for a 1 cm of healthy cervical stroma between internal os and surgical anastomosis (56). This maximizes local tumor control and increases the success of pregnancy by reducing the risk of cervical incompetence, ascending infection, and premature delivery. Some centers accept 0.5 cm as the minimum margin (57).
Tumor-to-internal os distance is difficult to assess clinically, but can be determined accurately on MRI (69). The internal os is seen as the waist of the uterine contour where low SI cervical stroma changes into intermediate SI myometrium (sagittal T2WI) and as the entrance of uterine vessels (axial or coronal oblique T2WI) (Figures 4 and 5). MRI excels at identifying patients who are unlikely to undergo successful fertility-sparing surgery because of close proximity of the superior tumor margin to the internal os (65, 69–71). In a recent study, all patients with tumor within 5 mm of internal os and 60% of patients with tumor within 6–9 mm of internal os required radical hysterectomy due to close surgical margin. In contrast, all patients with tumor-to-internal os distance of at least 1 cm underwent successful fertility-preserving surgery (69). Thus, MRI can identify high-risk patients who may have a better chance at fertility preservation if managed with neoadjuvant chemotherapy plus conservative surgery approach. The addition of DWI and DCE-MRI to T2WI can aid the assessment of endocervical extent through enhanced conspicuity of tumor margins (32, 35, 37).
Some patients have had prior excisions. Cervical length, measured on sagittal T2WI from the internal to external os, should ideally be > 2.5 cm and minimum 2 cm (33, 72). If extra cone biopsy is contemplated, this information is important to avoid cervical incompetence and future preterm delivery.
4. Depth of cervical stromal invasion < 50% (some centers accept any degree of CSI)
Deep stromal invasion (invasion of outer third) is associated with a higher recurrence rate and shorter survival (73, 74). In patients considered for fertility preservation, it is preferable that the tumor involves less than half of stromal thickness. This criterion is not strictly enforced because clinical assessment is a challenge and MRI evaluation is imprecise (55, 69).
Stromal invasion is present when intermediate-SI tumor disrupts low-SI stroma (Figures 5 and 6) (61). When T2WI is used, the sensitivity for the detection of deep stromal invasion is moderate at best (ranging from 50 to 75%) probably because of the difficulty in differentiating tumor from postsurgical inflammation and edema (69). Literature suggests that DCE-MRI may aid the assessment because stromal invasion can appear as early focal enhancement within otherwise hypo-enhancing stroma (63, 64).
5. Absence of parametrial invasion
Tumor spread beyond the cervix is a contraindication to conservative surgery (56). MRI is superior to clinical examination for identifying parametrial invasion (54). On T2WI, the presence of an intact rim of low-SI stroma surrounding the cervical tumor virtually excludes parametrial invasion (6). Parametrial invasion is present if the tumor disrupts low-SI stroma either focally or diffusely, results in nodular or spiculated tumor-to-parametrial interface, or encases peri-uterine vessels (Figure 6) (75). Parametrial invasion may be overestimated occasionally due to post-procedural edema and inflammation.
6. Absence of lymph node metastases
Presence of nodal metastases is an important adverse prognostic factor with pelvic and para-aortic regions being most commonly involved (76). If intra-operative frozen sections from sentinel lymph node sampling or pelvic lymphadenectomy indicate metastatic lymph nodes, primary chemoradiotherapy is usually the optimal treatment approach (56, 59).
Size is the main imaging criterion to detect nodal metastases with 1.0 cm short axis diameter being the most commonly used threshold for both pelvic and para-aortic lymph nodes (52, 77). Other suspicious morphologic features include round shape, irregular borders, loss of fatty hilum, SI similar to primary tumor, and presence of necrosis (77). Similar to CT, conventional MRI has high specificity but only modest sensitivity for identifying nodal metastases; this is primarily due to the inability to detect metastasis in normal-sized lymph nodes (52, 77).
Functional techniques (DWI, MRI enhanced with lymph-node specific contrast agents, and PET/MRI) may improve the detection of nodal metastases independent of size (78–81). Lymph nodes appear as discreet high SI ovoid structures on high b-value DWI increasing their conspicuity and facilitating detection. A recent meta-analysis suggests that DWI with ADC values may potentially increase sensitivity, but lack of standardized DWI protocols, overlap in ADC values of reactive and malignant nodes, and variability in ADC cut-off values limit applicability in daily clinical practice (79).
PET/MRI including PET/MRI-DWI is increasingly used in clinical practice, but both clinical applications and technical optimization are ongoing. This approach has the potential to provide a “one-stop-shop” evaluation of the primary tumor and lymph nodes (80, 81).
Part V: Fertility-Sparing Management of Endometrial Cancer
Endometrial cancer is the fifth most common cancer in women worldwide (82). It is the most common gynecologic cancer in developed countries and its incidence is increasing (1, 82). Although it presents most frequently in postmenopausal women, up to 7% of cases occur in patients younger than 45 years (83). The main risk factor is prolonged unopposed exposure to endogenous or exogenous estrogens (84). Younger patients are more likely to be obese, nulliparous, or have underlying conditions such as polycystic ovarian syndrome, all of which elevate endogenous estrogens (85, 86).
Endometrial cancer is often symptomatic early on and most patients are diagnosed at Stage I (tumor confined to the uterine corpus) (87). The standard management for apparently early stage disease is total hysterectomy and bilateral salpingo-oophorectomy (secondary to hormonal sensitivity of most endometrial cancer) with nodal assessment (sentinel lymph node mapping algorithm or pelvic ± para-aortic lymphadenectomy) (88, 89).
Conservative Medical Management
Fertility-sparing treatment is increasingly accepted as an alternative for young women who have disease limited to the endometrium and want to maintain their reproductive potential (88, 89). Patients should be counseled that conservative management is not a standard treatment and close follow-up is a must (88, 89). MRI is essential to confirm eligibility by excluding myometrial invasion and tumor extension beyond the uterine corpus (90).
Conservative treatment consists of progestins administered orally or via a levonorgestrel-coated intrauterine device (91, 92). Assessment of treatment response is performed at 6 and 9 months with dilation and curettage (D&C) and MRI (88, 89, 93). Approximately 75% of patients respond to therapy, but 30–40% recur (94–97). Responders at 6 months are encouraged to attempt immediate pregnancy that has the added benefit of decreasing the rate of recurrence (91, 95). Maintenance therapy for additional 6 months is offered to women who want to delay pregnancy (91). Non-responders at 9 to 12 months and those who recur after initial response should be referred for total hysterectomy (91). Ovaries may be preserved in selected cases, i.e., patients younger than 45 years with no genetic predisposition for ovarian cancer such as BRCA mutation or Lynch Syndrome (89).
After completion of child bearing, patients should undergo total hysterectomy due to the high risk of recurrent endometrial cancer (88, 89).
Patient Eligibility Criteria (All Must Be Satisfied) and Role of MRI (Table 4)
Table 4.
Endometrial Cancer: Eligibility Criteria For Fertility-Sparing Management (All Criteria Must Be Met)
| Tumor Characteristics |
|
|
|
|
|
| Patient Characteristics |
|
|
|
|
|
| Criteria evaluated at MR Imaging |
|
|
|
Abbreviations: FIGO = FIGO - The International Federation of Gynecology and Obstetrics, D&C = dilation and curettage, MR = magnetic resonance
1. Tumor and Patient Characteristics
Patients must have a well-differentiated (FIGO grade 1) endometriod adenocarcinoma or premalignant conditions (atypical hyperplasia or endometrial intraepithelial neoplasia) (88, 89). Histologic characteristics must be obtained by D&C since this approach is more accurate than pipelle biopsy for the assessment of tumor grade (98).
Testing for Lynch Syndrome is advised for all women with endometrial cancer, particularly patients younger than 50 years who have significant family history of endometrial and/or colorectal cancer, or patients who have suspicious pathologic features at immunohistochemistry or microsatellite instability screening (88). It is controversial whether conservative management is appropriate for women with Lynch Syndrome because the predisposing factor is genetic (mutations in DNA mismatch repair genes), and hormonal therapy may be ineffective (91).
2. Endometrium-confined disease
◦ Absence of any myometrial invasion
MRI is critical to confirm that tumor is confined to the endometrium (Figure 7). On T2WI, endometrial cancer has the same or lower SI than the normal endometrium and stands out against low SI inner myometrium (junctional zone, JZ) (99, 100). On DCE-MRI, most tumors enhance homogenously, as well as slower and less intensely than the myometrium, with peak myometrial enhancement and maximal tumor-to-myometrium contrast occurring about 90–120 seconds after intravenous administration of contrast medium (101, 102). On DWI, tumor has high SI on high-b-value images and low SI on ADC maps relative to the myometrium (103–106).
Figure 7.
40-year-old woman with new onset menorrhagia and D&C demonstrating FIGO grade 1 endometrioid adenocarcinoma. A and B. Sagittal and axial oblique T2WI demonstrate intermediate-SI tumor in the endometrial cavity and preserved smooth interface between the tumor and low-SI junctional zone (white arrows). C and D. Sagittal and axial oblique fused (T2WI + DWI) images confirm smooth interface between the tumor and junctional zone (white arrows). E and F. Sagittal early (E) and delayed DCE-MRI (F) images show intact band of junctional zone enhancement (black arrows) and smooth tumor-to-myometrium interface (black arrow), respectively.
Diagnostic clues that are useful to confirm endometrium-confined disease include uninterrupted low-SI band of JZ on T2WI and a continuous rim of sub-endometrial enhancement (SEE, thin layer of early enhancement of inner JZ) on DCE-MRI acquired within first minute of intravenous administration of contrast medium (100, 101, 107). If SEE is not present, the inner surface of the enhanced myometrium should be smooth and sharply demarcated (Figure 7) (101).
Recent data suggest that DWI is also highly accurate for assessing the presence and depth of myometrial invasion (105, 106, 108, 109). DWI alone does not provide sufficient anatomic detail and is best interpreted in conjunction with T2WI (Figure 7). In fact, fused images (T2WI + DWI) are superior to DCE-MRI alone or T2+DCE-MRI for detecting any myometrial invasion (105). Thus, a combination of T2WI + DWI + DCE-MRI is recommended in routine clinical practice for the most comprehensive and accurate assessment (6).
Several pitfalls exist. First, myometrial invasion may be under- or overestimated in the presence of leiomyomas and adenomyosis which distort normal uterine zonal anatomy (36). Second, myometrial invasion may be overestimated in the uterine cornua, where the myometrium is thinner than elsewhere in the uterus. Third, some tumors enhance similar to the myometrium. Lastly, peri-tumoral enhancement (early irregular enhancement around the tumor) can disrupt SEE and falsely indicated myometrial invasion (107). These pitfalls may be avoided or diminished if T2WI, DWI, and DCE-MRI are interpreted in conjunction with each other (38, 107, 108, 110).
◦ Absence of cervical stromal invasion
Infiltration of the cervical stroma (Stage II) is associated with a higher risk of lymph node metastases and adverse prognosis (111, 112). On T2WI, cervical stromal invasion is suspected when intermediate-to-high-SI tumor disrupts low-SI cervical stroma (100). On DCE-MRI, stromal invasion is suggested when hypo-enhancing tumor interrupts continuous enhancement of cervical epithelium (± stoma), best seen 1.5 to 4 minutes after intravenous administration of contrast medium (100, 113). Both T2WI and DCE-MRI suffer from modest sensitivity with false positive findings observed when the tumor distends endocervical canal. On the other hand, a recent report suggests that DWI is both highly sensitive and more accurate than either T2WI or DCE-MRI (114). The absence of cervical stromal invasion is indicated by the smooth tumor-cervical stromal interface on high b-value images.
◦Absence of synchronous primary ovarian cancer or ovarian metastases
In approximately 10% of cases endometrial cancer may manifest with synchronous primary ovarian cancer or metastasis to the ovaries (115–118). The detection and differentiation between the two entities are critical for patient management and prognostication (117). Recent studies using targeted gene panels suggest that the majority of ovarian cancers in patients with endometrial cancer are in fact metastases to the ovaries (119, 120). Young women with ovarian involvement from either cause are not amenable to conservative management.
Careful MR imaging assessment of both adnexa is crucial if fertility-sparing management is being contemplated. Ovaries should be inspected for signs of malignancy as discussed further in Part VI of this manuscript. The distinction between synchronous primary ovarian carcinoma versus metastasis is an ongoing challenge at imaging (121). The presence of a large endometrial tumor and a small ovarian lesion may favor metastasis, whereas a small endometrial tumor and a large unilateral complex ovarian mass ± endometriosis raises the possibility of a synchronous primary ovarian malignancy (121).
◦Absence of lymph node metastasis
Lymph node metastases are rare in early-stage endometrial cancer (84, 89, 122, 123). When they occur, the most common sites of involvement are pelvic and para-aortic nodal regions. MRI assessment of lymph node status in endometrial cancer parallels and faces similar challenges as that of cervical cancer (124).
Part VI: Fertility-Sparing Management of Ovarian Tumors
Most ovarian cancers manifest in postmenopausal women as advanced-stage disease. However, a substantial subset occurs in reproductive age women, with the incidence rising with age (125). Majority of ovarian malignancies in young women are germ cell tumors and epithelial borderline tumors, with invasive epithelial ovarian cancers and sex-cord stromal tumors being less common (126, 127).
Conservative Management
Surgery is the cornerstone of the initial management of ovarian tumors. The goals are to resect the ovarian tumor to attain accurate histologic diagnosis, comprehensively stage apparently early stage disease, or cytoreduce advanced-stage disease (128). Preoperative imaging can aid the characterization, map the extent of disease, and guide surgical approach (i.e. laparoscopy versus laparotomy) (128). The review of intraoperative frozen-sections by an expert gynecologic pathologist is invaluable to tailor surgical procedures based on the histologic information. Fertility-sparing surgery, defined as unilateral salpingo-oophorectomy (USO) with preservation of the uterus and contralateral ovary, is possible for most young women who desire future fertility and have any stage malignant germ cell tumors, sex-cord stromal tumors, borderline tumors or have early stage epithelial carcinomas (129–133).
Malignant germ cell tumors
Five percent of germ cell tumors are malignant; the rest are benign mature cystic teratomas. Malignant germ cell tumors manifest in very young women (younger than 20 years) and are the most common ovarian malignancy in this age group (Figure 8, and Figure 9) (127). Dysgerminomas and immature teratomas are the most frequent, followed by yolk sac and mixed tumors (134). Most patients present with Stage I (ovary-confined) disease and have >90% 5-year overall survival; even advanced-stage disease has a good prognosis due to high chemosensitivity making fertility preservation possible (135).
Figure 8.
23-year-old woman with dysgerminoma who presented with low back pain and elevated serum LDH and β-HCG. A and B. Axial and sagittal T2WI show solid intermediate-SI adnexal mass with low-SI fibrovascular septa (white arrows). C and D. Axial DWI and ADC map demonstrate diffusion restriction. E. Axial contrast-enhanced image shows solid enhancing adnexal mass with avidly enhancing fibrovascular septa (black arrows).
Figure 9.
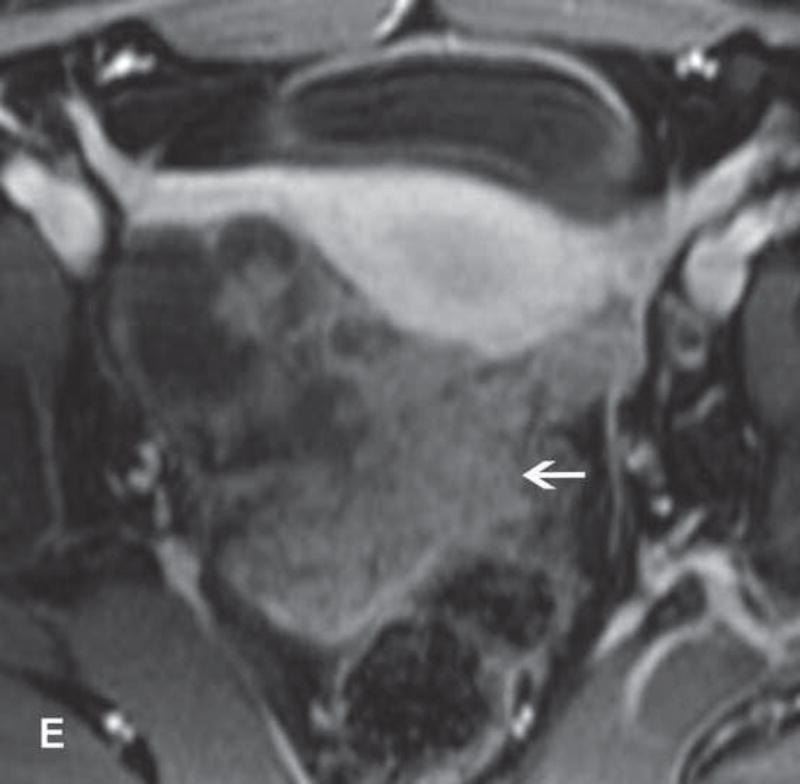
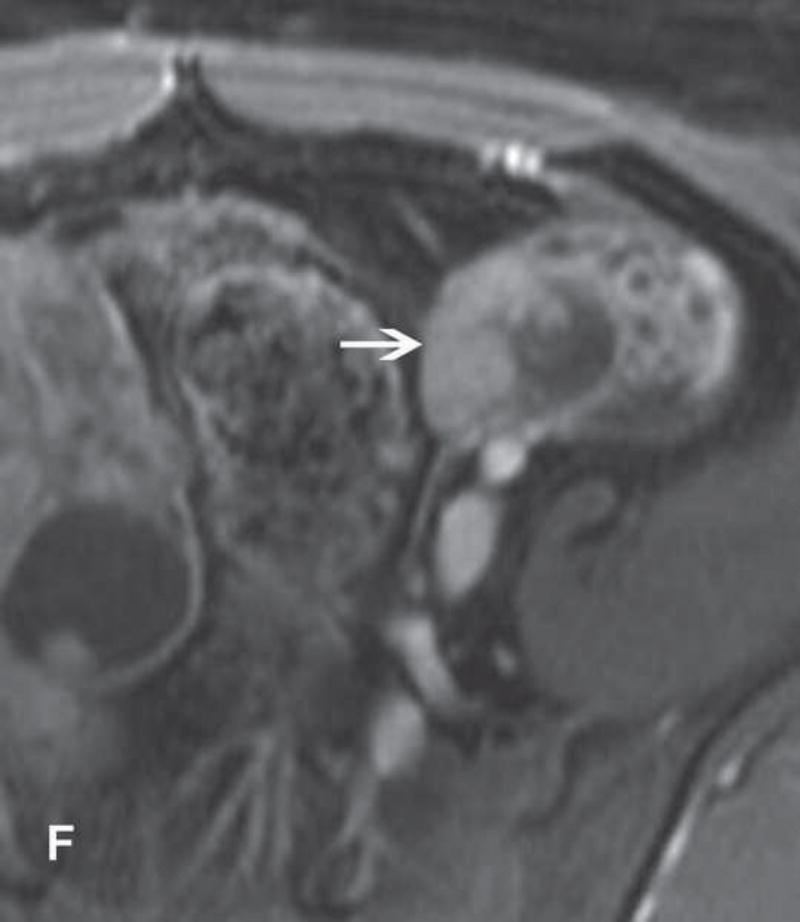
13-year-old teenager with immature cystic teratoma who presented with lower abdominal pain and no elevation in serum tumor markers. A and B. T1WI and FST1WI demonstrate left adnexal mass with small high-SI focus (white arrow) on T1WI (A) which becomes low-SI on FST1WI (white arrow) (B). C, D, and E. Axial T2WI, fused (T2WI + DWI), and contrast-enhanced images show complex predominantly cystic left adnexal mass with multiple cystic components and thickened and/or nodular septations with restricted diffusion and enhancement F. The same patient presented several years prior with right adnexal mass. Sagittal contrast-enhanced CT image obtained at that time shows a large cystic-solid right adnexal mass with several small foci of fat (white arrow) and small scattered calcifications (black arrow).
Conservative surgery for malignant germ cell tumors is considered appropriate regardless of histologic subtype and FIGO stage (129, 130). USO is combined with comprehensive peritoneal staging. Advanced-stage disease is debulked although extensive resections are avoided due to chemo-responsiveness. Lymphadenectomy can be omitted if lymph nodes are macroscopically normal (129). Adjuvant platinum-based chemotherapy is advised for most malignant germ cell tumors; exceptions are Stage I pure dysgerminoma and well-differentiated Stage I immature teratoma (129, 130).
Sex-cord stromal tumors
Most malignant sex-cord stromal tumors are comprised of granulosa cell tumors (70%) and Sertoli-Leydig tumors (132). Granulosa cell tumors (GCTs) are subdivided into adult (95%) and juvenile (5%) types. Adult type manifests mostly in perimenopausal women, whereas juvenile type predominates in pre-pubertal girls. Sertoli-Leydig tumors affect women younger than 30 years and are the most common androgen-producing ovarian tumors (with 30% being hormonally active) (Figure 10) (136). The majority of sex-cord stromal tumors are unilateral and ovary-confined at diagnosis with >90% 5-year overall survival rate for Stage I disease (132). Fertility-sparing surgery for patients with sex-cord stromal tumors is similar to that of malignant germ cell tumors (129, 130, 137). Endometrial curettage is mandatory in patients with granulosa cell tumors to rule out synchronous endometrial carcinoma (secondary to estrogen excess production by the tumor) (129). Adjuvant platinum-based chemotherapy is considered for patients with either high-risk Stage I disease or Stage > I disease (129, 132).
Figure 10.
25-year-old woman with Sertoli-Leydig tumor who presented with pelvic discomfort and elevated serum testosterone. A. Axial T2WI shows intermediate-SI predominantly solid mass with few small cystic areas. B and C. Axial fused (T2WI + DWI) and ADC images demonstrate diffusion restriction within the solid portion of the mass. D. Axial contrast-enhanced image shows enhancement within the solid portion of the mass.
Borderline ovarian tumors
Borderline ovarian tumors commonly manifest in premenopausal women (average age: 38 years) (138). Serous followed by mucinous borderline tumors are the most common histologic subtypes (139). While most mucinous borderline tumors are confined to a single ovary at presentation, serous borderline tumors may involve both ovaries (15–40%), develop peritoneal implants (35%), and at times involve lymph nodes (Figure 11) (139). Young women with borderline tumors are amenable to fertility-sparing surgery regardless of FIGO stage as long as there are no invasive peritoneal implants (i.e., low-grade serous ovarian cancer). Unilateral serous borderline tumors are approached with ovarian cystectomy or USO. Mucinous borderline tumors require USO due to their large size and to exclude intra-epithelial carcinoma (140–143) (144). Bilateral serous borderline tumors are managed with bilateral cystectomy or combined USO and contralateral cystectomy (140). These procedures are combined with appendectomy (if mucinous histology) and comprehensive peritoneal staging to exclude low-grade serous ovarian cancer (which is a contraindication to conservative fertility-sparing management).
Figure 11.
20-year-old woman with bilateral serous borderline ovarian tumors who presented with lower abdominal pain and elevated serum CA-125 level. A. Axial T2WI of the right adnexa shows cystic and solid right adnexal mass with large branching papillary projections (black arrow) extending into the pouch of Douglas. B. Axial T2WI of the left adnexa shows small cystic lesion with small intermediate-SI papillary projections (black arrow). C and D. Axial fused (T2WI + DWI) images demonstrate restricted diffusion within papillary projections (white arrows). E and F. Axial contrast-enhanced images show enhancement within these papillary projections (white arrows).
Epithelial ovarian cancers
Epithelial ovarian cancers are most common in postmenopausal women; however, around 12% of cases manifest in women younger than 45 years (125). Most patients present with advanced staged disease making these the most lethal of all gynecologic cancers (1). Young women with early stage disease and a strong desire for fertility may be offered fertility-sparing management. Patients with unilateral ovarian involvement (Stage IA or IC disease) and favorable histology (serous, mucinous, endometrioid, or mixed histologic subtypes) or Stage IA clear cell carcinoma (less favorable histology) may be candidates for USO plus comprehensive peritoneal staging and lymphadenectomy. Adjuvant platinum-based chemotherapy is required for Stage IA clear cell carcinoma and Stage IC tumors (131, 133). Bilateral ovarian involvement (Stage IB) and extra-ovarian spread preclude conservative management (131, 133).
General diagnostic approach based on clinical and imaging data
Patients with concerning symptoms and/or pelvic mass undergo pelvic ultrasonography and assessment of serum tumor markers (serum human chorionic gonadotropin (hCG), α-fetoprotein (AFP), lactate dehydrogenase (LDH), inhibin, and CA–125) (128, 145–148). Elevation of these markers can aid diagnosis; normal levels don’t rule out ovarian tumor. Elevated AFP or hCG are virtually diagnostic of malignant germ cell tumors (134). CA-125 can be raised with epithelial tumors (both borderline and invasive types), although non-neoplastic conditions including endometriosis, pelvic inflammatory disease, and uterine leiomyoma may also cause nonspecific elevation. Inhibin may serve as a useful marker of granulosa cell tumor (132).
Pelvic ultrasonography is the best imaging modality for the initial assessment of a suspected adnexal mass. When sonographic findings are suspicious for malignancy, a patient should be referred to a gynecologic oncologist and undergo staging CT, which is the current standard of care. Preoperative CT helps surgical planning by demonstrating the extent of disease including unilateral or bilateral ovarian involvement, presence and distribution of peritoneal implants, lymphadenopathy and distant metastases.
Up to 25% of adnexal mass cannot be characterized with US as definitely benign or definitely malignant (146, 147). In this setting, MRI is the most accurate and cost-effective imaging modality (7, 149). In addition to lesion characterization, MRI shows the presence of preserved ipsilateral ovarian tissue (important if cystectomy is being considered), evaluates contralateral ovary, detects and maps peritoneal implants, and demonstrates enlarged lymph nodes facilitating clinical decision-making with regard to fertility-sparing surgery.
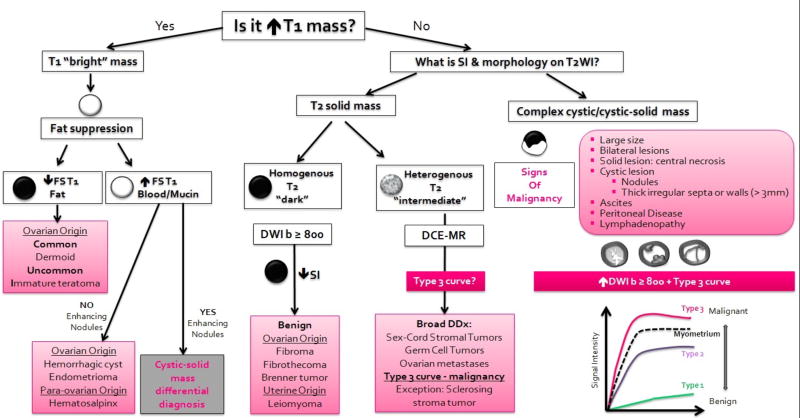
The European Society of Uroradiology has recently proposed an updated step-by-step algorithmic approach to assist the interpretation of multi-parametric MRI acquired to characterize sonographically indeterminate adnexal masses (Figure 12) (27). This approach helps to characterize adnexal masses as probably-definitely benign, probably-definitely malignant, or indeterminate (40). It relies on the evaluation of lesion origin, morphology and SI on conventional sequences (T1WI, FST1WI, T2WI), SI on high b-value DWI (≥800 sec/mm2), and enhancement characteristics/kinetics on DCE-MR (27).
Figure 12.
A diagram illustrates an algorithmic approach to the characterization of sonographically indeterminate adnexal masses using multi-parametric MRI. This approach is based on the recently updated ESUR guidelines (27).
In the first step, conventional T1WI and T2WI are reviewed to divide adnexal masses into one of three broad categories: T1 “bright” masses, T2 solid masses, and complex cystic or cystic-solid masses (27, 28). T1 “bright” masses require further assessment with fat saturated T1WI to differentiate fat from blood. If fat is observed within adnexal mass (high SI on TWI that suppresses after fat saturation), the differential diagnosis is narrowed to frequent mature cystic teratoma or rare malignant immature teratoma. Mature teratoma often presents as a cystic mass filled with fatty sebaceous fluid and may contain coarse tooth-like calcifications (best seen on CT) allowing confident diagnosis of a benign mass (134). In contrast, immature teratoma is typically a solid-cystic mass with small foci of fat, cysts filled with simple fluid, and scattered fine calcifications (best seen on CT) (134).
If a T1 “bright” mass contains blood products (high SI on T1WI that persists after fat saturation) and demonstrates no solid components, the differential diagnosis can be narrowed to benign entities such as a hemorrhagic cyst or endometrioma. If solid components are detected within a T1”bright” mass further evaluation is performed as for a complex cystic/cystic-solid mass (described below) (27).
If a T2 solid mass is identified, the most likely origin (i.e. ovarian, uterine, or other) should be determined. Findings suggestive of ovarian origin are the ovarian beak sign and absent normal ipsilateral ovary. Features indicative of uterine origin are the uterine claw sign, “bridging vessel” sign (if pedunculated leiomyoma), and normal ipsilateral ovary (27).
T2 solid masses are divided further into homogenous T2 “dark” masses (SI similar to that of skeletal muscle) or heterogeneous T2 “intermediate” masses (SI higher than that of skeletal muscle). If a homogenous T2 dark mass has low SI on high b-value DWI, benign etiology (ovarian fibroma or cystadenofibroma) is likely (27, 42). Heterogeneous T2 “intermediate” masses present a diagnostic challenge since differential diagnosis is broad and includes a variety of benign and malignant entities. Although confident diagnosis is not always possible, knowledge of patient age and serum tumor markers in combination with key MR imaging features can be useful. For example, dysgerminoma (most common malignant germ cell tumor) is suggested by young patient age, elevated LDH ± HCG, and heterogeneous T2 “intermediate” mass that contains fibro-vascular septa with low SI on T2WI and avid enhancement (134).
Purely cystic lesions or cystic lesions with few thin septa can be confidently diagnosed as benign (40). In contrast, complex cystic and cystic-solid masses require comprehensive assessment utilizing conventional sequences, DWI, and contrast-enhanced imaging (preferably DCE-MRI). This group includes adnexal masses caused by infection (pelvic inflammatory disease) and ovarian tumors including borderline tumors and invasive ovarian cancers (27, 28).
MR imaging features of overtly malignant ovarian masses are well established and include mixed cystic-solid mass, irregular or nodular thick septa and walls, intermediate SI solid components on T2WI, high SI on high b-value DWI, and contrast enhancement (40, 42, 149–151). The distinction between borderline and invasive tumors is an important and clinical relevant issue because epithelial ovarian cancer is amenable to conservative management only if confined to a single ovary whereas borderline tumors, particularly serous histology, are amenable to fertility-preservation even in the presence of bilateral ovarian involvement and peritoneal spread (130). Unfortunately, this differentiation presents an ongoing diagnostic challenge since these tumor types can have overlapping MR features such as a cystic-solid mass with intermediate SI solid component(s) on T2WI and contrast enhancement.
The presence of papillary projections (enhancing solid finger-like projections) is one architectural feature that is common to serous borderline tumors and suggests the diagnosis (152). On T2WI, papillary projections range from small low-to-intermediate SI lesions to larger lesions with characteristic internal branching low SI fibrous stalk and high SI peripheral clumps (Figure 11) (152). Emerging evidence also indicates that addition of DWI and DCE-MRI may facilitate the differentiation between borderline and invasive tumors. On DWI, solid components of borderline tumor have lower-SI on high b-value images and higher mean ADC values than invasive tumor (153). On DCE-MRI acquired using perfusion technique (as described in Part III), moderate initial enhancement followed by a plateau (Type 2 curve) may indicate borderline tumors, whereas rapid intense early enhancement with subsequent washout (Type 3 curve) may suggest malignant mass (27, 39–42). However, the advantage of perfusion imaging over traditional dynamic multiphase approach remains to be proven.
Conclusion
Fertility-sparing approaches are now included in various treatment guidelines (including ESMO and NCCN) and are increasing accepted as safe for appropriately selected reproductive age women with gynecologic cancer. MRI plays an important role in preoperative workup and proper selection of patients who are being considered for conservative fertility-sparing management of cancer. Radiologists should to be familiar with various fertility-sparing options and their eligibility criteria in order to provide comprehensive and clinically relevant imaging reports and to serve as effective consultants for their clinical colleagues.
Acknowledgments
1. Funding: This study was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
2. Authors thank Joanne Chin M.F.A. for her editorial assistance with the manuscript.
Abbreviations
- ADC
Apparent Diffusion Coefficient
- D & C
Dilation and Curettage
- DCE-MRI
Dynamic contrast-enhanced MRI
- DWI
Diffusion-weighted Imaging
- ESMO
European Society for Medical Oncology
- ESUR
European Society of Urogenital Radiology
- FDG
Fluorodeoxyglucose
- FIGO
International Federation of Gynecology and Obstetrics
- FST1WI
Fat saturated T1WI
- JZ
Junctional Zone
- LVSI
Lymphovascular Space Invasion
- MRI
Magnetic Resonance Imaging
- NCCN
National Comprehensive Cancer Network
- OHSS
Ovarian Hyperstimulation Syndrome
- PET/CT
Positron Emission Tomography-Computed Tomography
- PET/MRI
Positron Emission Tomography-Magnetic Resonance Imaging
- USO
Unilateral Salpingo-oophorectomy
- ROI
Region of Interest
- SEE
Subendometrial Enhancement
- SI
Signal Intensity
- T1WI
T1-weighted Images
- T2WI
T2-weighted Images
- US
Ultrasonography
Footnotes
Conflict of Interest:
Dr S Mcevoy declares that she has no conflict of interest.
Dr S Nougaret declares that she has no conflict of interest.
Dr N Abu-Rustum declares that he has no conflict of interest.
Dr H Vargas declares that he has no conflict of interest.
Dr E Sadowski declares that she has no conflict of interest.
Dr C Menias declares that she has no conflict of interest.
Dr F Shitano declares that she has no conflict of interest.
Dr S Fujii declares that he has no conflict of interest.
Mr R Sosa declares that he has no conflict of interest.
Dr J Escalon declares that she has no conflict of interest.
Dr E Sala declares that she has no conflict of interest.
Dr Y Lakhman declares that she has no conflict of interest.
Compliance with Ethical Standards:
2. Ethical approval: This review article does not contain any studies with human participants or animals performed by any of the authors. As a result, informed consent was not obtained.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Woodruff TK. The Oncofertility Consortium--addressing fertility in young people with cancer. Nature reviews Clinical oncology. 2010;7(8):466–75. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter J, Rowland K, Chi D, et al. Gynecologic cancer treatment and the impact of cancer-related infertility. Gynecologic oncology. 2005;97(1):90–5. doi: 10.1016/j.ygyno.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(19):2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384(9950):1302–10. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology. 2013;266(3):717–40. doi: 10.1148/radiol.12120315. [DOI] [PubMed] [Google Scholar]
- 7.Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H. Indeterminate ovarian mass at US: incremental value of second imaging test for characterization--meta-analysis and Bayesian analysis. Radiology. 2005;236(1):85–94. doi: 10.1148/radiol.2361041618. [DOI] [PubMed] [Google Scholar]
- 8.Noyes N, Knopman JM, Long K, Coletta JM, Abu-Rustum NR. Fertility considerations in the management of gynecologic malignancies. Gynecologic oncology. 2011;120(3):326–33. doi: 10.1016/j.ygyno.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Ovarian tissue cryopreservation: a committee opinion. Fertility and sterility. 2014;101(5):1237–43. doi: 10.1016/j.fertnstert.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 10.Bastings L, Beerendonk CC, Westphal JR, et al. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Human reproduction update. 2013;19(5):483–506. doi: 10.1093/humupd/dmt020. [DOI] [PubMed] [Google Scholar]
- 11.Baron KT, Babagbemi KT, Arleo EK, Asrani AV, Troiano RN. Emergent complications of assisted reproduction: expecting the unexpected. Radiographics : a review publication of the Radiological Society of North America, Inc. 2013;33(1):229–44. doi: 10.1148/rg.331125011. [DOI] [PubMed] [Google Scholar]
- 12.Zivi E, Simon A, Laufer N. Ovarian hyperstimulation syndrome: definition, incidence, and classification. Seminars in reproductive medicine. 2010;28(6):441–7. doi: 10.1055/s-0030-1265669. [DOI] [PubMed] [Google Scholar]
- 13.Kim IY, Lee BH. Ovarian hyperstimulation syndrome. US and CT appearances. Clinical imaging. 1997;21(4):284–6. doi: 10.1016/s0899-7071(96)00044-7. [DOI] [PubMed] [Google Scholar]
- 14.Jung SE, Byun JY, Lee JM, et al. MR imaging of maternal diseases in pregnancy. AJR American journal of roentgenology. 2001;177(6):1293–300. doi: 10.2214/ajr.177.6.1771293. [DOI] [PubMed] [Google Scholar]
- 15.Gelbaya TA. Short and long-term risks to women who conceive through in vitro fertilization. Human fertility (Cambridge, England) 2010;13(1):19–27. doi: 10.3109/14647270903437923. [DOI] [PubMed] [Google Scholar]
- 16.Zhang FL, Fu LL, Yang Y. Both thromboembolic stroke and cerebral venous thrombosis resulting from Ovarian Hyperstimulation Syndrome (OHSS) Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2016;36(2):267–8. doi: 10.3109/01443615.2015.1049987. [DOI] [PubMed] [Google Scholar]
- 17.Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. International journal of radiation oncology, biology, physics. 2005;62(3):738–44. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? The Lancet Oncology. 2005;6(4):209–18. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 19.Milgrom SA, Vargas HA, Sala E, Kelvin JF, Hricak H, Goodman KA. Acute effects of pelvic irradiation on the adult uterus revealed by dynamic contrast-enhanced MRI. The British journal of radiology. 2013;86(1031):20130334. doi: 10.1259/bjr.20130334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green DM, Sklar CA, Boice JD, Jr, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(14):2374–81. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irtan S, Orbach D, Helfre S, Sarnacki S. Ovarian transposition in prepubescent and adolescent girls with cancer. The Lancet Oncology. 2013;14(13):e601–8. doi: 10.1016/S1470-2045(13)70288-2. [DOI] [PubMed] [Google Scholar]
- 22.Oliver Perez MR, Magrina J, Garcia AT, Jimenez Lopez JS. Prophylactic salpingectomy and prophylactic salpingoophorectomy for adnexal high-grade serous epithelial carcinoma: A reappraisal. Surg Oncol. 2015;24(4):335–44. doi: 10.1016/j.suronc.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Gubbala K, Laios A, Gallos I, Pathiraja P, Haldar K, Ind T. Outcomes of ovarian transposition in gynaecological cancers; a systematic review and meta-analysis. Journal of ovarian research. 2014;7:69. doi: 10.1186/1757-2215-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto R, Okamoto K, Yukiharu T, et al. A study of risk factors for ovarian metastases in stage Ib–IIIb cervical carcinoma and analysis of ovarian function after a transposition. Gynecologic oncology. 2001;82(2):312–6. doi: 10.1006/gyno.2001.6277. [DOI] [PubMed] [Google Scholar]
- 25.Sella T, Mironov S, Hricak H. Imaging of transposed ovaries in patients with cervical carcinoma. AJR American journal of roentgenology. 2005;184(5):1602–10. doi: 10.2214/ajr.184.5.01841602. [DOI] [PubMed] [Google Scholar]
- 26.Ulaner GA, Lyall A. Identifying and distinguishing treatment effects and complications from malignancy at FDG PET/CT. Radiographics : a review publication of the Radiological Society of North America, Inc. 2013;33(6):1817–34. doi: 10.1148/rg.336125105. [DOI] [PubMed] [Google Scholar]
- 27.Forstner R, Thomassin-Naggara I, Cunha TM, et al. ESUR recommendations for MR imaging of the sonographically indeterminate adnexal mass: an update. European radiology. 2016 doi: 10.1007/s00330-016-4600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer JA, Ghattamaneni S. MR imaging of the sonographically indeterminate adnexal mass. Radiology. 2010;256(3):677–94. doi: 10.1148/radiol.10090397. [DOI] [PubMed] [Google Scholar]
- 29.deSouza NM, Dina R, McIndoe GA, Soutter WP. Cervical cancer: value of an endovaginal coil magnetic resonance imaging technique in detecting small volume disease and assessing parametrial extension. Gynecologic oncology. 2006;102(1):80–5. doi: 10.1016/j.ygyno.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 30.Downey K, Attygalle AD, Morgan VA, et al. Comparison of optimised endovaginal vs external array coil T2-weighted and diffusion-weighted imaging techniques for detecting suspected early stage (IA/IB1) uterine cervical cancer. European radiology. 2016;26(4):941–50. doi: 10.1007/s00330-015-3899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Downey K, Jafar M, Attygalle AD, et al. Influencing surgical management in patients with carcinoma of the cervix using a T2- and ZOOM-diffusion-weighted endovaginal MRI technique. British journal of cancer. 2013;109(3):615–22. doi: 10.1038/bjc.2013.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Downey K, Shepherd JH, Attygalle AD, et al. Preoperative imaging in patients undergoing trachelectomy for cervical cancer: validation of a combined T2- and diffusion-weighted endovaginal MRI technique at 3.0 T. Gynecologic oncology. 2014;133(2):326–32. doi: 10.1016/j.ygyno.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balleyguier C, Sala E, Da Cunha T, et al. Staging of uterine cervical cancer with MRI: guidelines of the European Society of Urogenital Radiology. European radiology. 2011;21(5):1102–10. doi: 10.1007/s00330-010-1998-x. [DOI] [PubMed] [Google Scholar]
- 34.Kinkel K, Forstner R, Danza FM, et al. Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. European radiology. 2009;19(7):1565–74. doi: 10.1007/s00330-009-1309-6. [DOI] [PubMed] [Google Scholar]
- 35.Charles-Edwards EM, Messiou C, Morgan VA, et al. Diffusion-weighted imaging in cervical cancer with an endovaginal technique: potential value for improving tumor detection in stage Ia and Ib1 disease. Radiology. 2008;249(2):541–50. doi: 10.1148/radiol.2491072165. [DOI] [PubMed] [Google Scholar]
- 36.Nougaret S, Tirumani SH, Addley H, Pandey H, Sala E, Reinhold C. Pearls and pitfalls in MRI of gynecologic malignancy with diffusion-weighted technique. AJR American journal of roentgenology. 2013;200(2):261–76. doi: 10.2214/AJR.12.9713. [DOI] [PubMed] [Google Scholar]
- 37.Akita A, Shinmoto H, Hayashi S, et al. Comparison of T2-weighted and contrast-enhanced T1-weighted MR imaging at 1.5 T for assessing the local extent of cervical carcinoma. European radiology. 2011;21(9):1850–7. doi: 10.1007/s00330-011-2122-6. [DOI] [PubMed] [Google Scholar]
- 38.Sala E, Crawford R, Senior E, et al. Added value of dynamic contrast-enhanced magnetic resonance imaging in predicting advanced stage disease in patients with endometrial carcinoma. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2009;19(1):141–6. doi: 10.1111/IGC.0b013e3181995fd9. [DOI] [PubMed] [Google Scholar]
- 39.Thomassin-Naggara I, Balvay D, Aubert E, et al. Quantitative dynamic contrast-enhanced MR imaging analysis of complex adnexal masses: a preliminary study. European radiology. 2012;22(4):738–45. doi: 10.1007/s00330-011-2329-6. [DOI] [PubMed] [Google Scholar]
- 40.Thomassin-Naggara I, Aubert E, Rockall A, et al. Adnexal masses: development and preliminary validation of an MR imaging scoring system. Radiology. 2013;267(2):432–43. doi: 10.1148/radiol.13121161. [DOI] [PubMed] [Google Scholar]
- 41.Thomassin-Naggara I, Balvay D, Rockall A, et al. Added Value of Assessing Adnexal Masses with Advanced MRI Techniques. BioMed research international. 2015;2015:785206. doi: 10.1155/2015/785206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomassin-Naggara I, Toussaint I, Perrot N, et al. Characterization of complex adnexal masses: value of adding perfusion- and diffusion-weighted MR imaging to conventional MR imaging. Radiology. 2011;258(3):793–803. doi: 10.1148/radiol.10100751. [DOI] [PubMed] [Google Scholar]
- 43.American Cancer Society. Global Cancer Facts & Figures. 3. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 44.Cancer of the Cervix Uteri - SEER Stat Fact Sheets [Internet] [May 16, 2016]; Available from: http://seer.cancer.gov/statfacts/html/cervix.html.
- 45.Covens A, Rosen B, Murphy J, et al. How important is removal of the parametrium at surgery for carcinoma of the cervix? Gynecologic oncology. 2002;84(1):145–9. doi: 10.1006/gyno.2001.6493. [DOI] [PubMed] [Google Scholar]
- 46.Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2012;23(Suppl 7):vii27–32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 47.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. [November 27, 2016];Cervical Cancer. Version 1.2017. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf.
- 48.Bentivegna E, Gouy S, Maulard A, Chargari C, Leary A, Morice P. Oncological outcomes after fertility-sparing surgery for cervical cancer: a systematic review. The Lancet Oncology. 2016;17(6):e240–53. doi: 10.1016/S1470-2045(16)30032-8. [DOI] [PubMed] [Google Scholar]
- 49.Xu L, Sun FQ, Wang ZH. Radical trachelectomy versus radical hysterectomy for the treatment of early cervical cancer: a systematic review. Acta obstetricia et gynecologica Scandinavica. 2011;90(11):1200–9. doi: 10.1111/j.1600-0412.2011.01231.x. [DOI] [PubMed] [Google Scholar]
- 50.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2009;105(2):107–8. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Qin Y, Peng Z, Lou J, Liu H, Deng F, Zheng Y. Discrepancies between clinical staging and pathological findings of operable cervical carcinoma with stage IB–IIB: a retrospective analysis of 818 patients. The Australian & New Zealand journal of obstetrics & gynaecology. 2009;49(5):542–4. doi: 10.1111/j.1479-828X.2009.01065.x. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell DG, Snyder B, Coakley F, et al. Early invasive cervical cancer: MRI and CT predictors of lymphatic metastases in the ACRIN 6651/GOG 183 intergroup study. Gynecologic oncology. 2009;112(1):95–103. doi: 10.1016/j.ygyno.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hricak H, Gatsonis C, Coakley FV, et al. Early invasive cervical cancer: CT and MR imaging in preoperative evaluation - ACRIN/GOG comparative study of diagnostic performance and interobserver variability. Radiology. 2007;245(2):491–8. doi: 10.1148/radiol.2452061983. [DOI] [PubMed] [Google Scholar]
- 54.Thomeer MG, Gerestein C, Spronk S, van Doorn HC, van der Ham E, Hunink MG. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: systematic review and meta-analysis. European radiology. 2013;23(7):2005–18. doi: 10.1007/s00330-013-2783-4. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell DG, Snyder B, Coakley F, et al. Early invasive cervical cancer: tumor delineation by magnetic resonance imaging, computed tomography, and clinical examination, verified by pathologic results, in the ACRIN 6651/GOG 183 Intergroup Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(36):5687–94. doi: 10.1200/JCO.2006.07.4799. [DOI] [PubMed] [Google Scholar]
- 56.Rob L, Skapa P, Robova H. Fertility-sparing surgery in patients with cervical cancer. The Lancet Oncology. 2011;12(2):192–200. doi: 10.1016/S1470-2045(10)70084-X. [DOI] [PubMed] [Google Scholar]
- 57.Rob L, Charvat M, Robova H, et al. Less radical fertility-sparing surgery than radical trachelectomy in early cervical cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2007;17(1):304–10. doi: 10.1111/j.1525-1438.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 58.Abu-Rustum NR, Sonoda Y. Fertility-sparing surgery in early-stage cervical cancer: indications and applications. Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8(12):1435–8. doi: 10.6004/jnccn.2010.0107. [DOI] [PubMed] [Google Scholar]
- 59.Plante M. Evolution in fertility-preserving options for early-stage cervical cancer: radical trachelectomy, simple trachelectomy, neoadjuvant chemotherapy. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2013;23(6):982–9. doi: 10.1097/IGC.0b013e318295906b. [DOI] [PubMed] [Google Scholar]
- 60.Plante M. Bulky Early-Stage Cervical Cancer (2–4 cm Lesions): Upfront Radical Trachelectomy or Neoadjuvant Chemotherapy Followed by Fertility-Preserving Surgery: Which Is the Best Option? International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2015;25(4):722–8. doi: 10.1097/IGC.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 61.Noel P, Dube M, Plante M, St-Laurent G. Early cervical carcinoma and fertility-sparing treatment options: MR imaging as a tool in patient selection and a follow-up modality. Radiographics : a review publication of the Radiological Society of North America Inc. 2014;34(4):1099–119. doi: 10.1148/rg.344130009. [DOI] [PubMed] [Google Scholar]
- 62.Wakefield JC, Downey K, Kyriazi S, deSouza NM. New MR techniques in gynecologic cancer. AJR American journal of roentgenology. 2013;200(2):249–60. doi: 10.2214/AJR.12.8932. [DOI] [PubMed] [Google Scholar]
- 63.Seki H, Azumi R, Kimura M, Sakai K. Stromal invasion by carcinoma of the cervix: assessment with dynamic MR imaging. AJR American journal of roentgenology. 1997;168(6):1579–85. doi: 10.2214/ajr.168.6.9168730. [DOI] [PubMed] [Google Scholar]
- 64.Yamashita Y, Takahashi M, Sawada T, Miyazaki K, Okamura H. Carcinoma of the cervix: dynamic MR imaging. Radiology. 1992;182(3):643–8. doi: 10.1148/radiology.182.3.1535875. [DOI] [PubMed] [Google Scholar]
- 65.Sahdev A, Sohaib SA, Wenaden AE, Shepherd JH, Reznek RH. The performance of magnetic resonance imaging in early cervical carcinoma: a long-term experience. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2007;17(3):629–36. doi: 10.1111/j.1525-1438.2007.00829.x. [DOI] [PubMed] [Google Scholar]
- 66.Hricak H, Yu KK. Radiology in invasive cervical cancer. AJR American journal of roentgenology. 1996;167(5):1101–8. doi: 10.2214/ajr.167.5.8911159. [DOI] [PubMed] [Google Scholar]
- 67.Hricak H, Lacey CG, Sandles LG, Chang YC, Winkler ML, Stern JL. Invasive cervical carcinoma: comparison of MR imaging and surgical findings. Radiology. 1988;166(3):623–31. doi: 10.1148/radiology.166.3.3340756. [DOI] [PubMed] [Google Scholar]
- 68.Exner M, Kuhn A, Stumpp P, et al. Value of diffusion-weighted MRI in diagnosis of uterine cervical cancer: a prospective study evaluating the benefits of DWI compared to conventional MR sequences in a 3T environment. Acta Radiol. 2016;57(7):869–77. doi: 10.1177/0284185115602146. [DOI] [PubMed] [Google Scholar]
- 69.Lakhman Y, Akin O, Park KJ, et al. Stage IB1 cervical cancer: role of preoperative MR imaging in selection of patients for fertility-sparing radical trachelectomy. Radiology. 2013;269(1):149–58. doi: 10.1148/radiol.13121746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peppercorn PD, Jeyarajah AR, Woolas R, et al. Role of MR imaging in the selection of patients with early cervical carcinoma for fertility-preserving surgery: initial experience. Radiology. 1999;212(2):395–9. doi: 10.1148/radiology.212.2.r99au01395. [DOI] [PubMed] [Google Scholar]
- 71.de Boer P, Adam JA, Buist MR, et al. Role of MRI in detecting involvement of the uterine internal os in uterine cervical cancer: systematic review of diagnostic test accuracy. European journal of radiology. 2013;82(9):e422–8. doi: 10.1016/j.ejrad.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 72.Sonoda Y, Abu-Rustum NR, Gemignani ML, et al. A fertility-sparing alternative to radical hysterectomy: how many patients may be eligible? Gynecologic oncology. 2004;95(3):534–8. doi: 10.1016/j.ygyno.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 73.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecologic oncology. 1990;38(3):352–7. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 74.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecologic oncology. 1999;73(2):177–83. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 75.Hricak H, Yu KK, Powell CB, Subak LL, Stem J, Arenson RL. Comparison of diagnostic studies in the pretreatment evaluation of stage Ib carcinoma of the cervix. Academic radiology. 1996;3(Suppl 1):S44–6. doi: 10.1016/s1076-6332(96)80479-x. [DOI] [PubMed] [Google Scholar]
- 76.Aoki Y, Sasaki M, Watanabe M, et al. High-risk group in node-positive patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Gynecologic oncology. 2000;77(2):305–9. doi: 10.1006/gyno.2000.5788. [DOI] [PubMed] [Google Scholar]
- 77.McMahon CJ, Rofsky NM, Pedrosa I. Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology. 2010;254(1):31–46. doi: 10.1148/radiol.2541090361. [DOI] [PubMed] [Google Scholar]
- 78.Rockall AG, Sohaib SA, Harisinghani MG, et al. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(12):2813–21. doi: 10.1200/JCO.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 79.Shen G, Zhou H, Jia Z, Deng H. Diagnostic performance of diffusion-weighted MRI for detection of pelvic metastatic lymph nodes in patients with cervical cancer: a systematic review and meta-analysis. The British journal of radiology. 2015;88(1052):20150063. doi: 10.1259/bjr.20150063. [DOI] [PubMed] [Google Scholar]
- 80.Stecco A, Buemi F, Cassara A, et al. Comparison of retrospective PET and MRI-DWI (PET/MRI-DWI) image fusion with PET/CT and MRI-DWI in detection of cervical and endometrial cancer lymph node metastases. La Radiologia medica. 2016;121(7):537–45. doi: 10.1007/s11547-016-0626-5. [DOI] [PubMed] [Google Scholar]
- 81.Queiroz MA, Kubik-Huch RA, Hauser N, et al. PET/MRI and PET/CT in advanced gynaecological tumours: initial experience and comparison. European radiology. 2015;25(8):2222–30. doi: 10.1007/s00330-015-3657-8. [DOI] [PubMed] [Google Scholar]
- 82.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer Journal international du cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 83.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012. based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [September 9, 2016]; Available from: http://seer.cancer.gov/csr/1975_2012/
- 84.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387(10023):1094–108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 85.Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Human reproduction (Oxford, England) 2012;27(5):1327–31. doi: 10.1093/humrep/des042. [DOI] [PubMed] [Google Scholar]
- 86.Navaratnarajah R, Pillay OC, Hardiman P. Polycystic ovary syndrome and endometrial cancer. Seminars in reproductive medicine. 2008;26(1):62–71. doi: 10.1055/s-2007-992926. [DOI] [PubMed] [Google Scholar]
- 87.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387(10023):1094–108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 88.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. [November 27, 2016];Uterine Neoplasms. Version 1.2017. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
- 89.Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2016;26(1):2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kinkel K, Kaji Y, Yu KK, et al. Radiologic staging in patients with endometrial cancer: a meta-analysis. Radiology. 1999;212(3):711–8. doi: 10.1148/radiology.212.3.r99au29711. [DOI] [PubMed] [Google Scholar]
- 91.Rodolakis A, Biliatis I, Morice P, et al. European Society of Gynecological Oncology Task Force for Fertility Preservation: Clinical Recommendations for Fertility-Sparing Management in Young Endometrial Cancer Patients. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2015;25(7):1258–65. doi: 10.1097/IGC.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 92.Minig L, Franchi D, Boveri S, Casadio C, Bocciolone L, Sideri M. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2011;22(3):643–9. doi: 10.1093/annonc/mdq463. [DOI] [PubMed] [Google Scholar]
- 93.Kim MK, Seong SJ, Song T, et al. Comparison of dilatation & curettage and endometrial aspiration biopsy accuracy in patients treated with high-dose oral progestin plus levonorgestrel intrauterine system for early-stage endometrial cancer. Gynecologic oncology. 2013;130(3):470–3. doi: 10.1016/j.ygyno.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 94.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. American journal of obstetrics and gynecology. 2012;207(4):266.e1–12. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 95.Park JY, Kim DY, Kim JH, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002) European journal of cancer (Oxford, England : 1990) 2013;49(4):868–74. doi: 10.1016/j.ejca.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 96.Tangjitgamol S, Manusirivithaya S, Hanprasertpong J. Fertility-sparing in endometrial cancer. Gynecologic and obstetric investigation. 2009;67(4):250–68. doi: 10.1159/000209324. [DOI] [PubMed] [Google Scholar]
- 97.Erkanli S, Ayhan A. Fertility-sparing therapy in young women with endometrial cancer: 2010 update. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2010;20(7):1170–87. doi: 10.1111/igc.0b013e3181e94f5a. [DOI] [PubMed] [Google Scholar]
- 98.Leitao MM, Jr, Kehoe S, Barakat RR, et al. Comparison of D&C and office endometrial biopsy accuracy in patients with FIGO grade 1 endometrial adenocarcinoma. Gynecologic oncology. 2009;113(1):105–8. doi: 10.1016/j.ygyno.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 99.Beddy P, O'Neill AC, Yamamoto AK, Addley HC, Reinhold C, Sala E. FIGO staging system for endometrial cancer: added benefits of MR imaging. Radiographics : a review publication of the Radiological Society of North America Inc. 2012;32(1):241–54. doi: 10.1148/rg.321115045. [DOI] [PubMed] [Google Scholar]
- 100.Manfredi R, Mirk P, Maresca G, et al. Local-regional staging of endometrial carcinoma: role of MR imaging in surgical planning. Radiology. 2004;231(2):372–8. doi: 10.1148/radiol.2312021184. [DOI] [PubMed] [Google Scholar]
- 101.Yamashita Y, Harada M, Sawada T, Takahashi M, Miyazaki K, Okamura H. Normal uterus and FIGO stage I endometrial carcinoma: dynamic gadolinium-enhanced MR imaging. Radiology. 1993;186(2):495–501. doi: 10.1148/radiology.186.2.8421757. [DOI] [PubMed] [Google Scholar]
- 102.Park SB, Moon MH, Sung CK, Oh S, Lee YH. Dynamic contrast-enhanced MR imaging of endometrial cancer: optimizing the imaging delay for tumour-myometrium contrast. European radiology. 2014;24(11):2795–9. doi: 10.1007/s00330-014-3327-2. [DOI] [PubMed] [Google Scholar]
- 103.Tamai K, Koyama T, Saga T, et al. Diffusion-weighted MR imaging of uterine endometrial cancer. Journal of magnetic resonance imaging : JMRI. 2007;26(3):682–7. doi: 10.1002/jmri.20997. [DOI] [PubMed] [Google Scholar]
- 104.Fujii S, Matsusue E, Kigawa J, et al. Diagnostic accuracy of the apparent diffusion coefficient in differentiating benign from malignant uterine endometrial cavity lesions: initial results. European radiology. 2008;18(2):384–9. doi: 10.1007/s00330-007-0769-9. [DOI] [PubMed] [Google Scholar]
- 105.Lin G, Ng KK, Chang CJ, et al. Myometrial invasion in endometrial cancer: diagnostic accuracy of diffusion-weighted 3.0-T MR imaging--initial experience. Radiology. 2009;250(3):784–92. doi: 10.1148/radiol.2503080874. [DOI] [PubMed] [Google Scholar]
- 106.Shen SH, Chiou YY, Wang JH, et al. Diffusion-weighted single-shot echo-planar imaging with parallel technique in assessment of endometrial cancer. AJR American journal of roentgenology. 2008;190(2):481–8. doi: 10.2214/AJR.07.2155. [DOI] [PubMed] [Google Scholar]
- 107.Fujii S, Kido A, Baba T, et al. Subendometrial enhancement and peritumoral enhancement for assessing endometrial cancer on dynamic contrast enhanced MR imaging. European journal of radiology. 2015;84(4):581–9. doi: 10.1016/j.ejrad.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 108.Beddy P, Moyle P, Kataoka M, et al. Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology. 2012;262(2):530–7. doi: 10.1148/radiol.11110984. [DOI] [PubMed] [Google Scholar]
- 109.Rechichi G, Galimberti S, Signorelli M, et al. Endometrial cancer: correlation of apparent diffusion coefficient with tumor grade, depth of myometrial invasion, and presence of lymph node metastases. AJR American journal of roentgenology. 2011;197(1):256–62. doi: 10.2214/AJR.10.5584. [DOI] [PubMed] [Google Scholar]
- 110.Utsunomiya D, Notsute S, Hayashida Y, et al. Endometrial carcinoma in adenomyosis: assessment of myometrial invasion on T2-weighted spin-echo and gadolinium-enhanced T1-weighted images. AJR American journal of roentgenology. 2004;182(2):399–404. doi: 10.2214/ajr.182.2.1820399. [DOI] [PubMed] [Google Scholar]
- 111.Turan T, Hizli D, Yilmaz SS, et al. What is the impact of cervical invasion on lymph node metastasis in patients with stage IIIC endometrial cancer? Arch Gynecol Obstet. 2012;285(4):1119–24. doi: 10.1007/s00404-011-2030-7. [DOI] [PubMed] [Google Scholar]
- 112.Tewari KS, Filiaci VL, Spirtos NM, et al. Association of number of positive nodes and cervical stroma invasion with outcome of advanced endometrial cancer treated with chemotherapy or whole abdominal irradiation: a Gynecologic Oncology Group study. Gynecologic oncology. 2012;125(1):87–93. doi: 10.1016/j.ygyno.2011.12.414. [DOI] [PubMed] [Google Scholar]
- 113.Seki H, Takano T, Sakai K. Value of dynamic MR imaging in assessing endometrial carcinoma involvement of the cervix. AJR American journal of roentgenology. 2000;175(1):171–6. doi: 10.2214/ajr.175.1.1750171. [DOI] [PubMed] [Google Scholar]
- 114.Lin G, Huang YT, Chao A, et al. Endometrial cancer with cervical stromal invasion: diagnostic accuracy of diffusion-weighted and dynamic contrast enhanced MR imaging at 3T. European radiology. 2016 doi: 10.1007/s00330-016-4583-0. [DOI] [PubMed] [Google Scholar]
- 115.Castro IM, Connell PP, Waggoner S, Rotmensch J, Mundt AJ. Synchronous ovarian and endometrial malignancies. American journal of clinical oncology. 2000;23(5):521–5. doi: 10.1097/00000421-200010000-00018. [DOI] [PubMed] [Google Scholar]
- 116.Gemer O, Bergman M, Segal S. Ovarian metastasis in women with clinical stage I endometrial carcinoma. Acta obstetricia et gynecologica Scandinavica. 2004;83(2):208–10. doi: 10.1111/j.0001-6349.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- 117.Bese T, Sal V, Kahramanoglu I, et al. Synchronous Primary Cancers of the Endometrium and Ovary With the Same Histopathologic Type Versus Endometrial Cancer With Ovarian Metastasis: A Single Institution Review of 72 Cases. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2016;26(2):394–406. doi: 10.1097/IGC.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 118.Walsh C, Holschneider C, Hoang Y, Tieu K, Karlan B, Cass I. Coexisting ovarian malignancy in young women with endometrial cancer. Obstetrics and gynecology. 2005;106(4):693–9. doi: 10.1097/01.AOG.0000172423.64995.6f. [DOI] [PubMed] [Google Scholar]
- 119.Anglesio MS, Wang YK, Maassen M, et al. Synchronous Endometrial and Ovarian Carcinomas: Evidence of Clonality. Journal of the National Cancer Institute. 2016;108(6):djv428. doi: 10.1093/jnci/djv428. [DOI] [PubMed] [Google Scholar]
- 120.Schultheis AM, Ng CK, De Filippo MR, et al. Massively Parallel Sequencing-Based Clonality Analysis of Synchronous Endometrioid Endometrial and Ovarian Carcinomas. Journal of the National Cancer Institute. 2016;108(6):djv427. doi: 10.1093/jnci/djv427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Willmott F, Allouni KA, Rockall A. Radiological manifestations of metastasis to the ovary. J Clin Pathol. 2012;65(7):585–90. doi: 10.1136/jclinpath-2011-200390. [DOI] [PubMed] [Google Scholar]
- 122.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. Journal of the National Cancer Institute. 2008;100(23):1707–16. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 123.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–36. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luomaranta A, Leminen A, Loukovaara M. Magnetic resonance imaging in the assessment of high-risk features of endometrial carcinoma: a meta-analysis. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2015;25(5):837–42. doi: 10.1097/IGC.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 125.Howlader NNA, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: [November 7, 2016]. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 126.Hermans AJ, Kluivers KB, Janssen LM, et al. Adnexal masses in children, adolescents and women of reproductive age in the Netherlands: A nationwide population-based cohort study. Gynecologic oncology. 2016;143(1):93–7. doi: 10.1016/j.ygyno.2016.07.096. [DOI] [PubMed] [Google Scholar]
- 127.Quirk JT, Natarajan N, Mettlin CJ. Age-specific ovarian cancer incidence rate patterns in the United States. Gynecologic oncology. 2005;99(1):248–50. doi: 10.1016/j.ygyno.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 128.Gershenson DM. Treatment of ovarian cancer in young women. Clinical obstetrics and gynecology. 2012;55(1):65–74. doi: 10.1097/GRF.0b013e318248045b. [DOI] [PubMed] [Google Scholar]
- 129.Colombo N, Peiretti M, Garbi A, Carinelli S, Marini C, Sessa C. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii20–6. doi: 10.1093/annonc/mds223. [DOI] [PubMed] [Google Scholar]
- 130.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. [April 27, 2017];Ovarian Cancer Version 1.2017. https://www.nccn.org/professionals/physician_gls/PDF/ovarian.pdf.
- 131.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 132.Colombo N, Parma G, Zanagnolo V, Insinga A. Management of ovarian stromal cell tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(20):2944–51. doi: 10.1200/JCO.2007.11.1005. [DOI] [PubMed] [Google Scholar]
- 133.Satoh T, Hatae M, Watanabe Y, et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: a proposal for patient selection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(10):1727–32. doi: 10.1200/JCO.2009.24.8617. [DOI] [PubMed] [Google Scholar]
- 134.Shaaban AM, Rezvani M, Elsayes KM, et al. Ovarian malignant germ cell tumors: cellular classification and clinical and imaging features. Radiographics. 2014;34(3):777–801. doi: 10.1148/rg.343130067. [DOI] [PubMed] [Google Scholar]
- 135.Mangili G, Sigismondi C, Gadducci A, et al. Outcome and risk factors for recurrence in malignant ovarian germ cell tumors: a MITO-9 retrospective study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2011;21(8):1414–21. doi: 10.1097/IGC.0b013e3182236582. [DOI] [PubMed] [Google Scholar]
- 136.Heo SH, Kim JW, Shin SS, et al. Review of ovarian tumors in children and adolescents: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America Inc. 2014;34(7):2039–55. doi: 10.1148/rg.347130144. [DOI] [PubMed] [Google Scholar]
- 137.Brown J, Sood AK, Deavers MT, Milojevic L, Gershenson DM. Patterns of metastasis in sex cord-stromal tumors of the ovary: can routine staging lymphadenectomy be omitted? Gynecologic oncology. 2009;113(1):86–90. doi: 10.1016/j.ygyno.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 138.Kennedy AW, Hart WR. Ovarian papillary serous tumors of low malignant potential (serous borderline tumors). A long-term follow-up study, including patients with microinvasion, lymph node metastasis, and transformation to invasive serous carcinoma. Cancer. 1996;78(2):278–86. doi: 10.1002/(SICI)1097-0142(19960715)78:2<278::AID-CNCR14>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 139.Morice P, Uzan C, Fauvet R, Gouy S, Duvillard P, Darai E. Borderline ovarian tumour: pathological diagnostic dilemma and risk factors for invasive or lethal recurrence. The Lancet Oncology. 2012;13(3):e103–15. doi: 10.1016/S1470-2045(11)70288-1. [DOI] [PubMed] [Google Scholar]
- 140.Vasconcelos I, de Sousa Mendes M. Conservative surgery in ovarian borderline tumours: a meta-analysis with emphasis on recurrence risk. European journal of cancer (Oxford, England : 1990) 2015;51(5):620–31. doi: 10.1016/j.ejca.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 141.Darai E, Fauvet R, Uzan C, Gouy S, Duvillard P, Morice P. Fertility and borderline ovarian tumor: a systematic review of conservative management, risk of recurrence and alternative options. Human reproduction update. 2013;19(2):151–66. doi: 10.1093/humupd/dms047. [DOI] [PubMed] [Google Scholar]
- 142.Uzan C, Muller E, Kane A, et al. Prognostic factors for recurrence after conservative treatment in a series of 119 patients with stage I serous borderline tumors of the ovary. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2014;25(1):166–71. doi: 10.1093/annonc/mdt430. [DOI] [PubMed] [Google Scholar]
- 143.Palomba S, Falbo A, Del Negro S, et al. Ultra-conservative fertility-sparing strategy for bilateral borderline ovarian tumours: an 11-year follow-up. Human reproduction (Oxford, England) 2010;25(8):1966–72. doi: 10.1093/humrep/deq159. [DOI] [PubMed] [Google Scholar]
- 144.Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Siriaunkgul S. Mucinous tumor of low malignant potential ("borderline" or "atypical proliferative" tumor) of the ovary: a study of 171 cases with the assessment of intraepithelial carcinoma and microinvasion. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2011;30(3):218–30. doi: 10.1097/PGP.0b013e3181fcf01a. [DOI] [PubMed] [Google Scholar]
- 145.Kinkel K, Hricak H, Lu Y, Tsuda K, Filly RA. US characterization of ovarian masses: a meta-analysis. Radiology. 2000;217(3):803–11. doi: 10.1148/radiology.217.3.r00dc20803. [DOI] [PubMed] [Google Scholar]
- 146.Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ (Clinical research ed) 2010;341:c6839. doi: 10.1136/bmj.c6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Van Calster B, Timmerman D, Valentin L, et al. Triaging women with ovarian masses for surgery: observational diagnostic study to compare RCOG guidelines with an International Ovarian Tumour Analysis (IOTA) group protocol. BJOG : an international journal of obstetrics and gynaecology. 2012;119(6):662–71. doi: 10.1111/j.1471-0528.2012.03297.x. [DOI] [PubMed] [Google Scholar]
- 148.Van Calster B, Van Hoorde K, Froyman W, et al. Practical guidance for applying the ADNEX model from the IOTA group to discriminate between different subtypes of adnexal tumors. Facts Views Vis Obgyn. 2015;7(1):32–41. [PMC free article] [PubMed] [Google Scholar]
- 149.Sohaib SA, Mills TD, Sahdev A, et al. The role of magnetic resonance imaging and ultrasound in patients with adnexal masses. Clinical radiology. 2005;60(3):340–8. doi: 10.1016/j.crad.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 150.Sohaib SA, Sahdev A, Van Trappen P, Jacobs IJ, Reznek RH. Characterization of adnexal mass lesions on MR imaging. AJR American journal of roentgenology. 2003;180(5):1297–304. doi: 10.2214/ajr.180.5.1801297. [DOI] [PubMed] [Google Scholar]
- 151.Hricak H, Chen M, Coakley FV, et al. Complex adnexal masses: detection and characterization with MR imaging--multivariate analysis. Radiology. 2000;214(1):39–46. doi: 10.1148/radiology.214.1.r00ja3939. [DOI] [PubMed] [Google Scholar]
- 152.Zhao SH, Qiang JW, Zhang GF, et al. MRI appearances of ovarian serous borderline tumor: pathological correlation. Journal of magnetic resonance imaging : JMRI. 2014;40(1):151–6. doi: 10.1002/jmri.24339. [DOI] [PubMed] [Google Scholar]
- 153.Zhao SH, Qiang JW, Zhang GF, et al. Diffusion-weighted MR imaging for differentiating borderline from malignant epithelial tumours of the ovary: pathological correlation. European radiology. 2014;24(9):2292–9. doi: 10.1007/s00330-014-3236-4. [DOI] [PubMed] [Google Scholar]