Abstract
Very little is known about the influence of early life exposures on adult cancer risk. The purpose of this narrative review was to summarize the epidemiologic evidence relating early life tobacco use, obesity, diet, and physical activity to adult cancer risk; describe relevant theoretical frameworks and methodological strategies for studying early life exposures; and discuss policies and research initiatives focused on early life. Our findings suggest that in utero exposures may indirectly influence cancer risk by modifying biological pathways associated with carcinogenesis; however, more research is needed to firmly establish these associations. Initiation of exposures during childhood and adolescence may impact cancer risk by increasing duration and lifetime exposure to carcinogens and/or by acting during critical developmental periods. To expand the evidence base, we encourage the use of life course frameworks, causal inference methods such as Mendelian randomization, and statistical approaches such as group-based trajectory modeling in future studies. Further, we emphasize the need for objective exposure biomarkers and valid surrogate endpoints to reduce misclassification. With the exception of tobacco use, there is insufficient evidence to support the development of new cancer prevention policies; however, we highlight existing policies that may reduce the burden of these modifiable risk factors in early life.
Keywords: adult cancer risk, diet, early life exposures, methodological strategies, obesity, physical activity, policies and research initiatives, tobacco use
INTRODUCTION
Exposure to established modifiable cancer risk factors such as tobacco use, obesity, diet, physical activity, infectious agents (refer to the review by Vedham et al. (1)), and ultraviolet radiation (refer to the review by Balk et al. (2)) can begin in early life (Table 1). Currently, there are major gaps in our understanding of how these exposures in early life, either alone or in combination with other environmental and genetic factors, influence subsequent cancer risk (3, 4). Such gaps are due, in part, to the significant methodological challenges to studying early life exposures, such as the limited validity and reliability of long-term exposure measurements and long induction periods between early life exposures and cancer outcomes in adulthood (3). Thus, to date, epidemiologic research has focused primarily on older adult populations; very little is known about the etiological relevance of exposure to modifiable risk factors in other stages of the life course, and whether temporal factors, such as stage of development and/or duration of exposure, modify the influence of early life exposures on adult cancer risk (5). Epidemiologic studies designed to address these significant knowledge gaps could provide important insights into etiology and help to identify critical points for targeting interventions that may achieve the greatest benefit for cancer prevention (3, 4).
Table 1.
Prevalence of the Most Common Modifiable Risk Factors for Cancer in Adulthood and Early Life in the United Statesa
| Risk Factor | Cancers Caused by Risk Factor, %a | Cancer Sitesb | Examples of Risk Factor Prevalence | ||
|---|---|---|---|---|---|
| Adulthood | Childhood and Adolescence | In Utero | |||
| Tobacco | 33 | Lung, mouth, larynx, pharynx, esophagus, stomach, colon/rectum, pancreas, kidney, bladder, cervix, ovary, myeloid leukemia | 18% use tobacco.c | 40% of children are exposed to ETS in the homed; 7.4% in middle school and 25.3% of high school students use tobacco.f |
10.7% of women smoke during pregnancy.e |
| Overweight and obesity | 20 | Breast, endometrium, colon/rectum, kidney, pancreas, esophagus, gallbladder, ovary, thyroid, possibly prostate | 66% are overweight or obese.g | 22.8%–34.5% are overweight or obese.g | 51.3% of reproductive age women are overweight or obese.g |
| Diet | 5 | Breast, colon/rectum, possibly pancreas | 67.1%–95.2% do not meet recommendations for fruit/vegetable intakeh; 80.2%–91.5% exceed recommendations for sugar/fat intake.h |
39.6%–98.4% do not meet recommendations for fruit/vegetable intakeh; 71.6%–99.9% exceed recommendations for sugar/fat intake.h |
86.0%–91.5% of reproductive age women exceed recommendations for sugar/fat intake.h 74.8% of reproductive age women do not meet recommendations for fruit/vegetable intake.h |
| Physical inactivityh,i | 5 | Breast, endometrium, colon | 50.8% do not meet recommendations for physical activity.i | 58.0%–92.0% do not meet recommendations for daily physical activity.j | 48.4% of reproductive age women do not meet recommendations for physical activity. |
Abbreviation: ETS, environmental tobacco smoke.
a Adapted from Colditz et al. (4).
b Source: American Cancer Society (7).
c Source: US Department of Health and Human Services (8).
d Source: Homa et al. (281).
e Source: Tong et al. (280).
f Source: Singh et al. (252).
g Source: Ogden et al. (33).
h Source: National Cancer Institute (282).
i Source: Centers for Disease Control and Prevention (283).
j Source: National Physical Activity Plan Alliance (284).
The purpose of this narrative review is 3-fold. First, we synthesize the current state of epidemiologic evidence linking early life exposures and adult cancer risk. A systematic evaluation of all cancer risk factors is beyond the scope of this narrative review; therefore, we limit our focus to the most common and modifiable cancer risk factors, namely, tobacco use, obesity, diet, and physical activity, which account for approximately 50% of all cancers (Table 1) (4, 6, 7). Next, to facilitate the epidemiologic study of early life exposures and adult cancer risk, we describe theoretical frameworks and methodological strategies that may address some of the current challenges associated with studying early life exposures. Finally, based on the summary of evidence presented, we identify potential policies and research initiatives that address early life exposures.
METHODS
For this narrative review, we focused on peer-reviewed, epidemiologic studies relating tobacco use, obesity, diet, and physical activity during periods of early life, namely, in utero, childhood, and adolescence (<21 years), to adult cancer risk. We used a life-course epidemiology framework to guide our literature search, focusing on evidence for associations of these risk factors with cancer and/or well-established cancer risk factors (e.g., obesity in adulthood). We conducted MEDLINE searches for English-language epidemiologic studies published in the peer-reviewed literature through July 2016, including combinations of the following terms: “pregnancy,” “in utero,” “gestational,” “prenatal,” “birth weight,” “fetal growth,” “childhood,” “adolescence,” “youth,” “early-life,” “offspring,” “tobacco,” “smoking,” “body size,” “adiposity,” “weight gain,” “obesity,” “diet,” “nutrition,” “breastfeeding,” “physical activity,” “exercise,” “puberty,” “menarche,” “cancer,” and “carcinogenesis.” We did not restrict by country of origin. We identified additional relevant articles in the References section of articles identified by our search process. We prioritized systematic reviews, meta-analyses, and population-based, prospective cohort studies. We also included government reports, including the Surgeon General's reports on tobacco use (8, 9). Theoretical frameworks and methodological strategies described focus on issues addressed during workshops held by the National Cancer Institute and the Centers for Disease Control and Prevention on this topic (3, 10), as well as methods from the causal inference literature (11, 12). On the basis of our review of the literature, we identified current research initiatives and US-based policies and/or programs that address the early life exposures.
EPIDEMIOLOGIC EVIDENCE LINKING EARLY LIFE EXPOSURES TO ADULT CANCER RISK
Tobacco
Over 90% of adult smokers try their first cigarette before 18 years of age, making adolescence and young adulthood a critical period for lung cancer risk (8). Young age of smoking initiation increases lung cancer risk by increasing cumulative exposure to tobacco smoke and may also represent a critical developmental period for enhanced lung cancer susceptibility (8, 13). With respect to other cancers, the evidence is less clear; cigarette smoking in young adulthood likely influences the early stages of colorectal carcinogenesis, supported by the consistent association of smoking and adenomas (8). For breast cancer, the timing of smoking relative to critical periods of breast development (e.g., pregnancy) may be important (8, 14), although the current evidence is insufficient to support this hypothesis (8).
Childhood tobacco smoke exposure occurs primarily through environmental tobacco smoke (ETS). With respect to childhood ETS exposure and lung cancer risk, findings from the meta-analysis of 24 studies included in the latest Surgeon General's report on ETS published in 2006 suggested some heterogeneity across studies but an overall null association (9). More recently, findings from 2 case-control studies with independent validation suggested a positive association of childhood ETS exposure with lung cancer risk, with potential effect modification by genetic variants involved in lung physiology and immunity (15, 16). More research is needed to determine whether gene-environment interactions can help to clarify the heterogeneity observed within and across studies (17).
Exposure to tobacco smoke in utero occurs indirectly from maternal smoking and/or maternal ETS exposure. Although some studies suggest a modest association between in utero exposure to paternal ETS and childhood leukemia (9, 18–22), current evidence does not support an association with adult cancer risk (9, 15, 16, 23, 24). Experimental and epidemiologic evidence suggests potential associations of maternal smoking with risk of obesity and metabolic syndrome in child offspring, which may increase their risk for certain types of cancer later in life. Indeed, findings from a recent systematic review and meta-analysis of 39 epidemiologic studies suggest an association of maternal smoking with childhood obesity, independent of important confounders such as socioeconomic status, maternal age, and body mass index (25). Prenatal ETS exposure has also been associated with aberrant fetal hormonal and growth factor signaling and early onset puberty, which could have implications for offspring risk of hormone-associated cancers later in life (26–28). Two recent systematic reviews and meta-analyses reported an association of prenatal ETS exposure with early onset puberty in both males and females (29, 30); however, according to a meta-analysis of 11 case-control studies, prenatal ETS exposure was not associated with risk of testicular cancer (30). Importantly, a majority of the studies included in the systematic reviews and meta-analyses ascertained prenatal ETS exposure through maternal self-report, which could be subject to recall bias and/or misclassification (31, 32).
In summary, the association between adolescent exposure to tobacco smoke and lung cancer risk is well-established. More research is needed to determine the independent effects of childhood and in utero ETS exposure on risk of lung and other cancers in adulthood. Incorporation of gene-environment interaction analyses may help to clarify the heterogeneity observed within and across studies of lung cancer (17).
Overweight and obesity
Nearly one-third of children and adolescents are overweight or obese (33), putting them at greater risk for chronic diseases, including certain cancers, later in life (34, 35). Childhood obesity and adolescent obesity have been associated with decreased risk of premenopausal breast cancer and benign breast disease, irrespective of adult body mass index (36, 37). Although the health risks associated with obesity far outweigh any potential benefit with respect to premenopausal breast cancer, this association provides important clues about how adiposity during a critical window of breast development (e.g., puberty) can influence subsequent cancer risk (36). With respect to other obesity-related cancers, US prospective cohort and population-based case-control studies have consistently demonstrated an association of weight gain since the age of 18 years with risk of endometrial cancer (38–44). Some of these studies also reported a positive association of obesity in adolescence with endometrial cancer risk, mediated by obesity in adulthood (38, 41, 45). The evidence for colorectal cancer is also fairly consistent, with most studies supporting an association of obesity in adolescence and weight gain from early to later adulthood with increased risk of colorectal cancer, although a majority were restricted to men only (46–49). In studies that included both sexes, 1 reported a stronger association among women compared with men (50), while others reported a stronger association among men (51, 52). In a large cohort study of older women assessing body mass index at multiple time periods, only weight gain since age 18 years and adult body mass index were positively associated with colorectal cancer risk (53). Data from prospective studies for other cancer types remain sparse, but they suggest potential associations between early life obesity and risk of esophageal cancer (54, 55), pancreatic cancer (56), and multiple myeloma (57). A pooled analysis of 20 cohort studies found that elevated body mass index at ages 18–21 years was a more significant predictor of pancreatic cancer mortality than body mass index gain in adulthood (58). In support of some of these findings, a series of population-based studies of school health records from over 140,000 children linked to Danish Cancer Registry data has demonstrated a positive association of childhood body mass index with risk of endometrial, esophageal, liver, and thyroid cancer in adulthood (59–62) but not with prostate cancer (63, 64). Of note, these studies were unable to adjust for adult body mass index.
Current evidence does not support an association between in utero exposure to maternal obesity and/or gestational weight gain with adult cancer risk. However, maternal obesity, like gestational diabetes (65–67), has been consistently associated with increased infant and early childhood growth (68–77). Experimental evidence suggests that this association may be mediated by higher levels of maternal glucose and fatty acids crossing the placenta, leading to increased fetal insulin production and adipose tissue accumulation (66, 78, 79), as well as potential deregulation of hypothalamic and neuroendocrine pathways responsible for long-term energy balance (80–82). This hypothesis is supported by evidence from early studies of women who underwent bariatric surgery between pregnancies, reporting a decreased prevalence of overweight and obesity and lower blood glucose, insulin, lipid, and triglyceride levels in offspring born after surgery compared with their siblings born prior to surgery (76, 77). Data from recent meta-analyses and additional prospective studies suggest that excess gestational weight gain may also influence early childhood adiposity (83–86), although the evidence is less consistent in studies among older children (87–91). Several epidemiologic studies have evaluated the association of birth weight with adult cancer risk. Recent systematic reviews and meta-analyses suggest a positive dose-response relationship between birth weight and breast cancer risk (92), a modest positive association of birth weight with total and aggressive/lethal prostate cancer (93), and an inverse relationship between birth weight and testicular cancer (94). Some prospective studies have also shown a positive association between birth weight and risk of colon cancer and adenomas (95–97), while others have demonstrated a J-shaped (98) or inverse (99) relationship between birth weight and colorectal cancer risk. These discrepancies could be attributed to use of self-reported birth weight in some studies (96–98) and/or lack of adjustment for potential confounders in adulthood (95, 98, 99).
In summary, childhood obesity and adolescent obesity have been associated with a decreased risk of premenopausal breast cancer, as well as a suggestive increased risk of several other cancers, including endometrial, colorectal, and pancreatic cancers. More work is needed to better clarify the etiologically relevant time windows for early life obesity and adult cancer risk. There is limited evidence that exposure to maternal obesity and/or gestational weight gain in utero directly influences adult cancer risk, but there is strong evidence that these exposures are associated with early life and potentially adult adiposity, thereby indirectly increasing risk for many cancers.
Diet
Childhood and adolescent dietary exposures have been extensively studied in relation to breast cancer risk (100, 101). Studies of Japanese migrants were some of the first to indicate an association of childhood exposure to a “Westernized” diet with adult breast cancer risk, providing preliminary support for a role for early life dietary exposures in breast carcinogenesis (102). A number of studies conducted among Asian and Asian-American women have reported a protective effect of childhood and adolescent soy intake against breast cancer risk, with stronger evidence for premenopausal breast cancer (103–107). In the Nurses’ Health Study II, adolescent intakes of fiber, vegetables, and nuts were associated with reduced risk of premenopausal breast cancer (108, 109) and benign breast disease (110, 111), while adolescent intakes of fat and red meat have been associated with increased breast density and premenopausal breast cancer (112–114). These findings have been replicated in some (115–120), but not all (121, 122), studies. Certain high-fat foods have been associated with increased estrogen production and early menarche, a marker of prolonged estrogen exposure, which could have important implications for breast and other hormone-related cancers (123–126). In a randomized trial designed to reduce fat intake in children, females in the intervention arm had reduced estradiol and progesterone concentrations at 5 years of follow-up (123); however, these associations did not persist into young adulthood (124). Two recent prospective studies of young girls reported an association of caffeinated beverage consumption with early menarche; however, findings were inconsistent in regard to sugar-sweetened and artificially sweetened beverage consumption (127, 128).
With respect to other cancers, findings from the Nurses’ Health Study II have reported associations between adolescent fish and poultry consumption (vs. red meat) and consumption of fruits, vegetables, and fish, with reduced risk of colorectal and rectal adenomas, respectively (129, 130). In the Boyd Orr cohort, childhood dairy intake was associated with colorectal cancer risk in adulthood, irrespective of meat, fruit and vegetable intake, and socioeconomic status (131). In the National Institutes of Health (NIH)-AARP Diet and Health Study, adolescent intakes of vegetables and vitamin A were associated with decreased colorectal cancer risk, independent of adult diet, whereas the protective effect for some nutrients, like calcium, was strongest when consumed in both adolescence and adulthood (132). In this same study, both adolescent and adult consumption of red and processed meat was associated with increased colorectal cancer risk, suggesting a cumulative effect (132). In a separate study from the same cohort, adolescent intake of foods rich in iodine was associated with thyroid cancer risk, whereas diet during midlife had no effect (133).
Although the literature does not support a direct relationship between in utero dietary exposures and adult cancer risk, there is a large body of research supporting a direct relationship between certain maternal dietary exposures (e.g., folic acid and long-chain fatty acids) and pre-and postnatal growth and development (134–136). Studies of prenatal famine exposure during the Dutch Hunger Winter following World War II were some of the first to demonstrate long-term effects of caloric restriction on offspring obesity and chronic disease risk (137, 138). Maternal overnutrition, particularly high-fat intake, has also been associated with offspring obesity risk in several prospective studies (139–142), potentially mediated through gestational diabetes and/or increased fetal exposure to fatty acids (143, 144). Some studies of specific maternal dietary patterns (140, 145–149), but not all (150–153), have reported associations with offspring growth and adiposity. To avoid potential misclassification associated with assessment of recalled dietary and nutrient exposures, some studies have explored the relationship between measures of overall maternal diet quality in relation to offspring growth and obesity risk (142, 154–156). For example, in a recent prospective study of adherence to the 2010 Dietary Guidelines for Americans, Shapiro et al. (156) found that nonadherence was related to increased neonatal fat mass, likely driven by total and saturated fat intake. In addition to maternal diet, numerous long-term beneficial effects of breastfeeding have been documented for both the mother and child, including decreased risk of offspring overweight and diabetes and of diabetes and breast and ovarian cancers in mothers (157–161).
In summary, childhood and adolescent diets have been most thoroughly studied with respect to breast and colorectal cancers; diets that are higher in fruits and vegetables, and lower in red meat and fat, appear to be associated with a lower risk of these cancers. More work is needed to characterize the influence of childhood and adolescent diets on other adult cancers. There is no evidence that exposure to specific maternal dietary factors in utero directly influences adult cancer risk, but there is evidence that maternal diet is associated with early life growth and potentially later-life adiposity, thereby indirectly increasing risk for many cancers.
Physical activity
As with diet, childhood and adolescent physical activities have been most thoroughly studied with respect to breast cancer. In the Nurses’ Health Study II, adolescent and lifetime physical activities were associated with reduced risk of premenopausal breast cancer (162, 163), with 1 study suggesting a stronger effect for women who had a long interval (<20 years) between menarche and first pregnancy (164). Despite these findings, most studies, including those conducted within the NIH-AARP and Women's Health Initiative cohorts, have not found an association of early life physical activity with reduced breast cancer risk (165–169). With respect to other cancers, vigorous physical activity during adolescence has been linked to reduced risk of Hodgkin lymphoma in women (170) and renal cell cancer (171–173). Some, but not all, studies have found an association between childhood and adolescent physical activity and reduced endometrial cancer risk (174–176).
Although the literature does not support an association of maternal physical activity with offspring cancer risk, the protective effects of exercise during pregnancy against excessive gestational weight gain and gestational diabetes are well-established (177–183), which could potentially mitigate the risk of offspring obesity (182, 184–190). The direct effects of maternal physical activity on offspring size and growth are unclear; some systematic reviews and meta-analyses have reported a modest association with lower birth weight and reduced risk of delivering a large-for-gestational-age infant (191–193), but not all (177, 194). In 1 small, community-based randomized trial, moderate-intensity exercise in late pregnancy was associated with lower birth weight and reduced fetal insulin-like growth factor I/II levels in cord blood (195).
In summary, there is inconsistent evidence that physical activity in adolescence may be protective against breast cancer. More work is needed to better characterize the influence of physical activity in adolescence on other adult cancers. Childhood physical activity has largely been unstudied with respect to adult cancer risk. There is no evidence that exposure to maternal physical activity in utero directly influences adult cancer risk, but there is evidence that maternal physical activity can reduce excess gestational weight gain, thereby indirectly decreasing risk for childhood adiposity and potentially adult cancer risk.
THEORETICAL FRAMEWORKS AND METHODOLOGICAL STRATEGIES FOR STUDYING EARLY LIFE
Epidemiologic studies of early life exposures and cancer risk require unique design and analytical considerations. The long time interval presents a challenge for reliable and accurate exposure measurement and increases the possibility of misclassification and confounding by later-life exposures (196). Here we describe theoretical frameworks and methodological strategies that may be particularly useful for studying early life exposures and adult cancer risk.
Multistage models of carcinogenesis
The 2-stage clonal expansion model assumes 3 phases of carcinogenesis: 1) the acquisition by a susceptible stem cell of 1 or more genetic or epigenetic changes (“initiation”); 2) clonal expansion of initiated cells (“promotion”); and 3) malignant transformation of 1 of the initiated clones via additional genetic and/or epigenetic changes (197). Early life exposures, such as tobacco smoke and diet, may affect different phases of this multistage process and, in some cases, may exert differential effects (198, 199). The widely used Cox proportional hazards model offers flexibility in assessing temporal aspects of an exposure-outcome relationship; if nonproportionality is suspected, time-dependent variables can be modeled with an interaction term for the covariate of interest and time (200–202). An alternative, fully parametric approach is the mathematical 2-stage clonal expansion model, which is biologically based and can incorporate complex temporal exposure patterns, including age at initiation/cessation and intensity into the analyses (for detailed methods, refer to Moolgavkar and Luebeck (199)). Rather than a hazard ratio, this approach uses likelihood-based methods to estimate individual hazard functions for specific exposure histories, which can be converted into relative risks by taking the ratio of the estimated hazard functions. This model has been used to show that smoking affects mainly the promotion of lung carcinogenesis (203), to estimate optimal colorectal cancer screening schedules (204, 205), and to clarify the dual effects of folate supplementation on colorectal carcinogenesis (206, 207).
Life-course epidemiology models
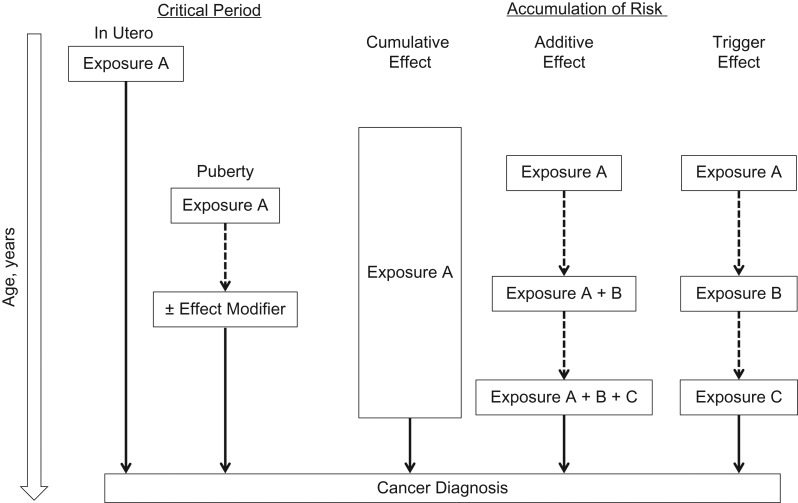
The life-course approach to chronic disease epidemiology, proposed by Ben-Shlomo and Kuh (5), provides a framework for developing hypotheses about the different pathways through which early life exposures may influence cancer risk (Figure 1). For example, during “critical” or “sensitive” developmental periods (e.g., in utero and puberty), exposures may modify processes related to growth and development by inducing permanent changes in tissue differentiation, metabolism, and gene expression that directly or indirectly influence cancer risk (5, 208). Retrospective ascertainment of early life exposure data is particularly prone to both nondifferential and differential misclassification due to long recall times and/or recall bias, which can attenuate effect estimates and result in spurious associations, respectively (3, 209). Moreover, including misclassified early life exposures in a multivariable model with later life exposures, presumably measured with greater precision and accuracy, can distort relative risk estimates, particularly if those exposures are strongly correlated (209). The identification of objective and reliable exposure biomarkers may address some of the challenges related to early life exposure measurement, although very few have been identified (3). Epigenetic modifications, such as DNA methylation, are critical for regulating cellular processes during development and responses to endogenous and exogenous exposures that are maintained over time. There has been growing interest in evaluating DNA methylation changes as biomarkers of early life exposures (refer to the report by Ladd-Acosta (210)). For example, several studies have reported an association of maternal cigarette smoking with DNA methylation changes in cord blood (211–217), with some evidence suggesting postnatal stability of these changes in children (212, 218–222) and adults (223–226). Although promising, large gaps in our understanding of the long-term stability, tissue specificity, and functional relevance of these DNA methylation changes limit their current utility as long-term biomarkers (227). Initiatives such the Roadmap Epigenomics Mapping Consortium may address some of these fundamental questions (228). In addition to understanding the biology of DNA methylation, researchers continue to develop epidemiologic methods related to quality control assessment, site-specific versus region-based analyses, and the most appropriate statistical models for detecting associations (227). Use of functional annotation tools, such as the University of California, Santa Cruz, Genome Browser (https://genome.ucsc.edu), may lead to hypotheses regarding the functional significance of DNA methylation changes to help inform some of these methods (227).
Figure 1.
Life-course epidemiology frameworks. The “critical period model” depicts how early life exposures can act during a critical period of development (e.g., in utero or puberty) and have lasting effects on future cancer risk, with or without the presence of effect modifiers later in life. In contrast, the “accumulation of risk models” depict how early life exposures can contribute to future cancer risk by increasing the duration of lifetime exposure (“cumulative effect”), leading to other exposures later in life such that each exposure increases cancer risk in a cumulative fashion (“additive effect,” exposures A + B + C) or indirectly by increasing the likelihood of a causal exposure (“trigger effect,” exposure C) later in life.
In addition to biomarkers of early life exposures, surrogate endpoints could potentially overcome some of the limitations associated with long-term exposure measurement (196). A surrogate endpoint can be defined as a measurable marker of preclinical carcinogenesis that lies on a causal pathway linking an exposure to cancer risk or, in some cases, is closely linked to a component of the causal pathway (229). Types of markers include histological changes, cellular processes (e.g., proliferation and apoptosis), molecular markers, infectious agents, cytokines, hormones, and prognostic factors (e.g., tumor recurrence) (229). Although the rapid advancement of high-throughput technologies has enhanced our ability to measure new markers, relatively few examples of valid surrogate endpoints exist in the cancer literature (e.g., colorectal adenoma, cervical intraepithelial neoplasia) (229). The presence of alternative causal pathways poses a major challenge to validating surrogate endpoints; if a potential surrogate is not a necessary component of the carcinogenic pathway, an estimate of its association with an exposure may not accurately reflect the association between the exposure and the true cancer endpoint (230). This is particularly problematic if an exposure influences an alternative pathway that offsets the effect of the exposure on the surrogate (229). To overcome some of these limitations, putative surrogates, ideally measured over multiple time points, should be integrated into studies with cancer outcomes so that they can be evaluated (229).
Early life exposures may also gradually accumulate over the life course such that their cumulative effect increases cancer risk (5) (Figure 1); however, this is often difficult to measure in epidemiologic studies (3). The tobacco literature defines cumulative exposure as the product of smoking duration and intensity (i.e., “pack-years”); however, the term, pack-years, does not account for other temporal factors such as intensity and age at smoking initiation/cessation (231). Expanded models that include parameters for smoking duration and intensity are difficult to interpret as they necessarily incorporate pack-year associations when either 1 of those parameters is conditioned on (232). To address this issue, Lubin and Caporaso (233) developed a model that includes pack-years as the primary exposure and incorporates variables related to smoking intensity as effect modifiers, which enables estimation of cumulative exposure at varying intensities (e.g., smoking 2 packs/day for 40 years vs. 4 packs/day for 20 years). Vlaanderen et al. (234) refined this model to include time since quitting as an effect modifier and cubic splines for flexible modeling of smoking intensity. Together, these methods have informed lung cancer etiology and have helped to identify potential targets for intervention (231). Extending these approaches to other exposures may provide insight into whether the use of cumulative exposure alone, or in combination with other time- and intensity-related variables, is justified.
Exposures may also be linked over time as “chains of risk” with “additive” or “trigger” effects (5) (Figure 1). In the additive effect model, each subsequent exposure increases cancer risk. In the trigger effect model, only the most proximal (“final link in the chain”) exposure is causal, and all other exposures indirectly influence cancer risk (5). Longitudinal studies with repeated measures provide an opportunity to assess temporal exposure patterns; however, traditional linear models (e.g., analysis of variance and growth curves) are limited by equal variance assumptions and balanced data requirements, and they are not equipped to model complex temporal patterns of exposure in relation to cancer risk (235). To overcome some of these limitations, finite mixture modeling approaches like growth mixture (236) and group-based trajectory modeling (237) have been developed to analyze populations composed of distinct exposure trajectories that are not easily distinguished by measured characteristics (for a review, refer to the article by Nagin and Odgers (235)). Both group-based trajectory and growth mixture models can be adapted to allow the probability of trajectory group membership to vary by fixed and/or time-varying covariates (235). By use of the growth mixture modeling approach, 2 or more growth curve models are established a priori to estimate population variability in developmental trajectories and interpreted in a similar way as a single growth curve model, with each representing a separate subpopulation following a different trajectory curve (235). On the other hand, group-based trajectory modeling approximates an unknown distribution of trajectories within the population. A useful feature of this approach is the ability to estimate the probabilities linking trajectory group memberships across different exposures (e.g., childhood diet and obesity in young adulthood) (235, 237). These methods have been applied in 2 recent prospective studies assessing trajectories of body shape in early life with adult cancer and cause-specific mortality (238, 239).
Additional approaches to strengthen causal inference
The use of negative controls may help to strengthen causal inference in studies of early life exposures, particularly if residual confounding is suspected (11, 12). A negative control is defined as an exposure that is unrelated to the outcome of interest or an outcome that is unlikely to be caused by the exposure of interest, but that shares similar sources of bias and confounding (12). Negative control associations can be compared with the main effect estimate to rule out confounding and strengthen causal inference (12). For example, paternal exposures can serve as negative controls for studies of maternal exposures and offspring health (240); if there is a direct intrauterine effect on offspring health, then the association should be much stronger for the maternal exposure compared with the paternal exposure (12, 240). Negative controls have been utilized in studies assessing the influence of maternal diet during pregnancy on offspring dietary behaviors (153) and associations of maternal obesity with cord blood DNA methylation levels (241).
Mendelian randomization is a special type of instrumental variable analysis that involves using genetic variants as proxies for exposures of interest (242, 243). This approach is attractive for studies of early life exposures because of the following: 1) genetic variants are randomly inherited and, thus, exposure status is not influenced by other confounding factors; 2) use of germline variants is not subject to bias due to reverse causation; and 3) a genetic variant may represent long-term, cumulative exposure (244). Mendelian randomization has been used in studies of maternal smoking, demonstrating an association of a variant in the CHRNA5-A3-B4 gene cluster that is strongly associated with heavy smoking and the inability to give up smoking during pregnancy, with low birth weight (245). A recent study utilized this approach to calculate a genetic score derived from single-nucleotide polymorphisms associated with Tanner stage in adolescent boys, demonstrating that later pubertal development reduces the risk of aggressive prostate cancer (28). Importantly, Mendelian randomization is appropriate only if the genetic variant is strongly associated with the exposure of interest; a genetic variant that influences more than 1 phenotype (i.e., pleiotropic), particularly if it is associated with both the exposure and outcome of interest, can introduce bias (244). Furthermore, if the genetic variant is correlated with other genetic variants (i.e., linkage disequilibrium), spurious associations may arise (244). Careful study design and analytical techniques (246, 247) can minimize these issues.
POLICY AND PUBLIC HEALTH–RELATED INTERVENTIONS AND RESEARCH INITIATIVES
Despite the large body of evidence linking early life exposure to the risk factors presented in this narrative review to numerous adverse health outcomes in both youth and adulthood, more research is needed to firmly establish associations with adult cancer risk. With the exception of youth tobacco exposure, our findings do not signify the need for specialized cancer prevention interventions, but they do provide additional evidence to support the need for ongoing efforts to reduce the burden of these risk factors in early life and to emphasize important gaps in the literature. Here, we provide examples of public health interventions and policies aimed at reducing early life exposure to these risk factors. We also provide examples of recent efforts to build the evidence base of the health consequences of early life risk factors via research initiatives.
Some of the most effective federal- and state-level policies and initiatives have been focused on reducing ETS exposure and tobacco use among youth, including taxation, comprehensive smoke-free laws, and mass-media campaigns (248–251). However, in recent years, the rate of decline has slowed, with approximately 7.4% of middle school and 25.3% of high school students reporting current use of any tobacco product in 2015 (252). Moreover, use of flavored and alternative tobacco products is on the rise, with electronic cigarettes being the most common (252). In light of these trends, the US Food and Drug Administration recently extended its regulatory authority to cover all tobacco products, including e-cigarettes. The new rule also restricts youth access to such products by preventing retailers from selling to youth under the age of 18 years and prohibiting the sale of tobacco products in vending machines (except in adult-only facilities) (253). Other public policy initiatives, such as legislation to prohibit smoking in motor vehicles, and the promotion of smoke-free homes through educational campaigns (254–256) have been enacted in only a few states, highlighting the need for improvement (257, 258). In 2015, the US Department of Housing and Urban Development proposed a smoking ban in multiunit housing, with the potential to impact over 750,000 children and adolescents, if enacted (254). Newer strategies focused on sales restrictions and harm reduction have also been proposed (254). For example, a federal bill that raises the minimum tobacco age to 21 years, which the Institute of Medicine reports could reduce youth smoking initiation by 25% nationwide, is currently under Congressional review (259).
National- and state-level policies targeting obesity-related behaviors in youth have been implemented primarily in schools, which play a critical role in shaping the health behaviors of children and adolescents (260). For example, as part of the 2010 Healthy, Hunger-Free Kids Act, the US Department of Agriculture implemented new “Smart Snacks in School” nutrition standards in 2014 for foods and beverages sold at school (261, 262). More research is needed to determine the impact of these policies on eating behaviors and the prevalence of overweight and obesity among youth (262, 263).
Interventions delivered in clinical settings can also play an important role in supporting healthy behaviors relevant to cancer prevention; however, for many interventions, there is limited evidence demonstrating their effectiveness in improving health behaviors (264). For example, preconception care interventions, such as tobacco cessation and obesity prevention, have the potential to improve the health of 2 generations (265, 266) and are strongly recommended by the American Congress of Obstetrics and Gynecologists and the Institute of Medicine (267, 268). Yet, despite these recommendations, evidence for the effectiveness of most preconception care services is lacking (266), and even those with a strong-evidence base, such as tobacco cessation interventions, are not widely implemented in routine practice (267, 269, 270). Federal coverage mandates for preconception services under the Affordable Care Act, such as tobacco-cessation counseling and pharmacotherapy for pregnant women on Medicaid (271), offer promising strategies to improve uptake, although data suggest that more work is needed to increase provider awareness of these benefits (271, 272). Because many of the modifiable risk factors covered in this narrative review tend to track with time, delivery of evidence-based preventive services to children and adolescents provides an opportunity to intervene early to reduce or modify the burden of established cancer risk factors in adulthood (260). The US Preventive Services Task Force issues guidelines for preventive service recommendations for children and adolescents; however, these recommendations are not integrated across domains, and some important topics, such as physical activity and dietary counseling, are not included because of insufficient evidence (273). Further, delivery of preventive care to adolescents is relatively low (260, 274). Recent changes in the US health-care system expanding coverage for preventive services without cost sharing offer opportunities to improve utilization of preventive care services among youth (275). Lack of the high-quality evidence required for the development of guidelines and recommendations underscores the need for more research to fill these important gaps.
Building the evidence base of the health consequences of early life risk factors may require innovative use of existing data from cohorts with relevant intermediate exposures (e.g., puberty), as well as prospective studies that are designed to capture cancer risk factors in early life. To this end, several funding opportunities have been established. For example, the National Institutes of Health recently launched a 7-year initiative called the “Environmental Influences on Child Health Outcomes” (ECHO) program to understand the effects of environmental exposures on child health and development, with obesity as 1 of 4 key outcomes (276). In addition, the National Cancer Institute has funding opportunities for studies evaluating the role of early life factors in carcinogenesis and whether markers associated with early life exposures can be measured and developed for use in cancer prevention strategies (277). To address the need for surrogate cancer endpoints, a “Pre-Cancer Genome Atlas” has been proposed to complement The Cancer Genome Atlas (278, 279). Collectively, these efforts have the potential to bolster our understanding of the role of early life exposures in adult cancer risk.
CONCLUSIONS
The purpose of this narrative review was to emphasize early life as an important, yet understudied period with respect to cancer research. Although there is consistent evidence linking certain early life exposures in adolescence to adult cancer risk (e.g., tobacco use and lung cancer), more research is needed for a majority of the exposures described in this narrative review. With respect to in utero and early childhood exposures, much of the evidence is indirect, suggesting potential associations through the modification of biological and/or behavioral pathways that may influence the trajectory of cancer risk over the life course. Collectively, this dearth of evidence emphasizes significant gaps in our knowledge regarding the period of early life with respect to adult cancer risk. Conceptual models, such as the life-course framework, may help to sharpen biologically based hypotheses about relevant time periods for cancer etiology, while statistical approaches, such as group-based trajectory modeling, have the potential to reveal novel associations and/or hidden heterogeneities within prospectively collected data; they also may help to identify critical points for targeting primary prevention strategies. In addition, objective molecular markers, such as DNA methylation, have the potential to improve early life exposure assessment and to elucidate mechanisms linking early life exposures with adult cancer outcomes (210, 227). The modifiable cancer risk factors described in this narrative review are also risk factors for other chronic diseases. Although the current evidence does not indicate the need for specific cancer prevention interventions in early life, it does support the need for the public health interventions that encourage overall health and well-being in young people. Continued research on early life exposures has the potential to provide new insights into cancer etiology and to inform primary prevention strategies to reduce the prevalence of cancer risk factors in the early stages of life, a time when these strategies might achieve the greatest benefit (4).
ACKNOWLEDGMENTS
Authors affiliation: Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Megan A. Clarke, Corinne E. Joshu).
This work was supported by P30 CA006973. M.A.C. was additionally supported by the National Cancer Institute, T32 CA009314.
Conflict of interest: none declared.
Abbreviations
- ETS
environmental tobacco smoke
REFERENCES
- 1. Vedham V, Verma M, Mahabir S. Early-life exposures to infectious agents and later cancer development. Cancer Med. 2015;4(12):1908–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balk SJ; Council on Environmental Health, Section on Dermatology . Ultraviolet radiation: a hazard to children and adolescents. Pediatrics. 2011;127(3):e791–e817. [DOI] [PubMed] [Google Scholar]
- 3. Mahabir S, Aagaard K, Anderson LM, et al. Challenges and opportunities in research on early-life events/exposures and cancer development later in life. Cancer Causes Control. 2012;23(6):983–990. [DOI] [PubMed] [Google Scholar]
- 4. Colditz GA, Wolin KY, Gehlert S. Applying what we know to accelerate cancer prevention. Sci Transl Med. 2012;4(127):127rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–293. [PubMed] [Google Scholar]
- 6. Stein CJ, Colditz GA. Modifiable risk factors for cancer. Br J Cancer. 2004;90(2):299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Cancer Society, Inc Cancer Prevention & Early Detection Facts & Figures 2015 –2016. Atlanta, GA: American Cancer Society, Inc; 2015. [Google Scholar]
- 8. Department of Health and Human Services The health consequences of smoking—50 years of progress: a report of the Surgeon General. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/#fullreport. Published 2014. Accessed October 21, 2015.
- 9. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention The health consequences of involuntary exposure to tobacco smoke. http://www.ncbi.nlm.nih.gov/books/NBK44321/. Published 2006. Accessed July 14, 2016.
- 10. Holman DM, Rodriguez JL, Peipins L, et al. Highlights from a workshop on opportunities for cancer prevention during preadolescence and adolescence. J Adolesc Health. 2013;52(5 suppl):S8–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davey Smith G, Leary S, Ness A, et al. Challenges and novel approaches in the epidemiological study of early life influences on later disease. Adv Exp Med Biol. 2009;646:1–14. [DOI] [PubMed] [Google Scholar]
- 12. Gage SH, Munafò MR, Davey Smith G. Causal inference in Developmental Origins of Health and Disease (DOHaD) research. Annu Rev Psychol. 2016;67:567–585. [DOI] [PubMed] [Google Scholar]
- 13. Wiencke JK, Kelsey KT. Teen smoking, field cancerization, and a “critical period” hypothesis for lung cancer susceptibility. Environ Health Perspect. 2002;110(6):555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russo IH. Cigarette smoking and risk of breast cancer in women. Lancet. 2002;360(9339):1033–1034. [DOI] [PubMed] [Google Scholar]
- 15. Robles AI, Yang P, Jen J, et al. A DRD1 polymorphism predisposes to lung cancer among those exposed to secondhand smoke during childhood. Cancer Prev Res (Phila). 2014;7(12):1210–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olivo-Marston SE, Yang P, Mechanic LE, et al. Childhood exposure to secondhand smoke and functional mannose binding lectin polymorphisms are associated with increased lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3375–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simonds NI, Ghazarian AA, Pimentel CB, et al. Review of the gene-environment interaction literature in cancer: what do we know. Genet Epidemiol. 2016;40(5):356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee KM, Ward MH, Han S, et al. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res. 2009;33(2):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Milne E, Greenop KR, Scott RJ, et al. Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol. 2012;175(1):43–53. [DOI] [PubMed] [Google Scholar]
- 20. Orsi L, Rudant J, Ajrouche R, et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: the ESTELLE study. Cancer Causes Control. 2015;26(7):1003–1017. [DOI] [PubMed] [Google Scholar]
- 21. Rudant J, Menegaux F, Leverger G, et al. Childhood hematopoietic malignancies and parental use of tobacco and alcohol: the ESCALE study (SFCE). Cancer Causes Control. 2008;19(10):1277–1290. [DOI] [PubMed] [Google Scholar]
- 22. Metayer C, Zhang L, Wiemels JL, et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1600–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brownson RC, Alavanja MC, Hock ET, et al. Passive smoking and lung cancer in nonsmoking women. Am J Public Health. 1992;82(11):1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boffetta P, Trédaniel J, Greco A. Risk of childhood cancer and adult lung cancer after childhood exposure to passive smoke: a meta-analysis. Environ Health Perspect. 2000;108(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rayfield S, Plugge E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J Epidemiol Community Health. 2016;71(2):162–173. [DOI] [PubMed] [Google Scholar]
- 26. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease. http://www.ncbi.nlm.nih.gov/books/NBK53022/. Published 2010. Accessed June 12, 2016.
- 27. Collaborative Group on Hormonal Factors in Breast Cancer Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonilla C, Lewis SJ, Martin RM, et al. Pubertal development and prostate cancer risk: Mendelian randomization study in a population-based cohort. BMC Med. 2016;14:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Håkonsen LB, Ernst A, Ramlau-Hansen CH. Maternal cigarette smoking during pregnancy and reproductive health in children: a review of epidemiological studies. Asian J Androl. 2014;16(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yermachenko A, Dvornyk V. A meta-analysis provides evidence that prenatal smoking exposure decreases age at menarche. Reprod Toxicol. 2015;58:222–228. [DOI] [PubMed] [Google Scholar]
- 31. Dietz PM, Homa D, England LJ, et al. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2011;173(3):355–359. [DOI] [PubMed] [Google Scholar]
- 32. Russell T, Crawford M, Woodby L. Measurements for active cigarette smoke exposure in prevalence and cessation studies: why simply asking pregnant women isn't enough. Nicotine Tob Res. 2004;6(suppl 2):S141–S151. [DOI] [PubMed] [Google Scholar]
- 33. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. [DOI] [PubMed] [Google Scholar]
- 36. Colditz GA, Bohlke K, Berkey CS. Breast cancer risk accumulation starts early: prevention must also. Breast Cancer Res Treat. 2014;145(3):567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baer HJ, Tworoger SS, Hankinson SE, et al. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171(11):1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dougan MM, Hankinson SE, Vivo ID, et al. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. Int J Cancer. 2015;137(3):625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park SL, Goodman MT, Zhang ZF, et al. Body size, adult BMI gain and endometrial cancer risk: the multiethnic cohort. Int J Cancer. 2010;126(2):490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stevens VL, Jacobs EJ, Patel AV, et al. Body weight in early adulthood, adult weight gain, and risk of endometrial cancer in women not using postmenopausal hormones. Cancer Causes Control. 2014;25(3):321–328. [DOI] [PubMed] [Google Scholar]
- 41. Le Marchand L, Wilkens LR, Mi MP. Early-age body size, adult weight gain and endometrial cancer risk. Int J Cancer. 1991;48(6):807–811. [DOI] [PubMed] [Google Scholar]
- 42. Weiderpass E, Persson I, Adami HO, et al. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden). Cancer Causes Control. 2000;11(2):185–192. [DOI] [PubMed] [Google Scholar]
- 43. Xu WH, Xiang YB, Zheng W, et al. Weight history and risk of endometrial cancer among Chinese women. Int J Epidemiol. 2006;35(1):159–166. [DOI] [PubMed] [Google Scholar]
- 44. Chang SC, Lacey JV Jr, Brinton LA, et al. Lifetime weight history and endometrial cancer risk by type of menopausal hormone use in the NIH-AARP Diet and Health Study. Cancer Epidemiol Biomarkers Prev. 2007;16(4):723–730. [DOI] [PubMed] [Google Scholar]
- 45. Yang TY, Cairns BJ, Allen N, et al. Postmenopausal endometrial cancer risk and body size in early life and middle age: prospective cohort study. Br J Cancer. 2012;107(1):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Le Marchand L, Wilkens LR, Mi MP. Obesity in youth and middle age and risk of colorectal cancer in men. Cancer Causes Control. 1992;3(4):349–354. [DOI] [PubMed] [Google Scholar]
- 47. Lee IM, Paffenbarger RS. Quetelet's index and risk of colon cancer in college alumni. J Natl Cancer Inst. 1992;84(17):1326–1331. [DOI] [PubMed] [Google Scholar]
- 48. Levi Z, Kark JD, Barchana M, et al. Measured body mass index in adolescence and the incidence of colorectal cancer in a cohort of 1.1 million males. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2524–2531. [DOI] [PubMed] [Google Scholar]
- 49. Kantor ED, Udumyan R, Signorello LB, et al. Adolescent body mass index and erythrocyte sedimentation rate in relation to colorectal cancer risk. Gut. 2016;65(8):1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang X, Wu K, Giovannucci EL, et al. Early life body fatness and risk of colorectal cancer in US women and men—results from two large cohort studies. Cancer Epidemiol Biomarkers Prev. 2015;24(4):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Campbell PT, Cotterchio M, Dicks E, et al. Excess body weight and colorectal cancer risk in Canada: associations in subgroups of clinically defined familial risk of cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1735–1744. [DOI] [PubMed] [Google Scholar]
- 52. Han X, Stevens J, Truesdale KP, et al. Body mass index at early adulthood, subsequent weight change and cancer incidence and mortality. Int J Cancer. 2014;135(12):2900–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oxentenko AS, Bardia A, Vierkant RA, et al. Body size and incident colorectal cancer: a prospective study of older women. Cancer Prev Res (Phila). 2010;3(12):1608–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thrift AP, Shaheen NJ, Gammon MD, et al. Obesity and risk of esophageal adenocarcinoma and Barrett's esophagus: a Mendelian randomization study. J Natl Cancer Inst. 2014;106(11):dju252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Levi Z, Kark JD, Shamiss A, et al. Body mass index and socioeconomic status measured in adolescence, country of origin, and the incidence of gastroesophageal adenocarcinoma in a cohort of 1 million men. Cancer. 2013;119(23):4086–4093. [DOI] [PubMed] [Google Scholar]
- 56. Stolzenberg-Solomon RZ, Schairer C, Moore S, et al. Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2013;98(4):1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hofmann JN, Moore SC, Lim U, et al. Body mass index and physical activity at different ages and risk of multiple myeloma in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2013;177(8):776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Genkinger JM, Kitahara CM, Bernstein L, et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann Oncol. 2015;26(11):2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cook MB, Freedman ND, Gamborg M, et al. Childhood body mass index in relation to future risk of oesophageal adenocarcinoma. Br J Cancer. 2015;112(3):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Berentzen TL, Gamborg M, Holst C, et al. Body mass index in childhood and adult risk of primary liver cancer. J Hepatol. 2014;60(2):325–330. [DOI] [PubMed] [Google Scholar]
- 61. Kitahara CM, Gamborg M, Berrington de González A, et al. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res. 2014;74(1):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aarestrup J, Gamborg M, Ulrich LG, et al. Childhood body mass index and height and risk of histologic subtypes of endometrial cancer. Int J Obes (Lond). 2016;40(7):1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cook MB, Gamborg M, Aarestrup J, et al. Childhood height and birth weight in relation to future prostate cancer risk: a cohort study based on the Copenhagen School Health Records Register. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2232–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aarestrup J, Gamborg M, Cook MB, et al. Childhood body mass index and the risk of prostate cancer in adult men. Br J Cancer. 2014;111(1):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition—an old hypothesis with new importance. Int J Epidemiol. 2013;42(1):7–29. [DOI] [PubMed] [Google Scholar]
- 66. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 67. Hartling L, Dryden DM, Guthrie A, et al. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the US Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. 2013;159(2):123–129. [DOI] [PubMed] [Google Scholar]
- 68. Oken E. Maternal and child obesity: the causal link. Obstet Gynecol Clin North Am. 2009;36(2):361–377. [DOI] [PubMed] [Google Scholar]
- 69. Boney CM, Verma A, Tucker R, et al. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. [DOI] [PubMed] [Google Scholar]
- 70. Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–1133. [DOI] [PubMed] [Google Scholar]
- 71. Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start Study. Am J Clin Nutr. 2015;101(2):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thompson AL. Intergenerational impact of maternal obesity and postnatal feeding practices on pediatric obesity. Nutr Rev. 2013;71(suppl 1):S55–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Patro B, Liber A, Zalewski B, et al. Maternal and paternal body mass index and offspring obesity: a systematic review. Ann Nutr Metab. 2013;63(1-2):32–41. [DOI] [PubMed] [Google Scholar]
- 74. Yu Z, Han S, Zhu J, et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8(4):e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ruager-Martin R, Hyde MJ, Modi N. Maternal obesity and infant outcomes. Early Hum Dev. 2010;86(11):715–722. [DOI] [PubMed] [Google Scholar]
- 76. Smith J, Cianflone K, Biron S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009;94(11):4275–4283. [DOI] [PubMed] [Google Scholar]
- 77. Kral JG, Biron S, Simard S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118(6):e1644–e1649. [DOI] [PubMed] [Google Scholar]
- 78. Zhu Y, Olsen SF, Mendola P, et al. Growth and obesity through the first 7 y of life in association with levels of maternal glycemia during pregnancy: a prospective cohort study. Am J Clin Nutr. 2016;103(3):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boyle KE, Patinkin ZW, Shapiro AL, et al. Mesenchymal stem cells from infants born to obese mothers exhibit greater potential for adipogenesis: the Healthy Start BabyBUMP project. Diabetes. 2016;65(3):647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92(2):287–298. [DOI] [PubMed] [Google Scholar]
- 81. Remmers F, Delemarre-van de Waal HA. Developmental programming of energy balance and its hypothalamic regulation. Endocr Rev. 2011;32(2):272–311. [DOI] [PubMed] [Google Scholar]
- 82. Bouret SG. Early life origins of obesity: role of hypothalamic programming. J Pediatr Gastroenterol Nutr. 2009;48(suppl 1):S31–S38. [DOI] [PubMed] [Google Scholar]
- 83. Mamun AA, Mannan M, Doi SA. Gestational weight gain in relation to offspring obesity over the life course: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2014;15(4):338–347. [DOI] [PubMed] [Google Scholar]
- 84. Nehring I, Lehmann S, von Kries R. Gestational weight gain in accordance to the IOM/NRC criteria and the risk for childhood overweight: a meta-analysis. Pediatr Obes. 2013;8(3):218–224. [DOI] [PubMed] [Google Scholar]
- 85. Diesel JC, Eckhardt CL, Day NL, et al. Is gestational weight gain associated with offspring obesity at 36 months. Pediatr Obes. 2015;10(4):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Houghton LC, Ester WA, Lumey LH, et al. Maternal weight gain in excess of pregnancy guidelines is related to daughters being overweight 40 years later. Am J Obstet Gynecol. 2016;215(2):246.e1–246.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Branum AM, Parker JD, Keim SA, et al. Prepregnancy body mass index and gestational weight gain in relation to child body mass index among siblings. Am J Epidemiol. 2011;174(10):1159–1165. [DOI] [PubMed] [Google Scholar]
- 88. Crozier SR, Inskip HM, Godfrey KM, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. Am J Clin Nutr. 2010;91(6):1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Oken E, Rifas-Shiman SL, Field AE, et al. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112(5):999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ehrenthal DB, Maiden K, Rao A, et al. Independent relation of maternal prenatal factors to early childhood obesity in the offspring. Obstet Gynecol. 2013;121(1):115–121. [DOI] [PubMed] [Google Scholar]
- 91. Sridhar SB, Darbinian J, Ehrlich SF, et al. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol. 2014;211(3):259.e1–259.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Silva Idos S, De Stavola B, McCormack V, et al. Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PLoS Med. 2008;5(9):e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhou CK, Sutcliffe S, Welsh J, et al. Is birthweight associated with total and aggressive/lethal prostate cancer risks? A systematic review and meta-analysis. Br J Cancer. 2016;114(7):839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cook MB, Akre O, Forman D, et al. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer—experiences of the son. Int J Epidemiol. 2010;39(6):1605–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Smith NR, Jensen BW, Zimmermann E, et al. Associations between birth weight and colon and rectal cancer risk in adulthood. Cancer Epidemiol. 2016;42:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Spracklen CN, Wallace RB, Sealy-Jefferson S, et al. Birth weight and subsequent risk of cancer. Cancer Epidemiol. 2014;38(5):538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Morois S, Mesrine S, Besemer F, et al. Risks of colon and rectal adenomas are differentially associated with anthropometry throughout life: the French E3N prospective cohort. Int J Epidemiol. 2011;40(5):1269–1279. [DOI] [PubMed] [Google Scholar]
- 98. Sandhu MS, Luben R, Day NE, et al. Self-reported birth weight and subsequent risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(9):935–938. [PubMed] [Google Scholar]
- 99. Nilsen TIL, Romundstad PR, Troisi R, et al. Birth size and colorectal cancer risk: a prospective population based study. Gut. 2005;54(12):1728–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mahabir S. Association between diet during preadolescence and adolescence and risk for breast cancer during adulthood. J Adolesc Health. 2013;52(5 suppl):S30–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Frazier AL, Rosenberg SM. Preadolescent and adolescent risk factors for benign breast disease. J Adolesc Health. 2013;52(5 suppl):S36–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85(22):1819–1827. [DOI] [PubMed] [Google Scholar]
- 103. Korde LA, Wu AH, Fears T, et al. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1050–1059. [DOI] [PubMed] [Google Scholar]
- 104. Wu AH, Wan P, Hankin J, et al. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23(9):1491–1496. [DOI] [PubMed] [Google Scholar]
- 105. Wu AH, Yu MC, Tseng CC, et al. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lee SA, Shu XO, Li H, et al. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women's Health Study. Am J Clin Nutr. 2009;89(6):1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Messina M, Hilakivi-Clarke L. Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutr Cancer. 2009;61(6):792–798. [DOI] [PubMed] [Google Scholar]
- 108. Farvid MS, Eliassen AH, Cho E, et al. Dietary fiber intake in young adults and breast cancer risk. Pediatrics. 2016;137(3):e20151226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Farvid MS, Chen WY, Michels KB, et al. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: population based cohort study. BMJ. 2016;353:i2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Su X, Tamimi RM, Collins LC, et al. Intake of fiber and nuts during adolescence and incidence of proliferative benign breast disease. Cancer Causes Control. 2010;21(7):1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Baer HJ, Schnitt SJ, Connolly JL, et al. Adolescent diet and incidence of proliferative benign breast disease. Cancer Epidemiol Biomarkers Prev. 2003;12(11 pt 1):1159–1167. [PubMed] [Google Scholar]
- 112. Farvid MS, Cho E, Chen WY, et al. Adolescent meat intake and breast cancer risk. Int J Cancer. 2015;136(8):1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Linos E, Willett WC, Cho E, et al. Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2146–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bertrand KA, Burian RA, Eliassen AH, et al. Adolescent intake of animal fat and red meat in relation to premenopausal mammographic density. Breast Cancer Res Treat. 2016;155(2):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tseng M, Olufade TO, Evers KA, et al. Adolescent lifestyle factors and adult breast density in US Chinese immigrant women. Nutr Cancer. 2011;63(3):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jung S, Goloubeva O, Klifa C, et al. Dietary fat intake during adolescence and breast density among young women. Cancer Epidemiol Biomarkers Prev. 2016;25(6):918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jones JA, Hartman TJ, Klifa CS, et al. Dietary energy density is positively associated with breast density among young women. J Acad Nutr Diet. 2015;115(3):353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Berkey CS, Willett WC, Tamimi RM, et al. Vegetable protein and vegetable fat intakes in pre-adolescent and adolescent girls, and risk for benign breast disease in young women. Breast Cancer Res Treat. 2013;141(2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liu Y, Colditz GA, Cotterchio M, et al. Adolescent dietary fiber, vegetable fat, vegetable protein, and nut intakes and breast cancer risk. Breast Cancer Res Treat. 2014;145(2):461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Boeke CE, Tamimi RM, Berkey CS, et al. Adolescent carotenoid intake and benign breast disease. Pediatrics. 2014;133(5):e1292–e1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sellers TA, Vachon CM, Pankratz VS, et al. Association of childhood and adolescent anthropometric factors, physical activity, and diet with adult mammographic breast density. Am J Epidemiol. 2007;166(4):456–464. [DOI] [PubMed] [Google Scholar]
- 122. Potischman N, Weiss HA, Swanson CA, et al. Diet during adolescence and risk of breast cancer among young women. J Natl Cancer Inst. 1998;90(3):226–233. [DOI] [PubMed] [Google Scholar]
- 123. Dorgan JF, Hunsberger SA, McMahon RP, et al. Diet and sex hormones in girls: findings from a randomized controlled clinical trial. J Natl Cancer Inst. 2003;95(2):132–141. [DOI] [PubMed] [Google Scholar]
- 124. Dorgan JF, Liu L, Klifa C, et al. Adolescent diet and subsequent serum hormones, breast density, and bone mineral density in young women: results of the Dietary Intervention Study in Children follow-up study. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jiménez-Pavón D, Sesé MA, Huybrechts I, et al. Dietary and lifestyle quality indices with/without physical activity and markers of insulin resistance in European adolescents: the HELENA Study. Br J Nutr. 2013;110(10):1919–1925. [DOI] [PubMed] [Google Scholar]
- 126. Cheng G, Buyken AE, Shi L, et al. Beyond overweight: nutrition as an important lifestyle factor influencing timing of puberty. Nutr Rev. 2012;70(3):133–152. [DOI] [PubMed] [Google Scholar]
- 127. Carwile JL, Willett WC, Spiegelman D, et al. Sugar-sweetened beverage consumption and age at menarche in a prospective study of US girls. Hum Reprod. 2015;30(3):675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mueller NT, Jacobs DR, MacLehose RF, et al. Consumption of caffeinated and artificially sweetened soft drinks is associated with risk of early menarche. Am J Clin Nutr. 2015;102(3):648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nimptsch K, Malik VS, Fung TT, et al. Dietary patterns during high school and risk of colorectal adenoma in a cohort of middle-aged women. Int J Cancer. 2014;134(10):2458–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nimptsch K, Bernstein AM, Giovannucci E, et al. Dietary intakes of red meat, poultry, and fish during high school and risk of colorectal adenomas in women. Am J Epidemiol. 2013;178(2):172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. van der Pols JC, Bain C, Gunnell D, et al. Childhood dairy intake and adult cancer risk: 65-y follow-up of the Boyd Orr cohort. Am J Clin Nutr. 2007;86(6):1722–1729. [DOI] [PubMed] [Google Scholar]
- 132. Ruder EH, Thiébaut ACM, Thompson FE, et al. Adolescent and mid-life diet: risk of colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr. 2011;94(6):1607–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Braganza MZ, Potischman N, Park Y, et al. Adolescent and mid-life diet and subsequent risk of thyroid cancer in the NIH-AARP Diet and Health Study. Int J Cancer. 2015;137(10):2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Honein MA, Paulozzi LJ, Mathews TJ, et al. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981–2986. [DOI] [PubMed] [Google Scholar]
- 135. Cetin I, Alvino G, Cardellicchio M. Long chain fatty acids and dietary fats in fetal nutrition. J Physiol. 2009;587(pt 14):3441–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Delgado-Noguera MF, Calvache JA, Bonfill Cosp X, et al. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst Rev. 2015;(7):CD007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485–491. [DOI] [PubMed] [Google Scholar]
- 138. Painter RC, De Rooij SR, Bossuyt PM, et al. A possible link between prenatal exposure to famine and breast cancer: a preliminary study. Am J Hum Biol. 2006;18(6):853–856. [DOI] [PubMed] [Google Scholar]
- 139. Much D, Brunner S, Vollhardt C, et al. Effect of dietary intervention to reduce the n-6/n-3 fatty acid ratio on maternal and fetal fatty acid profile and its relation to offspring growth and body composition at 1 year of age. Eur J Clin Nutr. 2013;67(3):282–288. [DOI] [PubMed] [Google Scholar]
- 140. Blumfield ML, Hure AJ, MacDonald-Wicks LK, et al. Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am J Clin Nutr. 2012;96(5):1032–1041. [DOI] [PubMed] [Google Scholar]
- 141. Murrin C, Shrivastava A, Kelleher CC, et al. Maternal macronutrient intake during pregnancy and 5 years postpartum and associations with child weight status aged five. Eur J Clin Nutr. 2013;67(6):670–679. [DOI] [PubMed] [Google Scholar]
- 142. Crume TL, Brinton JT, Shapiro A, et al. Maternal dietary intake during pregnancy and offspring body composition: the Healthy Start Study. Am J Obstet Gynecol. 2016;215(5):609.e1–609.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth—a review. Placenta. 2002;23(suppl A):S28–S38. [DOI] [PubMed] [Google Scholar]
- 144. Schoenaker DA, Mishra GD, Callaway LK, et al. The role of energy, nutrients, foods, and dietary patterns in the development of gestational diabetes mellitus: a systematic review of observational studies. Diabetes Care. 2016;39(1):16–23. [DOI] [PubMed] [Google Scholar]
- 145. Blumfield ML, Nowson C, Hure AJ, et al. Lower protein-to-carbohydrate ratio in maternal diet is associated with higher childhood systolic blood pressure up to age four years. Nutrients. 2015;7(5):3078–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Knudsen VK, Orozova-Bekkevold IM, Mikkelsen TB, et al. Major dietary patterns in pregnancy and fetal growth. Eur J Clin Nutr. 2008;62(4):463–470. [DOI] [PubMed] [Google Scholar]
- 147. Moore VM, Davies MJ, Willson KJ, et al. Dietary composition of pregnant women is related to size of the baby at birth. J Nutr. 2004;134(7):1820–1826. [DOI] [PubMed] [Google Scholar]
- 148. Andreasyan K, Ponsonby AL, Dwyer T, et al. Higher maternal dietary protein intake in late pregnancy is associated with a lower infant ponderal index at birth. Eur J Clin Nutr. 2007;61(4):498–508. [DOI] [PubMed] [Google Scholar]
- 149. Renault KM, Carlsen EM, Nørgaard K, et al. Intake of carbohydrates during pregnancy in obese women is associated with fat mass in the newborn offspring. Am J Clin Nutr. 2015;102(6):1475–1481. [DOI] [PubMed] [Google Scholar]
- 150. Poon AK, Yeung E, Boghossian N, et al. Maternal dietary patterns during third trimester in association with birthweight characteristics and early infant growth. Scientifica (Cairo). 2013;2013:786409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Bouwland-Both MI, Steegers-Theunissen RP, Vujkovic M, et al. A periconceptional energy-rich dietary pattern is associated with early fetal growth: the Generation R Study. BJOG. 2013;120(4):435–445. [DOI] [PubMed] [Google Scholar]
- 152. van den Broek M, Leermakers ET, Jaddoe VW, et al. Maternal dietary patterns during pregnancy and body composition of the child at age 6 y: the Generation R Study. Am J Clin Nutr. 2015;102(4):873–880. [DOI] [PubMed] [Google Scholar]
- 153. Brion MJ, Ness AR, Rogers I, et al. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: exploring parental comparisons and prenatal effects. Am J Clin Nutr. 2010;91(3):748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ferland S, O'Brien HT. Maternal dietary intake and pregnancy outcome. J Reprod Med. 2003;48(2):86–94. [PubMed] [Google Scholar]
- 155. Rodríguez-Bernal CL, Rebagliato M, Iñiguez C, et al. Diet quality in early pregnancy and its effects on fetal growth outcomes: the Infancia y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. Am J Clin Nutr. 2010;91(6):1659–1666. [DOI] [PubMed] [Google Scholar]
- 156. Shapiro AL, Kaar JL, Crume TL, et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes (Lond). 2016;40(7):1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. [DOI] [PubMed] [Google Scholar]
- 158. Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. 2015;26(12):2398–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Owen CG, Martin RM, Whincup PH, et al. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr. 2005;82(6):1298–1307. [DOI] [PubMed] [Google Scholar]
- 162. Boeke CE, Eliassen AH, Oh H, et al. Adolescent physical activity in relation to breast cancer risk. Breast Cancer Res Treat. 2014;145(3):715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Maruti SS, Willett WC, Feskanich D, et al. A prospective study of age-specific physical activity and premenopausal breast cancer. J Natl Cancer Inst. 2008;100(10):728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Liu Y, Tobias DK, Sturgeon KM, et al. Physical activity from menarche to first pregnancy and risk of breast cancer. Int J Cancer. 2016;139(6):1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Margolis KL, Mucci L, Braaten T, et al. Physical activity in different periods of life and the risk of breast cancer: the Norwegian-Swedish Women's Lifestyle and Health cohort study. Cancer Epidemiol Biomarkers Prev. 2005;14(1):27–32. [PubMed] [Google Scholar]
- 166. Peters TM, Moore SC, Gierach GL, et al. Intensity and timing of physical activity in relation to postmenopausal breast cancer risk: the prospective NIH-AARP Diet and Health Study. BMC Cancer. 2009;9:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Pronk A, Ji BT, Shu XO, et al. Physical activity and breast cancer risk in Chinese women. Br J Cancer. 2011;105(9):1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA. 2003;290(10):1331–1336. [DOI] [PubMed] [Google Scholar]
- 169. Kobayashi LC, Janssen I, Richardson H, et al. Moderate-to-vigorous intensity physical activity across the life course and risk of pre- and post-menopausal breast cancer. Breast Cancer Res Treat. 2013;139(3):851–861. [DOI] [PubMed] [Google Scholar]
- 170. Keegan TH, Glaser SL, Clarke CA, et al. Body size, physical activity, and risk of Hodgkin's lymphoma in women. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1095–1101. [DOI] [PubMed] [Google Scholar]
- 171. Moore SC, Chow WH, Schatzkin A, et al. Physical activity during adulthood and adolescence in relation to renal cell cancer. Am J Epidemiol. 2008;168(2):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Tavani A, Zucchetto A, Dal Maso L, et al. Lifetime physical activity and the risk of renal cell cancer. Int J Cancer. 2007;120(9):1977–1980. [DOI] [PubMed] [Google Scholar]
- 173. Menezes RJ, Tomlinson G, Kreiger N. Physical activity and risk of renal cell carcinoma. Int J Cancer. 2003;107(4):642–646. [DOI] [PubMed] [Google Scholar]
- 174. Olson SH, Vena JE, Dorn JP, et al. Exercise, occupational activity, and risk of endometrial cancer. Ann Epidemiol. 1997;7(1):46–53. [DOI] [PubMed] [Google Scholar]
- 175. Matthews CE, Xu WH, Zheng W, et al. Physical activity and risk of endometrial cancer: a report from the Shanghai Endometrial Cancer Study. Cancer Epidemiol Biomarkers Prev. 2005;14(4):779–785. [DOI] [PubMed] [Google Scholar]
- 176. Schmid D, Behrens G, Keimling M, et al. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30(5):397–412. [DOI] [PubMed] [Google Scholar]
- 177. da Silva SG, Ricardo LI, Evenson KR, et al. Leisure-time physical activity in pregnancy and maternal-child health: a systematic review and meta-analysis of randomized controlled trials and cohort studies. Sports Med. 2016;47(2):295–317. [DOI] [PubMed] [Google Scholar]
- 178. Tobias DK, Zhang C, van Dam RM, et al. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care. 2011;34(1):223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Yin Y, Li X, Tao T, et al. Physical activity during pregnancy and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2014;48(4):290–295. [DOI] [PubMed] [Google Scholar]
- 180. Muktabhant B, Lawrie TA, Lumbiganon P, et al. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;(6):CD007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Streuling I, Beyerlein A, Rosenfeld E, et al. Physical activity and gestational weight gain: a meta-analysis of intervention trials. BJOG. 2011;118(3):278–284. [DOI] [PubMed] [Google Scholar]
- 182. Barakat R, Cordero Y, Coteron J, et al.. Exercise during pregnancy improves maternal glucose screen at 24–28 weeks: a randomised controlled trial. Br J Sports Med. 2012;46(9):656–661. [DOI] [PubMed] [Google Scholar]
- 183. Russo LM, Nobles C, Ertel KA, et al. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(3):576–582. [DOI] [PubMed] [Google Scholar]
- 184. Hopkins SA, Cutfield WS. Exercise in pregnancy: weighing up the long-term impact on the next generation. Exerc Sport Sci Rev. 2011;39(3):120–127. [DOI] [PubMed] [Google Scholar]
- 185. Clapp JF. Exercise during pregnancy. A clinical update. Clin Sports Med. 2000;19(2):273–286. [DOI] [PubMed] [Google Scholar]
- 186. Clapp JF, Kim H, Burciu B, et al. Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. Am J Obstet Gynecol. 2002;186(1):142–147. [DOI] [PubMed] [Google Scholar]
- 187. Gavard JA, Artal R. Effect of exercise on pregnancy outcome. Clin Obstet Gynecol. 2008;51(2):467–480. [DOI] [PubMed] [Google Scholar]
- 188. Oken E, Ning Y, Rifas-Shiman SL, et al. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol. 2006;108(5):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hopkins SA, Artal R. The role of exercise in reducing the risks of gestational diabetes mellitus. Womens Health (Lond). 2013;9(6):569–581. [DOI] [PubMed] [Google Scholar]
- 190. Brett KE, Ferraro ZM, Holcik M, et al. Prenatal physical activity and diet composition affect the expression of nutrient transporters and mTOR signaling molecules in the human placenta. Placenta. 2015;36(2):204–212. [DOI] [PubMed] [Google Scholar]
- 191. Leet T, Flick L. Effect of exercise on birthweight. Clin Obstet Gynecol. 2003;46(2):423–431. [DOI] [PubMed] [Google Scholar]
- 192. Sanabria-Martínez G, García-Hermoso A, Poyatos-León R, et al. Effects of exercise-based interventions on neonatal outcomes: a meta-analysis of randomized controlled trials. Am J Health Promot. 2016;30(4):214–223. [DOI] [PubMed] [Google Scholar]
- 193. Wiebe HW, Boulé NG, Chari R, et al. The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstet Gynecol. 2015;125(5):1185–1194. [DOI] [PubMed] [Google Scholar]
- 194. Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev. 2006;(3):CD000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Hopkins SA, Baldi JC, Cutfield WS, et al. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J Clin Endocrinol Metab. 2010;95(5):2080–2088. [DOI] [PubMed] [Google Scholar]
- 196. Rothman KJ. Induction and latent periods. Am J Epidemiol. 1981;114(2):253–259. [DOI] [PubMed] [Google Scholar]
- 197. Moolgavkar SH. Commentary: Multistage carcinogenesis and epidemiological studies of cancer. Int J Epidemiol. 2015;45(3):645–649. [DOI] [PubMed] [Google Scholar]
- 198. Moolgavkar SH, Luebeck EG. The role of somatic mutations and cell replication kinetics in quantitative cancer risk assessment. Prog Clin Biol Res. 1991;369:469–479. [PubMed] [Google Scholar]
- 199. Moolgavkar SH, Luebeck EG. Multistage carcinogenesis and the incidence of human cancer. Genes Chromosomes Cancer. 2003;38(4):302–306. [DOI] [PubMed] [Google Scholar]
- 200. Bellera CA, MacGrogan G, Debled M, et al. Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol. 2010;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Ahmed FE, Vos PW, Holbert D. Modeling survival in colon cancer: a methodological review. Mol Cancer. 2007;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14(15):1707–1723. [DOI] [PubMed] [Google Scholar]
- 203. Hazelton WD, Clements MS, Moolgavkar SH. Multistage carcinogenesis and lung cancer mortality in three cohorts. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1171–1181. [DOI] [PubMed] [Google Scholar]
- 204. Jeon J, Meza R, Moolgavkar SH, et al. Evaluation of screening strategies for pre-malignant lesions using a biomathematical approach. Math Biosci. 2008;213(1):56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Jeon J, Meza R, Hazelton WD, et al. Incremental benefits of screening colonoscopy over sigmoidoscopy in average-risk populations: a model-driven analysis. Cancer Causes Control. 2015;26(6):859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ulrich CM, Potter JD. Folate supplementation: too much of a good thing. Cancer Epidemiol Biomarkers Prev. 2006;15(2):189–193. [DOI] [PubMed] [Google Scholar]
- 207. Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention. Gut. 2006;55(10):1387–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Biro FM, Deardorff J. Identifying opportunities for cancer prevention during preadolescence and adolescence: puberty as a window of susceptibility. J Adolesc Health. 2013;52(5 suppl):S15–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Phillips AN, Smith GD. How independent are “independent” effects? Relative risk estimation when correlated exposures are measured imprecisely. J Clin Epidemiol. 1991;44(11):1223–1231. [DOI] [PubMed] [Google Scholar]
- 210. Ladd-Acosta C. Epigenetic signatures as biomarkers of exposure. Curr Environ Health Rep. 2015;2(2):117–125. [DOI] [PubMed] [Google Scholar]
- 211. Joubert BR, Håberg SE, Nilsen RM, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2012;120(10):1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Novakovic B, Ryan J, Pereira N, et al. Postnatal stability, tissue, and time specific effects of AHRR methylation change in response to maternal smoking in pregnancy. Epigenetics. 2014;9(3):377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. de Vocht F, Simpkin AJ, Richmond RC, et al. Assessment of offspring DNA methylation across the lifecourse associated with prenatal maternal smoking using Bayesian mixture modelling. Int J Environ Res Public Health. 2015;12(11):14461–14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Joubert BR, Felix JF, Yousefi P, et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98(4):680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Küpers LK, Xu X, Jankipersadsing SA, et al. DNA methylation mediates the effect of maternal smoking during pregnancy on birthweight of the offspring. Int J Epidemiol. 2015;44(4):1224–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Markunas CA, Xu Z, Harlid S, et al. Identification of DNA methylation changes in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2014;122(10):1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Reese SE, Zhao S, Wu MC, et al. DNA methylation score as a biomarker in newborns for sustained maternal smoking during pregnancy [published online ahead of print June 21, 2016]. Environ Health Perspect. (doi:10.1289/EHP333). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ladd-Acosta C, Shu C, Lee BK, et al. Presence of an epigenetic signature of prenatal cigarette smoke exposure in childhood. Environ Res. 2016;144(pt A):139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Rzehak P, Saffery R, Reischl E, et al. Maternal smoking during pregnancy and DNA-methylation in children at age 5.5 years: epigenome-wide-analysis in the European Childhood Obesity Project (CHOP) Study. PLoS One. 2016;11(5):e0155554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Lee KW, Richmond R, Hu P, et al. Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ Health Perspect. 2015;123(2):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Breton CV, Siegmund KD, Joubert BR, et al. Prenatal tobacco smoke exposure is associated with childhood DNA CpG methylation. PLoS One. 2014;9(6):e99716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Richmond RC, Simpkin AJ, Woodward G, et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet. 2015;24(8):2201–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Shenker NS, Ueland PM, Polidoro S, et al. DNA methylation as a long-term biomarker of exposure to tobacco smoke. Epidemiology. 2013;24(5):712–716. [DOI] [PubMed] [Google Scholar]
- 224. Zeilinger S, Kühnel B, Klopp N, et al. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One. 2013;8(5):e63812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Philibert RA, Beach SR, Lei MK, et al. Changes in DNA methylation at the aryl hydrocarbon receptor repressor may be a new biomarker for smoking. Clin Epigenetics. 2013;5(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Sun YV, Smith AK, Conneely KN, et al. Epigenomic association analysis identifies smoking-related DNA methylation sites in African Americans. Hum Genet. 2013;132(9):1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Bakulski KM, Fallin MD. Epigenetic epidemiology: promises for public health research. Environ Mol Mutagen. 2014;55(3):171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Schatzkin A, Gail M. The promise and peril of surrogate end points in cancer research. Nat Rev Cancer. 2002;2(1):19–27. [DOI] [PubMed] [Google Scholar]
- 230. Schatzkin A, Freedman LS, Dorgan J, et al. Surrogate end points in cancer research: a critique. Cancer Epidemiol Biomarkers Prev. 1996;5(12):947–953. [PubMed] [Google Scholar]
- 231. Thomas DC. Invited commentary: is it time to retire the “pack-years” variable? Maybe not. Am J Epidemiol. 2014;179(3):299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Lubin JH, Caporaso NE. Misunderstandings in the misconception on the use of pack-years in analysis of smoking. Br J Cancer. 2013;108(5):1218–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;15(3):517–523. [DOI] [PubMed] [Google Scholar]
- 234. Vlaanderen J, Portengen L, Schüz J, et al. Effect modification of the association of cumulative exposure and cancer risk by intensity of exposure and time since exposure cessation: a flexible method applied to cigarette smoking and lung cancer in the SYNERGY Study. Am J Epidemiol. 2014;179(3):290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 236. Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55(2):463–469. [DOI] [PubMed] [Google Scholar]
- 237. Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol Methods. 2001;6(1):18–34. [DOI] [PubMed] [Google Scholar]
- 238. Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Song M, Hu FB, Wu K, et al. Trajectory of body shape in early and middle life and all cause and cause specific mortality: results from two prospective US cohort studies. BMJ. 2016;353:i2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Taylor AE, Davey Smith G, Munafò MR. Negative controls to investigate maternal exposures during pregnancy. Am J Epidemiol. 2014;180(9):959–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Sharp GC, Lawlor DA, Richmond RC, et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2015;44(4):1288–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. 2015;44(2):496–511. [DOI] [PubMed] [Google Scholar]
- 243. Relton CL, Davey Smith G. Mendelian randomization: applications and limitations in epigenetic studies. Epigenomics. 2015;7(8):1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33(1):30–42. [DOI] [PubMed] [Google Scholar]
- 245. Freathy RM, Ring SM, Shields B, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18(15):2922–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Bowden J, Davey Smith G, Haycock PC, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Duke JC, Alexander TN, Zhao X, et al. Youth's awareness of and reactions to The Real Cost National Tobacco Public Education Campaign. PLoS One. 2015;10(12):e0144827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Campaigns: the real cost. https://betobaccofree.hhs.gov/campaigns/the-real-cost/index.html. Published 2014. Accessed August 3, 2016.
- 250. Farrelly MC, Nonnemaker J, Davis KC, et al. The influence of the national truth campaign on smoking initiation. Am J Prev Med. 2009;36(5):379–384. [DOI] [PubMed] [Google Scholar]
- 251. Hawkins SS, Bach N, Baum CF. Impact of tobacco control policies on adolescent smoking. J Adolesc Health. 2016;58(6):679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011–2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361–367. [DOI] [PubMed] [Google Scholar]
- 253. Food and Drug Administration, Department of Health and Human Services Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Final rule. Fed Regist. 2016;81(90):28973–29106. [PubMed] [Google Scholar]
- 254. Farber HJ, Nelson KE, Groner JA, et al. Public policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136(5):998–1007. [DOI] [PubMed] [Google Scholar]
- 255. Farrelly MC, Loomis BR, Kuiper N, et al. Are tobacco control policies effective in reducing young adult smoking. J Adolesc Health. 2014;54(4):481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Farrelly MC, Loomis BR, Han B, et al. A comprehensive examination of the influence of state tobacco control programs and policies on youth smoking. Am J Public Health. 2013;103(3):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Ott W, Klepeis N, Switzer P. Air change rates of motor vehicles and in-vehicle pollutant concentrations from secondhand smoke. J Expo Sci Environ Epidemiol. 2008;18(3):312–325. [DOI] [PubMed] [Google Scholar]
- 258. Kabir Z, Manning PJ, Holohan J, et al. Second-hand smoke exposure in cars and respiratory health effects in children. Eur Respir J. 2009;34(3):629–633. [DOI] [PubMed] [Google Scholar]
- 259. National Academy of Sciences Public health implications of raising the minimum age of legal access to tobacco products. http://www.nationalacademies.org/hmd/Reports/2015/TobaccoMinimumAgeReport.aspx. Published March 12, 2015. Updated August 19, 2015. Accessed July 16, 2016. [PubMed]
- 260. Banspach S, Zaza S, Dittus P, et al. CDC grand rounds: adolescence—preparing for lifelong health and wellness. MMWR Morb Mortal Wkly Rep. 2016;65(30):759–762. [DOI] [PubMed] [Google Scholar]
- 261. United States Department of Agriculture Smart snacks in school resources. http://healthymeals.nal.usda.gov/smartsnacks. Published 2015. Accessed November 5, 2015.
- 262. Sanchez-Vaznaugh EV, Sánchez BN, Crawford PB, et al. Association between competitive food and beverage policies in elementary schools and childhood overweight/obesity trends: differences by neighborhood socioeconomic resources. JAMA Pediatr. 2015;169(5):e150781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Terry-McElrath YM, O'Malley PM, Johnston LD. Potential impact of national school nutritional environment policies: cross-sectional associations with US secondary student overweight/obesity, 2008–2012. JAMA Pediatr. 2015;169(1):78–85. [DOI] [PubMed] [Google Scholar]
- 264. Whitlock EP, Orleans CT, Pender N, et al. Evaluating primary care behavioral counseling interventions: an evidence-based approach. Am J Prev Med. 2002;22(4):267–284. [DOI] [PubMed] [Google Scholar]
- 265. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-6):1–23. [PubMed] [Google Scholar]
- 266. Temel S, van Voorst SF, Jack BW, et al. Evidence-based preconceptional lifestyle interventions. Epidemiol Rev. 2014;36(1):19–30. [DOI] [PubMed] [Google Scholar]
- 267. The American Congress of Obstetrics and Gynecologists Committee opinion: obesity in pregnancy. http://www.acog.org/resources-and-publications/committee-opinions/committee-on-obstetric-practice/obesity-in-pregnancy. Published 2013. Accessed November 10, 2015.
- 268. The American Congress of Obstetrics and Gynecologists Committee opinion: the importance of preconception care in the continuum of women's health care. http://www.acog.org/resources-and-publications/committee-opinions/committee-on-gynecologic-practice/the-importance-of-preconception-care-in-the-continuum-of-womens-health-care. Published September 2005. Accessed November 10, 2015.
- 269. Yamamoto A, McCormick MC, Burris HH. US provider-reported diet and physical activity counseling to pregnant and non-pregnant women of childbearing age during preventive care visits. Matern Child Health J. 2014;18(7):1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Siu AL; US Preventive Services Task Force . Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(8):622–634. [DOI] [PubMed] [Google Scholar]
- 271. Centers for Medicare and Medicaid Services, Department of Health and Human Services New Medicaid tobacco cessation services. http://downloads.cms.gov/cmsgov/archived-downloads/SMDL/downloads/smd11-007.pdf. Published 2011. Accessed July 16, 2016.
- 272. McMenamin SB, Halpin HA, Ganiats TG. Medicaid coverage of tobacco-dependence treatment for pregnant women: impact of the Affordable Care Act. Am J Prev Med. 2012;43(4):e27–e29. [DOI] [PubMed] [Google Scholar]
- 273. Elster A. Guidelines for adolescent preventive services. http://www.uptodate.com/contents/guidelines-for-adolescent-preventive-services. Published 2015. Updated February 17, 2017.
- 274. Melnyk BM, Grossman DC, Chou R, et al. USPSTF perspective on evidence-based preventive recommendations for children. Pediatrics. 2012;130(2):e399–e407. [DOI] [PubMed] [Google Scholar]
- 275. American Academy of Pediatrics Bright Futures: prevention and health promotion for infants, children, adolescents, and their families. https://brightfutures.aap.org/Pages/default.aspx. Published 2015.
- 276. National Institutes of Health Environmental Influences on Child Health Outcomes (ECHO) program. https://www.nih.gov/echo. Accessed August 4, 2016.
- 277. Epidemiology and Genomics Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute Early life exposures and cancer. http://epi.grants.cancer.gov/early_life_exposures/. Published 2016. Updated May 10, 2016.
- 278. National Cancer Institute The cancer genome atlas home page. Cancer genome atlas. http://cancergenome.nih.gov/. Accessed August 8, 2016.
- 279. Campbell JD, Mazzilli SA, Reid ME, et al. The case for a Pre-Cancer Genome Atlas (PCGA). Cancer Prev Res (Phila). 2016;9(2):119–124. [DOI] [PubMed] [Google Scholar]
- 280. Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ. 2013;62(6):1–19. [PubMed] [Google Scholar]
- 281. Homa DM, Neff LJ, King BA, et al. Vital signs: disparities in nonsmokers’ exposure to secondhand smoke—United States, 1999–2012. MMWR Morb Mortal Wkly Rep. 2015;64(4):103–108. [PMC free article] [PubMed] [Google Scholar]
- 282. National Cancer Institute Usual dietary intakes: food intakes, US population, 2007–10. http://epi.grants.cancer.gov/diet/usualintakes/pop/2007-10/. Updated May 20, 2015.
- 283. National Center for Health Statistics, Centers for Disease Control and Prevention FastStats—exercise and physical activity. http://www.cdc.gov/nchs/fastats/exercise.htm. Published 2016. Accessed August 14, 2016.
- 284. National Physical Activity Plan Alliance 2014 United States report card on physical activity for children and youth. http://www.physicalactivityplan.org/projects/reportcard.html. Published 2014.