Abstract
Maresin 1 (MAR1), which is derived from docosahexaenoic acid biosynthesized by macrophages, has been reported to improve insulin resistance. Recently, it has been documented that MAR1 could ameliorate inflammation and insulin resistance in obese mice. These findings led us to investigate the effects of MAR1 on hepatic lipid metabolism. We found that MAR1 could stimulate AMP-activated protein kinase (AMPK), thereby augmenting sarcoendoplasmic reticulum Ca2+-ATPase 2b (SERCA2b) expression. This stimulation suppressed lipid accumulation by attenuating the endoplasmic reticulum (ER) stress in hepatocytes under hyperlipidemic conditions. Attenuation was mitigated by knockdown of AMPK or thapsigargin, a SERCA2b inhibitor. We also demonstrated that MAR1 administration resulted in increased hepatic AMPK phosphorylation and Serca2b mRNA expression, whereas hepatic ER stress was reduced in high-fat diet (HFD)-fed mice. Moreover, MAR1 treatment suppressed hepatic lipid synthesis, thereby attenuating hepatic steatosis in HFD-fed mice. In conclusion, our results suggest that MAR1 ameliorates hepatic steatosis via AMPK/SERCA2b-mediated suppression of ER stress. Therefore, MAR1 may be an effective therapeutic strategy for treating non-alcoholic fatty liver disease (NAFLD) via regulation of ER stress-induced hepatic lipogenesis.
Keywords: AMP-activated kinase (AMPK), endoplasmic reticulum stress (ER stress), adiponectin, autophagy, fatty acid metabolism, Maresin 1, non-alcoholic fatty liver disease, sarco-endoplasmic reticulum (ER) Ca2+-ATPase 2b
Introduction
Non-alcoholic fatty liver disease (NAFLD),3 a type of fatty liver related to overnutrition, is associated with most common metabolic disorders, such as obesity, dyslipidemia, and diabetes. Maresin 1 (MAR1), a product of docosahexaenoic acid (DHA) (1), is a novel pro-resolving lipid mediator derived from omega-3. MAR1 has potent anti-inflammatory effects (2) and improves insulin resistance (3). During insulin resistance, various inflammatory cytokines, such as monocyte chemotactic protein 1 (MCP-1) (2), tumor necrosis factor α (4), and interleukin-1β (Il-1β) (5), could impair insulin signaling in various organs. However, the effect of MAR1 on high-fat diet (HFD)-induced hepatic steatosis remains elusive.
Disruption of endoplasmic reticulum (ER) homeostasis, termed as ER stress, has been detected in liver and adipose tissue of obese animal models (6) and in humans with NAFLD and/or obesity (7, 8). Ozcan et al. (6) found that animals with increased expression of chaperones and hepatic ER stress markers have a higher potential for hepatic steatosis. One possible explanation is that excessive ER stress in hepatocytes results in lipogenesis and hepatic steatosis. ER calcium disequilibrium is considered to be one of the initial and pivotal events contributing to ER stress-mediated apoptosis (9). The normal function of sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) is to reuptake Ca2+ from the cytosol into the ER lumen, whereas SERCA dysfunction results in Ca2+ release from the ER lumen, leading to alteration of ER homeostasis and subsequent ER stress (10). In mammals, three different isoforms (SERCA1–3) have at least two additional sub-isoforms. The main isoform of SERCA2 in the liver is SERCA2b (11, 12). SERCA2b overexpression attenuated thapsigargin- and tunicamycin-induced ER stress in hepatocytes (13). However, the effects of MAR1 on hepatocytes through mechanisms related to ER stress and SERCA have not been fully elucidated. AMPK-activated protein kinase (AMPK) has been shown to maintain energy homeostasis and to attenuate ER stress (14). Furthermore, 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), a specific AMPK activator, increased SERCA activity (15). Therefore, this study mainly focused on the suppression of ER stress and attenuation of NAFLD by the AMPK–SERCA-mediated pathway.
Our first goal was to investigate the effects of MAR1 on lipid metabolism and hepatic steatosis under hyperlipidemic conditions. Our second goal was to confirm the underlying mechanism of MAR1-mediated protective effects on palmitate-induced ER stress and lipid accumulation by demonstrating the AMPK–SERCA2b-dependent pathway in mouse primary hepatocytes. Finally, we wanted to evaluate the effects of MAR1 on hepatic AMPK phosphorylation, Serca2b mRNA expression, ER stress, and hepatic steatosis in high-fat diet (HFD)-fed mice in vivo.
Results
MAR1 attenuates palmitate-induced TG accumulation caused by ER stress in hepatocytes
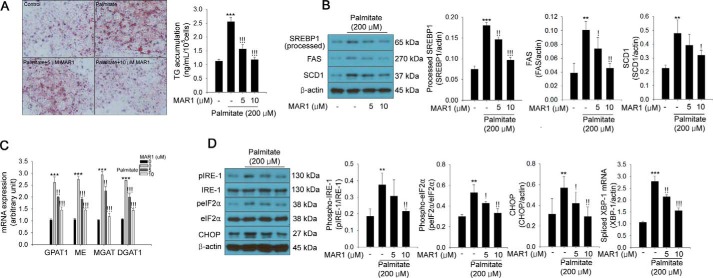
Mouse serum MAR1 levels were ∼1.5 μm 1 h after MAR1 injection (1 μg/mouse) (Fig. S1A). Therefore, we first tested the inhibitory effects of MAR1 at the concentration range of 0–10 μm on palmitate-induced TG accumulation in mouse primary hepatocytes. We found that palmitate-induced TG accumulation and expression of lipogenesis-associated genes (including SREBP1, FAS, and SCD1) were suppressed by MAR1 in a dose-dependent manner in mouse primary hepatocytes (Fig. 1, A and B). Furthermore, palmitate-induced mRNA expression of TG synthesis-associated genes (such as Gpat1, Me, Mgat, and Dgat1) was also attenuated by MAR1 in a dose-dependent manner (Fig. 1C). Because palmitate induces ER stress, thereby resulting in lipid accumulation via SREBP1-mediated signaling (16, 17), we investigated the effect of MAR1 on palmitate-induced ER stress. As shown in Fig. 1D, treatment of hepatocytes with MAR1 significantly attenuated palmitate-induced ER stress-associated gene expression in a dose-dependent manner as well (Fig. 1D).
Figure 1.
MAR1 ameliorates palmitate-induced TG accumulation and expression of lipogenic genes in primary hepatocytes. A, Oil Red-O staining in hepatocytes in the presence of 200 μm palmitate and MAR1 (0–10 μm) for 24 h. TG accumulation was quantitated by isopropyl alcohol extraction. Western blot analysis of SREBP1 (processed), FAS, and SCD1 expression (B) and quantitative RT-PCR of Gpat1, Me, Mgat, and Dgat1 mRNA expression (C) in hepatocytes in the presence of 200 μm palmitate and MAR1 (0–10 μm) are shown. D, Western blot analysis and quantitative RT-PCR of ER stress markers in hepatocytes in the presence of 200 μm palmitate and MAR1 (0–10 μm) for 24 h. Means ± S.D. were calculated from three independent experiments. One-way ANOVA with Tukey post hoc was performed. ***, p < 0.001 and **, p < 0.01 when compared with expression levels in the controls. !!!, p < 0.001, !!, p < 0.01, and !, p < 0.05 when compared with palmitate treatment.
MAR1 suppresses palmitate-induced ER stress and TG accumulation through AMPK activation
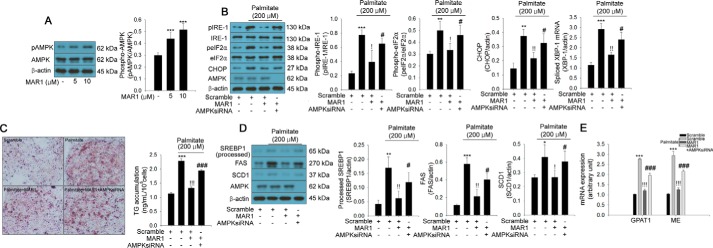
Because AMPK has been reported to attenuate ER stress and TG accumulation in hepatocytes (17), we therefore tested the effect of MAR1 on AMPK phosphorylation. Notably, MAR1 was able to induce AMPK phosphorylation in a dose-dependent manner in primary hepatocytes (Fig. 2A). Furthermore, siRNA-mediated silencing of AMPK abrogated the inhibitory effects of MAR1 on palmitate-induced ER stress and TG accumulation (Fig. 2, B–E). It was reported that G-protein-coupled receptor 120 (GPR120) has been implicated in the anti-inflammatory and insulin-sensitizing effects of omega-3 (18). Omega-3 ameliorates inflammation and ER stress through the GPR120-mediated pathway (19). Therefore, we examined whether GPR120 contributes to MAR1-mediated AMPK phosphorylation. AH7614, a selective GPR120 antagonist, abrogated the effects of MAR1 on AMPK phosphorylation (Fig. S2).
Figure 2.
AMPK contributes to the effects of MAR1 on palmitate-induced TG accumulation and ER stress. A, Western blot analysis and quantitative RT-PCR of ER stress markers in hepatocytes in the presence of 200 μm palmitate and MAR1 (0–10 μm) for 24 h. Western blot analysis of ER stress markers' phosphorylation and expression and quantitative RT-PCR of spliced Xbp-1 mRNA expression (B) and Oil Red-O staining (C) in transfected hepatocytes with sequence-scrambled siRNA or AMPK siRNA in the presence of 200 μm palmitate and MAR1 (10 μm) for 24 h are shown. TG accumulation was quantitated by a TG assay kit. Western blot analysis (D) and quantitative RT-PCR (E) of lipogenic markers in transfected hepatocytes with sequence-scrambled siRNA or AMPK siRNA in the presence of 200 μm palmitate and MAR1 (10 μm) for 24 h are shown. Means ± S.D. were calculated from three independent experiments. One-way ANOVA with Tukey post hoc was performed. ***, p < 0.001 and **, p < 0.01 when compared with expression levels in control or sequence-scrambled siRNA. !!!, p < 0.001 and !!, p < 0.01 when compared with palmitate treatment. ###, p < 0.001 and #, p < 0.05 when compared with palmitate plus MAR1 treatment.
AMPK-mediated induction of SERCA2b expression contributes to the suppressive effect of MAR1 on ER stress and TG accumulation
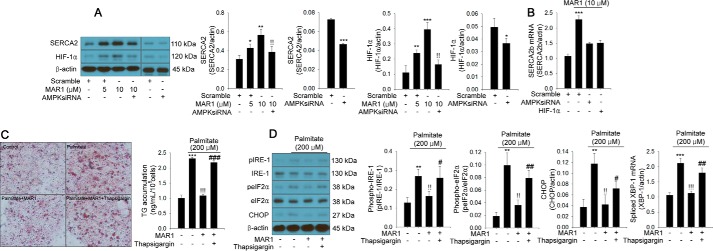
To elucidate the AMPK-mediated protective mechanism of MAR1 on ER stress, we further addressed the effects of MAR1 on the expression of SERCA2b and autophagy in primary hepatocytes. We found that SERCA2 expression was up-regulated by MAR1 treatment (Fig. 3A). Thereafter, we investigated whether MAR1-induced AMPK could contribute to MAR1-mediated up-regulation of SERCA2b expression, and we also examined whether SERCA2b is involved in the suppressive effects of MAR1 on ER stress and TG accumulation. As shown in Fig. 3A, siRNA-mediated silencing of AMPK markedly reduced the effect of MAR1 on SERCA2 expression (Fig. 3A). Hypoxia-inducible transcription factor-1α (HIF-1α) has been reported to modulate Serca2b expression (20). Therefore, we further examined the effects of AMPK on HIF-1α expression and HIF-1α on MAR1-mediated induction of SERCA2b mRNA expression. MAR1 induced HIF-1α expression in a dose-dependent manner. However, AMPK siRNA reversed this effect (Fig. 3A). siRNA-mediated suppression of AMPK and HIF-1α expression abrogated MAR1-induced Serca2b mRNA expression (Fig. 3B). Additionally, thapsigargin, a SERCA2b inhibitor and siRNA-mediated suppression of SERCA2b, significantly reduced the effects of MAR1 on palmitate-induced TG accumulation and ER stress markers (Fig. 3, C and D; Fig. S3).
Figure 3.
AMPK-mediated induction of SERCA2b expression is involved in the effects of MAR1 on palmitate-induced ER stress and TG accumulation in primary hepatocytes. Western blot analysis of SERCA2 and HIF-1α expression (A) and quantitative RT-PCR of Serca2b mRNA expression (B) in transfected hepatocytes with sequence-scrambled siRNA, AMPK siRNA, and HIF-1α siRNA in the presence of palmitate (200 μm) and MAR1 (0–10 μm) for 24 h are shown. Oil Red-O staining (C) and Western blot analysis and quantitative RT-PCR expression (D) of ER stress markers in treated hepatocytes with thapsigargin (30 nm) (26) in the presence of 200 μm palmitate and MAR1 (10 μm) for 24 h are shown. TG accumulation was quantitated by a TG assay kit. Means ± S.D. were calculated from three independent experiments. One-way ANOVA with Tukey post hoc was performed. ***, p < 0.001, **, p < 0.01, and *, p < 0.05 when compared with expression levels in control or sequence-scrambled siRNA. !!!, p < 0.001 and !!, p < 0.01 when compared with palmitate or MAR1 treatment. ###, p < 0.001, ##, p < 0.01, and #, p < 0.05 when compared with palmitate plus MAR1 treatment.
MAR1 administration stimulates phosphorylation of AMPK and SERCA2b expression, leading to attenuation of hepatic steatosis in HFD-fed mice
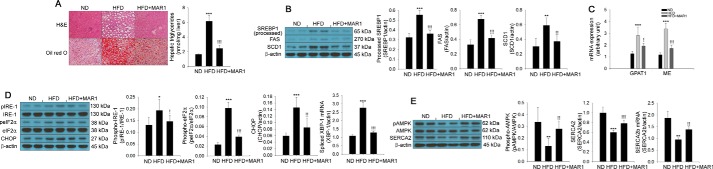
Based on the results of in vitro models, we subsequently assessed the effect of MAR1 on lipid accumulation in mice. To this end, we performed histological analysis by staining with H&E and Oil Red-O. In this mouse model, HFD treatment for 8 weeks increased hepatic TG accumulation and lipogenesis-associated gene expression in the liver. However, intraperitoneally administered MAR1 for 8 weeks markedly restored these changes (Fig. 4, A–C). Similar to the TG accumulation in the liver, hepatic ER stress was also attenuated by MAR1 administration (Fig. 4D). Furthermore, HFD induced suppression of AMPK phosphorylation; SERCA2 protein and Serca2b mRNA expression in the liver were markedly reversed by MAR1 treatment (Fig. 4E). MAR1 administration significantly reduced HFD-induced serum-free fatty acids and TG levels (Fig. S1, B and C). MAR1 treatment did not affect calorie intake, although it significantly decreased the body weight gain in the HFD (Fig. S4, A and B). Moreover, MAR1 administration markedly reduced the weight of liver and epididymal adipose tissue in HFD-fed mice (Fig. S4, C and D).
Figure 4.
MAR1 administration attenuates hepatic steatosis and both AMPK phosphorylation and SERCA2b expression. A, H&E and Oil Red-O staining on liver sections of experimental animals: ND, HFD, and HFD plus MAR1 (HFD+MAR1). TG accumulation was quantitated by a TG assay kit. Western blot analysis (B) and quantitative RT-PCR (C) of lipogenic markers are shown. ER stress markers' phosphorylation and expression and quantitative RT-PCR of spliced Xbp-1 mRNA expression (D) in liver of experimental mice are shown. Western blot analysis of AMPK phosphorylation and SERCA2 expression and quantitative RT-PCR of Serca2b mRNA expression (E) in livers of experimental mice are shown. Means ± S.D. were obtained from five individual animals. One-way ANOVA with Tukey post hoc was performed. ***, p < 0.001, **, p < 0.01, and *, p < 0.05 when compared with ND treatment. !!!, p < 0.001, !!, p < 0.01, and !, p < 0.05 when compared with the HFD.
Discussion
Disruption of ER homeostasis in the liver could contribute considerably to the alteration of lipid metabolism, thereby leading to hepatic steatosis (21) and apoptosis (22). Therefore, appropriate regulation of hepatic ER stress appears to be a promising therapeutic strategy for treating hepatic diseases, including NAFLD. To the best of our knowledge, this investigation demonstrates for the first time that MAR1 can ameliorate lipid-induced hepatic ER stress and steatosis in in vitro and in vivo models through AMPK-mediated induction of SERCA2b. First, we have shown that MAR1-induced AMPK activation protected against palmitate-induced ER stress and TG accumulation in mouse primary hepatocytes. Second, MAR1 markedly induced Serca2b mRNA expression through the AMPK-mediated pathway. Finally, MAR1 administration increased AMPK phosphorylation and SERCA2b activity, thereby attenuating HFD-induced ER stress and TG accumulation in the mouse liver.
Chronic low-grade inflammation has been associated with the development of obesity-mediated metabolic disorders. The resolution of inflammation is coordinated and regulated by a large panel of mediators (23). One of them involves maresins, specialized pro-resolving mediators (SPM) with anti-inflammatory properties derived from DHA. Specifically, SPM exert protective effects against necroinflammatory hepatic injury by suppressing hepatic COX-2 expression and reducing oxidative burden (24). MAR1 also exerts its protective effect against sepsis-induced inflammatory response via reducing serum lipopolysaccharide through inhibition of NFκB activity (25). MAR1 improves insulin sensitivity and attenuates adipose tissue inflammation in diet-induced obese mice through up-regulation of adiponectin, GLUT4, and Akt phosphorylation in white adipose tissue (3). So far, there have been no studies that were focused on the cellular effects and the underlying mechanisms of MAR1 on lipid-induced hepatic ER stress and steatosis.
Previous studies have demonstrated that disturbed ER homeostasis-induced apoptosis (27) and lipid accumulation (28) in the liver may contribute to the development of NAFLD. Furthermore, elevated ER stress has been detected in the adipose tissue and livers of patients with NAFLD (7). ER stress has been reported to be associated with obesity, hepatic lipid metabolism, insulin resistance, and diabetes (29, 30). Therefore, investigating the mechanisms that could regulate hepatic ER stress may help to develop the effective therapeutic approaches for treating NAFLD. To support this idea, obesity-related metabolic disorders, such as atherosclerosis (31), diabetes (32), and NAFLD (33), are ameliorated by ER stress suppression. In this study, we found that MAR1 significantly mitigated hyperlipidemia-induced ER stress markers (e.g. IRE-1, eIF2α, and CHOP) and TG accumulation in both in vitro and in vivo models.
Based on these results, we next investigated MAR1-mediated mechanisms by which palmitate-induced ER stress is attenuated. Activation of AMPK has been reported to be involved in the development of NAFLD (34–38). Omega-3 fatty acids, including eicosapentaenoic acid and DHA, have been reported to activate AMPK and consequently improve lipid metabolism in the liver and skeletal muscle (39, 40). In this study, we demonstrated that MAR1 markedly augmented AMPK phosphorylation. Additionally, we showed that siRNA-mediated suppression of AMPK markedly reduced the effect of MAR1 on palmitate-induced ER stress and TG accumulation in primary hepatocytes. These results suggest that AMPK plays an important role in MAR1 suppression of hepatic steatosis. However, it remains elusive whether MAR1 activates AMPK directly or indirectly in hepatocytes. Therefore, further studies are needed to identify the specific receptors for MAR1.
Altered Ca2+ homeostasis in the ER causes ER stress, which can partially attenuate disruptions to homeostasis. However, when the defense system does not respond properly, various signals can result in apoptosis, lipid accumulation, and inflammatory responses. Therefore, the potential role of ER stress in the development of NAFLD supports the therapeutic relevance of approaches that maintain Ca2+ homeostasis and attenuate ER burden to reduce ER stress. It is documented that SERCAs are essential to modulate the movement of calcium across the ER membrane. Thus, SERCAs play an important role in maintaining Ca2+ homeostasis and cell viability. Activation of ER SERCA ameliorates imbalanced calcium homeostasis during ER stress, leading to attenuation of ER stress-induced apoptosis and lipid accumulation in hepatocytes (41). In this study, we found that MAR1 stimulated SERCA expression and Serca2b mRNA expression through AMPK-dependent pathway in hepatocytes. Furthermore, thapsigargin, a SERCA2 inhibitor, abrogated the suppressive effects of MAR1 on ER stress and lipid accumulation in both in vitro and in vivo models. These results suggest that SERCA2 may contribute to the suppressive effects of MAR1-induced AMPK activation on hepatic ER stress and lipid accumulation.
Herein, MAR1 administration significantly reduced HFD-induced serum-free fatty acids and TG levels in mice. These results suggest that MAR1 may ameliorate hepatic steatosis not only through direct effects on liver but also attenuation of hyperlipidemia. We found that MAR1 administration reduced liver and epididymal adipose tissue weight and body weight, although it did not affect the calorie intake in mice. MAR1 treatment increased fatty acid oxidation-associated genes such as Cpt1 and Fabp3 mRNA expression in differentiated 3T3-L1 cells and epididymal adipose tissue of mice (Fig. S5, A and B). Furthermore, MAR1 treatment augmented the levels of serum adiponectin and fibroblast growth factor 21 (FGF21) (Fig. S5, C and D) that were reported to stimulate fatty acid oxidation in adipocytes (42, 43). These results suggest that fatty acid oxidation may contribute to MAR1-mediated protective effects on hepatic steatosis and loss of body weight. Further studies in fatty acid oxidation-associated genes in hepatocytes and adiponectin and FGF21-knockout animal models are needed to elucidate the involvement of fatty acid oxidation in MAR1-associated protective effects on hepatic steatosis and body weight loss as an additional signal transduction pathway in mice.
Conclusions
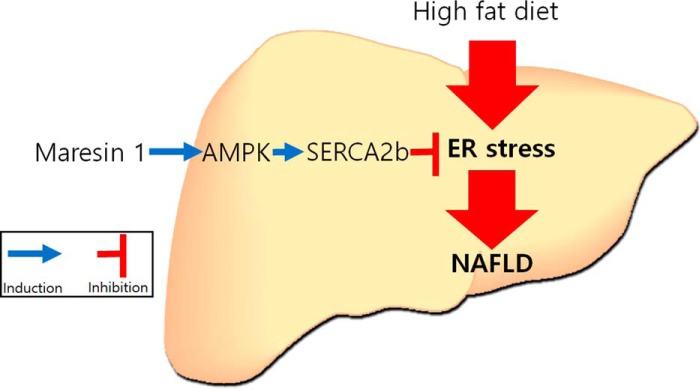
We demonstrated that MAR1 suppresses lipid-induced ER stress through AMPK-mediated induction of SERCA2b expression, thereby attenuating hepatic steatosis and lipid metabolism (Fig. 5). Our results suggest that MAR1-mediated regulation of lipid-induced ER stress through the AMPK–SERCA2b pathway is a novel effective therapeutic strategy for treating NAFLD.
Figure 5.
Schematic diagram of the effects of MAR1 on NAFLD.
Experimental procedures
Cell cultures, reagents, and antibodies
The mouse primary hepatocytes were purchased from ZenBio (Research Triangle Park, NC). Cells were cultured on collagen type-1–coated 6-well plates with Hepatocyte Plating Medium (ZenBio). After 12 h of attachment, the medium was exchanged with Hepatocyte Maintenance Medium (ZenBio) supplemented with 10% fetal bovine serum (Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C. Mycoplasma was not detected in hepatocytes. MAR1 (Cayman Chemical, Ann Arbor, MI) and thapsigargin (Sigma) were dissolved in ethanol. Sodium palmitate (Sigma) conjugated to 2% BSA (fatty acid-free grade; Sigma) was dissolved in Dulbecco's modified Eagle's medium. The final concentration of ethanol did not affect cell viability. In all experiments, cells were treated with palmitate-BSA and MAR1 for 24 h and 2% BSA/ethanol was used as a control. AH7614 (Sigma) was dissolved in dimethyl sulfoxide (DMSO). Anti-phospho-IRE-1 (1:1000), anti-IRE-1 (1:2500), anti-phospho eIF2α (1:1000), anti-eIF2α (1:1000), anti-CHOP (1:1000), anti-phospho-AMPK (1:1000), and anti-AMPK (1:2500) were purchased from Cell Signaling (Beverly, MA). Anti-SREBP1 (1:2500), anti-FAS (1:2500), anti-SCD1 (1:2500), HIF-1α (1:1000), SERCA2 (1:1000), and anti-β-actin (1:2500) were supplied from Santa Cruz Biotechnology.
Animals, feeding, and treatments
This study was approved by the Institutional Animal Care and Use Committee of Korea University, Seoul, Republic of Korea. Animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication, 8th Ed., 2011). A control (n = 5) and two experimental groups (5 animals each) of 8-week-old male C57BL/6J (B6) mice were fed a normal diet (ND; Brogaarden, Gentofte, Denmark) and a HFD (Research Diets, New Brunswick, NJ), respectively, for 8 weeks. The HFD plus MAR1 group (n = 5) was administered MAR1 intraperitoneally (35 μg/kg/day) for 8 weeks. The ND group was injected with ethanol and used as a control. Post-administration, all animals were sacrificed under anesthesia after overnight fasting (12 h).
Western blot analysis
Hepatocytes were harvested, and proteins were extracted with lysis buffer (PRO-PREP; Intron Biotechnology, Seoul, Republic of Korea) for 60 min at 4 °C. Protein samples (30 μg) were subjected to 12% SDS-PAGE and transferred to a nitrocellulose membrane (Amersham Biosciences). The membrane was probed with primary antibody followed by secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology). The samples were detected with enhanced chemiluminescence kits.
RNA extraction and quantitative real-time PCR
Total RNA from harvested hepatocytes was isolated using TRIzol reagent (Invitrogen). Gene expression was measured by quantitative real-time PCR (qPCR) using the fluorescent TaqMan 5′-nuclease assay on an Applied Biosystems 7000 sequence detection system (Foster City, CA). qPCR was performed using cDNA with 2× TaqMan Master Mix and 20× premade TaqMan gene expression assays (Applied Biosystems). qPCR conditions were 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min using PCR primers for mouse Serca2b (Applied Biosystems; Mm01201431_m1), mouse glycerol-3-phosphate acyltransferase 1 (Gpat1) (Applied Biosystems; Mm00833328_m1), mouse maleic enzyme 1 (Me) (Applied Biosystems; Mm00782380_s1), mouse (monoacylglycerol acyltransferase 1 (Mgat) (Applied Biosystems; Mm00487690_m1), mouse diacylglycerol acyltransferase 1 (Dgat1) (Applied Biosystems; Mm00515643_m1), and mouse fatty acid-binding protein 3 (Fabp3) (Applied Biosystems; Mm02342495_m1). The mRNA of carnitine palmitoyltransferase 1a (CPT1) was quantified using the following primers: 5′-CCATCCTGTCCTGACAAGGTTTAG-3′ and 5′-CCTCACTTCTGTTACAGCTAGCAC-3′. The mRNA of spliced Xbp-1 was quantified using the following primers: 5′-GCAGCAAGTGGTGGATTTGGA-3′ and 5′-CTGCACCTGCTGCGGACTCAG-3′ (44). The mRNA of β-actin was quantified as an endogenous control, using the following primers: 5′-CGATGCTCCCCGGGCTGTAT-3′ and 5′-TGGGGTACTTCAGGGTCAGG-3′.
Transient transfection for gene silencing
At 70% cell confluence, 0–20 nmol/liter small interfering (si) RNA oligonucleotides for AMPKα1/2, HIF-1α, and SERCA2 (45) were purchased from Santa Cruz Biotechnology and transfected to suppress gene expression. A sequence-scrambled siRNA was used as a control. Transfection was performed with Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's specifications.
Histological analysis
Hepatocytes and mouse liver sections were stained using the Oil Red-O method to quantify accumulated cellular neutral lipids, including TG. After fixation with 10% formalin for 40 min, hepatocytes were stained with the Oil Red-O solution (Sigma) for 1 h at 37 °C. Oil Red-O-stained TG content was quantified by adding isopropyl alcohol to each sample. The mixtures were gently agitated for 8 min at 25 °C. Finally, 100 μl of isopropyl-extracted sample was analyzed by a spectrophotometer at 510 nm.
TG measurement
Total lipids were extracted using a 2:1 chloroform/methanol (2:1, v/v) mixture. The organic layer was dried and immediately dissolved in 60% methanol. The extracted TG were measured using a colorimetric TG assay kit according to the manufacturer's directions with modification (Biovision, Milpitas, CA).
Enzyme linked immunosorbent assay (ELISA)
Mouse serum adiponectin and FGF21 were measured with each ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Statistical analysis
All statistical analyses were performed using the SPSS/PC statistical program (version 12.0 for Windows; SPSS, Chicago, IL). Results were presented as absolute values (means ± S.D.). All experiments were performed at least three times. One-way ANOVA with Tukey post hoc was used for data analysis.
Author contributions
T. W. J., H.-C. K., A. M. A., and J. H. J. conceptualization; T. W. J. resources; T. W. J. data curation; T. W. J. and H.-C. K. formal analysis; T. W. J. funding acquisition; T. W. J. and J. H. J. investigation; T. W. J. visualization; T. W. J. methodology; T. W. J., H.-C. K., and A. M. A. writing-original draft; T. W. J. project administration; A. M. A. writing-review and editing; J. H. J. validation.
Supplementary Material
This work was supported by the Basic Science Research Program through National Research Foundation of Korea Grant 2017R1D1A1B03028892 funded by the Korean Ministry of Education. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S5.
- NAFLD
- non-alcoholic fatty liver disease
- AMPK
- AMP-activated protein kinase
- SERCA
- sarco/endoplasmic reticulum Ca3+-ATPase
- DHA
- docosahexaenoic acid
- ANOVA
- analysis of variance
- ER
- endoplasmic reticulum
- HFD
- high-fat diet
- qPCR
- quantitative real-time PCR
- TG
- triglyceride
- SPM
- specialized pro-resolving mediator
- ND
- normal diet
- FAS
- fatty-acid synthase
- ME
- maleic enzyme.
References
- 1. Serhan C. N., Dalli J., Karamnov S., Choi A., Park C. K., Xu Z. Z., Ji R. R., Zhu M., and Petasis N. A. (2012) Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 26, 1755–1765 10.1096/fj.11-201442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sell H., Dietze-Schroeder D., Kaiser U., and Eckel J. (2006) Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology 147, 2458–2467 10.1210/en.2005-0969 [DOI] [PubMed] [Google Scholar]
- 3. Martínez-Fernández L., Gonznález-Muniesa P., Laiglesia L. M., Sáinz N., Prieto-Hontoria P. L., Escoté X., Odriozola L., Corrales F. J., Arbones-Mainar J. M., Martínez J. A., and Moreno-Aliaga M. J. (2017) Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J. 31, 2135–2145 10.1096/fj.201600859R [DOI] [PubMed] [Google Scholar]
- 4. Peraldi P., Hotamisligil G. S., Buurman W. A., White M. F., and Spiegelman B. M. (1996) Tumor necrosis factor (TNF)-α inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J. Biol. Chem. 271, 13018–13022 10.1074/jbc.271.22.13018 [DOI] [PubMed] [Google Scholar]
- 5. Jager J., Grémeaux T., Cormont M., Le Marchand-Brustel Y., and Tanti J. F. (2007) Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148, 241–251 10.1210/en.2006-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Görgün C., Glimcher L. H., and Hotamisligil G. S. (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 10.1126/science.1103160 [DOI] [PubMed] [Google Scholar]
- 7. Boden G., Duan X., Homko C., Molina E. J., Song W., Perez O., Cheung P., and Merali S. (2008) Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57, 2438–2444 10.2337/db08-0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma N. K., Das S. K., Mondal A. K., Hackney O. G., Chu W. S., Kern P. A., Rasouli N., Spencer H. J., Yao-Borengasser A., and Elbein S. C. (2008) Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J. Clin. Endocrinol. Metab. 93, 4532–4541 10.1210/jc.2008-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mekahli D., Bultynck G., Parys J. B., De Smedt H., and Missiaen L. (2011) Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 3, a004317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shore G. C., Papa F. R., and Oakes S. A. (2011) Signaling cell death from the endoplasmic reticulum stress response. Curr. Opin. Cell Biol. 23, 143–149 10.1016/j.ceb.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ashby M. C., and Tepikin A. V. (2001) ER calcium and the functions of intracellular organelles. Semin. Cell Dev. Biol. 12, 11–17 10.1006/scdb.2000.0212 [DOI] [PubMed] [Google Scholar]
- 12. Vangheluwe P., Raeymaekers L., Dode L., and Wuytack F. (2005) Modulating sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium 38, 291–302 10.1016/j.ceca.2005.06.033 [DOI] [PubMed] [Google Scholar]
- 13. Park S. W., Zhou Y., Lee J., Lee J., and Ozcan U. (2010) Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc. Natl. Acad. Sci. U.S.A. 107, 19320–19325 10.1073/pnas.1012044107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hardie D. G. (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8, 774–785 10.1038/nrm2249 [DOI] [PubMed] [Google Scholar]
- 15. Dong Y., Zhang M., Wang S., Liang B., Zhao Z., Liu C., Wu M., Choi H. C., Lyons T. J., and Zou M. H. (2010) Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes 59, 1386–1396 10.2337/db09-1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fang D. L., Wan Y., Shen W., Cao J., Sun Z. X., Yu H. H., Zhang Q., Cheng W. H., Chen J., and Ning B. (2013) Endoplasmic reticulum stress leads to lipid accumulation through upregulation of SREBP-1c in normal hepatic and hepatoma cells. Mol. Cell. Biochem. 381, 127–137 10.1007/s11010-013-1694-7 [DOI] [PubMed] [Google Scholar]
- 17. Li H., Min Q., Ouyang C., Lee J., He C., Zou M. H., and Xie Z. (2014) AMPK activation prevents excess nutrient-induced hepatic lipid accumulation by inhibiting mTORC1 signaling and endoplasmic reticulum stress response. Biochim. Biophys. Acta 1842, 1844–1854 10.1016/j.bbadis.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., and Olefsky J. M. (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 10.1016/j.cell.2010.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moura-Assis A., Afonso M. S., de Oliveira V., Morari J., Dos Santos G. A., Koike M., Lottenberg A. M., Ramos Catharino R., Velloso L. A., Sanchez Ramos da Silva A., de Moura L. P., Ropelle E. R., Pauli J. R., and Cintra D. E. (2017) Flaxseed oil rich in omega-3 protects aorta against inflammation and endoplasmic reticulum stress partially mediated by GPR120 receptor in obese, diabetic and dyslipidemic mice models. J. Nutr. Biochem. 53, 9–19 [DOI] [PubMed] [Google Scholar]
- 20. Kopach O., Maistrenko A., Lushnikova I., Belan P., Skibo G., and Voitenko N. (2016) HIF-1α-mediated upregulation of SERCA2b: the endogenous mechanism for alleviating the ischemia-induced intracellular Ca2+ store dysfunction in CA1 and CA3 hippocampal neurons. Cell Calcium 59, 251–261 10.1016/j.ceca.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 21. Flamment M., Kammoun H. L., Hainault I., Ferré P., and Foufelle F. (2010) Endoplasmic reticulum stress: a new actor in the development of hepatic steatosis. Curr. Opin. Lipidol. 21, 239–246 10.1097/MOL.0b013e3283395e5c [DOI] [PubMed] [Google Scholar]
- 22. Malhi H., Guicciardi M. E., and Gores G. J. (2010) Hepatocyte death: a clear and present danger. Physiol. Rev. 90, 1165–1194 10.1152/physrev.00061.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rius B., López-Vicario C., González-Périz A., Morán-Salvador E., García-Alonso V., Clária J., and Titos E. (2012) Resolution of inflammation in obesity-induced liver disease. Front. Immunol. 3, 257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. González-Périz A., Planagumà A., Gronert K., Miquel R., López-Parra M., Titos E., Horrillo R., Ferré N., Deulofeu R., Arroyo V., Rodés J., and Clària J. (2006) Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 20, 2537–2539 10.1096/fj.06-6250fje [DOI] [PubMed] [Google Scholar]
- 25. Li R., Wang Y., Ma Z., Ma M., Wang D., Xie G., Yin Y., Zhang P., and Tao K. (2016) Maresin 1 mitigates inflammatory response and protects mice from sepsis. Mediators Inflamm. 2016, 3798465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sehgal P., Szalai P., Olesen C., Praetorius H. A., Nissen P., Christensen S. B., Engedal N., and Møller J. V. (2017) Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response. J. Biol. Chem. 292, 19656–19673 10.1074/jbc.M117.796920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wei Y., Wang D., Topczewski F., and Pagliassotti M. J. (2006) Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 291, E275–E281 10.1152/ajpendo.00644.2005 [DOI] [PubMed] [Google Scholar]
- 28. Werstuck G. H., Lentz S. R., Dayal S., Hossain G. S., Sood S. K., Shi Y. Y., Zhou J., Maeda N., Krisans S. K., Malinow M. R., and Austin R. C. (2001) Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Invest. 107, 1263–1273 10.1172/JCI11596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hotamisligil G. S. (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 10.1016/j.cell.2010.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glimcher L. H., and Lee A. H. (2009) From sugar to fat: how the transcription factor XBP1 regulates hepatic lipogenesis. Ann. N.Y. Acad. Sci. 1173, Suppl. 1, E2–E9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haas M. J., Jafri M., Wehmeier K. R., Onstead-Haas L. M., and Mooradian A. D. (2016) Inhibition of endoplasmic reticulum stress and oxidative stress by vitamin D in endothelial cells. Free Radic. Biol. Med. 99, 1–10 10.1016/j.freeradbiomed.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 32. Wu J., and Kaufman R. J. (2006) From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 13, 374–384 10.1038/sj.cdd.4401840 [DOI] [PubMed] [Google Scholar]
- 33. Kammoun H. L., Chabanon H., Hainault I., Luquet S., Magnan C., Koike T., Ferré P., and Foufelle F. (2009) GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 119, 1201–1215 10.1172/JCI37007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith B. K., Marcinko K., Desjardins E. M., Lally J. S., Ford R. J., and Steinberg G. R. (2016) Treatment of nonalcoholic fatty liver disease: role of AMPK. Am. J. Physiol. Endocrinol. Metab. 311, E730–E740 10.1152/ajpendo.00225.2016 [DOI] [PubMed] [Google Scholar]
- 35. Jung T. W., Hong H. C., Hwang H. J., Yoo H. J., Baik S. H., and Choi K. M. (2015) C1q/TNF-related protein 9 (CTRP9) attenuates hepatic steatosis via the autophagy-mediated inhibition of endoplasmic reticulum stress. Mol. Cell. Endocrinol. 417, 131–140 10.1016/j.mce.2015.09.027 [DOI] [PubMed] [Google Scholar]
- 36. Lee Y. H., Yun M. R., Kim H. M., Jeon B. H., Park B. C., Lee B. W., Kang E. S., Lee H. C., Park Y. W., and Cha B. S. (2016) Exogenous administration of DLK1 ameliorates hepatic steatosis and regulates gluconeogenesis via activation of AMPK. Int. J. Obes. 40, 356–365 10.1038/ijo.2015.173 [DOI] [PubMed] [Google Scholar]
- 37. Ford R. J., Fullerton M. D., Pinkosky S. L., Day E. A., Scott J. W., Oakhill J. S., Bujak A. L., Smith B. K., Crane J. D., Blümer R. M., Marcinko K., Kemp B. E., Gerstein H. C., and Steinberg G. R. (2015) Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem. J. 468, 125–132 10.1042/BJ20150125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jung T. W., Youn B. S., Choi H. Y., Lee S. Y., Hong H. C., Yang S. J., Yoo H. J., Kim B. H., Baik S. H., and Choi K. M. (2013) Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem. Pharmacol. 86, 960–969 10.1016/j.bcp.2013.07.034 [DOI] [PubMed] [Google Scholar]
- 39. Deng X., Dong Q., Bridges D., Raghow R., Park E. A., and Elam M. B. (2015) Docosahexaenoic acid inhibits proteolytic processing of sterol regulatory element-binding protein-1c (SREBP-1c) via activation of AMP-activated kinase. Biochim. Biophys. Acta 1851, 1521–1529 10.1016/j.bbalip.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 40. Figueras M., Olivan M., Busquets S., López-Soriano F. J., and Argilés J. M. (2011) Effects of eicosapentaenoic acid (EPA) treatment on insulin sensitivity in an animal model of diabetes: improvement of the inflammatory status. Obesity 19, 362–369 10.1038/oby.2010.194 [DOI] [PubMed] [Google Scholar]
- 41. Zhang J., Li Y., Jiang S., Yu H., and An W. (2014) Enhanced endoplasmic reticulum SERCA activity by overexpression of hepatic stimulator substance gene prevents hepatic cells from ER stress-induced apoptosis. Am. J. Physiol. Cell Physiol. 306, C279–C290 10.1152/ajpcell.00117.2013 [DOI] [PubMed] [Google Scholar]
- 42. Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., Eto K., Akanuma Y., Froguel P., Foufelle F., Ferre P., et al. (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 10.1038/nm788 [DOI] [PubMed] [Google Scholar]
- 43. Chau M. D., Gao J., Yang Q., Wu Z., and Gromada J. (2010) Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc. Natl. Acad. Sci. U.S.A. 107, 12553–12558 10.1073/pnas.1006962107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bai Y., Hassler J., Ziyar A., Li P., Wright Z., Menon R., Omenn G. S., Cavalcoli J. D., Kaufman R. J., and Sartor M. A. (2014) Novel bioinformatics method for identification of genome-wide non-canonical spliced regions using RNA-Seq data. PLoS One 9, e100864 10.1371/journal.pone.0100864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Y., Zhou D., Phung S., Warden C., Rashid R., Chan N., and Chen S. (2017) SGK3 sustains ERα signaling and drives acquired aromatase inhibitor resistance through maintaining endoplasmic reticulum homeostasis. Proc. Natl. Acad. Sci. U.S.A. 114, E1500–E1508 10.1073/pnas.1612991114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.