Abstract
Background:
Dementia is among the leading causes of severe and long-term disability worldwide, decreasing the quality of life of individuals and families. Moreover, it induces an enormous economic burden on societies. The most prevalent cause of dementia is Alzheimer's disease (AD). Because current treatment options for AD are limited, deep brain stimulation (DBS) has been considered.
Methods:
The aim of this review is to survey the current understanding regarding the effects of DBS in AD and possibly shed light on the mechanisms of DBS in AD. We searched PubMed and Cochrane for various studies in English literature describing DBS in patients with AD and relevant preclinical studies. All related studies published from December 2013 to March 2017 were included in this review.
Results:
Our understanding of the neural circuitry underlying learning and memory in both rodent models and human patients has grown over the past years and provided potential therapeutic targets for DBS such as the fornix and the nucleus basalis of Meynert. Clinical results indicate that DBS is most beneficial for patients who are in the early stages of AD. Potential mechanisms of action of DBS in AD comprise long-term structural plasticity, including hippocampal enlargement as well as enhanced neurotransmitter release.
Conclusion:
It is still premature to conclude that DBS can be used in the treatment of AD, and the field will wait for the results of ongoing and future clinical trials.
Keywords: Alzheimer's disease, deep brain stimulation, dementia, memory, fornix, nucleus basalis of Meynert
INTRODUCTION
Patients with dementia suffer from progressive cognitive decline. The most prevalent cause of dementia is Alzheimer's disease (AD) as it constitutes 50–80% of the cases. Dementia is a serious socioeconomic threat for ageing societies.[24,30] The mean life expectancy after diagnosis of AD is approximately 7 years.[4] At present, only symptomatic treatments are available, including NMDA receptor antagonist and acetylcholinesterase inhibitors.[1] The major drawback of these drugs, however, is that only a limited number of patients benefit from their temporary therapeutic effect. These limitations have triggered researchers to consider neuromodulation-based approaches in memory-related disorders. The field of neuromodulation is receiving more and more interest due to some key advances in this field.[35]
One of the neuromodulation approaches considered for AD is deep brain stimulation (DBS).[9,21,22] The key principle of DBS is to modulate the activity of neural elements by implanted electrodes in a key brain region with an internal pulse generator. The rationale for using DBS in AD is that, in addition to being a neurodegenerative disorder, AD can be considered a neural circuit disorder because it affects several integrated cortical and subcortical pathways, especially those involved in memory and cognition.[28]
Previously, we have reviewed relevant studies, which have been published until 2013.[13] Our main conclusion was that the use of DBS in patients with memory loss has placed special emphasis on stimulating the fornix or the nucleus basalis of Meynert (NBM), although exact mechanisms of action were yet to be elucidated. However, potential mechanisms underlying memory enhancement were hypothesized to include the release of specific neurotransmitters and neuroplasticity.
In the last few years, new data has appeared on DBS in AD [Table 1]. Here, we will discuss these studies and analyze the clinical outcomes in light of the stimulated target as well as the functional and anatomical changes in the brain. In particular, we will review relevant preclinical and clinical literature published after 2013 and evaluate the progress of our current understanding.
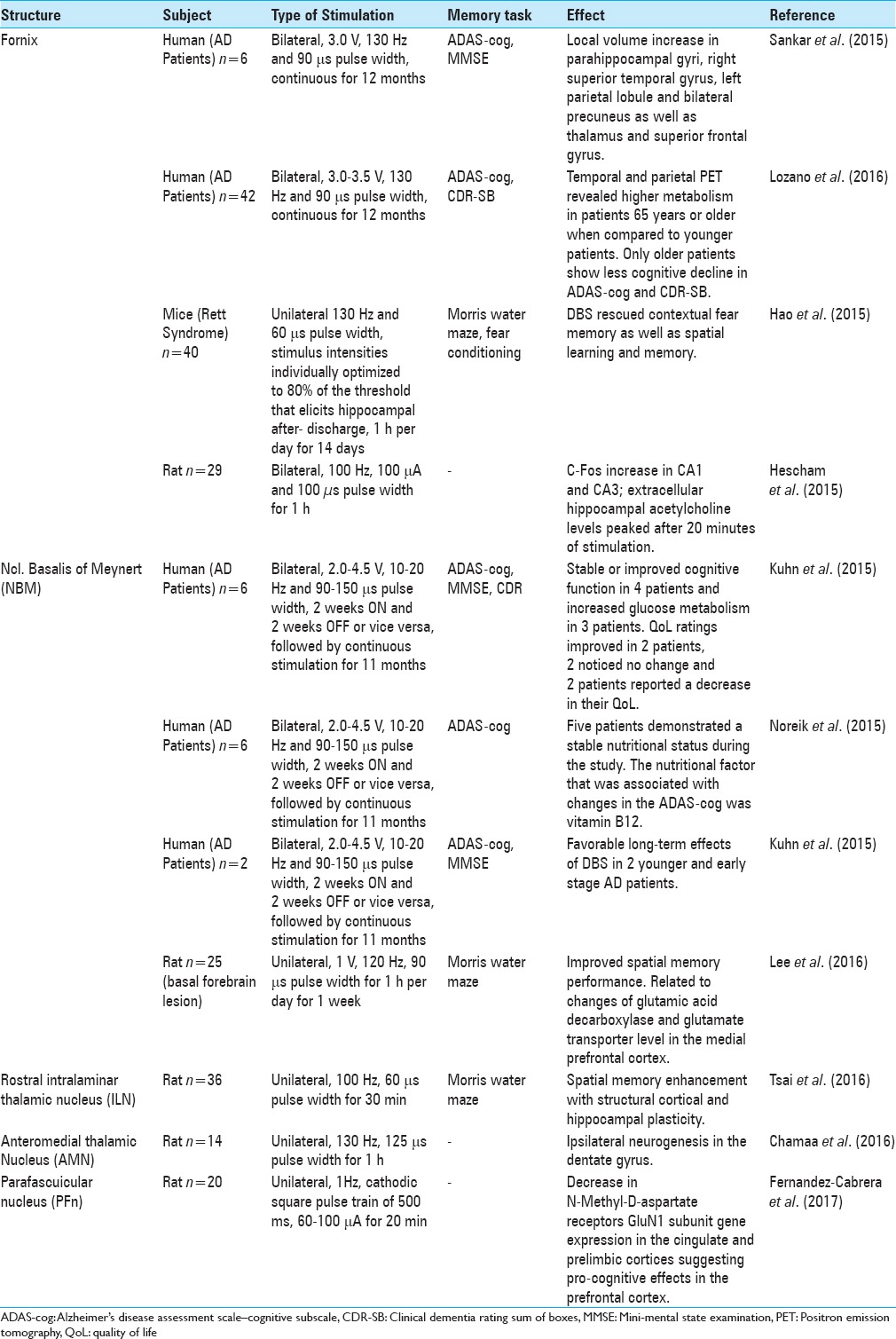
Table 1.
Summary of studies from December 2013 to March 2017 organized based on the structure targeted by DBS
LITERATURE SEARCH
We searched PubMed and Cochrane library for various clinical and preclinical studies in English literature with the search terms “Deep Brain Stimulation,” “memory loss,” “cognitive impairment,” “dementia,” and “Alzheimer's Disease.” Key words were used independently and in different combinations. Relevant articles were chosen from review papers, original research articles, and book chapters about DBS and AD. Articles of interest within the reference lists of selected articles were also considered. Studies describing DBS in AD patients using the fornix or NBM as the target structure were included. Clinical outcomes were Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS-cog), Mini-Mental State Examination (MMSE), and/or Clinical Dementia Rating Scale Sum of Boxes (CDR-SB). Preclinical studies targeting the fornix, NBM, or different thalamic nuclei were also included. Outcome measures were performance in behavioral tests (e.g. Morris water maze and fear conditioning). Articles aimed to study the effect of DBS in other neurodegenerative diseases or other forms of dementia [e.g. Parkinson's Disease dementia, vascular dementia, Huntington's disease dementia, alcohol related dementia, Creutzfeldt–Jakob disease, Lewy-body dementia] were excluded. Moreover, case reports and articles written in languages other than English were also excluded. To update our previous review,[13] which described relevant studies up to 2013, we considered all related studies published from December 2013 to March 2017 in the present review.
CLINICAL STUDIES
Thus far, only two different brain targets have been implicated for DBS in AD patients. These targets include the fornix[22,25] and the NBM.[8,18,20]
As described in our previous review, the idea of using fornix DBS for memory restoration was found incidentally by Hamani et al.[9] while treating a patient suffering from morbid obesity. On the basis of that study, a phase I trial was performed by Laxton et al.[22] in six patients with mild AD. The site of implantation of electrodes was the fornix/hypothalamus. Patients underwent 12 months of high frequency stimulation at 3 V, 130 Hz, and 90 μs pulse width. The study showed that stimulation of the fornix/hippocampus resulted in enhanced entorhinal and hippocampal neural activity. In addition, impaired glucose metabolism in both the temporal and partial lobes was reversed after chronic stimulation. However, the authors have not correlated the changes in glucose metabolism to an improvement in clinical outcome in the present study. Now, a study has been published describing that fornix DBS resulted in structural changes within the circuit of Papez in these patients.[32] MRI of hippocampus, fornix, and mammillary bodies was measured at baseline as well as 1-year after the procedure to evaluate structural changes. The authors found that DBS can significantly reduce the rate of hippocampal atrophy when compared to the age-, sex-, and severity-matched group of AD patients (n = 25) not receiving DBS.[32] Two patients with the best clinical response (improvement of the ADAS-cog and MMSE scores) to fornix DBS even showed a bilateral volume increase of the hippocampus of 5.6% and 8.2%, respectively. In one of the two patients, hippocampal volume was preserved 3 years after diagnosis. The mean hippocampal atrophy rate in AD patients has been estimated to be approximately 4–5% per year,[2] although the averages found in literature are highly variable. Several quantitative human MRI studies have suggested that the hippocampus can enlarge when performing physical exercise or when recovering from neurological disease states (for review see[7]). The average increase of hippocampal volume in healthy volunteers, who engaged in voluntary physical exercise, for example, was in the magnitude of 0.2–1.4%.[38] To our knowledge, hippocampal enlargement in AD patients has not yet been reported in literature. The unexpected 5–8% increase the authors observed in the aforementioned study appear to be striking and might even bring hippocampal volumes back to pre-disease levels. Further studies are indicated to determine what causes this volume increase. Possible mechanisms include synaptic plasticity, neurogenesis, gliogenesis, or even increased vascularization in the hippocampal region.
Glucose metabolism also changed in DBS patients in accordance with volume changes of the hippocampus and mammillary bodies. Deformation-based morphometry (DBM) showed local enlargement in regions that are typically atrophied in AD patients such as parahippocampal gyri, right superior temporal gyrus, left parietal lobule, and bilateral precuneus.[32] However, there was also expansion of areas that are not usually influenced by AD such as the thalamus and superior frontal gyrus. The authors hypothesized that these changes might represent mechanical re-expansion in areas deformed by the initial neurosurgical implantation procedure given the close proximity of the thalamus and superior frontal gyrus to the implanted DBS electrodes.
Following the abovementioned phase I trial, Lozano and colleagues launched the ADvance study, a double-blinded randomized controlled study in which 42 AD patients were implanted with fornix DBS electrodes in different centers across the U.S. and Canada. The aim of the study was to investigate the long and short-term safety of DBS. Outcome measures were neuropsychological tests such as the ADAS-cog, clinical dementia ratings, and glucose metabolism.[31] The study included 42 patients between the ages of 45 and 85 with an ADAS-cog score between 12 and 24. Patients underwent bilateral implantation of DBS electrodes and were followed at 2, 6, and 12 weeks interval after the procedure. The authors concluded that fornix DBS simulation can be accurately performed across neurosurgeons.
The outcomes of the phase II trial of the ADvance study have recently been published.[25] Twenty-one patients with “off” stimulation as a sham control group and 21 patients with “on” stimulation at 2 weeks after surgery with 130 Hz, between 3.0 and 3.5 V and 90 μs pulse width for a period of 12 months were included in this study. Age analysis was associated with clinical outcomes, for instance, preoperative positron emission tomography (PET) scan revealed significant lower metabolism in the younger patients compared to the older patients in temporal and parietal areas. After 6 months, PET imaging outcomes demonstrated a great reversal increase of the impaired glucose metabolism in several brain regions in fornix DBS group of patients more than 65 years of age in comparison to the patients younger than 65 years. However, the increase in glucose metabolism decreased again after 12 months of chronic stimulation. Younger patients (<65 years) receiving fornix DBS indicated cognitive decline in the 13-item version of the ADAS-cog and Clinical Dementia Rating Scale Sum of Boxes (CDR-SB). The authors suggested that this may be related to the greater brain atrophy, metabolic deficit, and malignant course of AD, or different genetic and clinical phenotypes that were less responsive to neural network modulation. The authors concluded that patients 65 years of age and older with mild AD responded to fornix DBS. The study findings are making grounds towards better understanding of the mechanism of fornix DBS and adjustment towards optimal dose to obtain maximum benefit.
Another potential target for DBS in AD patients is the NBM because it has cholinergic projections to hippocampus and neocortex. In AD, NBM degenerates, which results in reduction of cholinergic transmission, and subsequently decline of cognition in patients.[3]
Recently, 6 mild-moderate AD patients were enrolled in a German study and received bilateral low frequency DBS of the NBM.[18] DBS parameters were individually based. The study design consisted of two phases – a randomized sham-controlled stimulation phase of 1 month followed by 11 months of continued open stimulation. During the first phase, patients received either 2 weeks of stimulation (ON) followed by 2 weeks without stimulation (OFF) or vice versa. For ethical reasons, the authors switched into an open-label study after 11 months with a 24-hour isolation phase before the crossover. Primary outcome was clinical improvement and was assessed based on neuropsychological tests, PET scans, and electroencephalography (EEG). Two patients deteriorated in the ADAS-cog score, 3 patients remained stable and 1 patient improved. The mean MMSE score for the rate of decline was decreased by 0.5 points. In total, scores improved for 3 patients and worsened for the other 3 patients. The mean score of the Clinical Dementia Rating remained stable over the 12-month follow-up period. PET scans showed an increase of glucose metabolism in 3 out of 4 patients on 12-months follow-up. On average, quality of life (QoL) dropped by 0.2 (5.7 to 5.5) after 12 months. Two patients reported an improved QoL, 2 noticed no change, and 2 reported a decrease in their QoL. Of note, even early in the disease course, AD is characterized by the fact that patients are unable to understand the impact of their disease on daily functioning, a clinical phenomenon known as anosognosia.[39] In line with this, caregivers would usually rate patients’ QoL worse than patients themselves,[17] so the aforementioned QoL changes should be treated with caution.
In a next publication, the nutritional status of these patients was assessed before receiving NBM DBS and after 1 year to analyze potential associations between changes in cognition and nutritional status.[29] The hypothesis was that DBS can counteract the deterioration of nutritional status and progressive weight loss normally observed in AD. Indeed, the authors found that all but one patient gained body weight during the study period. This was reflected in a stable or improved body composition, assessed by bioelectrical impedance analysis, in 5 of the 6 patients. The nutritional factor that was associated with changes in the ADAS-cog was vitamin B12.
The same group performed a follow-up study in 2 patients, different from the 6 patients recruited in the pilot study based on the hypothesis that earlier intervention results in a better outcome. They targeted the same location (NBM) in 2 younger patients (61 and 67 years) with an earlier stage of AD.[19] After 26 months of follow-up, 1 patient remained stable in the ADAS-cog and even improved in the MMSE. An enhancement of cognitive functions is very unusual in patients with AD, especially after such a long follow-up period. The other patient showed a global improvement during the first year of treatment, but a slight deterioration of the ADAS-cog became apparent after 26 months. The major conclusion of the study stated that NBM DBS modulates the cholinergic input and has a favorable long-term cognitive effect with regard to ADAS-cog and MMSE scores in younger patients with less advanced AD stages. Caution needs to be applied, however, because of the small sample size and to their definition of “young.” In the aforementioned ADvance trial, younger patients were considered to be <65 years of age and did not respond to fornix DBS, whereas patients ≥65 years benefited from the treatment. The reasons for this are not yet understood. The authors state that younger patients have a more severe pathology when compared to older patients.[25] In addition, a great portion of young patients could be falsely diagnosed with AD. Moreover, genetics and phenotypes could have played a role in the response of AD to DBS. Further research is warranted to explore the relationship between DBS and AD with age as a factor.
PRECLINICAL STUDIES
Different brain targets have been implicated for DBS in animal models which showed enhancement of memory functions. These sites include the fornix,[14] entorhinal cortex,[34] NBM,[15] and anterior thalamic nucleus.[10,36] Preclinical studies have been used to investigate neuroanatomical, neurophysiological, and neurochemical changes within the memory circuits.
Recently, the effects of fornix DBS in a mouse model of Rett syndrome has been evaluated.[11] Rett syndrome is the leading cause of intellectual disability in females and the mouse model, which reproduces the broad phenotype of this disorder, shows clear deficits in hippocampus-dependent learning and memory and hippocampal synaptic plasticity. Unilateral fimbria-fornix DBS was able to rescue contextual fear memory as well as spatial learning and memory in these mice. In parallel, the authors were able to restore hippocampal long-term potentiation in vivo and hippocampal neurogenesis. Because cholinergic signaling plays a role in Rett syndrome, the authors hypothesized that fornix DBS can enhance hippocampal memory through cholinergic modulation. However, they were unable to corroborate this hypothesis in the present study.
Interestingly, in the same year, a neurochemical study was published in which the effects of fornix DBS with regard to hippocampal neurotransmitter release was described.[12] Rats were stimulated bilaterally in the fornix with 100 Hz, 100 μA, and 100 μs pulse width for 1 h whereas the sham group was only connected to the cable and not stimulated. In addition, a microdialysis probe was implanted in the dorsal hippocampus for continuous monitoring of neurotransmitters. First, c-Fos immunohistochemical analysis revealed a significant increase in the CA1 and CA3 subfield of the hippocampus in stimulated animals when compared to sham. Second, neurochemical analysis revealed that fornix DBS led to an increase in hippocampal acetylcholine levels. In particular, acetylcholine levels were elevated after 20 min of stimulation, but then declined despite ongoing DBS. Therefore, the authors concluded that intermittent stimulation might be needed to sustain high levels of acetylcholine. Notably, hippocampal glutamate levels were not affected by DBS.
With regard to neurochemical effects of NBM stimulation in rats with basal forebrain cholinergic neurons degeneration, gamma-aminobutyric acid (GABA), and glutamate seem to play a role in restoring memory loss.[23] The degeneration of basal forebrain cholinergic neurons is preferentially vulnerable in AD and is associated to spatial learning and memory impairment. Stimulation was unilateral, bipolar and parameters were 1 V, 90 μs at 120 Hz for 1 h per day for 1 week. In a spatial memory test, the DBS group with basal forebrain lesion showed an equivalent performance to controls without lesion, while sham animals performed significantly worse. Moreover, NBM DBS seemed to regulate levels of glutamic acid decarboxylase, which is involved in the synthesis of GABA and glutamate. In sham animals, glutamic acid decarboxylase decreased in the medial prefrontal cortex, whereas expression of glutamate transporters increased in the medial prefrontal cortex and hippocampus.
Since 2013, various animal studies have been published investigating DBS in different thalamic nuclei. In line with this, DBS of the rostral intralaminar thalamic nucleus (ILN) improved the acquisition of spatial memory when compared to sham and control rats.[37] Stimulation parameters were 1.5 mA, 60 μs at 100 Hz. Only a single 30 min train of stimulation was delivered to each animal on the first day of the Morris water maze. It was shown that DBS rats had more c-Fos immunoreactive neurons in layer IV of somatosensory cortex and hippocampal dentate gyrus than control rats, indicating that DBS enhanced structural alterations, which lead to increased synaptic connectivity. The number of dendritic spines also increased (58%) on CA1 hippocampal pyramidal neurons of DBS rats when compared to controls. Significant upsurge of dendritic spines (55–69%) was also seen in layer III of the somatosensory cortex as well as the proximal apical dendrites of layer V pyramidal neurons (16%) in comparison to control rats. These results indicate that the enhanced cognitive performance of ILN DBS rats might be due to modulation of hippocampal neuronal plasticity and intracortical functional and dendritic connectivity.
In another study, the effect of biphasic unilateral stimulation of the anteromedial thalamic nucleus on neurogenesis was investigated in awake and unrestrained rats.[5] Rats (n = 6) were stimulated for 1 h with 100 μA, 125 μs, 130 Hz, whereas sham rats (n = 4) were not stimulated. The authors showed a significant increase of the cell proliferation marker 5-Bromo-2-deoxyuridine (BrdU) in response to stimulation in the unilateral subgranular zone of the dentate gyrus compared with the contralateral side and sham rats (irrespective of the gender). The increase in unilateral neurogenesis reached 76% of neural progenitor cells.
Recently, DBS of the parafascicular thalamic nucleus has shown to affect NMDA receptor GluN1 subunit gene expression in the prefrontal cortex.[6] In this study, 20 naïve Wistar rats were stimulated for 20 min at 1 Hz cathodic square pulse trains of 500 ms and a current intensity ranging from 60 to 100 μA depending on the rats’ behavior (agitation, motor stereotypies, or other abnormal behavior were avoided). The results showed that parafascicular thalamic nucleus DBS induced a decrease in NMDAR GluN1 subunit gene expression in the cingulate and prelimbic cortices, but no significant differences were found in the density of NMDA or GABAB receptors. Because the authors have shown previously that parafascicular thalamic nucleus DBS can restore memory loss in NBM lesioned rats,[33] these findings suggest that pro-cognitive effects might be dependent on NMDAR GluN1 subunits in the prefrontal cortex.
DISCUSSION
Our understanding of the neural circuitry underlying learning and memory in both rodent models and human patients has grown over the past years and provided us with potential therapeutic targets for DBS. Phase I trials of DBS for AD, targeting either the fornix[22] or NBM,[18] have shown that DBS is safe and well tolerated in AD patients, with promising early data for cognitive improvement. Functional imaging revealed that DBS can modulate neuronal activity within memory circuits and alter pathological cortical physiology. Consistent with these promising early trials, the clinicaltrials.gov registry shows four clinical trials of DBS in either fornix or NBM for AD, which are either ongoing or have been completed recently. These results will shed more light on the therapeutic effects of DBS in AD patients.
Furthermore, an increasing amount of preclinical studies aim to elucidate underlying mechanisms of action [Figure 1]. In our previous review, mechanisms such as increased neurotransmitter release, release of growth factors, neurogenesis and neurotransmitter respecification have been outlined.[13] Since then, preclinical studies have collected more evidence with regard to increased neurotransmitter release[12] and neurogenesis.[5] Moreover, enhanced synaptic plasticity and long-term potentiation have been described.[11] There are of course some limitations of translational studies in predicting human clinical outcome, which can be attributed to the disease model itself, the small sample size and the experimental design.[16] Nevertheless, in line with these preclinical findings, remarkable new clinical evidence suggests that fornix DBS can affect long-term structural plasticity, including hippocampal enlargement.[32]
Figure 1.
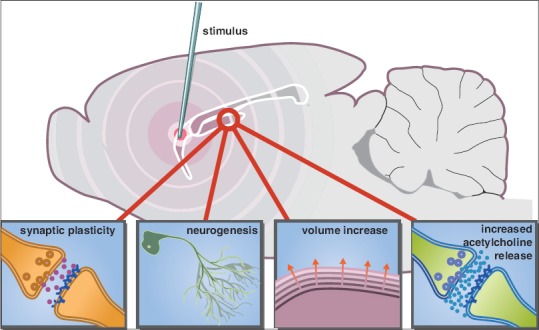
An updated schematic representation of the potential mechanisms involved in enhancing memory functions by deep brain stimulation. Stimulation of a target area within the memory circuit (e.g. fornix) can modulate the hippocampus through synaptic plasticity, neurogenesis, volume increase, and increased acetylcholine release
A cost-effectiveness study found that the clinical and economic thresholds required for DBS to be considered cost-effective for AD are relatively low.[27] Compared to standard treatment, DBS needs a success rate of only 3% to overcome effects of potential surgical complications on quality of life. At a success rate of 20%, DBS can be considered cost-effective for mild AD. Above 80% success rate, DBS is both clinically more effective and more cost-effective than standard treatment.[27]
Although the discussed therapeutics have shown promise in improving memory in AD, it is important to acknowledge that AD patients suffer from overall cognitive deterioration as well as non-cognitive symptoms and not solely memory impairment. Therefore, it is difficult to determine if improving memory will enhance quality of life. Moreover, it should be noted, that DBS does not prevent neurodegeneration in AD. Symptoms are only alleviated temporarily and in specific patient groups. Studies have shown that improvement in cognitive functioning and memory can last for a few years and deterioration can occur afterwards. In addition, it remains unclear which inclusion criteria should be considered for AD patients to benefit from DBS. With regard to the age of the patients, it is possible that the cognitive decline in young AD patients is related to DBS itself as has been observed in some PD cases.[26] However, in our opinion, this seems unlikely because young people with AD have a more severe progression, which could explain the structural, metabolic, and genetic abnormalities of their course of the disease compared with isolated AD pathology in elderly patients. More research needs to be done to establish the criteria needed for the treatment approach in patients with AD. Despite the promising data presented in this review, studies exploring the use of DBS in AD have limitations. At present, most published studies are done on a small sample size which can lead to over-interpretation of data. In addition, measured clinical outcomes are inherently subjective and biased. At present, there are multiple groups focusing on different targets in the brain, which makes drawing solid conclusions rather difficult. Furthermore, most available research remains in the preclinical phase, which gives no guaranteed outcomes in the translational setting. An important limitation of this approach remains that DBS is considered an invasive surgery with multiple risks associated with it, such as bleeding, infection, and possibly personality changes. Other side effects remain unknown and are yet to be discovered.
Nevertheless, because the clinical burden of dementia is considerable and the efficacy of current medical treatments limited, further investigation is warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Majed Aldehri, Email: m.aldehri@maastrichtuniversity.nl.
Yasin Temel, Email: y.temel@maastrichtuniversity.nl.
Ibrahim Alnaami, Email: imalnaami@kku.edu.sa.
Ali Jahanshahi, Email: a.jahanshahianvar@maastrichtuniversity.nl.
Sarah Hescham, Email: sarah.hescham@maastrichtuniversity.nl.
REFERENCES
- 1.Association As. 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7:208. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C, et al. A meta-analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiol Aging. 2009;30:1711–23. doi: 10.1016/j.neurobiolaging.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartus RT, Dean Rr, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 4.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Pub Health. 1998;88:1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamaa F, Sweidan W, Nahas Z, Saade N, Abou-Kheir W. Thalamic Stimulation in Awake Rats Induces Neurogenesis in the Hippocampal Formation. Brain Stimulat. 2016;9:101–8. doi: 10.1016/j.brs.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Cabrera MR, Selvas A, Miguéns M, Higuera-Matas A, Vale-Martínez A, Ambrosio E, et al. Parafascicular thalamic nucleus deep brain stimulation decreases NMDA receptor GluN1 subunit gene expression in the prefrontal cortex. Neuroscience. 2017;348:73–82. doi: 10.1016/j.neuroscience.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 8.Freund HJ, Kuhn J, Lenartz D, Mai JK, Schnell T, Klosterkoetter J, et al. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol. 2009;66:781–5. doi: 10.1001/archneurol.2009.102. [DOI] [PubMed] [Google Scholar]
- 9.Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63:119–23. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- 10.Hamani C, Stone SS, Garten A, Lozano AM, Winocur G. Memory rescue and enhanced neurogenesis following electrical stimulation of the anterior thalamus in rats treated with corticosterone. Exp Neurol. 2011;232:100–4. doi: 10.1016/j.expneurol.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Hao S, Tang B, Wu Z, Ure K, Sun Y, Tao H, et al. Forniceal deep brain stimulation rescues hippocampal memory in Rett syndrome mice. Nature. 2015;526:430–4. doi: 10.1038/nature15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hescham S, Jahanshahi A, Schweimer JV, Mitchell SN, Carter G, Blokland A, et al. Fornix deep brain stimulation enhances acetylcholine levels in the hippocampus. Brain Struct Funct. 2016;221:4281–6. doi: 10.1007/s00429-015-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hescham S, Lim LW, Jahanshahi A, Blokland A, Temel Y. Deep brain stimulation in dementia-related disorders. Neurosci Biobehav Rev. 2013;37:2666–75. doi: 10.1016/j.neubiorev.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Hescham S, Lim LW, Jahanshahi A, Steinbusch HW, Prickaerts J, Blokland A, et al. Deep brain stimulation of the forniceal area enhances memory functions in experimental dementia: The role of stimulation parameters. Brain Stimulat. 2013;6:72–77. doi: 10.1016/j.brs.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Hotta H, Kagitani F, Kondo M, Uchida S. Basal forebrain stimulation induces NGF secretion in ipsilateral parietal cortex via nicotinic receptor activation in adult, but not aged rats. Neurosci Res. 2009;63:122–8. doi: 10.1016/j.neures.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med. 2010;16:1210–4. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- 17.Kahle-Wrobleski K, Ye W, Henley D, Hake AM, Siemers E, Chen Y-F, et al. Assessing quality of life in Alzheimer's disease: Implications for clinical trials. Alzheimers Dement. 2017;6:82–90. doi: 10.1016/j.dadm.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn J, Hardenacke K, Lenartz D, Gruendler T, Ullsperger M, Bartsch C, et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer's dementia. Mol Psychiatry. 2015;20:353–60. doi: 10.1038/mp.2014.32. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn J, Hardenacke K, Shubina E, Lenartz D, Visser-Vandewalle V, Zilles K, et al. Deep Brain Stimulation of the Nucleus Basalis of Meynert in Early Stage of Alzheimer's Dementia. Brain Stimulat. 2015;8:838–9. doi: 10.1016/j.brs.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn J, Hardenacke K, Shubina E, Lenartz D, Visser-Vandewalle V, Zilles K, et al. Deep Brain Stimulation of the Nucleus Basalis of Meynert in Early Stage of Alzheimer's Dementia. Brain Stimul. 2015;8:838–9. doi: 10.1016/j.brs.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Laxton AW, Lozano AM. Deep brain stimulation for the treatment of Alzheimer disease and dementias. World Neurosurg. 2013;80:S28 e21–28. doi: 10.1016/j.wneu.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. 2010;68:521–34. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 23.Lee JE, Jeong DU, Lee J, Chang WS, Chang JW. The effect of nucleus basalis magnocellularis deep brain stimulation on memory function in a rat model of dementia. BMC Neurol. 2016;16:1. doi: 10.1186/s12883-016-0529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobo A, Launer L, Fratiglioni L, Andersen K, Di Carlo A, Breteler M, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology. 2000;54(Suppl 5):S4–9. [PubMed] [Google Scholar]
- 25.Lozano AM, Fosdick L, Chakravarty MM, Leoutsakos J-M, Munro C, Oh E, et al. A phase II study of fornix deep brain stimulation in mild Alzheimer's disease. J Alzheimers Dis. 2016;54:777–87. doi: 10.3233/JAD-160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massano J, Garrett C. Deep brain stimulation and cognitive decline in Parkinson's disease: A clinical review. Front Neurol. 2012;3:66. doi: 10.3389/fneur.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirsaeedi-Farahani K, Halpern CH, Baltuch GH, Wolk DA, Stein SC. Deep brain stimulation for Alzheimer disease: A decision and cost-effectiveness analysis. J Neurol. 2015;262:1191–7. doi: 10.1007/s00415-015-7688-5. [DOI] [PubMed] [Google Scholar]
- 28.Mirzadeh Z, Bari A, Lozano AM. The rationale for deep brain stimulation in Alzheimer's disease. J Neural Transm (Vienna) 2016;123:775–83. doi: 10.1007/s00702-015-1462-9. [DOI] [PubMed] [Google Scholar]
- 29.Noreik M, Kuhn J, Hardenacke K, Lenartz D, Bauer A, Bührle CP, et al. Changes in nutritional status after deep brain stimulation of the nucleus basalis of Meynert in Alzheimer's disease – Results of a phase I study. J Nutr Health Aging. 2015;19:812–8. doi: 10.1007/s12603-015-0595-8. [DOI] [PubMed] [Google Scholar]
- 30.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–32. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponce FA, Asaad WF, Foote KD, Anderson WS, Rees Cosgrove G, Baltuch GH, et al. Bilateral deep brain stimulation of the fornix for Alzheimer's disease: Surgical safety in the ADvance trial. J Neurosurg. 2016;125:75–84. doi: 10.3171/2015.6.JNS15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sankar T, Chakravarty MM, Bescos A, Lara M, Obuchi T, Laxton AW, et al. Deep brain stimulation influences brain structure in Alzheimer's disease. Brain Stimulat. 2015;8:645–54. doi: 10.1016/j.brs.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sos-Hinojosa H, Guillazo-Blanch G, Vale-MartÍnez A, Nadal R, Morgado-Bernal I, MartÍ-Nicolovius M. Parafascicular electrical stimulation attenuates nucleus basalis magnocellularis lesion-induced active avoidance retention deficit. Behav Brain Res. 2003;144:37–48. doi: 10.1016/s0166-4328(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 34.Stone SS, Teixeira CM, DeVito LM, Zaslavsky K, Josselyn SA, Lozano AM, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–84. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temel Y, Jahanshahi A. Treating brain disorders with neuromodulation. Science. 2015;347:1418–9. doi: 10.1126/science.aaa9610. [DOI] [PubMed] [Google Scholar]
- 36.Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg. 2008;108:132–8. doi: 10.3171/JNS/2008/108/01/0132. [DOI] [PubMed] [Google Scholar]
- 37.Tsai S-T, Chen L-J, Wang Y-J, Chen S-Y, Tseng G-F. Rostral Intralaminar Thalamic Deep Brain Stimulation Triggered Cortical and Hippocampal Structural Plasticity and Enhanced Spatial Memory. Stereotact Funct Neurosurg. 2016;94:108–17. doi: 10.1159/000444759. [DOI] [PubMed] [Google Scholar]
- 38.Varma VR, Chuang Y-f, Harris GC, Tan EJ, Carlson MC. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2015;25:605–15. doi: 10.1002/hipo.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson RS, Boyle PA, Yu L, Barnes LL, Sytsma J, Buchman AS, et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 2015;85:984–91. doi: 10.1212/WNL.0000000000001935. [DOI] [PMC free article] [PubMed] [Google Scholar]


