Abstract
Patient-reported outcomes (PROs) are increasingly used to monitor treatment-related symptoms and physical function decrements in cancer clinical trials. As more patients enter survivorship, it is important to capture PRO physical function throughout trials to help restore pretreatment levels of function. We completed a systematic review of PRO physical function measures used in cancer clinical trials and evaluated their psychometric properties on the basis of guidelines from the US Food and Drug Administration. Five databases were searched through October 2015: PubMed/MEDLINE, EMBASE, CINAHL (Cumulative Index of Nursing and Allied Health Literature), Health and Psychosocial Instruments, and Cochrane. From an initial total of 10,233 articles, we identified 108 trials that captured PRO physical function. Within these trials, approximately 67% used the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire and 25% used the Medical Outcomes Study Short Form 36. Both the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire and Medical Outcomes Study Short Form 36 instruments generically satisfy most Food and Drug Administration requirements, although neither sought direct patient input as part of item development. The newer Patient-Reported Outcomes Measurement Information System physical function short form may be a brief, viable alternative. Clinicians should carefully consider the psychometric properties of these measures when incorporating PRO instrumentation into clinical trial design to provide a more comprehensive understanding of patient function.
Keywords: clinical outcome assessment, health status, neoplasms, patient-reported outcomes
INTRODUCTION
Symptom monitoring via patient-reported outcome (PRO) measures, defined as any unfiltered report of the status of a patient's health condition that comes directly from the patient (1), is rapidly becoming commonplace in oncology clinical trials (2–5). Real-time capture of treatment-related symptoms and physical function decrements integrates the patient voice into trial conduct and can assist clinicians in understanding the patient's experiences (2–4, 6).
Because of the rapid increase in number of cancer survivors (7), it is especially important to accurately capture PRO physical function (i.e., physical abilities such as walking or reaching that are considered essential for maintaining independence) throughout the conduct of a clinical trial. Availability of such PRO information would allow clinical trialists to monitor potential treatment-related changes from baseline and make recommendations and informed referrals to rehabilitation specialists (e.g., physiatrists, physical therapists) to facilitate the restoration of any losses in physical function as the patient enters survivorship.
A number of well-established and newer measures have been developed to assess PRO physical function in oncology (e.g., European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) (8); Medical Outcomes Study Short Form 36 (SF-36) (9)). In support of this shift toward emphasizing PROs, the National Institutes of Health has completed 2 separate large psychometric initiatives. The first initiative developed a lay-language version of a clinician-based system for documenting and grading adverse events (Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events) (10–12). The Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events gives cancer patients the opportunity to provide information about their symptoms/adverse events (e.g., frequency, intensity, and interference with functioning) in addition to the clinician rating. It is important to capture both clinicians’ and patients’ perceptions of adverse events because prior studies have shown a low correlation between the 2 (13, 14).
The second National Institutes of Health initiative involved development and evaluation of a series of item banks to capture nuanced, treatment-related symptom and quality-of-life information (i.e., the Patient-Reported Outcomes Measurement Information System (PROMIS)) (15–18). PROMIS measures are intended to be used across health conditions and thus are not cancer specific like the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events. With numerous choices to include in clinical trial design, it is important to identify all potential PRO physical function measures and compare their psychometric properties in cancer patients to inform clinicians in the selection of tools.
The Food and Drug Administration (FDA) provides regulatory recommendations for the psychometric properties of PRO instruments to be used in drug development, entitled Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims (19). This 2009 guidance contains specific criteria for a PRO measure to meet to be used as a clinical outcome assessment for drug development. These criteria include a conceptual framework for the PRO measure, reliability, content validity, construct validity, and ability to detect clinically relevant score changes. Additionally, the FDA has a rigorous formal qualification procedure that reviews the psychometric properties of a candidate PRO measure that is to be potentially used as clinical outcome assessment in a drug development trial relative to the criteria outlined in the FDA PRO guidance (20). Although not all clinical trials in oncology involve medical product development, the psychometric recommendations included in this FDA PRO guidance are reasonable milestones for any measure that is meant to capture this patient information in the clinical trial setting.
The purpose of this article is to systematically identify and review existing PRO measures that have been used to capture physical function in oncology trials. The psychometric properties of these measures will be summarized with respect to criteria outlined in the FDA PRO Guidance, with existing gaps in measurement identified and recommendations made for clinician use in future trials in oncology.
METHODS
Search strategy
A comprehensive electronic literature search for articles was conducted in the following 5 databases: PubMed/MEDLINE (National Library of Medicine); EMBASE (Elsevier); CINAHL (Cumulative Index of Nursing and Allied Health Literature; Elton B. Stephens Co. (EBSCO)); Health and Psychosocial Instruments (OVID; Wolters Kluwer); and CENTRAL (Cochrane Central Register of Controlled Trials; Wiley), through October 2015. No date or language restrictions were applied.
Five main components made up the search strategy used in PubMed/MEDLINE and EMBASE, where a combination of keywords and controlled vocabulary were used (Medical Subject Headings (MeSH) and Emtree, respectively) to describe 1) physical function, 2) cancer, 3) PROs, 4) types of PRO measurement instruments, and 5) properties of PRO measurement instruments.
Synonyms for terms/concepts describing each component part were first searched on individually and combined with the Boolean operator OR. Each individual component search set was then combined with the Boolean operator AND, resulting in a final set of citations that included all of the main component concepts. The CINAHL, Health and Psychosocial Instruments, and CENTRAL searches used only the first 4 components of the strategy: 1) physical function, 2) cancer, 3) PROs, and 4) types of PRO measurement instruments, as the search strategy for these comparatively smaller databases was translated using only keywords. The search terms were as follows: (((instrumentation[sh] OR methods[sh] OR Validation Studies[pt] OR Comparative Study[pt] OR “psychometrics” [MeSH] OR psychometr*[tiab] OR clinimetr*[tw] OR clinometr*[tw] OR “outcome assessment (health care)”[MeSH] OR “outcome assessment”[tiab] OR “outcome measure*”[tw] OR “observer variation”[MeSH] OR “observer variation”[tiab] OR “Health Status Indicators”[MeSH] OR “reproducibility of results”[MeSH] OR reproducib*[tiab] OR “discriminant analysis”[MeSH] OR reliab*[tiab] OR unreliab*[tiab] OR valid*[tiab] OR coefficient[tiab] OR homogeneity[tiab] OR homogeneous[tiab] OR “internal consistency”[tiab] OR (cronbach*[tiab] AND (alpha[tiab] OR alphas[tiab])) OR (item[tiab] AND (correlation*[tiab] OR selection*[tiab] OR reduction*[tiab])) OR agreement[tiab] OR precision[tiab] OR imprecision[tiab] OR “precise values”[tiab] OR test-retest[tiab] OR (test[tiab] AND retest[tiab]) OR (reliab*[tiab] AND (test[tiab] OR retest[tiab])) OR stability[tiab] OR interrater[tiab] OR inter-rater[tiab] OR intrarater[tiab] OR intra-rater[tiab] OR intertester[tiab] OR inter-tester [tiab] OR intratester[tiab] OR intra-tester[tiab] OR interobserver[tiab] OR inter-observer[tiab] OR intraobserver[tiab] OR intraobserver[tiab] OR intertechnician[tiab] OR inter-technician[tiab] OR intratechnician[tiab] OR intra-technician[tiab] OR interexaminer[tiab] OR interexaminer[tiab] OR intraexaminer[tiab] OR intra-examiner[tiab] OR interassay[tiab] OR inter-assay[tiab] OR intraassay[tiab] OR intraassay[tiab] OR interindividual[tiab] OR inter-individual[tiab] OR intraindividual[tiab] OR intra-individual[tiab] OR interparticipant[tiab] OR inter-participant[tiab] OR intraparticipant[tiab] OR intraparticipant[tiab] OR kappa[tiab] OR kappa's[tiab] OR kappas[tiab] OR repeatab*[tiab] OR ((replicab*[tiab] OR repeated[tiab]) AND (measure[tiab] OR measures[tiab] OR findings[tiab] OR result[tiab] OR results[tiab] OR test[tiab] OR tests[tiab])) OR generaliza*[tiab] OR generalisa*[tiab] OR concordance[tiab] OR (intraclass[tiab] AND correlation*[tiab]) OR discriminative[tiab] OR “known group”[tiab] OR factor analysis[tiab] OR factor analyses[tiab] OR dimension*[tiab] OR subscale*[tiab] OR (multitrait[tiab] AND scaling[tiab] AND (analysis[tiab] OR analyses[tiab])) OR item discriminant[tiab] OR interscale correlation*[tiab] OR error[tiab] OR errors[tiab] OR “individual variability”[tiab] OR (variability[tiab] AND (analysis[tiab] OR values[tiab])) OR (uncertainty[tiab] AND (measurement[tiab] OR measuring[tiab])) OR “standard error of measurement”[tiab] OR sensitiv*[tiab] OR responsive*[tiab] OR ((minimal[tiab] OR minimally [tiab] OR clinical[tiab] OR clinically[tiab]) AND (important[tiab] OR significant[tiab] OR detectable[tiab]) AND (change[tiab] OR difference[tiab])) OR (small*[tiab] AND (real[tiab] OR detectable[tiab]) AND (change[tiab] OR difference[tiab])) OR meaningful change[tiab] OR “ceiling effect”[tiab] OR “floor effect”[tiab] OR “Item response model”[tiab] OR IRT[tiab] OR Rasch[tiab] OR “Differential item functioning”[tiab] OR DIF[tiab] OR “computer adaptive testing”[tiab] OR “item bank”[tiab] OR “cross-cultural equivalence”[tiab]))). For the fifth component (i.e., properties of PRO measurement instruments), search terms from a validated search filter were used (21).
Selection strategy
Studies were deemed eligible for inclusion if they described an oncology clinical trial that included a patient-reported measure of physical function. Both cancer-specific measures and general physical function measures for any health condition were accepted.
Screening process
After removal of duplicates, all titles were randomly assigned to 2 coders (coauthors) and independently reviewed for eligibility. For the abstract reviewing stage, articles were considered if both independent coauthor reviewers reached consensus on eligibility in the prior round. In instances of disagreement, a third coauthor reviewer arbitrated the article. For full-text review, the randomly assigned coders consisted of a primary reviewer and a secondary reviewer for the purposes of verification and quality assurance. Both reviewers independently completed standardized coding forms to extract predetermined information from each potentially eligible article. All reviewers then met as a group and compared full-text article reviews to resolve any potential discrepancies and make final decisions regarding article inclusion. Reference lists from the included full-text articles were also searched to determine whether they should also be considered for inclusion.
Psychometric review
All PRO measures of physical function determined from abstract coding were then reviewed on the basis of the psychometric criteria contained in the FDA PRO Guidance (i.e., conceptual framework, reliability, content validity, construct validity, and ability to detect clinically relevant score changes) (19). Table 1 is a summary of the FDA recommendations.
Table 1.
Summary of FDA Recommendations for the Psychometric Properties of a Patient-Reported Outcomes Instrument
| Property | FDA PRO Guidance Recommendations |
|---|---|
| Conceptual framework | Should be confirmed by using empirical evidence during instrument development |
| Explicit statement of relationship among instruments’ concepts, domains, and items | |
| Response options should be clear and appropriate. | |
| Reliability | Instrument should demonstrate test-retest reliability. |
| Instrument should demonstrate internal consistency. | |
| Content validity | Must encompass most important and comprehensive outcomes for patients |
| Patient input should be sought for item generation. | |
| Patient input should be sought until point of saturation. | |
| Construct validity | Obtained results should be consistent with preexisting hypotheses. |
| Instrument should have the ability to differentiate between clinically distinct groups. | |
| Clinical relevance of score changes | Instrument should be equally sensitive to gains and losses in health status. |
| Instrument should be sensitive to change at all points for the clinical population. |
Abbreviations: FDA, Food and Drug Administration; PRO, patient-reported outcome.
RESULTS
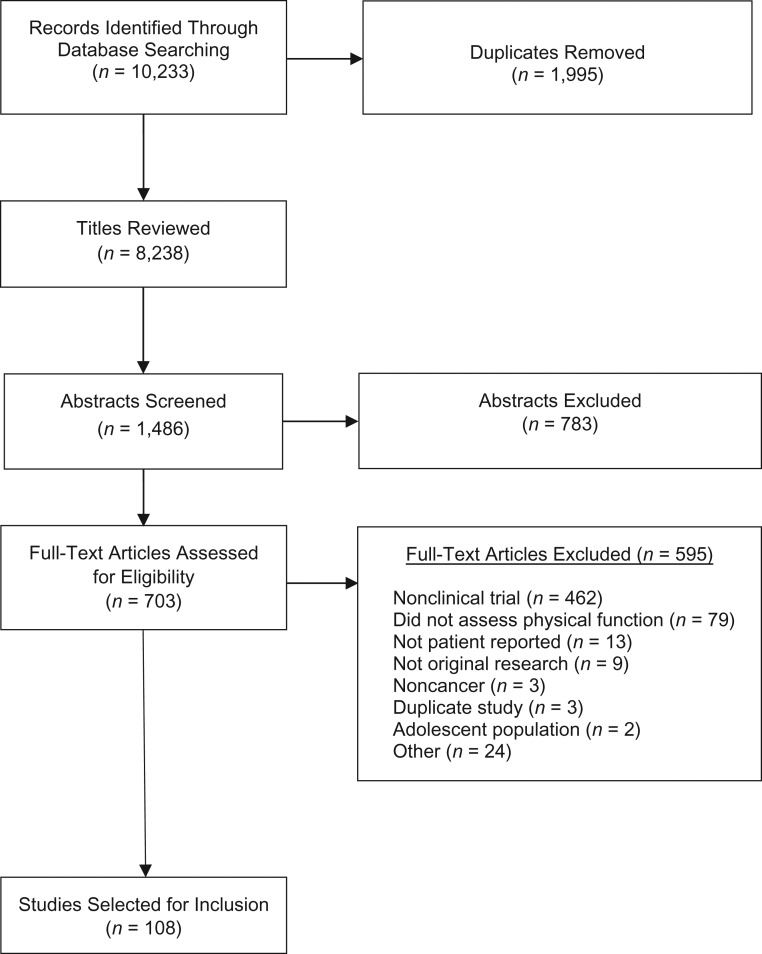
The initial electronic literature search yielded a total of 10,233 titles. After duplicates were removed, 8,238 records remained. Following the process of title screening, 2 of the primary authors independently reviewed each of the remaining 1,486 unique article abstracts. Of the 703 articles retained for full-text review, 659 were full-text, 35 were conference proceeding abstracts, and 9 were dissertations. A total of 595 articles were excluded during this phase, the majority of which did not describe findings from clinical trials (n = 462). Additional reasons for article exclusion during full-text review included the following: physical function was not assessed (n = 79), a patient-reported instrument was not used to capture physical function (n = 13), the article described nonoriginal research (n = 9), the study population was noncancer (n = 3), duplicate study (n = 3), the study population comprised adolescents (n = 2), and other (n = 24). A total of 108 articles met eligibility criteria and were included in this review (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow chart.
Characteristics of included studies
Approximately two-thirds of the included studies (67%) utilized the EORTC QLQ-C30 to capture patient-reported physical function (8, 22–87). The EORTC QLQ-C30 is a 30-item PRO measure designed to capture physical, social, emotional, and cognitive well-being; symptoms as they relate to cancer and its treatment; and overall global health and quality of life. The majority (n = 28) of the items are scored by using a 4-point numerical rating scale ranging from 1 (not at all) to 4 (very much). This measure has been validated in over 90 languages and is currently being validated for use in computer-adaptive testing. With respect to the capture of physical function, the first 5 items of the EORTC QLQ-C30 query patients on whether they have had trouble doing strenuous activities, taking a long walk, taking a short walk, whether they need to stay in bed or a chair during the day, and whether they need assistance with daily activities, such as eating or dressing.
Twenty-seven studies (25%) made use of the Medical Outcomes Study Short Form 12 (SF-12) (88, 89), Short Form 20 (SF-20) (90), or SF-36 (91–114). Five included studies made use of both the EORTC QLQ-C30 and SF-36 questionnaires (115–119). The SF-36 is a 36-item measure of general health, mental health, vitality, and pain, as well as physical role functioning, emotional role functioning, social role functioning, and physical function (9). Ten items are specific to physical function and include content related to performing moderate or vigorous activities, lifting or carrying groceries, climbing 1 or several flights of stairs, bending, walking 100 yards (91.4 m) or several hundred yards, walking more than a mile (1.6 km), or bathing or dressing oneself. These items are rated by using a 3-point numerical rating scale to assess functional limitation, ranging from 1 (yes, limited a lot), 2 (yes, limited a little), to 3 (not limited at all). The SF-20 and SF-12 are shorter versions of the SF-36 that include physical function scales with 6 and 2 items, respectively.
Of the remaining 8 (7%) trials, 2 used the Disabilities of the Arm, Shoulder, and Hand (120) Questionnaire (121, 122), 1 made use of The Care Notebook (123, 124), 1 utilized a Linear Analog Scale of Assessment (125), and 4 made use of unidentified or locally developed measures of physical function (126–129).
FDA PRO guidance psychometric review
The EORTC QLQ-C30 and SF-36 were then reviewed by using the FDA PRO Guidance Criteria. Table 2 is a display of the established conceptual framework, reliability, construct validity, content validity, and ability to capture clinically relevant score changes for the EORTC QLQ-C30 and SF-36, respectively. Both the EORTC and SF-36 have established conceptual frameworks. The EORTC QLQ-C30 physical function subscale has reasonable internal consistency (before treatment Cronbach α = 0.68; during treatment Cronbach α = 0.71) and high test-retest reliability (Pearson r = 0.91). The SF-36 physical function subscale has both high internal consistency (Cronbach α = 0.94) and high test-retest reliability at 1-week (Pearson r = 0.74) and 4-week (Pearson r = 0.85) intervals.
Table 2.
Psychometric Review of Patient-Reported Outcomes Physical Function Measures
| PRO Physical Function Measure | % Used in Clinical Trial Review | FDA PRO Guidance Recommendations | ||||
|---|---|---|---|---|---|---|
| Conceptual Framework | Reliability | Content Validity | Construct Validity | Clinical Relevance of Score Changes | ||
| EORTC QLQ-C30 (8) | 67 | 5 items representing various aspects of physical function. 4-point NRS indicating degree of impaired physical function. | Internal consistency (before treatment, Cronbach α = 0.68; during treatment, Cronbach α = 0.71); test-retest reliability (Pearson r = 0.91) | No formal patient input for item development; however, patients were debriefed on item comprehension. | Physical function interscale correlation was statistically significant (P < 0.01). No differences in physical function were observed on the basis of disease stage. | Physical function scale was sensitive to change over time on the basis of performance status groups (P < 0.001). |
| SF-36 (9) | 25 | 10 items representing various aspects of physical function. 3-point NRS indicating level of limitation. | Internal consistency (Cronbach α = 0.94); test-retest reliability (1 week, Pearson r = 0.74; 4 weeks, Pearson r = 0.85) | No formal patient input for item development. Items have been refined through expert consultation. | Physical function correlated 0.90 with physical component of SF-36. Physical function scale successfully discriminates between those who have physical limitations and healthy controls. | Minimally important difference was established by using a distribution-based approach. A 3-point lower physical function T score is associated with being unable to work and a higher 1-year mortality risk. |
| PROMIS physical function SF (136, 139) | 0 | 10 items measuring physical function of upper extremity, lower extremity, central region, and activities of daily living. 5-point NRS indicating level of physical function. | In cancer patients 6–13 months postdiagnosis, internal consistency was high (Cronbach α = 0.94); test-retest reliability (not determined) | Cognitive interviewing techniques were used to establish patient comprehension. | Physical function SF correlated 0.96 with full PROMIS physical function item bank and 0.88 with the SF-36 physical function scale. The physical function SF discriminates well between those who have high and low physical function and performs well consistently across race/ethnic and age groups. | Minimally important difference was established in advanced-stage cancer patients. Recommended T-score minimally important difference range for 10-item physical functioning is 4.0–6.0 for advanced cancer patients. |
Abbreviations: EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; FDA, Food and Drug Administration; NRS, numerical rating scale; PRO, patient-reported outcome; PROMIS, Patient-Reported Outcomes Measurement Information System; SF, short form; SF-36, Medical Outcomes Study Short Form 36.
Both the EORTC QLQ-C30 and SF-36 physical function scales have high interscale correlations, an indicator of construct validity. For content validity, the FDA PRO Guidance recommends the use of cognitive interviewing techniques (130, 131) for item development. The SF-36 and EORTC QLQ-C30 did not include any formal patient input for item development; however, the EORTC QLQ-C30 did include patient debriefing to assess item comprehension.
With respect to clinically relevant score changes, the EORTC QLQ-C30 physical function scale is sensitive to changes over time on the basis of patient performance status. Minimally important differences have been established for the SF-36, with a 3-point reduction in physical function T score associated with being unable to work, as well as a 1-year mortality risk.
DISCUSSION
Given that there are currently over 14.5 million cancer survivors in the United States, with that number expected to triple by the year 2030 (7), it is important to accurately monitor the patient symptomatic experience from the time of trial enrollment and throughout their treatment to provide these individuals with the best care possible and prepare them for survivorship. PROs have been repeatedly shown to be a feasible, acceptable, and reliable source of monitoring this information (4, 132, 133), and there is a high level of acceptability for clinicians to incorporate this patient-reported information into their decision-making (6, 134). Physical function is a quintessential area of focus for patients participating in clinical trials, as many treatments may negatively impact a patient's ability to perform moderate or routine everyday physical activities, ultimately leading to a reduced quality of life. We systematically reviewed clinical trials in oncology to understand the current methods of capturing patient-reported physical function and evaluated the resulting measures relative to established psychometric guidelines (19).
Almost 90% (n = 96) of the reviewed trials included use of the physical function subscales of the EORTC QLQ-C30 or SF-36 to capture PRO information on this domain. In evaluating these subscales relative to the regulatory psychometric recommendations of the FDA PRO Guidance (19), we found that both generically satisfy the minimum requirements for conceptual framework, reliability, construct validity, and establishment of clinically relevant score changes. However, content validity is limited because both the EORTC QLQ-C30 and SF-36 did not directly involve patients in the process of item generation during initial development. Because concepts such as “limitations” and “strenuous” may be highly subjective, particularly in older patients and/or those with more advanced disease, these measures would potentially benefit from the use of qualitative techniques to determine whether the physical function domain items are understood and perceived similarly across patients regardless of demographical or disease type. Both the EORTC QLQ-C30 and SF-36 have been since successfully translated into numerous languages, thus minimizing concern about patient item comprehension.
Approximately 7% of the included trials did not make use of standardized measures that satisfy the FDA PRO Guidance recommendations. While single items or linear analog scale assessment methods may be suitable for the capture of physical function (135), locally developed measures should meet minimum psychometric standards before being included as part of clinical trial conduct.
A shortcoming of both the EORTC QLQ-C30 and SF-36 is that their physical function subscales have not been validated to be administered independently of their core instrument. Although it was not included in any of the reviewed clinical trials, the relatively recently developed Patient-Reported Outcomes Measurement Information System Physical Function (PROMIS-PF) short form (136) may be a viable alternative to monitor this information as part of clinical trial conduct in that it was developed and validated across a wide range of patients with cancer, including varied demographical characteristics (e.g., age, race, ethnicity) and disease type/severity as a brief, stand-alone tool for use across cancer patient subpopulations (17, 137–139). Unlike the EORTC QLQ-C30 and SF-36, the PROMIS-PF did include patient input as part of item development (16, 18, 140), which enhances content validity.
The PROMIS-PF short form (Web Appendix 1, available at http://aje.oxfordjournals.org/) consists of 10 items that assess function in a given patient's lower and upper extremities, as well as their central region in addition to various activities of daily living. These items are scored by using a 5-point numerical rating scale that either asks patients to indicate how limited their activity is (1 = cannot do, 5 = not at all limited) or whether the patient is able to do a given activity (1 = unable to do, 5 = can do without any difficulty). We have included a summary of the psychometric properties of PROMIS-PF relative to the regulatory psychometric recommendations of the FDA PRO Guidance as part of Table 2. PROMIS-PF generically satisfies (17, 137–139) all minimum psychometric criteria of the FDA PRO Guidance, with the exception of not having established test-retest reliability in patients with cancer.
Our findings are potentially affected by our method of defining physical function. In characterizing physical function as physical abilities that are considered essential for maintaining independence, we have subsequently reduced the number of PRO measures that have been established to capture this domain. For example, a number of the reviewed clinical trials incorporated the well-validated and widely used Functional Assessment of Cancer Therapy–General (141) scale to assess “physical well-being.” Although the Functional Assessment of Cancer Therapy–General scale is used to capture health-related quality of life in patients with cancer, its physical well-being domain does not meet our definition of capturing physical function, as its 7 items ask patients about energy level, nausea, pain, meeting the needs of the family, general bother, generally feeling ill, and being forced to spend time in bed. Because these items do not address the core attributes of physical functioning, such as the ability to conduct activities of daily life or limitations on walking, we excluded any trials that exclusively used this tool. An additional limitation may be related to the lack of concordance between patient- and clinician-based reports of physical function (14). Patients of advanced age or with late-stage disease may artificially limit their physical function, or they may have varied subjective definitions of concepts such as “limitations” or “strenuous.”
The present review provides evidence that the vast majority of clinical trials in oncology that have opted to monitor patient-reported physical function have used well-accepted and psychometrically valid methods of capturing this information. When several quality-of-life domains such as emotional, physical, and social functioning are of interest, the EORTC QLC-C30 and SF-36 are reasonable measures because the subscales have been validated as a package. However, if the only PRO domain of interest is physical function, the PROMIS-PF short form may be a viable alternative option that would be of minimal burden to patients. Regardless of which of these 3 measures is used, it is nonetheless essential that clinical trialists incorporate psychometrically sound instrumentation when monitoring PROs to ensure that accurate and reliable patient data are being incorporated into their decision-making process to ultimately improve long-term outcomes for patients.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Psychiatry and Behavioral Sciences, Memorial Sloan Kettering Cancer Center, New York, New York (Thomas M. Atkinson, Daniel F. Storfer, Rebecca M. Saracino, Thomas A. D'Agostino, Denise Pergolizzi, Yuelin Li); Lineberger Comprehensive Cancer Center at the University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Angela M. Stover, Ethan Basch); Information Systems, Memorial Sloan Kettering Library, Memorial Sloan Kettering Cancer Center, New York, New York (Konstantina Matsoukas); and Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, New York (Ethan Basch).
This project was supported by a National Institutes of Health research training grant (T32 CA009461-25), as well as a National Institutes of Health support grant (P30 CA08748-50), which provides partial support for the Behavioral Research Methods Core Facility used in conducting this investigation.
We wish to thank Dr. Jamie S. Ostroff for her support of this project.
Conflict of interest: none declared.
Abbreviations
- EORTC QLQ-C30
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire
- FDA
Food and Drug Administration
- MeSH
Medical Subject Headings
- PRO
patient-reported outcome
- PROMIS-PF
Patient-Reported Outcomes Measurement Information System Physical Function
- SF-36
Medical Outcomes Study Short Form 36
REFERENCES
- 1. Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362(10):865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basch E. New frontiers in patient-reported outcomes: adverse event reporting, comparative effectiveness, and quality assessment. Annu Rev Med. 2014;65:307–317. [DOI] [PubMed] [Google Scholar]
- 3. Basch E, Abernethy AP, Mullins CD, et al. . Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–4255. [DOI] [PubMed] [Google Scholar]
- 4. Basch E, Deal AM, Kris MG, et al. . Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basch E, Geoghegan C, Coons SJ, et al. . Patient-reported outcomes in cancer drug development and US regulatory review: perspectives from industry, the food and drug administration, and the patient. JAMA Oncol. 2015;1(3):375–379. [DOI] [PubMed] [Google Scholar]
- 6. Basch E, Wood WA, Schrag D, et al. . Feasibility and clinical impact of sharing patient-reported symptom toxicities and performance status with clinical investigators during a phase 2 cancer treatment trial. Clin Trials. 2016;13(3):331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Cancer Society Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 8. Aaronson NK, Ahmedzai S, Bergman B, et al. . The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 9. Ware JE, Snow KK, Kosinski M, et al. . SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: Health Institute, New England Medical Centre; 1993. [Google Scholar]
- 10. Basch E, Reeve BB, Mitchell SA, et al. . Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9):dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dueck AC, Mendoza TR, Mitchell SA, et al. . Validity and reliability of the US National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hay JL, Atkinson TM, Reeve BB, et al. . Cognitive interviewing of the US National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Qual Life Res. 2014;23(1):257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atkinson TM, Li Y, Coffey CW, et al. . Reliability of adverse symptom event reporting by clinicians. Qual Life Res. 2012;21(7):1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atkinson TM, Ryan SJ, Bennett AV, et al. . The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cella D, Riley W, Stone A, et al. . The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeWalt DA, Rothrock N, Yount S, et al. . Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45(5 suppl 1):S12–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yost KJ, Eton DT, Garcia SF, et al. . Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia SF, Cella D, Clauser SB, et al. . Standardizing patient-reported outcomes assessment in cancer clinical trials: a Patient-Reported Outcomes Measurement Information System initiative. J Clin Oncol. 2007;25(32):5106–5112. [DOI] [PubMed] [Google Scholar]
- 19. Department of Health and Human Services Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Published December 2009. Accessed December 20, 2016.
- 20. Department of Health and Human Services Guidance for industry and FDA staff. Qualification process for drug development tools. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm230597.pdf. Published January 2014. Accessed December 1, 2016.
- 21. Terwee CB, Jansma EP, Riphagen II, et al. . Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Qual Life Res. 2009;18(8):1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dhillon HM, van der Ploeg HP, Bell ML, et al. . The impact of physical activity on fatigue and quality of life in lung cancer patients: a randomised controlled trial protocol. BMC Cancer. 2012;12:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Issell BF, Gotay CC, Pagano I, et al. . Using quality of life measures in a phase I clinical trial of noni in patients with advanced cancer to select a phase II dose. J Diet Suppl. 2009;6(4):347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jensen BT, Jensen JB, Laustsen S, et al. . Multidisciplinary rehabilitation can impact on health-related quality of life outcome in radical cystectomy: secondary reported outcome of a randomized controlled trial. J Multidiscip Healthc. 2014;7:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johansson JE, Wersäll P, Brandberg Y, et al. . Efficacy of epoetin beta on hemoglobin, quality of life, and transfusion needs in patients with anemia due to hormone-refractory prostate cancer: a randomized study. Scand J Urol Nephrol. 2001;35(4):288–294. [DOI] [PubMed] [Google Scholar]
- 26. Jordhøy MS, Fayers P, Loge JH, et al. . Quality of life in palliative cancer care: results from a cluster randomized trial. J Clin Oncol. 2001;19(18):3884–3894. [DOI] [PubMed] [Google Scholar]
- 27. Kang SB, Park JW, Jeong SY, et al. . Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11(7):637–645. [DOI] [PubMed] [Google Scholar]
- 28. Kassam Z, Mackay H, Buckley CA, et al. . Evaluating the impact on quality of life of chemoradiation in gastric cancer. Curr Oncol. 2010;17(4):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kessler PA, Bloch-Birkholz A, Leher A, et al. . Evaluation of quality of life of patients with oral squamous cell carcinoma. Comparison of two treatment protocols in a prospective study. Radiother Oncol. 2004;70(3):275–282. [DOI] [PubMed] [Google Scholar]
- 30. King PM, Blazeby JM, Ewings P, et al. . Detailed evaluation of functional recovery following laparoscopic or open surgery for colorectal cancer within an enhanced recovery programme. Int J Colorectal Dis. 2008;23(8):795–800. [DOI] [PubMed] [Google Scholar]
- 31. Kopp I, Bauhofer A, Koller M. Understanding quality of life in patients with colorectal cancer: comparison of data from a randomised controlled trial, a population based cohort study and the norm reference population. Inflamm Res. 2004;53(suppl 2):S130–S135. [DOI] [PubMed] [Google Scholar]
- 32. Kornblith AB, Herndon JE, Silverman LR, et al. . Impact of azacytidine on the quality of life of patients with myelodysplastic syndrome treated in a randomized phase III trial: a Cancer and Leukemia Group B study. J Clin Oncol. 2002;20(10):2441–2452. [DOI] [PubMed] [Google Scholar]
- 33. Koukouli S, Stamou A, Alegakis A, et al. . Psychometric properties of the QLQ-C30 (version 3.0) in a sample of ambulatory Cretan cancer patients. Eur J Cancer Care (Engl). 2009;18(5):447–456. [DOI] [PubMed] [Google Scholar]
- 34. Lang I, Inbar MJ, Kahan Z, et al. . Quality of life (QOL) results from the TURANDOT trial comparing two bevacizumab (BEV)-containing regimens as first-line treatment for HER2-negative metastatic breast cancer (mBC) [abstract]. Cancer Res. 2012;72(24 suppl):P5-17-03. [Google Scholar]
- 35. Lara PN Jr, Douillard JY, Nakagawa K, et al. . Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(22):2965–2971. [DOI] [PubMed] [Google Scholar]
- 36. Lee SJ, Richardson PG, Sonneveld P, et al. . Bortezomib is associated with better health-related quality of life than high-dose dexamethasone in patients with relapsed multiple myeloma: results from the APEX study. Br J Haematol. 2008;143(4):511–519. [DOI] [PubMed] [Google Scholar]
- 37. Leppert W, Majkowicz M, Forycka M, et al. . Quality of life assessment in advanced cancer patients treated at home, an inpatient unit, and a day care center. Onco Targets Ther. 2014;7:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin KY, Shun SC, Lai YH, et al. . Comparison of the effects of a supervised exercise program and usual care in patients with colorectal cancer undergoing chemotherapy. Cancer Nurs. 2014;37(2):E21–E29. [DOI] [PubMed] [Google Scholar]
- 39. Lips IM, van Gils CH, van der Heide UA, et al. . Health-related quality of life 3 years after high-dose intensity-modulated radiotherapy with gold fiducial marker-based position verification. BJU Int. 2009;103(6):762–767. [DOI] [PubMed] [Google Scholar]
- 40. Macquart-Moulin G, Viens P, Palangie T, et al. . High-dose sequential chemotherapy with recombinant granulocyte colony-stimulating factor and repeated stem-cell support for inflammatory breast cancer patients: does impact on quality of life jeopardize feasibility and acceptability of treatment. J Clin Oncol. 2000;18(4):754–764. [DOI] [PubMed] [Google Scholar]
- 41. Di Maio M, Leighl NB, Gallo C, et al. . Quality of life analysis of TORCH, a randomized trial testing first-line erlotinib followed by second-line cisplatin/gemcitabine chemotherapy in advanced non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1830–1844. [DOI] [PubMed] [Google Scholar]
- 42. Marchand V, Bourdin S, Charbonnel C, et al. . No impairment of quality of life 18 months after high-dose intensity-modulated radiotherapy for localized prostate cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2010;77(4):1053–1059. [DOI] [PubMed] [Google Scholar]
- 43. van der Meulen IC, May AM, de Leeuw JR, et al. . Long-term effect of a nurse-led psychosocial intervention on health-related quality of life in patients with head and neck cancer: a randomised controlled trial. Br J Cancer. 2014;110(3):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Minniti G, Scaringi C, Baldoni A, et al. . Health-related quality of life in elderly patients with newly diagnosed glioblastoma treated with short-course radiation therapy plus concomitant and adjuvant temozolomide. Int J Radiat Oncol Biol Phys. 2013;86(2):285–291. [DOI] [PubMed] [Google Scholar]
- 45. Morak MJ, Pek CJ, Kompanje EJ, et al. . Quality of life after adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled study. Cancer. 2010;116(4):830–836. [DOI] [PubMed] [Google Scholar]
- 46. Ng SS, Leung WW, Wong CY, et al. . Quality of life after laparoscopic vs open sphincter-preserving resection for rectal cancer. World J Gastroenterol. 2013;19(29):4764–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. . Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. J Clin Oncol. 2009;27(21):3547–3556. [DOI] [PubMed] [Google Scholar]
- 48. Nuemi G, Devilliers H, Le Malicot K, et al. . Construction of quality of life change patterns: example in oncology in a phase III therapeutic trial (FFCD 0307). Health Qual Life Outcomes. 2015;13:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oliva EN, Nobile F, Alimena G, et al. . Quality of life in elderly patients with acute myeloid leukemia: patients may be more accurate than physicians. Haematologica. 2011;96(5):696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Osoba D, Aaronson NK, Muller M, et al. . Effect of neurological dysfunction on health-related quality of life in patients with high-grade glioma. J Neurooncol. 1997;34(3):263–278. [DOI] [PubMed] [Google Scholar]
- 51. Osoba D, Burchmore M. Health-related quality of life in women with metastatic breast cancer treated with trastuzumab (Herceptin). Semin Oncol. 1999;26(4 suppl 12):84–88. [PubMed] [Google Scholar]
- 52. Osoba D, Slamon DJ, Burchmore M, et al. . Effects on quality of life of combined trastuzumab and chemotherapy in women with metastatic breast cancer. J Clin Oncol. 2002;20(14):3106–3113. [DOI] [PubMed] [Google Scholar]
- 53. Osoba D, Zee B, Pater J, et al. . Psychometric properties and responsiveness of the EORTC quality of life questionnaire (QLQ-C30) in patients with breast, ovarian and lung cancer. Qual Life Res. 1994;3(5):353–364. [DOI] [PubMed] [Google Scholar]
- 54. Pavel M, Unger N, Borbath I, et al. . Quality-of-life (QoL) assessments in patients (pts) with pancreatic neuroendocrine tumors (pNET) enrolled in the open-label, phase 3b, multicenter, expanded access study of everolimus in pts with advanced NET [abstract]. Eur J Cancer. 2013;49(suppl 2):S619. [Google Scholar]
- 55. Poorkiani M, Abbaszadeh A, Hazrati M, et al. . The effect of rehabilitation on quality of life in female breast cancer survivors in Iran. Indian J Med Paediatr Oncol. 2010;31(4):105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pucciarelli S, Del Bianco P, Efficace F, et al. . Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg. 2011;253(1):71–77. [DOI] [PubMed] [Google Scholar]
- 57. Rathod S, Gupta T, Ghosh-Laskar S, et al. . Quality-of-life (QOL) outcomes in patients with head and neck squamous cell carcinoma (HNSCC) treated with intensity-modulated radiation therapy (IMRT) compared to three-dimensional conformal radiotherapy (3D-CRT): evidence from a prospective randomized study. Oral Oncol. 2013;49(6):634–642. [DOI] [PubMed] [Google Scholar]
- 58. Reck M, von Pawel J, Macha HN, et al. . Efficient palliation in patients with small-cell lung cancer by a combination of paclitaxel, etoposide and carboplatin: quality of life and 6-years’-follow-up results from a randomised phase III trial. Lung Cancer. 2006;53(1):67–75. [DOI] [PubMed] [Google Scholar]
- 59. Rees J, Hurt CN, Gollins S, et al. . Patient-reported outcomes during and after definitive chemoradiotherapy for oesophageal cancer. Br J Cancer. 2015;113(4):603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Reni M, Bonetto E, Cordio S, et al. . Quality of life assessment in advanced pancreatic adenocarcinoma: results from a phase III randomized trial. Pancreatology. 2006;6(5):454–463. [DOI] [PubMed] [Google Scholar]
- 61. Revicki DA, van den Eertwegh AJ, Lorigan P, et al. . Health related quality of life outcomes for unresectable stage III or IV melanoma patients receiving ipilimumab treatment. Health Qual Life Outcomes. 2012;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ringash J, Au HJ, Siu LL, et al. . Quality of life in patients with K-RAS wild-type colorectal cancer: the CO.20 phase 3 randomized trial. Cancer. 2014;120(2):181–189. [DOI] [PubMed] [Google Scholar]
- 63. Sadighi S, Mohagheghi MA, Montazeri A, et al. . Quality of life in patients with advanced gastric cancer: a randomized trial comparing docetaxel, cisplatin, 5-FU (TCF) with epirubicin, cisplatin, 5-FU (ECF). BMC Cancer. 2006;6:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schadendorf D, Amonkar MM, Milhem M, et al. . Functional and symptom impact of trametinib versus chemotherapy in BRAF V600E advanced or metastatic melanoma: quality-of-life analyses of the METRIC study. Ann Oncol. 2014;25(3):700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schadendorf D, Amonkar MM, Stroyakovskiy D, et al. . Health-related quality of life impact in a randomised phase III study of the combination of dabrafenib and trametinib versus dabrafenib monotherapy in patients with BRAF V600 metastatic melanoma. Eur J Cancer. 2015;51(7):833–840. [DOI] [PubMed] [Google Scholar]
- 66. Soffietti R, Kocher M, Abacioglu UM, et al. . A European organisation for research and treatment of cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65–72. [DOI] [PubMed] [Google Scholar]
- 67. Sorbe BG, Horvath G, Andersson H, et al. . External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma: a prospective, randomized study–quality-of-life analysis. Int J Gynecol Cancer. 2012;22(7):1281–1288. [DOI] [PubMed] [Google Scholar]
- 68. Steindorf K, Schmidt ME, Klassen O, et al. . Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol. 2014;25(11):2237–2243. [DOI] [PubMed] [Google Scholar]
- 69. Steinmann D, Paelecke-Habermann Y, Geinitz H, et al. . Prospective evaluation of quality of life effects in patients undergoing palliative radiotherapy for brain metastases. BMC Cancer. 2012;12:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stephens RJ, Thompson LC, Quirke P, et al. . Impact of short-course preoperative radiotherapy for rectal cancer on patients’ quality of life: data from the Medical Research Council CR07/National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. J Clin Oncol. 2010;28(27):4233–4239. [DOI] [PubMed] [Google Scholar]
- 71. Sundstrøm S, Bremnes RM, Brunsvig P, et al. . Palliative thoracic radiotherapy in locally advanced non-small cell lung cancer: can quality-of-life assessments help in selection of patients for short- or long-course radiotherapy. J Thorac Oncol. 2006;1(8):816–824. [PubMed] [Google Scholar]
- 72. Svensson H, Einbeigi Z, Johansson H, et al. . Quality of life in women with metastatic breast cancer during 9 months after randomization in the TEX trial (epirubicin and paclitaxel w/o capecitabine). Breast Cancer Res Treat. 2010;123(3):785–793. [DOI] [PubMed] [Google Scholar]
- 73. Taphoorn MJ, Bent MJ, Mauer ME, et al. . Health-related quality of life in patients treated for anaplastic oligodendroglioma with adjuvant chemotherapy: results of a European organisation for research and treatment of cancer randomized clinical trial. J Clin Oncol. 2007;25(36):5723–5730. [DOI] [PubMed] [Google Scholar]
- 74. Thorsen L, Skovlund E, Stromme SB, et al. . Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005;23(10):2378–2388. [DOI] [PubMed] [Google Scholar]
- 75. Vadiraja HS, Rao MR, Nagarathna R, et al. . Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complement Ther Med. 2009;17(5-6):274–280. [DOI] [PubMed] [Google Scholar]
- 76. Van Bokhorst-de Van der Schuer MA, Langendoen SI, Vondeling H, et al. . Perioperative enteral nutrition and quality of life of severely malnourished head and neck cancer patients: a randomized clinical trial. Clin Nutr. 2000;19(6):437–444. [DOI] [PubMed] [Google Scholar]
- 77. Velikova G, Barrett-Lee P, Bloomfield D, et al. . Quality of life results of the UK TACT2 trial: more intensive chemotherapy for early breast cancer has a measurable impact on patient-reported symptoms and functioning (CRUK/05/019) [abstract]. Eur J Cancer. 2014;50(suppl 2):S108–S110. [Google Scholar]
- 78. Verelst SG, Termorshuizen F, Uyl-de Groot CA, et al. . Effect of thalidomide with melphalan and prednisone on health-related quality of life (HRQoL) in elderly patients with newly diagnosed multiple myeloma: a prospective analysis in a randomized trial. Ann Hematol. 2011;90(12):1427–1439. [DOI] [PubMed] [Google Scholar]
- 79. Versmessen H, Vinh-Hung V, Van Parijs H, et al. . Health-related quality of life in survivors of stage I–II breast cancer: randomized trial of post-operative conventional radiotherapy and hypofractionated tomotherapy. BMC Cancer. 2012;12:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Voskuil DW, van Nes JG, Junggeburt JM, et al. . Maintenance of physical activity and body weight in relation to subsequent quality of life in postmenopausal breast cancer patients. Ann Oncol. 2010;21(10):2094–2101. [DOI] [PubMed] [Google Scholar]
- 81. Wardley A, Davidson N, Barrett-Lee P, et al. . Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration. Br J Cancer. 2005;92(10):1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wisløff F, Hjorth M. Health-related quality of life assessed before and during chemotherapy predicts for survival in multiple myeloma. Nordic Myeloma Study Group. Br J Haematol. 1997;97(1):29–37. [DOI] [PubMed] [Google Scholar]
- 83. Yeo W, Mo FK, Koh J, et al. . Quality of life is predictive of survival in patients with unresectable hepatocellular carcinoma. Ann Oncol. 2006;17(7):1083–1089. [DOI] [PubMed] [Google Scholar]
- 84. Yuste Sanchez MJ, Lacomba MT, Sanchez BS, et al. . Health related quality of life improvement in breast cancer patients: secondary outcome from a simple blinded, randomised clinical trial. Breast. 2015;24(1):75–81. [DOI] [PubMed] [Google Scholar]
- 85. Zeng J, Liu JS. Quality of life after three kinds of esophagectomy for cancer. World J Gastroenterol. 2012;18(36):5106–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ziefle S, Egberts F, Heinze S, et al. . Health-related quality of life before and during adjuvant interferon-α treatment for patients with malignant melanoma (DeCOG-trial). J Immunother. 2011;34(4):403–408. [DOI] [PubMed] [Google Scholar]
- 87. Zittoun R, Suciu S, Watson M, et al. . Quality of life in patients with acute myelogenous leukemia in prolonged first complete remission after bone marrow transplantation (allogeneic or autologous) or chemotherapy: a cross-sectional study of the EORTC-GIMEMA AML 8A trial. Bone Marrow Transplant. 1997;20(4):307–315. [DOI] [PubMed] [Google Scholar]
- 88. Miller J, Smith A, Kouba E, et al. . Prospective evaluation of short-term impact and recovery of health related quality of life in men undergoing robotic assisted laparoscopic radical prostatectomy versus open radical prostatectomy. J Urol. 2007;178(3 pt 1):854–858. [DOI] [PubMed] [Google Scholar]
- 89. Nilssen SR, Morkved S, Overgard M, et al. . Does physiotherapist-guided pelvic floor muscle training increase the quality of life in patients after radical prostatectomy? A randomized clinical study. Scand J Urol Nephrol. 2012;46(6):397–404. [DOI] [PubMed] [Google Scholar]
- 90. Kiebert GM, Curran D, Aaronson NK, et al. . Quality of life after radiation therapy of cerebral low-grade gliomas of the adult: results of a randomised phase III trial on dose response (EORTC trial 22844). Eur J Cancer. 1998;34(12):1902–1909. [DOI] [PubMed] [Google Scholar]
- 91. Alibhai SM, Breunis H, Timilshina N, et al. . Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28(34):5038–5045. [DOI] [PubMed] [Google Scholar]
- 92. Brown JC, Schmitz KH. Weight lifting and physical function among survivors of breast cancer: a post hoc analysis of a randomized controlled trial. J Clin Oncol. 2015;33(19):2184–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Doorenbos A, Given B, Given C, et al. . Physical functioning: effect of behavioral intervention for symptoms among individuals with cancer. Nurs Res. 2006;55(3):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Galvao DA, Spry N, Denham J, et al. . A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65(5):856–864. [DOI] [PubMed] [Google Scholar]
- 95. Griffith K, Wenzel J, Shang J, et al. . Impact of a walking intervention on cardiorespiratory fitness, self-reported physical function, and pain in patients undergoing treatment for solid tumors. Cancer. 2009;115(20):4874–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kemeny NE, Niedzwiecki D, Hollis DR, et al. . Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24(9):1395–1403. [DOI] [PubMed] [Google Scholar]
- 97. Kennedy RH, Francis A, Dutton S, et al. . EnROL: a multicentre randomised trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme. BMC Cancer. 2012;12:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kopec JA, Yothers G, Ganz PA, et al. . Quality of life in operable colon cancer patients receiving oral compared with intravenous chemotherapy: results from National Surgical Adjuvant Breast and Bowel Project Trial C-06. J Clin Oncol. 2007;25(4):424–430. [DOI] [PubMed] [Google Scholar]
- 99. Lengacher CA, Johnson-Mallard V, Post-White J, et al. . Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261–1272. [DOI] [PubMed] [Google Scholar]
- 100. McQuellon RP, Loggie BW, Fleming RA, et al. . Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27(1):65–73. [DOI] [PubMed] [Google Scholar]
- 101. Milbury K, Spelman A, Vence L, et al. . Baseline depressive symptoms moderate the effectiveness of expressive writing on subjective and biological outcome measures for patients with renal cell carcinoma [abstract]. Psychosom Med. 2014;76(3):A126. [Google Scholar]
- 102. Milbury K, Spelman A, Wood C, et al. . Randomized controlled trial of expressive writing for patients with renal cell carcinoma. J Clin Oncol. 2014;32(7):663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Newton-Bishop JA, Nolan C, Turner F, et al. . A quality-of-life study in high-risk (thickness > = or 2 mm) cutaneous melanoma patients in a randomized trial of 1-cm versus 3-cm surgical excision margins. J Investig Dermatol Symp Proc. 2004;9(2):152–159. [DOI] [PubMed] [Google Scholar]
- 104. Park SW, Kim TN, Nam JK, et al. . Recovery of overall exercise ability, quality of life, and continence after 12-week combined exercise intervention in elderly patients who underwent radical prostatectomy: a randomized controlled study. Urology. 2012;80(2):299–305. [DOI] [PubMed] [Google Scholar]
- 105. Roeloffzen EM, Lips IM, van Gellekom MP, et al. . Health-related quality of life up to six years after (125)I brachytherapy for early-stage prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76(4):1054–1060. [DOI] [PubMed] [Google Scholar]
- 106. Saad IA, Botega NJ, Toro IF. Predictors of quality-of-life improvement following pulmonary resection due to lung cancer. Sao Paulo Med J. 2007;125(1):46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Samue SR, Maiya GA, Babu AS, et al. . Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res. 2013;137(3):515–520. [PMC free article] [PubMed] [Google Scholar]
- 108. Saquib N, Pierce JP, Saquib J, et al. . Poor physical health predicts time to additional breast cancer events and mortality in breast cancer survivors. Psychooncology. 2011;20(3):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tehrani AM, Farajzadegan Z, Rajabi FM, et al. . Belonging to a peer support group enhances the quality of life and adherence rate in patients affected by breast cancer: a non-randomized controlled clinical trial. J Res Med Sci. 2011;16(5):658–665. [PMC free article] [PubMed] [Google Scholar]
- 110. Wyatt G, Sikorskii A, Rahbar MH, et al. . Health-related quality-of-life outcomes: a reflexology trial with patients with advanced-stage breast cancer. Oncol Nurs Forum. 2012;39(6):568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wyatt G, Sikorskii A, Tamkus D, et al. . Quality of life among advanced breast cancer patients with and without distant metastasis. Eur J Cancer Care (Engl). 2013;22(2):272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhao L, Portier K, Stein K, et al. . Exploratory factor analysis of the Cancer Problems in Living Scale: a report from the American Cancer Society's Studies of Cancer Survivors. J Pain Symptom Manage. 2009;37(4):676–686. [DOI] [PubMed] [Google Scholar]
- 113. Moinpour C, Berry DL, Ely B, et al. . Preliminary quality-of-life outcomes for SWOG-9346: intermittent androgen deprivation in patients with hormone-sensitive metastatic prostate cancer (HSM1PC)-phase III [abstract]. J Clin Oncol. 2012;30(suppl):15. [Google Scholar]
- 114. Moinpour CM, Savage MJ, Troxel A, et al. . Quality of life in advanced prostate cancer: results of a randomized therapeutic trial. J Natl Cancer Inst. 1998;90(20):1537–1544. [DOI] [PubMed] [Google Scholar]
- 115. Lindholm E, Daneryd P, Körner U, et al. . Effects of recombinant erythropoietin in palliative treatment of unselected cancer patients. Clin Cancer Res. 2004;10(20):6855–6864. [DOI] [PubMed] [Google Scholar]
- 116. Lips I, Dehnad H, Kruger AB, et al. . Health-related quality of life in patients with locally advanced prostate cancer after 76 Gy intensity-modulated radiotherapy vs. 70 Gy conformal radiotherapy in a prospective and longitudinal study. Int J Radiat Oncol Biol Phys. 2007;69(3):656–661. [DOI] [PubMed] [Google Scholar]
- 117. Nout RA, van de Poll-Franse LV, Lybeert ML, et al. . Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the post operative radiation therapy in endometrial carcinoma 1 (PORTEC-1) trial. J Clin Oncol. 2011;29(13):1692–1700. [DOI] [PubMed] [Google Scholar]
- 118. Sancho A, Carrera S, Arietaleanizbeascoa M, et al. . Supervised physical exercise to improve the quality of life of cancer patients: the EFICANCER randomised controlled trial. BMC Cancer. 2015;15(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Travier N, Guillamo E, Oviedo GR, et al. . Is quality of life related to cardiorespiratory fitness in overweight and obese breast cancer survivors. Women Health. 2015;55(5):505–524. [DOI] [PubMed] [Google Scholar]
- 120. Beaton DE, Katz JN, Fossel AH, et al. . Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder, and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2):128–146. [PubMed] [Google Scholar]
- 121. Kopec JA, Colangelo LH, Land SR, et al. . Relationship between arm morbidity and patient-reported outcomes following surgery in women with node-negative breast cancer: NSABP protocol B-32. J Support Oncol. 2013;11(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lee SA, Kang JY, Kim YD, et al. . Effects of a scapula-oriented shoulder exercise programme on upper limb dysfunction in breast cancer survivors: a randomized controlled pilot trial. Clin Rehabil. 2010;24(7):600–613. [DOI] [PubMed] [Google Scholar]
- 123. Oizumi S, Kobayashi K, Inoue A, et al. . Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of North East Japan Study Group 002 Trial. Oncologist. 2012;17(6):863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kobayashi K, Green J, Shimonagayoshi M, et al. . Validation of the care notebook for measuring physical, mental and life well-being of patients with cancer. Qual Life Res. 2005;14(4):1035–1043. [DOI] [PubMed] [Google Scholar]
- 125. Rummans TA, Clark MM, Sloan JA, et al. . Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: a randomized controlled trial. J Clin Oncol. 2006;24(4):635–642. [DOI] [PubMed] [Google Scholar]
- 126. Johansson E, Steineck G, Holmberg L, et al. . Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12(9):891–899. [DOI] [PubMed] [Google Scholar]
- 127. Kolligs FT, Bilbao JI, Jakobs T, et al. . Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35(6):1715–1721. [DOI] [PubMed] [Google Scholar]
- 128. Leitgeb C, Pecherstorfer M, Fritz E, et al. . Quality of life in chronic anemia of cancer during treatment with recombinant human erythropoietin. Cancer. 1994;73(10):2535–2542. [DOI] [PubMed] [Google Scholar]
- 129. Morita S, Kobayashi K, Eguchi K, et al. . Influence of clinical parameters on quality of life during chemotherapy in patients with advanced non-small cell lung cancer: application of a general linear model. Jpn J Clin Oncol. 2003;33(9):470–476. [DOI] [PubMed] [Google Scholar]
- 130. Patrick DL, Burke LB, Gwaltney CJ, et al. . Content validity–establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2–assessing respondent understanding. Value Health. 2011;14(8):978–988. [DOI] [PubMed] [Google Scholar]
- 131. Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: SAGE Publications, Inc; 2005. [Google Scholar]
- 132. Basch E, Jia X, Heller G, et al. . Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Basch EM, Reeve BB, Mitchell SA, et al. . Electronic toxicity monitoring and patient-reported outcomes. Cancer J. 2011;17(4):231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Stover A, Irwin DE, Chen RC, et al. . Integrating patient-reported outcome measures into routine cancer care: cancer patients’ and clinicians’ perceptions of acceptability and value. EGEMS (Wash DC). 2015;3(1):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Locke DE, Decker PA, Sloan JA, et al. . Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients. J Pain Symptom Manage. 2007;34(6):628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rose M, Bjorner JB, Gandek B, et al. . The PROMIS physical function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol. 2014;67(5):516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Schalet BD, Hays RD, Jensen SE, et al. . Validity of PROMIS physical function measured in diverse clinical samples. J Clin Epidemiol. 2016;73:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jensen RE, Potosky AL, Reeve BB, et al. . Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res. 2015;24(10):2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jensen RE, Moinpour CM, Potosky AL, et al. . Responsiveness of 8 Patient-Reported Outcomes Measurement Information System (PROMIS) measures in a large, community-based cancer study cohort. Cancer. 2017;123(2):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bruce B, Fries JF, Ambrosini D, et al. . Better assessment of physical function: item improvement is neglected but essential. Arthritis Res Ther. 2009;11(6):R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cella DF, Tulsky DS, Gray G, et al. . The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.