Abstract
Interferon consensus sequence–binding protein (Icsbp) is required for terminating emergency granulopoiesis, an episodic event responsible for granulocyte production in response to infections and a key component of the innate immune response. Icsbp inhibits the expression of Stat3 and C/ebpβ, transcription factors essential for initiating and sustaining granulopoiesis, and activates transcription of Fanconi C (FANCC), a DNA repair protein. In prior studies, we noted accelerated bone marrow failure in Fancc−/− mice undergoing multiple episodes of emergency granulopoiesis, associated with apoptosis of bone marrow cells with unrepaired DNA damage. Additionally, we found increased expression of Fanconi C and F proteins during emergency granulopoiesis. These findings suggest that Icsbp protects the bone marrow from DNA damage by increasing activity of the Fanconi DNA repair pathway, but the mechanisms for FANCC activation during initiation of emergency granulopoiesis are unclear. In this study, we observed that Stat3 and C/ebpβ activate FANCC transcription and contribute to DNA repair. Our findings indicate that FancC expression is increased during Stat3- and C/ebpβ-induced initiation of emergency granulopoiesis by these transcription factors and is maintained through termination by Icsbp. Our work reveals that Stat3- and C/ebpβ-mediated FancC expression is a critical component for initiating and sustaining key innate immune responses.
Keywords: innate immunity, STAT transcription factor, DNA repair, CCAAT-enhancer-binding protein (C/EBP), stress response, Stat3
Introduction
The Fanconi DNA repair pathway rescues collapsed or stalled replication forks during S phase of the cell cycle, protects chromatin common fragile sites during DNA replication, and effects repair DNA interstrand cross-links (1–6). Fanconi proteins are categorized in three groups: core components, substrates, and effectors. Assembly of the core components (Fanconi A, B, C, E, F, L, M) into a complex with ubiquitin ligase activity is the first step of activating the Fanconi pathway. This complex activates the substrate components (Fanconi D2 and I) through monoubiquitination. These substrate proteins recruit effectors to sites of DNA damage (Fanconi D1, J, O, P, and Rad51) (1, 3). Fanconi anemia (FA)3 is an inherited disorder because of mutation of a gene in the Fanconi repair pathway. FA is clinically variable but classically involves skeletal abnormalities, bone marrow failure (BMF) during childhood, and progression to acute myeloid leukemia in subjects surviving BMF (1, 7, 8). In prior studies, we found that BMF and/or clonal progression were accelerated in a murine model of FA (Fancc−/− mice) by repeated stimulation of an emergency (stress) granulopoiesis response (9).
Emergency granulopoiesis is an episodic process for the production of granulocytes in response to infectious challenge and a key component of the innate immune response (10, 11). In contrast, steady-state granulopoiesis is a continuous process for replacement of granulocytes lost to normal programmed cell death. Studies using murine models with gene disruptions demonstrated that emergency granulopoiesis requires IL1β and IL1β-induced expression of G-CSF at levels that are 10-fold greater than the steady state (11, 12). In additional murine gene disruption studies, Stat3 and C/ebpβ were found to be necessary for initiation and maintenance of the emergency granulopoiesis response (13, 14). Also, such studies determined that termination of emergency granulopoiesis requires the leukemia suppressor Icsbp (also known as interferon regulatory factor 8 (Irf8)) and the proto-oncogene HoxA10 (15, 16). In contrast, steady-state granulopoiesis requires Stat5 and C/ebpα and is facilitated by G-CSF and GM-CSF (12).
In prior studies, we found that Icsbp activated FANCC and FANCF gene transcription during emergency granulopoiesis (encoding Fanconi C and F, respectively (FancC and FancF)) (9, 17). This was associated with Icsbp-dependent protection of bone marrow cells from DNA damage during in vivo stimulation of emergency granulopoiesis in mice or ex vivo stimulation of bone marrow cells with IL1β or G-CSF (9, 17). Also, we determined that Icsbp is required for decreased expression of Stat3 and C/ebpβ during termination of the emergency granulopoiesis response (15).
Emergency granulopoiesis is studied in mice by intraperitoneal (i.p.) injection with pathogens (Candida albicans or encapsulated bacteria) or an antigen/adjuvant mixture referred to as “Alum” (ovalbumin antigen and aluminum hydroxide adjuvant) (9, 11, 15, 18). In WT mice, this results in immediate release of mature granulocytes from the bone marrow, followed by enhanced commitment of hematopoietic stem cells to granulocytes, with maximal expansion of granulocyte/monocyte progenitors and differentiating granulocytes in the bone marrow by 2 weeks (9, 15). By 4 weeks, the process has terminated and steady state resumes.
We found that injecting Alum every 4 weeks repeated this process in WT mice without resulting in morbidity or mortality (9, 15). We performed repeated induction of emergency granulopoiesis to mimic repeated infectious challenges because of environmental exposure to bacterial and fungal pathogens, which occur on an ongoing basis in humans.
Although Alum injection induced immediate granulocyte release from the bone marrow of Fancc−/− mice, there was no subsequent expansion of myeloid progenitors or production of mature granulocytes (9). Instead, repeated emergency granulopoiesis attempts resulted in progressive pancytopenia and death in most Fancc−/− mice, associated with apoptosis of bone marrow hematopoietic stem cells and progenitors (9). Surviving mice developed clonal progression with a rapid rise of myeloid blasts in the bone marrow (9).
These studies suggest that Icsbp protects the bone marrow from DNA-damage as emergency granulopoiesis is terminating by increasing the activity of the Fanconi DNA repair pathway (9, 15, 17). However, the mechanisms to increase FANCC transcription during initiation of emergency granulopoiesis or at peak granulocyte production are not clear.
In this work, we investigate the roles of Stat3 and C/ebpβ in activation of the FANCC gene promoter. We determine that Stat3 and C/ebpβ increase FANCC transcription during initiation of emergency granulopoiesis, that Icsbp cooperates with these two transcription factors for maximal FANCC transcription during peak granulocyte production, and that Icsbp maintains FANCC expression as Stat3 and C/ebpβ levels fall during termination of this process. We also implicate a sustained increase in Stat3 and C/ebpβ proteins as the major mechanism for this activity during initiation and peak emergency granulopoiesis.
Results
Stat3 and C/ebpβ activate separate FANCC promoter cis elements
In prior studies, we identified an Icsbp binding cis element in the proximal FANCC promoter (−48 to −56 bp) (9). We hypothesize that Icsbp protects bone marrow stem and progenitor cells from DNA damage during termination of emergency granulopoiesis. Because Stat3 and C/ebpβ are required to initiate and sustain emergency granulopoiesis, we considered the possibility that these transcription factors activate the FANCC promoter early in this process.
To investigate this hypothesis, we assayed the promoter activity of a set of reporter constructs with −1.0 kb to −400 bp of the FANCC 5′ flank linked to a firefly luciferase reporter (or an empty control reporter vector) (Fig. 1A). We determined the activity of these constructs in U937 myeloid cells co-transfected with vectors to express Stat3 or C/ebpβ (versus an empty control expression vector). For these studies, we considered two isoforms of C/ebpβ: Lap and Lip. The former is a transcriptional activator, and the shorter Lip isoform is an antagonist of Lap (19). Because Stat3 and Stat5 share similar binding site consensus sequences, we tested both proteins. Icsbp was a positive control for activation of the FANCC promoter. All cells were co-transfected with an internal control reporter vector (with a CMV promoter and Renilla luciferase reporter) to normalize for transfection efficiency.
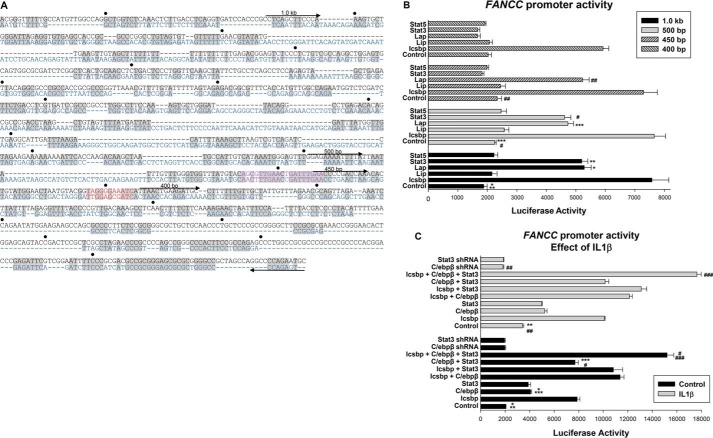
Figure 1.
C/ebpβ and Stat3 activate the FANCC promoter in myeloid cells. A, alignment of the human and murine FANCC promoters identified conserved consensus sequences for Stat and C/ebp binding. The human sequence is shown in black and the murine in blue. Conserved regions are indicated in gray. The tandem Stat consensus is shown in purple and the C/ebp consensus in red. Truncations for reporter assays are indicated. B, Stat3 and C/ebpβ-Lap activate discrete regions of the FANCC promoter, but C/ebpβ-Lip and Stat5 do not influence FANCC promoter activity. U937 cells transfected with a 1-kb FANCC promoter construct or truncated derivatives thereof were co-transfected with DNA plasmids driving the expression of each of the three different transcription factors indicated. Reporter constructs with truncations of the FANCC promoter were assayed for the effect of overexpressed Stat3, Stat5, C/ebpβ (Lap or Lip isoform), Icsbp (positive control), or empty vector. Statistically significant differences are indicated by *, **, ***, #, or ## (p < 0.01, n = 6 for all comparisons). C, Stat3, C/ebpβ-Lap, and Icsbp are not redundant for FANCC promoter activation. The 1.0 kb of FANCC promoter/luciferase reporter construct was assayed in U937 transfectants for the effect of combinations of overexpressed Stat3, C/ebpβ (Lap), or Icsbp or shRNA knockdown of Stat3 or C/ebpβ. Some transfectants were differentiated with IL1β prior to analysis. Statistically significant differences are indicated by *, **, ***, #, or ## (p < 0.01, n = 6 for all comparisons).
We found that Stat3, C/ebpβ-Lap, and Icsbp each significantly increased the reporter activity from constructs with 1.0 kb or 500 bp of the FANCC promoter (p < 0.001, n = 6) (Fig. 1B). Both Icsbp and C/ebpβ-Lap activated a construct with 450 bp of the FANCC promoter (p < 0.001, n = 6) but Stat3 did not. This suggested the presence of a Stat3-influenced cis element between 450 and 500 bp in the FANCC promoter.
Further truncation of the promoter to 400 bp abolished activation by C/ebpβ-Lap, identifying a potential cis element between −450 and −400 bp (Fig. 1B). None of the FANCC promoter constructs were activated or repressed by Stat5 or C/ebpβ-Lip. None of these proteins influenced the activity of an empty reporter control vector, and this was subtracted as background.
Stat3 and C/ebpβ are required for emergency granulopoiesis, and IL1β is the essential cytokine for this process. Therefore, we tested the effect of Stat3 and C/ebpβ on FANCC promoter activity during differentiation with IL1β (11). We assayed the activity of the 1.0 kb FANCC promoter/luciferase reporter construct in U937 cells co-transfected with vectors to overexpress or knock down Stat3 or C/ebpβ-Lap. We found that IL1β significantly increased FANCC promoter activity with or without overexpression of Stat3 or C/ebpβ-Lap (p < 0.0001, n = 6) (Fig. 1C). We also found that knockdown of either Stat3 or C/ebpβ significantly decreased FANCC promoter activity (p < 0.0001, n = 6), but only in IL1β-treated transfectants. This was consistent with a role for endogenous Stat3 and C/ebpβ in FANCC promoter activation during IL1β-induced differentiation.
We investigated possible additive or cooperative effects of these transcription factors on the FANCC promoter despite interacting with discrete binding sites. For these studies, we co-transfected U937 cells with the 1.0 FANCC promoter/firefly luciferase reporter vector and various combinations of vectors to overexpress Stat3, C/ebpβ-Lap, or Icsbp. Cells were co-transfected with the internal control reporter vector described above. We found that combination of any two of these transcription factors was additive for FANCC promoter activity (p < 0.001, n = 6), and the effect of all three was greater than any two (p < 0.001, n = 6) (Fig. 1C). The total amount of expression vector was maintained at a constant level in these studies (e.g. there is half the amount of Stat3 plasmid in Stat3 + C/ebpβ experiments compared with experiments with Stat3 alone). Therefore, these studies indicated cooperation, rather than redundancy, between the three transcription factors.
Stat3 and C/ebpβ bind to and activate discrete FANCC promoter cis elements
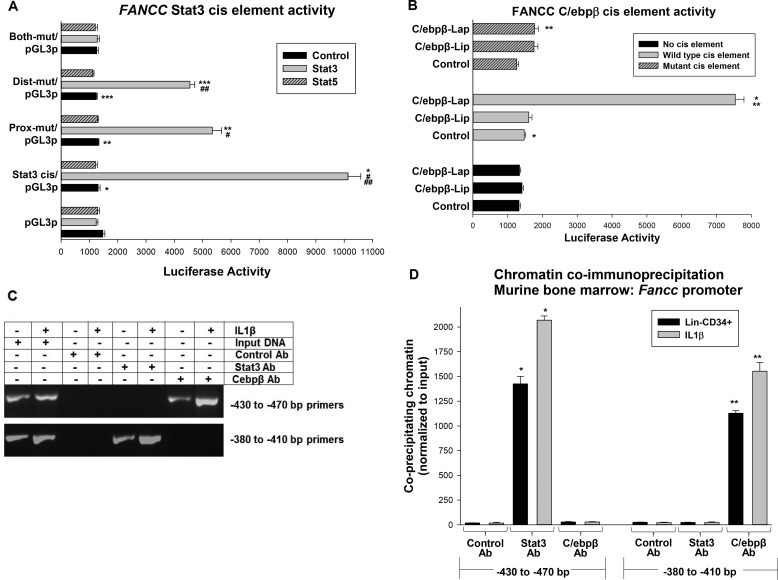
To confirm that Stat3 and C/ebpβ activated cis elements within the promoter regions defined above, we performed a sequence analysis (using Vista Tools for Comparative Software) (20, 21). We identified a tandem Stat consensus sequence from −442 to −464 bp in the FANCC promoter (separated by 2 bp). These sites were conserved in mouse and human but slightly divergent from the derived consensus for Stat3 binding (consensus, 5′-T(T/G)N4–5GAA-3′; proximal FANCC site, 5′-TGATTTGAA-3′; distal site, 5′-AGCGTTGAA-3′) (23, 24). We generated a reporter construct with one copy of this tandem site linked to a minimal promoter (−438 to −474 bp from the FANCC promoter in the pGL3-promoter vector). Additional constructs were generated with mutation of the proximal, distal, or both putative Stat3 binding sites. Reporter activity was assayed in U937 cells co-transfected with vectors to express Stat3, Stat5, or an empty control vector (and a reporter vector to function as an internal control as described above).
We found that Stat3 significantly increased the activity of the −438 to −474 bp FANCC/minimal promoter construct (p < 0.0001, n = 6) (Fig. 2A). Mutation of either consensus significantly decreased this activity (p < 0.001, n = 6), but activation by Stat3 was completely abolished by mutation of both (p = 0.6, n = 6 relative to minimal promoter/reporter control) (Fig. 2A). Stat5 had no effect on the −438 to −474 bp construct, and none of these proteins influenced the empty minimal promoter/luciferase reporter vector (Fig. 2A).
Figure 2.
Stat3 and C/ebpβ-Lap interact with discrete FANCC promoter cis elements. A, Stat3 activates tandem cis elements in the FANCC promoter. U937 cells were transfected with a minimal promoter/luciferase reporter construct (designated pGL3p) with −438 to −474 bp of FANCC 5′ flank or constructs with mutation in one or both of the Stat binding consensus sequences (or the minimal promoter/luciferase reporter control vector). Cells were co-transfected with vectors to overexpress Stat3 or Stat5 (versus control vector). Statistically significant differences are indicated by *, **, ***, #, or ## (p < 0.01, n = 6 for all comparisons). B, C/ebpβ-Lap activates a cis element in the FANCC promoter but C/ebpβ-Lip does not. U937 cells were transfected with a minimal promoter/luciferase reporter construct with three copies of the −385 to −408 bp of FANCC 5′ flank or a construct with mutation of the C/ebp consensus sequence (or the minimal promoter/luciferase reporter control vector). Cells were co-transfected with vectors to overexpress the Lap or Lip forms of C/ebpβ (versus the control vector). Statistically significant differences are indicated by * or ** (p < 0.001, n = 6 for all comparisons). C, Stat3 and C/ebpβ bind to the FANCC promoter. Murine bone marrow myeloid progenitor cells were analyzed by chromatin immunoprecipitation with antibodies to Stat3 or C/ebpβ or irrelevant control antibody. Some cells were differentiated for 24 h with IL1β prior to analysis. Co-precipitating chromatin was amplified by PCR with primers flanking the cis elements activated by Stat3 or C/ebpβ and separated by acrylamide gel electrophoresis. D, IL1β increases Stat3 or C/ebpβ binding to FANCC promoter cis elements. Some co-immunoprecipitated chromatin was analyzed by quantitative real-time PCR (data are presented using the standard curve method). Statistically significant differences are indicated by * or ** (p < 0.01, n = 4 for all comparisons).
We identified a potential C/ebp binding site between −392 and −403 in the FANCC promoter (consensus, 5′-T[TG]NNGNNAA[TG]-3′; FANCC sequence, 5′-TAGGGGAAATC-3′) (26). We generated a minimal promoter/reporter construct with three copies of the −385 to −408 bp FANCC promoter sequence. An additional construct was generated with mutation of this consensus. Reporter activity was assayed in U937 cells co-transfected with vectors to express C/ebpβ-Lap, C/ebpβ-Lip, or control vector (and the internal control reporter vector described above). C/ebpβ-Lap increased the activity of the −385 to −408 bp FANCC sequence significantly (p < 0.0001, n = 6) (Fig. 2B). C/ebpβ-Lip did not activate this FANCC sequence, and neither protein activated the minimal promoter/reporter control vector.
We investigated binding of Stat3 and C/ebpβ to these FANCC promoter regions by chromatin co-immunoprecipitation. For these studies, lysates of Lin−CD34+ murine bone marrow cells were immunoprecipitated with antibodies to Stat3, C/ebpβ, or an irrelevant control antibody (27). Some cells were differentiated with IL1β for 24 h prior to cross-linking and lysis. Co-precipitating chromatin was amplified by semiquantitative PCR (Fig. 2C) or quantitative real-time PCR (using SYBR Green and the standard curve method) (Fig. 2D).
We found specific co-precipitation of the −380 to −410 bp sequence with C/ebpβ and of the −430 to −470 bp sequence by Stat3 (Fig. 2C). Co-precipitation of both proteins was significantly increased by IL1β. Neither protein co-precipitated the irrelevant 5′ flank sequence (data not shown).
IL1β increased expression of FancC, Stat3, and C/ebpβ in murine bone marrow cells
Reporter assays in cell lines provide information regarding promoter activity but should be interpreted carefully because of the transformed nature of these cells. To investigate FancC expression in a non-transformed setting, and possible contributions by Stat3 or C/ebpβ, we performed studies in primary myeloid progenitor cells from murine bone marrow. For these studies, Lin−CD34+ cells were isolated, and some cells were differentiated with IL1β or G-CSF. The amount of G-CSF employed was consistent with serum levels in emergency granulopoiesis (9). Total cellular RNA was analyzed for gene expression by quantitative real-time PCR. The standard curve method was used, so data are presented as mRNA abundance according to this technique.
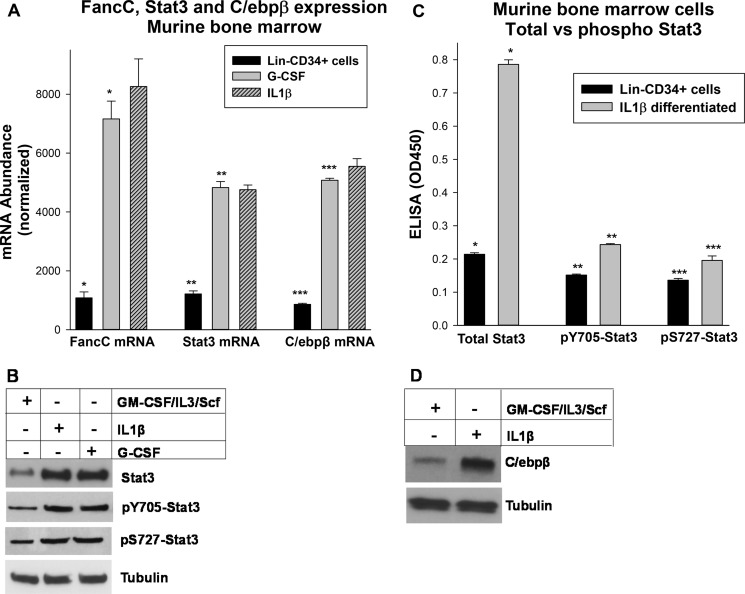
We found significantly increased FancC mRNA in response to treatment with either cytokine (p < 0.001, n = 4) (Fig. 3A). We also found a significant cytokine-induced increase in Stat3 and C/ebpβ mRNA in these cells (p < 0.001, n = 4) (Fig. 3A). The relative increase in expression of Stat3 and C/ebpβ in response to either IL1β or G-CSF was not significantly different (p = 0.2, n = 4).
Figure 3.
Expression of Stat3, C/ebpβ, and FancC increased IL1β- or G-CSF–differentiated cells. A, differentiation of murine bone marrow myeloid progenitor cells with IL1β or G-CSF increased Stat3, C/ebpβ, and FancC mRNA. Lin−CD34+ murine bone marrow cells were isolated, and some cells were differentiated with IL1β or G-CSF for 48 h prior to analysis. RNA expression was analyzed by real-time PCR. Statistically significant differences are indicated by *, **, or *** (p < 0.001, n = 6 for all comparisons). B and C, expression of total and phospho-Stat3 protein was increased by IL1β-differentiation. Lin−CD34+ cells were isolated from bone marrow mononuclear cells from the femora of mice. Some cells were differentiated with IL1β for 48 h prior to analysis. Cell lysates were analyzed by Western blot (B) or ELISA (C) for total Stat3, Tyr(P)-705–Stat3, or Ser(P)-727–Stat3. Statistically significant differences are indicated by *, **, or *** (p < 0.001, n = 6 for all comparisons). D, C/ebpβ-Lap protein was increased by IL1β differentiation of these cells. Cell lysates were also analyzed by Western blots serially probed with antibodies to C/ebpβ or tubulin (as a loading control). Representative blots are shown.
We next investigated the impact on Stat3 protein. For these studies, cells were analyzed with or without IL1β or G-CSF treatment for total Stat3, Tyr(P)-705–Stat3, or Ser(P)-727–Stat3 by Western blot. We found that either cytokine increased total and phospho-Stat3 protein (Fig. 3B).
To quantify these results, we performed an ELISA for total Stat3, Tyr(P)-705–Stat3, or Ser(P)-727–Stat3. We found that IL1β also significantly increased total Stat3 protein in this assay (p < 0.001, n = 3) (Fig. 3C). Although phosphotyrosine or phosphoserine Stat3 increased, the relative increase was significantly less than in total Stat3 protein (4-fold increase versus ∼50% increase) (Fig. 3C).
We similarly investigated the impact of emergency granulopoiesis on C/ebpβ protein (by Western blot). We found that IL1β increased C/ebpβ expression, consistent with our mRNA studies (Fig. 3D). In this experiment, only the C/ebpβ-Lap form was detected (35 kDa), not the smaller Lip form (∼20 kDa).
Abundance of Stat3 protein influenced FANCC promoter activity
We also investigated the roles of tyrosine or serine phosphorylation of Stat3 on activation of the FANCC cis element. For these experiments, we co-transfected U937 cells with a vector to express Y705F-Stat3 or S727A-Stat3 and the −482 to −517 bp FANCC minimal promoter/reporter vector (or control minimal promoter/reporter vector). Cells were also co-transfected with the internal control reporter vector as described above. Phosphorylation of Tyr-705 enhances transcriptional activation of some target genes by Stat3 (23). Phosphorylation of Ser-727 was found to either enhance or inhibit this effect in a context-dependent manner (25).
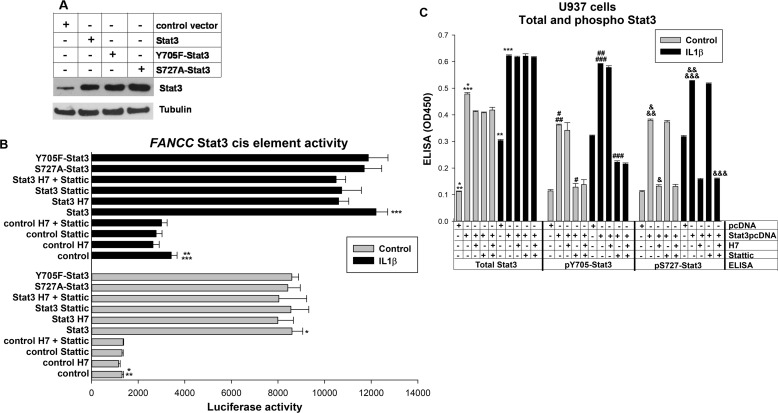
In initial studies, we performed Western blots to verify overexpression of these forms of Stat3 in U937 cells. We found that WT, Y705F, or S727A Stat3 were equivalently expressed under the assay conditions (Fig. 4A).
Figure 4.
Stat3 protein abundance contributes to FANCC promoter activation. A, Stat3, Y705F-Stat3, and S727A-Stat3 are equivalently overexpressed in U937 cells. U937 cells were transfected with vectors to overexpress various Stat proteins. Protein expression was determined by Western blots serially probed with antibodies for Stat3, Tyr(P)-705–Stat3, Ser(P)-727–Stat3, or tubulin (as a loading control). A representative blot is shown. B, increased abundance of Stat3 protein increases the activity of the FANCC promoter Stat3-binding cis element. U937 cells were transfected with a minimal promoter/luciferase reporter construct with −438 to −474 bp of FANCC 5′ flank (or the minimal promoter/luciferase reporter control vector). Cells were co-transfected with vectors to overexpress Stat3, S727A-Stat3, or Y705F-Stat3 (versus control vector). Some transfectants were treated with a serine kinase inhibitor (H7), a tyrosine kinase inhibitor (Stattic), or both, and some were differentiated with IL1β prior to analysis. Statistically significant differences are indicated by *, **, or *** (p < 0.01, n = 6 for all comparisons). C, H7 or Stattic alter the Stat3 phosphorylation state but not protein abundance. U937 cells were transfected and assayed under the conditions described for reporter gene assays. Total, Tyr(P)-705–Stat3, or Ser(P)-727–Stat3 were analyzed by ELISA. Statistically significant differences are indicated by *, **, ***, #, ##, ###, &, &&, or &&& (p < 0.01, n = 6 for all comparisons).
We also found that this FANCC cis element was equivalently activated by tyrosine mutant, serine mutant, or WT Stat3 (p ≥ 0.2, n = 6) (Fig. 4B). We found that IL1β increased the activity of the FANCC cis element with or without Stat3 overexpression, consistent with the effect of increased endogenous Stat3 in IL1β-treated cells.
We further investigated the impact of Stat3 phosphorylation on FANCC cis element activity in assays using an inhibitor of Stat3 serine phosphorylation (H7), tyrosine phosphorylation (Stattic), or both. We found that neither inhibitor altered the effect of overexpressed Stat3 on the FANCC cis element, individually or in combination (p > 0.1, n = 6 for all comparisons) (Fig. 4B). We verified the effect of these inhibitors on Stat3 in control ELISA experiments (Fig. 4C). None of these forms of overexpressed Stat3, nor treatment with any of the inhibitors, influenced the activity of the control minimal promoter-reporter vector (data not shown).
Phased expression of Stat3, C/ebpβ, and Icsbp sustains FancC expression during emergency granulopoiesis
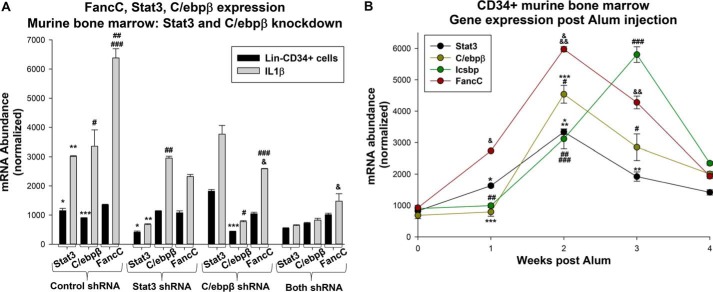
We also investigated the impact of endogenous Stat3 or C/ebpβ on FancC expression in differentiating murine bone marrow cells. For these studies, Lin−CD34+ cells were transduced with retroviral vectors to express shRNAs specific to Stat3, C/ebpβ, or both. We tested scrambled shRNAs for each of these proteins as controls, and none influenced the target protein or FancC mRNA, so they were combined for these experiments. Some cells were treated with IL1β, and FancC mRNA was quantified, with quantification of Stat3 and C/ebpβ expression as controls.
We found that knockdown of either protein significantly impaired FancC expression in differentiating cells (p < 0.001, n = 3) (Fig. 5A). The combined effect of knocking down both Stat3 and C/ebpβ was greater than either alone (p < 0.001, n = 3) (Fig. 5A). Because the total amount of plasmid was kept constant, the combined amount of shRNA in samples with knockdown of both Stat3 and C/ebpβ was half as much as in experiments with either alone.
Figure 5.
Stat3 and C/ebpβ influence FancC expression during emergency granulopoiesis. A, knockdown of Stat3 or C/ebpβ decreases IL1β-induced expression of Fanconi C. Murine bone marrow myeloid progenitor cells were transduced with vectors to express shRNAs specific for Stat3 or C/ebpβ (or scrambled control shRNA) and analyzed for FancC expression. Statistically significant differences are indicated by *, **, ***, #, ##, ###, or & (p < 0.01, n = 6 for all comparisons). B, expression of Stat3 and C/ebpβ increases early, Icsbp increases later, and FancC is increased throughout emergency granulopoiesis. Mice were injected with Alum (i.p.) to induce emergency granulopoiesis, and bone marrow Lin−CD34+ cells was collected 0, 1, 2, 3, or 4 weeks after injection. Gene expression was determined by quantitative real-time PCR. Statistically significant differences are indicated by *, **, ***, #, ##, ###, &, or && (p < 0.01, n = 6 for all comparisons).
Stat3, C/ebpβ, and Icsbp may activate FANCC transcription at different times during emergency granulopoiesis. Specifically, Stat3 and C/ebpβ are involved in initiating and maintaining emergency granulopoiesis, whereas Icsbp terminates this process (in part by decreasing Stat3 and C/ebpβ expression) (13–15). We investigated this hypothesis using an in vivo murine model of emergency granulopoiesis.
For these studies, we induced emergency granulopoiesis in mice by i.p. injection of Alum or saline (as a control for steady-state granulopoiesis, n = 6 mice per group) (9, 15, 18). Alum injection results in maximal expansion of myeloid progenitor cells and differentiating granulocytes in the bone marrow by 2 weeks and resumption of the steady state by 4 weeks after injection (9, 15, 18). To investigate mRNA expression of FancC, Stat3, C/ebpβ, and Icsbp at various points during this process, cohorts of mice were sacrificed 0, 1, 2, 3, and 4 weeks after Alum injection, and Lin−CD34+ bone marrow cells were analyzed.
We found significantly increased expression of Stat3 and C/ebpβ that was maximal 2 weeks after Alum injection (p < 0.0001, n = 3 relative to the steady state) and began to decrease at 3 weeks (p < 0.001, n = 3 for comparison of 1 versus 2 or 2 versus 3 weeks) (Fig. 5B). In contrast, Icsbp mRNA was maximally expressed 3 weeks after Alum injection (p < 0.001, n = 3 for comparison of 2 and 3 weeks) and was returning to steady-state levels at 4 weeks (Fig. 5B). FancC mRNA expression was significantly increased 1 week after Alum injection, maximal at 2 weeks (p < 0.0001, n = 3), and returning to baseline at 4 weeks (Fig. 5B).
Stat3 or C/ebpβ rescues DNA repair in a FancC-dependent manner
We were interested in determining the impact of Stat3 or C/ebpβ on DNA repair in cells exposed to the stress of emergency granulopoiesis. To investigate this, we used a plasmid-based DNA repair assay we employed previously to study the influence of Icsbp on this process. In this study, a reporter plasmid is treated with mitomycin C to generate DNA cross-links (with an untreated plasmid as a control) (9, 17). Damaged or undamaged reporter plasmids are transfected into U937 cells, and reporter activity represents the efficiency of DNA repair. Cells are co-transfected with a second reporter plasmid as an internal control for transduction efficiency (not MMC-treated).
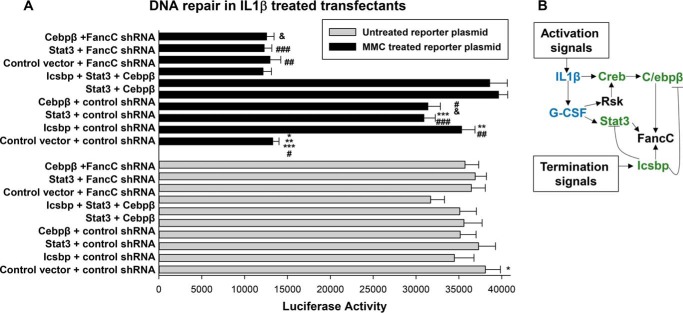
In prior studies, we determined that U937 cells efficiently repaired the MMC-treated plasmid, but treatment of U937 cells with differentiating agents (retinoic acid/dimethyl formamide or Ifnγ) significantly impaired this activity (9, 17). For this study, U937 cells were co-transfected with an MMC-treated or untreated control plasmid (CMV-firefly luciferase vector) and vectors to overexpress Icsbp, Stat3, C/ebpβ-Lap, combinations of these proteins, or empty control expression vector (with the TK-Renilla luciferase vector as a control for transfection efficiency). Transfectants were assayed after 24 h of treatment with IL1β.
We found significantly less firefly luciferase reporter activity from the MMC-treated reporter vector compared with the untreated control reporter vector in IL1β-treated transfectants (p < 0.001, n = 6) (Fig. 6A). Overexpression of Icsbp, Stat3, or C/ebpβ significantly increased the reporter activity of the MMC-treated plasmid in IL1β transfectants (p < 0.001, n = 6) but had no effect on the activity of reporter vectors that had not been MMC-treated (Fig. 6A). Assays with combinations of the three proteins demonstrated that effects were non-redundant because the total amount of expression plasmid was held constant in these experiments. In the absence of IL1β treatment of the transfectants, we found that the luciferase reporter activity from the MMC-treated plasmid was not significantly different than the activity of the untreated plasmid (data not shown), consistent with our prior studies (9, 17).
Figure 6.
Stat3 and C/ebpβ enhance DNA repair in a FancC-dependent manner in myeloid cells undergoing IL1β-induced differentiation. A, Icsbp, Stat3, and C/ebpβ rescue DNA repair during IL1β-induced differentiation of U937 cells, but this is reversed by FancC knockdown. U937 cells were co-transfected with a mitomycin C (MMC) cross-linked luciferase reporter plasmid (or untreated control luciferase reporter plasmid) or vectors to express Icsbp, Stat3, or C/ebpβ alone or in combination (or control vector). Other cells were transfected with vectors to express Icsbp, Stat3, or C/ebpβ and express FancC-specific shRNAs (or scrambled control shRNA). Some transfectants were treated for 24 h with IL1β before reporter activity was determined. Statistically significant differences are indicated by *, **, ***, #, or ## (p < 0.001, n = 6 for all comparisons). B, schematic of the regulation of FancC expression by Icsbp, Stat3, and C/ebpβ during emergency granulopoiesis. Cross-regulation of these transcription factors is also indicated.
We were interested in determining whether the effects of Icsbp, Stat3, or C/ebpβ on the activity of the MMC-damaged reporter plasmid required FancC expression. To examine this, we co-transfected U937 cells with MMC-treated or an untreated reporter plasmid; vectors to overexpress Icsbp, Stat3, or C/ebpβ; vectors to express shRNAs specific to FancC (or scrambled shRNA control); and a plasmid to control for transfection activity (as above). Transfectants were assayed after 24-h treatment with IL1β.
We found that knockdown of FancC prevented Icsbp, Stat3, or C/ebpβ overexpression from rescuing the reporter activity of the MMC-treated plasmid (Fig. 4A). These results suggested that these transcription factors were acting through a FancC-dependent mechanism to drive DNA cross-link repair.
Discussion
Emergency granulopoiesis is a high-risk/high-gain response to infectious challenge. During this process, rapid granulocyte production requires increased proliferation and faster differentiation of bone marrow stem and progenitor cells, resulting in genotoxic stress. This risk is further enhanced by the apoptosis resistance and cell cycle shortening that occur during this process (9, 15). In this study, we found Stat3 and C/ebpβ, essential transcription factors for initiating and sustaining emergency granulopoiesis, are involved in protecting the genome by increasing the expression of Fanconi C.
We also found that Stat3, C/ebpβ, and Icsbp are non-redundant for activation of the FANCC promoter and that IL1β increases FANCC promoter activity and enhances the effects of these transcription factors. Also, our study demonstrates that IL1β increases binding of Stat3 and C/ebpβ to their respective FANCC cis elements, perhaps because of the ability of IL1β to increase the expression of Stat3 and C/ebpβ mRNA and protein. We found that IL1β treatment decreases the ability of U937 myeloid cells to repair DNA cross-links and that DNA cross-link repair is rescued by Stat3 or C/ebpβ in a FancC-dependent manner, similar to our prior results with Icsbp and FancC (9).
Our studies suggest that different expression levels of Stat3, C/ebpβ, and Icsbp at various times during emergency granulopoiesis ensure sustained FancC expression throughout this process. At initiation of emergency granulopoiesis, Stat3 expression increases, followed by expression of C/ebpβ, correlating with increased FancC expression. All three transcription factors are expressed at peak granulocyte production during Alum-stimulated emergency granulopoiesis, correlating with maximal FancC expression and consistent with maximal protection from DNA damage at this point in the process.
We found that expression of FancC was still increased, relative to the steady state, 3 weeks after initiation of emergency granulopoiesis, despite decreasing Stat3 and C/ebpβ at this time. However, Icsbp expression was persistently elevated at this point. In prior studies, we found that emergency granulopoiesis failed to terminate in Icsbp−/− mice, associated with increased and sustained Stat3 and C/ebpβ expression (15). This implicated Icsbp in resetting Stat3 and C/ebpβ to steady-state levels. These results determined that Icsbp antagonizes FancC expression by decreasing Stat3 and C/ebpβ but compensates for this effect by activating the FANCC promoter until resumption of the steady state (Fig. 5B) (15, 22).
We found that Stat3, C/ebpβ, and Icsbp each activate different FANCC promoter cis elements. We previously found that activation of the FANCC promoter by Icsbp was enhanced by tyrosine phosphorylation of this protein during emergency granulopoiesis, identifying roles for enhanced expression and posttranslational modification in this process (9). In this study, we found that neither tyrosine nor serine phosphorylation of Stat3 enhances FANCC promoter activation, suggesting that protein expression is driving function. This is in contrast to some other Stat3 target genes, where tyrosine phosphorylation enhances transcriptional activation (23). The role of Ser(P)-727 in Stat3 function is controversial and may be context-dependent (25).
In addition to regulating FANCC transcription, Icsbp also enhances calpain activity through repression of the growth-specific arrest 2 (GAS2) gene, a calpain inhibitor (28). Because Stat3 is a calpain substrate, Icsbp may influence both Stat3 mRNA expression and Stat3 protein stability during termination of emergency granulopoiesis (29). Understanding cooperation versus antagonism between Icsbp and Stat3 is of interest to understand the innate immune response and a focus of ongoing investigations in the laboratory.
The Stat3-binding FANCC cis element identified in this work has a tandem binding consensus sequence, and we found that both copies were required for maximal cis element activity. This is consistent with interaction of Stat3 as a homodimer with such tandem binding sites in a number of pro-inflammatory genes (30).
Although this cis element was activated by Stat3, we found no effect of Stat5 on the FANCC promoter. Conversely, we found previously that Stat5 represses the distal IRF8 promoter in myeloid progenitor cells (31), but Stat3 had no effect. We also determined previously that Icsbp regulates Stat5 protein stability (through Gas2/calpain) but does not influence Stat5 mRNA (31). Therefore, these two Stat proteins play discrete roles during myelopoiesis and are differentially regulated by Icsbp.
We found that the C/ebpβ Lap isoform activated a cis element in the FANCC promoter but that the Lip isoform did not. These isoforms were originally described in regenerating liver cells, and Lip (liver inhibitory protein) antagonized the effects of Lap (liver activating protein) in these cells (19). In our study, we found that Lap was the dominant C/ebpβ isoform in myeloid cell lines and differentiating murine bone marrow progenitors. Overexpression of Lip did not repress FANCC promoter activity in myeloid cell line transfectants, but we also did not find an increase in Lip during termination of emergency granulopoiesis. This suggests that other mechanisms, such as general repression of CEBPB transcription by Icsbp-dependent events, may regulate C/ebpβ activity during emergency granulopoiesis.
Increased expression of Stat3 and C/ebpβ is found in chronic myeloid leukemia (CML) (32, 33). The function of these transcription factors in leukemogenesis may be consistent with their normal roles in expanding myeloid progenitor populations during emergency granulopoiesis. In contrast, Icsbp is a leukemia suppressor for CML, with decreased expression in this disease (34, 35). Therefore, regulation of emergency granulopoiesis may represent a paradigm for leukemia promotion versus suppression.
It is additionally possible that episodes of emergency granulopoiesis facilitate leukemogenesis under conditions with decreased Icsbp or enhanced expression of Stat3 or C/ebpβ. All three proteins enhance FancC expression, which would be anticipated to protect cells from DNA damage. However, we found significantly more FANCC promoter activity and FancC expression in the presence of all three transcription factors compared with Stat3 and C/ebpβ without Icsbp. It would be of interest to determine whether repeated episodes of emergency granulopoiesis enhance drug resistance or progression to blast crisis in CML. Studies are currently being performed in the laboratory to address this issue.
Experimental procedures
Protein expression vectors
The Icsbp/Irf8 cDNA was obtained from Dr. Ben Zion-Levi (Technion, Haifa, Israel) and subcloned into the mammalian expression vector pcDNA (Stratagene, La Jolla, CA), as described previously (36). Wildtype and Y705F mutant murine Stat3 cDNAs and C/ebpβ Lap and Lip cDNAs were obtained from Addgene and subcloned into the pcDNA (for expression in myeloid cell lines) and MSCV (for generation of retrovirus) vectors. FancC-specific shRNAs (and scrambled control shRNAs) were generated using the Promega website and subcloned into the pLKO retroviral vector.
Reporter constructs
The human FANCC 5′ flank (1.0 kb from the ATG codon) was generated by PCR from the U937 myeloid cell line. The genomic clone was sequenced to ensure identity with the sequence in the ENSEMBL database (37). This sequence and additional truncations (−500, −450, or −400 bp) were subcloned into the pGL3-basic reporter vector (Promega, expressing the firefly luciferase reporter gene). Other constructs were generated with one copy of the −387 to −403 bp FANCC promoter or with three copies of the −470 to −530 bp FANCC promoter subcloned into a minimal promoter-reporter vector (pGL3-promoter vector) (Promega, with the TK minimal promoter expressing firefly luciferase reporter gene). Some of the −387 to −403 bp FANCC promoter/minimal promoter/reporter constructs had mutation of the proximal, distal, or both Stat consensus binding sequences.
Myeloid cell line culture
The human myelomonocytic leukemia cell line U937 (38) was obtained from Andrew Kraft (University of Arizona, Tucson, AZ). Cells were maintained as described previously (38).
Transfections and reporter gene assays
U937 cells were transfected with FANCC promoter/luciferase reporter constructs (or empty control reporter vector) and various combinations of vectors to overexpress Stat3, C/ebpβ, or Icsbp (or empty expression vector) or specific shRNAs to knock down Stat3 or C/ebpβ (or scrambled shRNA control vectors). Cells were also co-transfected with an internal control plasmid to normalize for transfection efficiency (Promega Dual-Luciferase system, a CMV-luciferase reporter vector expressing Renilla luciferase). Transfectants were assayed for luciferase activity according to the instructions of the manufacturer. Some transfectants were treated with IL1β (50 ng/ml for 24 h) prior to harvesting.
Luciferase activity from control empty reporter vectors was not influenced by IL1β treatment or overexpression or knockdown of any of these proteins and was subtracted as background. All reporter assays were repeated six times in independent experiments (and samples were assayed in duplicate) for each condition.
The efficacy of IL1β differentiation of U937 cells was verified for various batches of cytokines used in these studies by determining enhanced FANCC promoter activity in transfection assays or expression of endogenous mRNA for FancC and gp91phox in cells treated with the cytokine. Some transfectants were studied after treatment for 24 h with H7 (a serine kinase inhibitor for Stat3 (40)) or Stattic (a specific inhibitor of Stat3 tyrosine phosphorylation (41)).
Mitomycin C treatment of plasmids and DNA repair assays
To generate DNA cross-links, purified plasmid DNA (CMV-firefly luciferase from Promega) was incubated with mitomycin C (40 μm) for 12 h at room temperature. Plasmid DNA was recovered by phenol:chloroform extraction followed by ethanol precipitation. DNA cross-linking was verified by non-denaturing agarose gel electrophoresis (17).
U937 cells were co-transfected with cross-linked or untreated control reporter plasmid, vectors to overexpress Icsbp, Stat3, or C/ebpβ (or control expression plasmid), and vectors expressing specific shRNAs to knock down FancC (or scrambled control). Cells were also transfected with the TK-Renilla luciferase vector as an internal control for transfection efficiency. Lysates were analyzed and simultaneously assayed for dual luciferase activity as described above. Luciferase reporter activities were determined after 24-h treatment with IL1β. Reporter assays were repeated six times in duplicate as described above.
Western blot and ELISA of lysate proteins
For Western blots, cells were lysed by boiling in 2× SDS sample buffer. Lysate proteins (50 μg) were separated by SDS-PAGE (10% acrylamide) and transferred to nitrocellulose, and filters were serially probed with antibodies as described previously (39). Each experiment was repeated at least three times with different sets of lysates, and a representative blot is shown.
In other experiments, total, serine-phosphorylated, or tyrosine-phosphorylated Stat3 proteins in cell lysates were quantified by commercially available ELISA (Abcam, Cambridge, MA). ELISAs were performed in duplicate on three independent sets of lysates, and the results were graphed as A450 (according to the instructions of the manufacturer).
Chromatin immunoprecipitation
Cells were incubated briefly in medium supplemented with formaldehyde, and lysates were sonicated to generate chromatin fragments with an average size of 500 bp and immunoprecipitated with Stat3, C/ebpβ, or irrelevant control antibody (Abcam) (26). Chromatin was amplified by quantitative real-time PCR using SYBR Green and the standard curve method (Thermo Fisher Scientific) according to the instructions of the manufacturer). Primers were designed flanking the Stat3 or C/ebp consensus sequences in the FANCC promoter. The standard curve was generated using total chromatin from murine bone marrow cells. Input chromatin (not precipitated) from each sample was analyzed to normalize data between the samples. At least three independent immunoprecipitation experiments were performed, and the samples were analyzed in triplicate.
Quantitative real-time PCR
RNA was isolated using TRIzol reagent (Gibco-BRL, Gaithersburg, MD) and tested for integrity by denaturing gel electrophoresis. Primers were designed with Applied Biosystems software, and real-time PCR was performed using SYBR Green and the standard curve method. The standard curve for these experiments was generated with cDNA from WT cells cultured in GM-CSF, IL3, and stem cell factor. The results were normalized to 18S and actin and presented as mRNA abundance with 1 ng of cDNA set as 1000 in the standard curve. At least three independent samples were evaluated in triplicate.
Animal use
The mice used for this study were C57 Black 6 and maintained in an approved and accredited animal facility at Northwestern University. The mice were housed in a specific pathogen-free, tightly regulated environment that included control of the flow of animals, equipment, and personnel and use of micro-isolator cages and husbandry procedures to minimize pathogen exposure and disease outbreak. All work was reviewed and approved by the Animal Care and Use Committees of Jesse Brown Veterans Affairs Medical Center and Northwestern University.
In vitro murine studies
For in vitro studies, bone marrow mononuclear cells were harvested by flushing femora repeatedly with Hanks' balanced salt solution until no additional cells were obtained. Washed cells were treated with ammonium–chloride–potassium buffer to lyse red blood cells and then washed extensively. Lin−CD34+ cells were separated using a magnetic bead–based, affinity chromatography–based technique according to the instructions of the manufacturer (Miltenyi Biotech, San Diego, CA). Cells were cultured (2 × 105/ml) for 48 h in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 1% penicillin-streptomycin, 10 ng/ml murine GM-CSF (R&D Systems Inc., Minneapolis, MN), 10 ng/ml murine recombinant IL3 (R&D Systems Inc.), and 100 ng/ml of stem cell factor (R&D Systems Inc.). Cells were maintained in GM-CSF, IL3, and stem cell factor for 24 h or stimulated with 50 ng/ml G-CSF (R&D Systems Inc.) or 20 ng/ml IL1β (R&D Systems Inc.) during this time period. Apoptotic cells were removed before analysis according to the instructions of the manufacturer (Miltenyi, Dead Cell Clean Up). Some cells were transduced with retroviral vectors prior to analysis according to techniques described in our prior work (9).
In vivo murine emergency granulopoiesis assay
WT mice (18–20 weeks of age) were injected i.p. with Alum or saline control (12 mice per group). Mice were randomly assigned to cohorts for injection of Alum or saline control. Alum was prepared as described previously (9, 15, 18), and a volume of 0.5 ml was injected.
Cohorts of mice (6 per group) were sacrificed weekly, and bone marrow was collected from both femora. Successful induction of emergency granulopoiesis in Alum-injected mice (compared with saline control) was verified by weekly peripheral blood granulocytes counts (using a Hemavet automated cell counter; Drew Scientific, Miami Lakes, FL). Blood count data were analyzed by an investigator who was blinded to the status of the mice as Alum versus saline injected.
Statistical analysis
Statistical significance was determined by unpaired two-tailed Student's t test (comparing two conditions) or analysis of variance (for more than two conditions) using SigmaPlot software. p < 0.02 was considered statistically significant. In all graphs, error bars represent ± S.E.
Author contributions
C. A. S., L. Broglie, L. H., L. Bei, W. H., and D. B. D. investigation; C. A. S., L. H., W. H., and E. A. E. methodology; W. H. and E. A. E. conceptualization; E. A. E. formal analysis; E. A. E. supervision; E. A. E. funding acquisition; E. A. E. project administration.
This work was supported by Department of Veterans Affairs Merit Review Grant BX002067; NIDDK, National Institutes of Health Grant R01-DK098812; and NCI, National Institutes of Health Grant R01-CA174205 (to E. A. E.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- FA
- Fanconi anemia
- BMF
- bone marrow failure
- IL
- interleukin
- GM-CSF
- granulocyte/macrophage colony-stimulating factor
- i.p.
- intraperitoneal(ly)
- CMV
- cytomegalovirus
- shRNA
- short hairpin RNA
- MMC
- mitomycin C
- CML
- chronic myeloid leukemia
- TK
- thymidine kinase
- G-CSF
- granulocyte colony stimulating factor.
References
- 1. Moldovan G. L., and D'Andrea A. D. (2009) How the Fanconi anemia pathway guards the genome. Annu. Rev. Genet. 43, 223–249 10.1146/annurev-genet-102108-134222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kee Y., and D'Andrea A. D. (2012) Molecular pathogenesis and clinical management of Fanconi anemia. J. Clin. Invest. 122, 3799–3806 10.1172/JCI58321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson L. H., and Hinz J. M. (2009) Cellular and molecular consequences of defective Fanconi anemia proteins in replication-coupled DNA repair: mechanistic insights. Mutat. Res. 668, 54–72 10.1016/j.mrfmmm.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang L. C., Stone S., Hoatlin M. E., and Gautier J. (2008) Fanconi anemia proteins stabilize replication forks. DNA Repair 7, 1973–1981 10.1016/j.dnarep.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arlt M. F., Durkin S. G., Ragland R. L., and Glover T. W. (2006) Common fragile sites as targets for chromosome rearrangements. DNA Repair (Amst.) 25, 1126–1135 10.1016/j.dnarep.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 6. Howlett N. G., Taniguchi T., Durkin S. G., D'Andrea A. D., and Glover T. W. (2005) The Fanconi anemia pathway is required for the DNA replication stress response and for the regulation of common fragile site stability. Hum. Mol. Genet. 14, 693–701 10.1093/hmg/ddi065 [DOI] [PubMed] [Google Scholar]
- 7. Auerbach A. D., and Allen R. G. (1991) Leukemia and pre-leukemia in Fanconi anemia patients. Cancer Genet. Cytogenet. 51, 1–12 10.1016/0165-4608(91)90002-C [DOI] [PubMed] [Google Scholar]
- 8. Cioc A. M., Wagner J. E., MacMillan M. L., DeFor T., and Hirsch B. (2010) Diagnosis of myelodysplastic syndrome among a cohort of 119 patients with Fanconi anemia. Am. J. Clin. Pathol. 133, 92–100 10.1309/AJCP7W9VMJENZOVG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu L., Huang W., Hjort E., and Eklund E. A. (2013) Increased Fanconi C expression contributes to the emergency granulopoiesis response. J. Clin. Invest. 123, 3952–3966 10.1172/JCI69032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lord B. I., Molineux G., Pojda Z., Souza L. M., Mermod J. J., and Dexter T. M. (1991) Myeloid cell kinetics in mice treated with recombinant interleukin-3, granulocyte colony-stimulating factor (CSF), or granulocyte-macrophage CSF in vivo. Blood 77, 2154–2159 [PubMed] [Google Scholar]
- 11. Ueda Y., Cain D. W., Kuraoka M., Kondo M., and Kelsoe G. (2009) IL1R type I dependent hematopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and neutrophilia. J. Immunol. 182, 6477–6484 10.4049/jimmunol.0803961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panopoulos A. D., and Watowich S. S. (2008) Granulocyte colony stimulating factor: molecular mechanisms of activation during steady state and emergency hematopoiesis. Cytokine 42, 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirai H., Zhang P., Dayaram T., Hetherington C. J., Mizuno S., Imanishi J., Akashi K., and Tenen D. G. (2006) C/EBPβ is required for “emergency” granulopoiesis. Nat. Immunol. 7, 732–739 10.1038/ni1354 [DOI] [PubMed] [Google Scholar]
- 14. Panopoulos A. D., Zhang L., Snow J. W., Jones D. M., Smith A. M., El Kasmi K. C., Liu F., Goldsmith M. A., Link D. C., Murray P. J., and Watowich S. S. (2006) Stat3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood 108, 3682–3690 10.1182/blood-2006-02-003012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu L., Huang W., Hjort E. E., Bei L., Platanias L. C., and Eklund E. A. (2016) Interferon consensus sequence binding protein is required to terminate emergency granulopoiesis. J. Biol. Chem. 291, 4107–4120 10.1074/jbc.M115.681361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H., Bei L., Shah C. A., Hu L., and Eklund E. A. (2015) HoxA10 terminates emergency granulopoiesis by increasing expression of Triad1. J. Immunol. 194, 5375–5387 10.4049/jimmunol.1401909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saberwal G., Horvath E., Hu L., Zhu C., Hjort E., and Eklund E. A. (2009) The interferon consensus sequence binding protein (ICSBP/IRF8) activates transcription of the FANCF gene during myeloid differentiation. J. Biol. Chem. 284, 33242–33254 10.1074/jbc.M109.010231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kool M., Pétrilli V., De Smedt T., Rolaz A., Hammad H., van Nimwegen M., Bergen I. M., Castillo R., Lambrecht B. N., and Tschopp J. (2008) Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 181, 3755–3759 10.4049/jimmunol.181.6.3755 [DOI] [PubMed] [Google Scholar]
- 19. Luedde T., Duderstadt M., Streetz K. L., Tacke F., Kubicka S., Manns M. P., and Trautwein C. (2004) C/EBP β isoforms LIP and LAP modulate progression of the cell cycle in the regenerating mouse liver. Hepatology 40, 356–365 10.1002/hep.20333 [DOI] [PubMed] [Google Scholar]
- 20. Frazer K. A., Pachter L., Poliakov A., Rubin E. M., and Dubchak I. (2004) VISTA: computational tools for comparative genomics. Nucleic Acids Res. 32, W273–9 10.1093/nar/gkh458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dubchak I., Brudno M., Loots G. G., Mayor C., Pachter L., Rubin E. M., and Frazer K. A. (2000) Active conservation of noncoding sequences revealed by 3-way species comparisons. Genome Res. 10, 1304–1306 10.1101/gr.142200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Resende C., Regalo G., Durães C., Pinto M. T., Wen X., Figueiredo C., Carneiro F., and Machado J. C. (2016) Interleukin-1B signaling leads to increased survival of gastric carcinoma cells through a CREB-C/EBPβ-associated mechanism. Gastric Cancer 19, 74–84 10.1007/s10120-014-0448-x [DOI] [PubMed] [Google Scholar]
- 23. Horvath C. M., Wen Z., and Darnell J. E. Jr, (1995) A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 9, 984–994 10.1101/gad.9.8.984 [DOI] [PubMed] [Google Scholar]
- 24. Ehret G. B., Reichenbach P., Schindler U., Horvath C. M., Fritz S., Nabholz M., and Bucher P. (2001) DNA binding specificity of different STAT proteins: comparison of in vitro specificity with natural target sites. J. Biol. Chem. 276, 6675–6688 10.1074/jbc.M001748200 [DOI] [PubMed] [Google Scholar]
- 25. Decker T., and Kovarik P. (2000) Serine phosphorylation of STATs. Oncogene 19, 2628–2637 10.1038/sj.onc.1203481 [DOI] [PubMed] [Google Scholar]
- 26. Osada S., Yamamoto H., Nishihara T., and Imagawa M. (1996) DNA binding specificity of the CCAAT/enhancer-binding protein transcription factor family. J. Biol. Chem. 271, 3891–3896 10.1074/jbc.271.7.3891 [DOI] [PubMed] [Google Scholar]
- 27. Weinmann A. S., and Farnham P. J. (2002) Identification of unknown target genes of human transcription factors using chromatin immunoprecipitation. Methods 26, 37–47 10.1016/S1046-2023(02)00006-3 [DOI] [PubMed] [Google Scholar]
- 28. Huang W., Zhou W., Saberwal G., Konieczna I., Horvath E., Katsoulidis E., Platanias L. C., and Eklund E. A. (2010) The interferon consensus sequence binding protein (ICSBP) decreases βcatenin-activity in myeloid cells by repressing GAS2 transcription. Mol. Cell Biol. 30, 4575–4594 10.1128/MCB.01595-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oda A., Wakao H., and Fujita H. (2002) Calpain is a signal transducer and activator of transcription (STAT) 3 and STAT5 protease. Blood 99, 1850–1852 10.1182/blood.V99.5.1850 [DOI] [PubMed] [Google Scholar]
- 30. Kisseleva T., Bhattacharya S., Braunstein J., and Schindler C. W. (2002) Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 285, 1–24 10.1016/S0378-1119(02)00398-0 [DOI] [PubMed] [Google Scholar]
- 31. Hjort E. E., Huang W., Hu L., and Eklund E. A. (2016) Bcr-abl regulates Stat5 through Shp2, the interferon consensus sequence binding protein (Icsbp/Irf8), growth arrest specific 2 (Gas2) and calpain. Oncotarget 7, 77635–77650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coppo P., Dusanter-Fourt I., Millot G., Nogueira M. M., Dugray A., Bonnet M. L., Mitjavila-Garcia M. T., Le Pesteur D., Guilhot F., Vainchenker W., Sainteny F., and Turhan A. G. (2003) Constitutive and specific activation of STAT3 by BCR-ABL in embryonic stem cells. Oncogene 22, 4102–4110 10.1038/sj.onc.1206607 [DOI] [PubMed] [Google Scholar]
- 33. Hayashi Y., Hirai H., Kamio N., Yao H., Yoshioka S., Miura Y., Ashihara E., Fujiyama Y., Tenen D. G., and Maekawa T. (2013) C/EBPβ promotes BCR-ABL-mediated myeloid expansion and leukemic stem cell exhaustion. Leukemia 27, 619–628 10.1038/leu.2012.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmidt M., Nagel S., Proba J., Thiede C., Ritter M., Waring J. F., Rosenbauer F., Huhn D., Wittig B., Horak I., and Neubauer A. (1998) Lack of interferon consensus sequence binding protein (ICSBP) transcripts in human myeloid leukemias. Blood 91, 22–29 [PubMed] [Google Scholar]
- 35. Hao S. X., and Ren R. (2000) Expression of interferon consensus sequence binding protein (ICSBP) is downregulated in Bcr-Abl-induced murine chronic myelogenous leukemia-like disease, and forced coexpression of ICSBP inhibits Bcr-Abl-induced myeloproliferative disorder. Mol. Cell. Biol. 20, 1149–1161 10.1128/MCB.20.4.1149-1161.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eklund E. A., Jalava A., and Kakar R. (1998) PU.1, interferon regulatory factor 1, and interferon consensus sequence binding protein cooperate to increase gp91PHOX expression. J. Biol. Chem. 273, 13957–13965 10.1074/jbc.273.22.13957 [DOI] [PubMed] [Google Scholar]
- 37. Yates A., Akanni W., Amode M. R., Barrell D., Billis K., Carvalho-Silva D., Cummins C., Clapham P., Fitzgerald S., Gil L., Girón C. G., Gordon L., Hourlier T., Hunt S. E., Janacek S. H., et al. (2016) Ensembl 2016. Nucleic Acids Res. 44, D710–6 10.1093/nar/gkv1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larrick J. W., Fischer D. G., Anderson S. J., and Koren H. S. (1980) Characterization of a human macrophage-like cell line stimulated to differentiate in vitro: a model of macrophage functions. J. Immunol. 125, 6–12 [PubMed] [Google Scholar]
- 39. Konieczna I., Horvath E., Wang H., Lindsey S., Saberwal G., Bei L., Huang W., Platanias L., and Eklund E. A. (2008) Constitutive activation of SHP2 cooperates with ICSBP-deficiency to accelerate progression to acute myeloid leukemia. J. Clin. Invest. 118, 853–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abe K., Hirai M., Mizuno K., Higashi N., Sekimoto T., Miki T., Hirano T., and Nakajima K. (2001) The YXXQ motif in gp 130 is crucial for STAT3 phosphorylation at Ser727 through an H7-sensitive kinase pathway. Oncogene 20, 3464–3474 10.1038/sj.onc.1204461 [DOI] [PubMed] [Google Scholar]
- 41. Schust J., Sperl B., Hollis A., Mayer T. U., and Berg T. (2006) Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 13, 1235–1242 10.1016/j.chembiol.2006.09.018 [DOI] [PubMed] [Google Scholar]