Abstract
Background
Temporomandibular disorder (TMD) represents a subgroup of painful orofacial disorders involving pain in the temporomandibular joint (TMJ) region, fatigue of the cranio-cervico-facial muscles (especially masticatory muscles), limitation of mandible movement, and the presence of a clicking sound in the TMJ. TMD is associated with multiple factors and systemic diseases. This study aimed to assess the prevalence of TMD in Nepalese subjects for the first time.
Methods
A total of 500 medical and dental students (127 men and 373 women) participated in this study from May 2016 to September 2016. The Fonseca questionnaire was used as a tool to evaluate the prevalence of TMD, and Fonseca's Anamnestic Index (FAI) was used to classify the severity of TMD.
Results
The majority of the participants with TMD had a history of head trauma, psychological stress, and dental treatment or dental problems. The prevalence of TMD in Nepalese students was mild to moderate.
Conclusions
The prevalence of TMD in Nepalese subjects was mild to moderate. The majority of the study subjects had eyesight problems, history of head trauma, psychological stress, and drinking alcohol and had received dental treatments.
Keywords: Dentistry, Nepalese, Prevalence, Temporomandibular Joint Disorders
INTRODUCTION
Temporomandibular disorder (TMD) is a term used to define a subgroup of painful orofacial disorders in the temporomandibular joint (TMJ) region, fatigue of the cranio-cervico-facial muscles (especially masticatory muscles), limitation of mandible movement, and the presence of a clicking sound in the TMJ. The etiology of TMDs has been linked to multiple factors, including traumatic injury, immune-mediated systemic disease, neoplastic growths, emotional stress, occlusal interferences, malpositioning or loss of teeth, postural changes, dysfunctions of the masticatory musculature and adjacent structures, extrinsic and intrinsic changes of TMJ structure, nonfunctional movements of the mandible (bruxing), tooth clenching habits, or a combination of such factors [1,2]. Prosthodontic rehabilitation, orthodontic treatment, orthognathic surgery, and mandibular fractures have been associated with TMJ changes and worsening of existing TMD [3].
Psychological factors are known to play a role in the etiology and persistence of TMD. In particular, a high incidence of exposure to stressful life events and elevated levels of anxiety and stress-related symptoms have been reported in patients with TMD [4].
The Fonseca questionnaire is a self-administered questionnaire that has been proposed as a low-cost, easily applied alternative TMD assessment tool for the non-patient population. It serves as a preliminary screening tool for TMD. Fonseca's questionnaire follows the characteristics of a multidimensional evaluation. It is composed of 10 questions that screen for the presence of pain in the TMJ, head, and back; pain while chewing, parafunctional habits, movement limitations, joint clicking, perception of malocclusion, and sensation of emotional stress [4]. Fonseca's anamnestic index (FAI) was used to classify TMD severity as ‘no dysfunction,’ ‘light dysfunction,’ ‘moderate dysfunction,’ or ‘severe dysfunction.’
The university setting provides an ideal context for studying the mental health of young adults. University students are often undergoing role transitions, such as moving away from home for the first time, residing with other students, and experiencing reduced adult supervision [5,6]. This study aimed to access the prevalence of TMD in Nepalese subjects for the first time.
METHODS
The objective of this cross-sectional study was to study the prevalence of TMD in Nepalese subjects. A total of 500 medical and dental students (127 men and 373 women) participated in this study from May 2016 to September 2016. After obtaining ethical approval from the Institutional Review Committee (IRC number: 28/16), all selected subjects who met the criteria were informed on the details of the study and requested to sign informed consent prior to the study.
The inclusion criteria of the study were: not receiving orthodontic treatment or treatment for TMD, no developmental anomalies of the face, and/or not having any severe or immunocompromising disease. The subjects were asked to complete a self-assessed questionnaire. It contained questions on demographic information and past medical, dental, and TMJ history. Then, after obtaining consent, the subjects were asked Fonseca's 10 questions where they were asked to select one answer from ‘yes,’ ‘no,’ or ‘sometimes’ [4]. Each ‘yes’ answer was assigned a value of 10, each ‘sometimes’ answer a value of 5, and each ‘no’ answer a value of 0. The values of the 10 answers were added for each participant. Then, according to Fonseca's Anamnestic Index (FAI), TMD severity was classified as without dysfunction (score between 0–15), mild dysfunction (score between 20–40), moderate dysfunction (score between 45–65), and severe dysfunction (score between 70–100).
Statistical analyses were performed using the SPSS Statistics Software (version 24 IBM Corp. Armonk, NY, USA) with a 5% level of significance. Descriptive statistics and frequency analyses were performed. Pearson chi-Square was used to examine the correlation between various dental treatments and TMJ dysfunction.
RESULTS
A total of 500 questionnaires was completed by the subjects, which included of 127 (25.40 %) men and 373 (74.60 %) women (Table 1). The mean age of subjects was 20.61 ± 1.66 years (Table 1).
Table 1. Subject characteristics.
| Subject Details | Frequency (%) |
|---|---|
| Total subjects | 500 (100) |
| Male | 127 (25.40) |
| Female | 373 (74.60) |
| Age | |
| Mean ± SD | 20.61 ± 1.66 years |
| Range | 17–27 years |
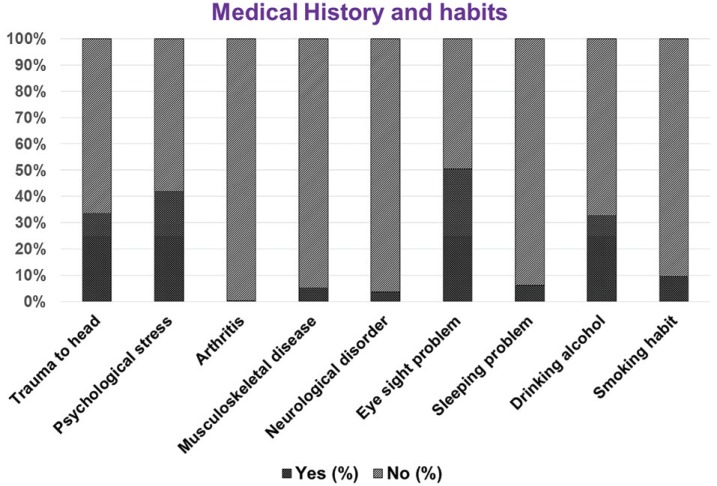
The results regarding medical history and the habits of the subjects are presented in Figure 1. Based on these results, 168 (33.6%) of the subjects had head trauma, 201 (42%) had psychological stress, 2 (0.4%) had arthritis, 26 (5.2%) had a musculoskeletal disease, 18 (3.6%) had a neurological disease, 252 (50.4%) had an eyesight problem, 31 (6.2%) had a sleeping problem, 164 (32.8%) had an alcohol drinking habit, and 48 (9.6%) had a smoking habit.
Fig. 1. Medical history and habits of the subjects (expressed in percentages).
The results of the TMJ-related Fonseca questions are shown in Table 2. The most frequently reported problem was neck pain or neck stiffness with 26.2% for the ‘yes’ and 31% for the ‘sometimes’ responses. The least frequently reported problem was difficulty opening the mouth with 1% for the ‘yes’ and 1.8% for the ‘sometimes’ responses.
Table 2. Results of Fonseca questions.
| SN | Fonseca Questions | Yes n (%) | Sometimes n (%) | No n (%) |
|---|---|---|---|---|
| 1 | Is it hard for you to open your mouth? | 5 (1) | 9 (1.8) | 486 (97.2) |
| 2 | Is it hard for you to move your mandible from side to side? | 9 (1.8) | 28 (5.6) | 463 (92.6) |
| 3 | Do you get tired/ muscular pain while chewing? | 15 (3) | 42 (8.4) | 443 (88.6) |
| 4 | Do you have frequent headaches? | 36 (7.2) | 134 (26.8) | 330 (66.0) |
| 5 | Do you have pain on the neck or neck stiffness? | 131 (26.2) | 155 (31.0) | 214 (42.8) |
| 6 | Do you have earaches or pain in cranio-mandibular joints? | 25 (5.0) | 52 (10.4) | 423 (84.6) |
| 7 | Have you noticed any TMJ clicking while chewing or when you open your mouth? | 8 (1.6) | 26 (5.2) | 466 (93.2) |
| 8 | Do you clench or grind your teeth? | 72 (14.4) | 76 (15.2) | 352 (70.4) |
| 9 | Do you feel your teeth do not articulate well? | 28 (5.6) | 62 (12.4) | 410 (82.0) |
| 10 | Do you consider yourself a tense (nervous) person? | 31 (6.2) | 45 (9.0) | 424 (84.8) |
TMJ: temporomandibular joint
Table 3 shows the results regarding TMD according to FAI. It was found that more than half of the subjects (69.4%) had no dysfunction, whereas, 26.6% had mild dysfunction, 3.4% had moderate dysfunction, and 0.6% had severe dysfunction.
Table 3. Results of temporomandibular disorder according to Fonseca's Anamnestic Index.
| TMJ Dysfunction | Number (%) | Mean age ± SD | 95% CI for Mean | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Without Dysfunction | 347 (69.4) | 20.56 ± 1.70 | 20.38 | 20.74 |
| Mild Dysfunction | 133 (26.6) | 20.68 ± 1.55 | 20.41 | 20.94 |
| Moderate Dysfunction | 17 (3.4) | 21.06 ± 1.81 | 20.12 | 21.99 |
| Severe Dysfunction | 3 (0.6) | 21.00 ± 1.00 | 18.52 | 23.48 |
TMJ: temporomandibular joint
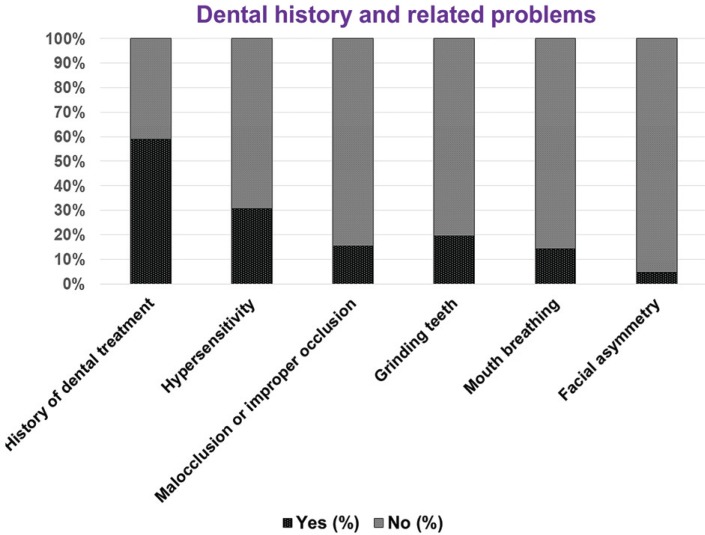
The details regarding dental treatments are shown in Table 4. In subjects with mild and moderate TMJ dysfunctions, the majority had received filling, root canal treatment, extraction, and orthodontic treatment. However, subjects with severe TMJ dysfunction had only received filling treatment. There was a significant correlation (P = 0.003) between the various dental treatments and TMJ dysfunction. The dental-related problems in the subjects are shown in Figure 2. It was found that 295 (59%) of the subjects reported dental-related problems. Major dental-related problems were hypersensitivity (30.8%), malocclusion (15.4%), and grinding teeth (19.6%).
Table 4. Details of dental treatments of the subjects.
| TMJ Dysfunction | Scaling and Polishing | Filling | RCT | Extraction | Orthodontic Treatment | Prosthetic Treatment | Pearson Chi-Square (P-value) |
|---|---|---|---|---|---|---|---|
| Mild Dysfunction | 5 | 32 | 19 | 14 | 21 | 2 | 0.003 |
| Moderate Dysfunction | 4 | 21 | 11 | 6 | 18 | 2 | |
| Severe Dysfunction | 0 | 9 | 0 | 0 | 0 | 0 |
TMJ: temporomandibular joint, RCT: root canal treatment.
Significant Correlation at P value < 0.05.
Fig. 2. Dental history and related problems of the subjects (expressed in percentages).
Table 5 shows the results of TMJ dysfunction in various clinical conditions. There were higher numbers of subjects with mild than with moderate and severe TMJ dysfunction in each clinical condition. The order of frequency of all studied clinical conditions was ‘no,’ ‘sometimes,’ and ‘yes,’ and there was a statistically significant difference (P < 0.01) among ‘yes,’ ‘sometimes,’ and ‘no’ for all the clinical conditions.
Table 5. Results of temporomandibular dysfunction in various clinical conditions.
| Conditions | Without dysfunction n (%) | Mild dysfunction n (%) | Moderate dysfunction n (%) | Severe dysfunction n (%) | P value |
|---|---|---|---|---|---|
| Hard to open mouth | < 0.001 | ||||
| Yes | 1 | 6 | 6 | 1 | |
| Sometimes | 9 | 27 | 6 | 2 | |
| No | 337 | 100 | 5 | 0 | |
| Hard to move mandible laterally | < 0.001 | ||||
| Yes | 0 | 2 | 5 | 1 | |
| Sometimes | 3 | 17 | 6 | 2 | |
| No | 334 | 114 | 6 | 0 | |
| Muscle pain or tired while chewing | < 0.001 | ||||
| Yes | 1 | 6 | 6 | 3 | |
| Sometimes | 13 | 23 | 6 | 0 | |
| No | 333 | 104 | 6 | 0 | |
| Frequent headache | < 0.001 | ||||
| Yes | 10 | 16 | 8 | 2 | |
| Sometimes | 67 | 58 | 8 | 1 | |
| No | 270 | 59 | 1 | 0 | |
| Neck stiffness | < 0.001 | ||||
| Yes | 60 | 60 | 9 | 2 | |
| Sometimes | 112 | 38 | 4 | 1 | |
| No | 175 | 35 | 4 | 0 | |
| Earache or TMJ joint pain | < 0.001 | ||||
| Yes | 3 | 21 | 1 | 0 | |
| Sometimes | 23 | 24 | 4 | 1 | |
| No | 321 | 88 | 12 | 2 | |
| TMJ clinking while chewing | < 0.001 | ||||
| Yes | 0 | 2 | 4 | 2 | |
| Sometimes | 10 | 11 | 5 | 0 | |
| No | 337 | 120 | 8 | 1 | |
| Grinding teeth | < 0.001 | ||||
| Yes | 26 | 33 | 10 | 3 | |
| Sometimes | 32 | 39 | 5 | 0 | |
| No | 289 | 61 | 2 | 0 | |
| Teeth not articulating well | < 0.001 | ||||
| Yes | 5 | 18 | 3 | 2 | |
| Sometimes | 27 | 27 | 8 | 0 | |
| No | 315 | 88 | 6 | 1 | |
| Nervous/tense person | < 0.001 | ||||
| Yes | 5 | 23 | 2 | 1 | |
| Sometimes | 17 | 24 | 4 | 0 | |
| No | 325 | 86 | 11 | 2 |
TMJ: temporomandibular joint
DISCUSSION
This study was the first to assess the severity and prevalence of TMD among university students in Nepal. The prevalence rate of TMD based on FAI has been studied extensively, and has been found to vary from approximately 40–70%, as reported by other investigators [7,8,9,10,11,12]. In our study, approximately 31% of subjects were found to have TMD, as classified by FAI (26.6% mild, 3.4% moderate, and 0.6% severe dysfunction). The prevalence of TMD based on FAI found in our study was lower than that reported by other studies performed in different populations: Saudi students (46.8%) [7], Pakistani students (92.2%) [8], Indian students (45.16%) [9], Brazilian students (53.2% [10] and 41.3% [11]), and Taiwanese students (42.9%) [12].
Our investigation additionally inspected the effect of significant medical and dental histories on the prevalence of TMD in Nepalese students. Some studies have found that ‘mental anxiety or stress,’ is significantly associated with TMD [5,6,7]. The majority of the subjects had psychological stress (42%). This is similar to the results reported by other studies [5,6,7] that have shown that approximately 33% of subjects with TMD had a history of mental anxiety. Nonetheless, it is challenging to quantify a related variable, for example, stress or nervousness. Additionally, despite the fact that endeavors have been made to investigate the prevalence of stress among patients with TMD, there is a requirement for long term investigations.
A study by Habib et al. [7] on TMD in Saudi subjects found psychological stress in 30.5% and direct restorations in 77% of total participants. However, in our study, eyesight problems, psychological stress, head trauma, and alcohol drinking habit were most frequently reported. More than half of the subjects (59%) had a history of dental treatment with dental problems such as hypersensitivity, malocclusion, and grinding teeth. We also found a significant correlation between the various dental treatments and TMJ dysfunction. It was found that severe TMD was present only in subjects who had fillings. Becker [13] mentioned that significant scientific evidence exists regarding occlusal causative factors for TMD. Improper or incorrect fillings lead to disorders of masticatory muscles and TMD [14]. Clinicians need current information for dental treatment requiring restorative procedures. Elimination of occlusal interferences is a simple method of TMD correction. Hence, maintaining proper occlusion is compulsory in occlusal fillings for successful restoration.
Park et al. [15] and Chi et al. [16] also found an association among dental problems, TMJ pain, and TMD. A study on TMD and related factors by Ebrahimi et al. [17] found the prevalent predisposing factors of TMD to be clenching, premature contact in protrusive movement, and bruxism. In our study, 20% of the study subjects showed grinding.
Neck pain or neck stiffness may be caused by abnormalities, inflammation, or injury to the neck, or referred pain from the masticatory muscles [16]. This study may be extended to see the correlations of TMD with neck pain or neck stiffness, eyesight problems, head trauma, and alcohol drinking habit.
The limitations of this study are that we used a brief questionnaire, a conveniently selected sample, and that the sample population comprised of only medical students from one region. In addition, in this descriptive study, the association of each medical and dental history and related problems with TMD is not considered. This study only provides information regarding the prevalence and severity of TMD in Nepalese medical students. Long-term studies should be conducted to investigate the associations of medical and dental history and related problems targeting a larger population in different regions.
It is important that TMD be diagnosed early to prevent future complications. Fonseca's questionnaire and FAI, as used in this study, serve as important tools for determining TMD. The prevalence of TMD in Nepalese subjects was mild to moderate. The majority of the study subjects had eyesight problems, history of head trauma, psychological stress, and drinking alcohol, and had received dental treatments.
ACKNOWLEDGEMENTS
We acknowledge the financial support received from the Dhulikhel Hospital/ Kathmandu University School of Medical Sciences.
Footnotes
DECLARATION OF INTERESTS: The authors declare no conflict of interest.
References
- 1.de Santis TO, Motta LJ, Biasotto-Gonzalez DA, Mesquita-Ferrari RA, Fernandes KP, de Godoy CH, et al. Accuracy study of the main screening tools for temporomandibular disorder in children and adolescents. J Bodyw Mov Ther. 2014;18:87–91. doi: 10.1016/j.jbmt.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Manfredini D, Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e26–e50. doi: 10.1016/j.tripleo.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein BH. Temporomandibular disorders: a review of current understanding. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:379–385. doi: 10.1016/s1079-2104(99)70048-x. [DOI] [PubMed] [Google Scholar]
- 4.Da Fonseca DM, Bonfante G, Valle AL, de Freitas SFT. Diagnóstico pela anamnese da disfunção craniomandibular. Rev Gauch de Odontol. 1994;4:23–32. [Google Scholar]
- 5.Bonjardim LR, Gavião MB, Pereira LJ, Castelo PM. Anxiety and depression in adolescents and their relationship with signs and symptoms of temporomandibular disorders. Int J Prosthodont. 2005;18:347–352. [PubMed] [Google Scholar]
- 6.Pesqueira AA, Zuim PR, Monteiro DR, Ribeiro Pdo P, Garcia AR. Relationship between psychological factors and symptoms of TMD in university undergraduate students. Acta Odontol Latinoam. 2010;23:182–187. [PubMed] [Google Scholar]
- 7.Habib SR, Al Rifaiy MQ, Awan KH, Alsaif A, Alshalan A, Altokais Y. Prevalence and severity of temporomandibular disorders among university students in Riyadh. Saudi Dent J. 2015;27:125–130. doi: 10.1016/j.sdentj.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahid A, Mian FI, Razzaq A, Bokhari SAH, Kaukab T, Iftikhar A, et al. Prevalence and severity of temporomandibular disorder (TMD) in undergraduate medical students using Fonseca's questionnaire. Pakistan Oral Dent J. 2014;31:38–41. [Google Scholar]
- 9.Modi P, Shaikh SS, Munde A. A cross sectional study of prevalence of temporomandibular disorders in university students. Int J Sci Res Publ. 2012;2:1–3. [Google Scholar]
- 10.Nomura K, Vitti M, Oliveira AS, Chaves TC, Semprini M, Siéssere S, et al. Use of the Fonseca's questionnaire to assess the prevalence and severity of temporomandibular disorders in Brazilian dental undergraduate. Braz Dent J. 2007;18:163–167. doi: 10.1590/s0103-64402007000200015. [DOI] [PubMed] [Google Scholar]
- 11.Conti PC, Ferreira PM, Pegoraro LF, Conti JV, Salvador MC. A cross sectional study of prevalence and etiology of signs and symptoms of temporomandibular disorders in high school and university students. J Orofac Pain. 1996;10:254–262. [PubMed] [Google Scholar]
- 12.Shiau YY, Chang C. An epidemiological study of temporomandibular disorders in university students of Taiwan. Community Dent Oral Epidemiol. 1992;20:43–47. doi: 10.1111/j.1600-0528.1992.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 13.Becker IM. Occlusion as a causative factor in TMD. Scientific basis to occlusal therapy. N Y State Dent J. 1995;61:54–57. [PubMed] [Google Scholar]
- 14.Joo SJ, Kang DW, Lee HS, Jin SY, Lee GJ. Re-restoration of temporomandibular joint disorder acquired after implant prosthetic restoration using T-Scan: a case report. J Korean Acad Prosthodont. 2016;54:431–437. [Google Scholar]
- 15.Park KS, Lee CH, Lee JW. Use of a botulinum toxin A in dentistry and oral and maxillofacial surgery. J Dent Anesth Pain Med. 2016;16:151–157. doi: 10.17245/jdapm.2016.16.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi SI, Kim HJ, Seo KS, Lee JH, Chang J. Local anesthesia of the temporomandibular joint to reduce pain during mouth opening for dental treatment in a patient with spinal muscular atrophy. J Dent Anesth Pain Med. 2016;16:137–140. doi: 10.17245/jdapm.2016.16.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebrahimi M, Dashti H, Mehrabkhani M, Arghavani M, Daneshvar-Mozafari A. Temporomandibular disorders and related factors in a group of Iranian adolescents: A cross-sectional survey. J Dent Res Dent Clin Dent Prospects. 2011;5:123–127. doi: 10.5681/joddd.2011.028. [DOI] [PMC free article] [PubMed] [Google Scholar]