Abstract
Background:
Utilizing the spine literature, we compared the complication and reoperation rates for laminectomy alone vs. instrumented fusions including minimally invasive (MI) transforaminal lumbar interbody fusion (TLIF) for the surgical management of multilevel degenerative lumbar disease with/without degenerative spondylolisthesis (DS).
Methods:
Epstein compared complication and reoperation rates over 2 years for 137 patients undergoing laminectomy alone undergoing 2-3 level (58 patients) and 4-6 level (79 patients) Procedures for lumbar stenosis with/without DS. Results showed no new postoperative neurological deficits, no infections, no surgery for adjacent segment disease (ASD), 4 patients (2.9%) who developed intraoperative cerebrospinal fluid (CSF) fistulas, no readmissions, and just 1 reopereation for a (postoperative day 7). These rates were compared to other literature for lumbar laminectomies vs. fusions (e.g. particularly MI TLIF) addressing pathology comparable to that listed above.
Results:
Some studies in the literature revealed an average 4.8% complication rate for laminectomy alone vs. 8.3% for decompressions/fusion; at 5 postoperative years, reoperation rates were 10.6% vs. 18.4%, respectively. Specifically, the MI TLIF literature complication rates ranged from 7.7% to 23.0% and included up to an 8.3% incidence of wound infections, 6.1% durotomies, 9.7% permanent neurological deficits, and 20.2% incidence of new sensory deficits. Reoperation rates (1.6–6%) for MI TLIF addressed instrumentation failure (2.3%), cage migration (1.26–2.4%), cage extrusions (0.8%), and misplaced screws (1.6%). The learning curve (e.g. number of cases required by a surgeon to become proficient) for MI TLIF was the first 33-44 cases. Furthermore, hospital costs for lumbar fusions were 2.6 fold greater than those for laminectomy alone, with overall neurosurgeon reimbursement quoted in one study as high as $142,075 per year.
Conclusions:
The spinal literature revealed lower complication and reoperation rates for lumbar laminectomy alone vs. higher rates for instrumented fusion, including MI TLIF, for degenerative lumbar disease with/without DS.
Keywords: Complication rates, fusions, laminectomy alone, minimally invasive, reoperation rates, transforaminal lumbar interbody fusion
INTRODUCTION
When reviewing the literature, we asked whether lower complication and reoperation rates would be associated with performing multilevel laminectomy alone vs. fusions [e.g., predominantly minimally invasive (MI) transforaminal lumbar interbody fusion (TLIF)] for degenerative lumbar disease with/without degenerative spondylolisthesis (DS) [Tables 1–6]. In a personal consecutive cohort series of 137 patients undergoing multilevel laminectomies without fusions, at 2 postoperative years, there were no new neurological deficits, no infections, 4 (2.9%) intraoperative cerebrospinal fluid (CSF) fistulas (e.g. only in for those undergoing 4-6 level lamienctomies), and just 1 (0.7%) reoperation (sterile seroma at 7 postoperative days without readmission) [Table 2].[16] In a review of 37 studies from PubMed/Medline involving 1156 patients with lumbar spinal stenosis and stable low-grade 1–II DS (1983–2015) undergoing decompressions alone, Scholler et al. documented reoperation rates of 16.3% for OL (open laminectomy: 19 studies) vs. 5.8% for MIL (minimally invasive laminotomy: 18 studies).[36] In another study (2013) addressing degenerative lumbar disease/DS, Lad et al. showed that the complication rate for laminectomy alone was 4.8% vs. 8.3% for instrumented fusions; 5 years later, the reoperation rate was 10.6% without vs. 18.4% with spinal instrumentation [Table 1].[21] Other studies documented a 5.6% incidence of adjacent segment disease (ASD) following lumbar decompressions with noninstrumented fusions vs. 18.5% for decompressions with spinal instrumentation.[11,13] The MI TLIF literature documented a 7.7–19.2% complication rate for degenerative lumbar disease, that increased to 13–23.04% when combined with degenerative spondylolisthesis (DS); complications included 0.2–8.3% wound infections, 3.9–6.1% durotomies, 0.2–9.7% permanent neurological deficits, and 20.2% new sensory deficits [Tables 3–5]. Reoperations rates for MI TLIF ranged from 1.6-6% and addressed instrumentation failure (2.3%), cage migration (1.3–2.4%), cage extrusions (0.8%), and misplaced screws (1.6%) [Table 3 and 5].[3,23,31,40] Four studies documented the “learning curve” for safely/effectively performing MI TLIF required from 33-44 of the initial cases vs. Ahn et al. finding of no such learning curve (0 cases) for becoming proficient in performing MI laminotomy alone (0%) [Table 6].[1,22,27,31,37] In addition, not only were the costs for fusions 2.6 fold greater than those for laminectomy alone, but physician reimbursement rates were also higher with fusions (e.g., TLIF/MI TLIF/PLIF/others average $142,075/year).[25,42] Here, we predomiantly reviewed the literature regarding complication and reoperation rates for performing laminectomy alone vs. MI TLIF. In particular, we asked whether for comparable degenerative lumbar disease/DS, whether the added morbidity of MI TLIF fusion was and is acceptable.
Table 1.
Literature summarizing complications, reoperation rates, and incidence of adjacent segment disease utilizing laminectomy for degenerative lumbar disease vs. decompressions/fusions

Table 6.
Learning curve for TLIF/MIS TLIF vs. MI laminectomy/diskectomy
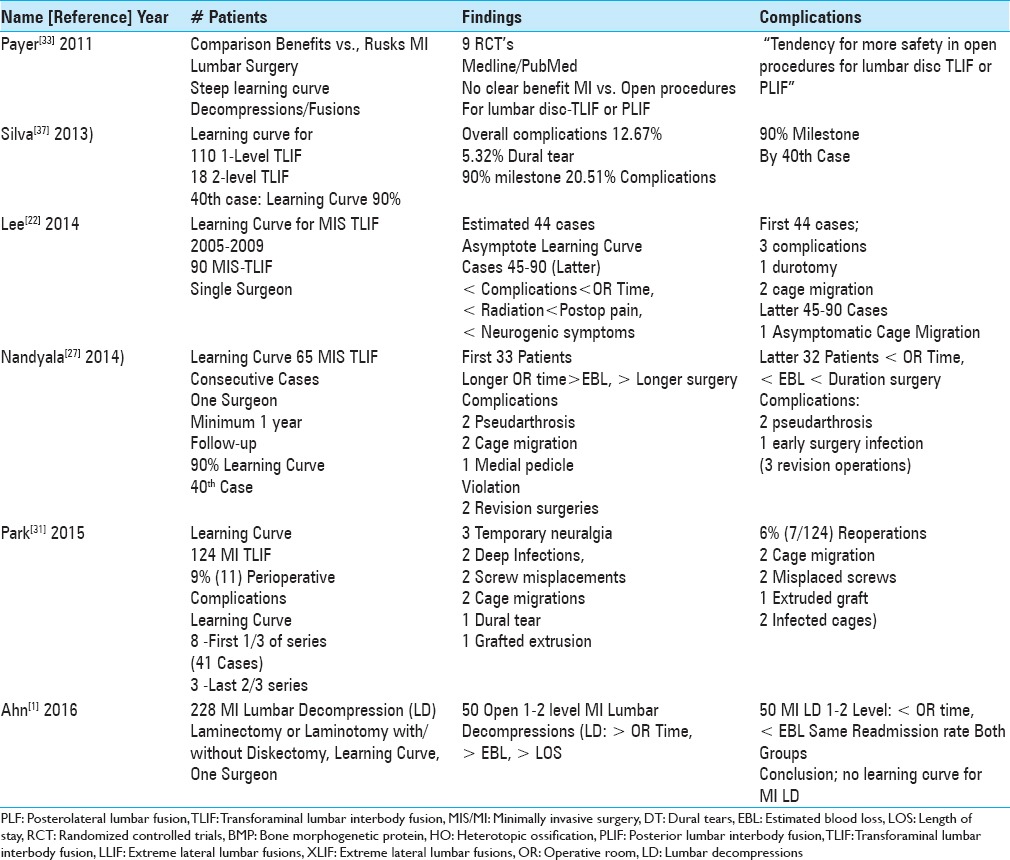
Table 2.
Epstein series clinical data following 2-3 (58 patients) vs. 4-6 level (79 patients) lumbar laminectomies without fusions[16]
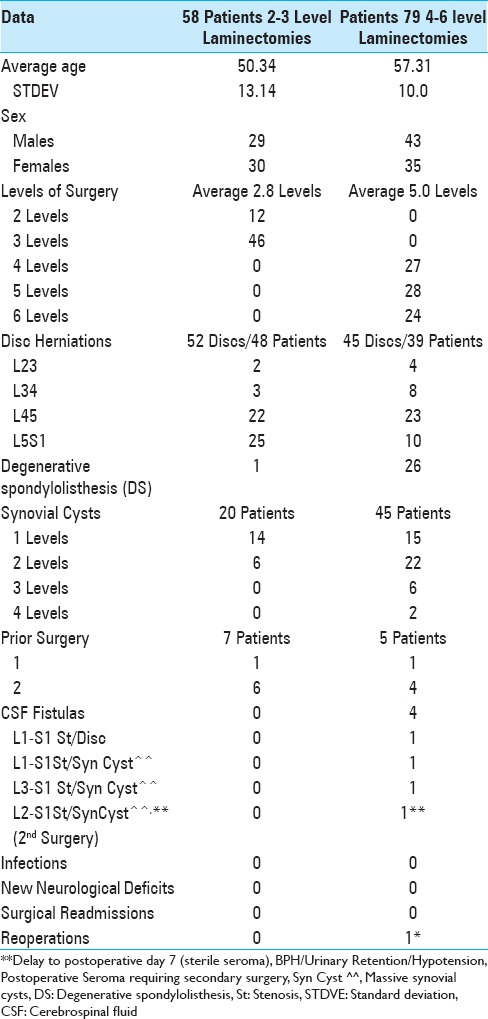
Table 3.
Summary complications/reoperations for TLIF/MI TLIF 2008-2013
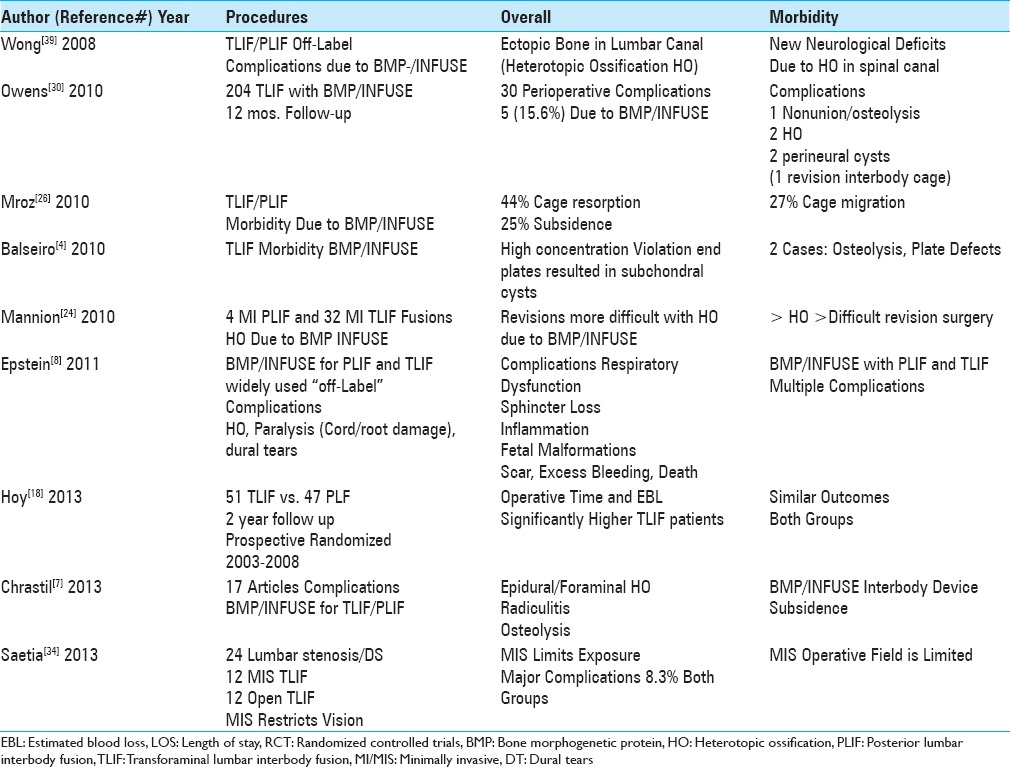
Table 5.
Summary of complications/reoperations for TLIF/MI TLIF 2016
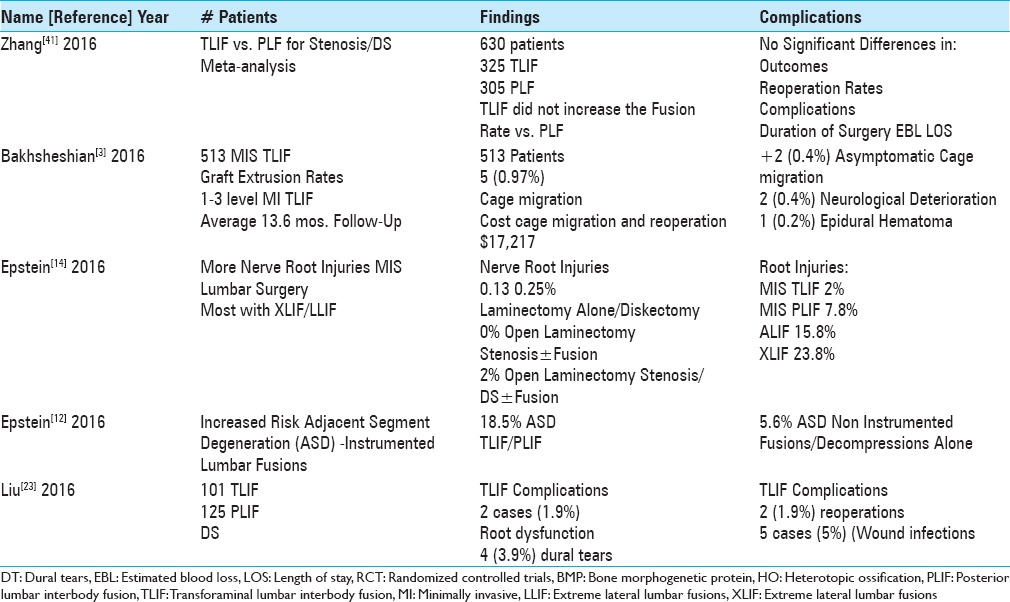
Trends toward more laminectomies/fusions vs. laminectomies alone for degenerative lumbar disease with/without degenerative spondylolisthesis (DS)
For lumbar degenerative disease with/without DS, several studies documented the increasing trend toward utilizing not only laminectomy for decompression but also adding instrumented fusions [Table 1].[2,29] Utilizing the Nationwide Inpatient Sample, Bae et al. (2013) examined the national trends for managing lumbar spinal stenosis (LSS) from 2004 to 2009 [Table 1].[2] The number of decompressions alone decreased from 58.5% to 49.2%, “simple fusions” (1–2 disc levels/single approach) increased from 21.5% to 31.2%, while the number of complex fusion (>2 disc levels/360 procedures) remained the same (6.7%). Of interest, the frequency with which bone morphogenetic protein (BMP)/INFUSE was used (largely “off-label” in the posterior lumbar spine) increased from 2004 to 2009, more than two fold (14.5% to 33.0% of fusions). There was also a 1.6 fold greater incidence of interbody fusions (28.5% to 45.1%). Notably, by 2009, 26.2% of patients with LSS without instability (without DS) were fused, while 82.7% of those with LSS/DS, and 67% of those with LSS/scoliosis had fusions. When Norton et al. (2015) evaluated 48,911 patients from the Nationwide Inpatient Sample Database (2001–2010) undergoing lumbar fusions for DS (237,383 procedures), more patients underwent posterolateral lumbar fusions (PLF), anterior lumbar interbody fusions (ALIF) with PLF, or ALIF alone; fewer had TLIF only or TLIF with PLF [Table 1].[29] Furthermore, PLF, typically performed in older patients, correlated with lower hospital charges, shorter length of stay (LOS), fewer complications, and reduced mortality rates vs. higher morality rates seen for TLIF with PLF or ALIF alone.
Complication and reoperation rates For laminectomy vs. fusions for degenerative lumbar disease with or without degenerative spondylolisthesis (DS)
The surgical literature showed lower complication and/or reoperation rates utilizing decompressions alone vs. decompressions and fusions for degenerative lumbar disease with/without DS [Table 1].[21,32,36] When Lad et al. (2013) evaluated lumbar decompressions performed between 2000 and 2009 utilizing the MarketScan database (the Thomson Reuters MarketScan Commercial Claims and Encounters and the Medicare Supplemental and Coordination of Benefits database containing 16,556 patients with a primary diagnosis of lumbar spondylolisthesis), the complication rate at 3 postoperative months was 4.8% for laminectomy without fusion vs. 8.3% with fusion; 5 years postoperatively, the reoperation rate for those undergoing laminectomy alone was 10.6% vs. 18.4% for instrumented arthrodeses.[21] Patil et al. (2014) also utilized the MarketScan Database (2007–2009; 16,556 patients) to identify 174 patients with degenerative lumbar disease/with DS undergoing laminectomy alone; at 1 postoperative year, the complication rate was 9.2% and the reoperation rate was 5.8%.[32] When Scholler et al. (2017) summarized the reoperation rates for 1156 patients with lumbar stenosis with low grade I–II DS in 37 studies obtained from Medline/PubMed (1983–2015), the total reoperation rate was 16.3% for open laminectomy (OL) vs. 5.8% for minimally invasive laminotomy (MIL); secondary fusions were warranted in 12.8% following OL and 3.3% after MIL [Table 1].[36]
Less adjacent segment disease (ASD) for decompressions/noninstrumented fusions vs. instrumented fusions for degenerative lumbar disease/degenerative spondylolisthesis (DS)
Several articles documented a lower incidence of ASD following laminectomy/laminectomy with noninstrumented fusion vs. decompressions with instrumented lumbar fusions (ASD) [Table 1].[6,11,13,15] In two review articles (2015, 2016), Epstein documented, that over 13 years, there was a 5.6% incidence of ASD with noninstrumented lumbar fusions vs. an 18.5% rate of ASD with spinal instrumenttion.[11,13] Yet the performance of an instrumented fusion did not significantly improve outcomes vs. noninstrumented fusions.[11,13] Bydon et al. (2016) demonstrated that, for 398 patients undergoing laminectomies alone without fusions, there was a 10% incidence of ASD following 1-level decompressions and a 9% frequency of ASD after 2-level decompressions.[6] In a personal clinical series (2016) involving 336 multilevel lumbar laminectomies (average, 4.7 levels) with noninstrumented fusions (average, 1.4 levels) for patients averaging 66.5 years of age with Grade I DS (154 patients) or Grade II DS (66 patients), Epstein documented that 9 patients (2.7%) required reoperations performed an average of 6.3 years after the index surgery.[15] These procedures addressed ASD attributed to stenosis/instability with grade I DS (7 patients) or grade II DS (1 patient), new disc herniations (2 patients), and a synovial cyst (1 patient).
Separate Epstein series showed reduced complication/reoperation rates with laminectomy alone without noninstrumented fusion for treating degenerative lumbar disease/degenerative spondylolisthesis (DS)
Epstein performed a prospective, consecutive cohort study regarding the efficacy of laminectomy alone for 137 patients with degenerative lumbar disease/with or without DS.[16] The study included 58 patients undergoing 2–3 level (average, 2.8 levels) and 79 patients undergoing 4–6 level (average, 5.0 level) laminectomies for stenosis with/wihtout DS [Table 2]. Patients in the two groups averaged 50.3 vs. 57.3 years of age, respectively, and underwent average 3.3 hour and 4.01 hour decompressive procedures. These operations additionally respectively addressed 52 vs. 45 discs, 20 vs. 45 single/multiple synovial cysts, and/or 1. vs. 26 instances of DS. Patients were respectively discharged an average of 2.4 and 3.3 days postoperatively. Over 2 postoperative years, none of the 137 patients developed new neurological deficits, there were no infections, 4 (2.9%) developed intraoperative CSF fistulas (e.g., all undergoing 4–6 level decompressions), there were no readmissions, and just 1 patient (0.7%) required a reoperation (e.g., 7 days postoperatively for a sterile seroma). Notably none developed ASD.
Complication rate of minimally invasive transforaminal lumbar interbody fusion (MI TLIF) for degenerative lumbar disease without degenerative spondylolisthesis (DS)
The overall complication rate for MI TLIF fusions addressing degenerative lumbar disease without DS ranged from 7.7% to 19.2% [Tables 3–5].[17,19,31,34,40] For 12 minimally invasive MI TLIF vs. 12 open TLIF, Saetia et al. (2013) noted an 8.3% complication rate in both groups (2008–2009) [Table 3].[34] Park et al. (2015) observed that, out of 124 MI TLIF, there was a 9% incidence of perioperative complications [Table 4].[31] For Giorgi et al. (2015), 7.7% of 182 MI TLIF developed postoperative complications.[17] Wong et al. (2015) observed a 15.6% complication rate out of 513 MI-TLIF.[40] In a meta-analysis of 54 studies involving MI TLIF (5454 patients; 6040 levels fused), Joseph et al. (2015) observed a 19.2% (1045 patients) complication rate.[19] In summary, the complication rates for MI TLIF fusions performed for patients with degenerative lumbar disease without DS were high, ranging up to 19.2%.
Table 4.
Summary of complications/reoperations for TLIF/MI TLIF 2014-2015
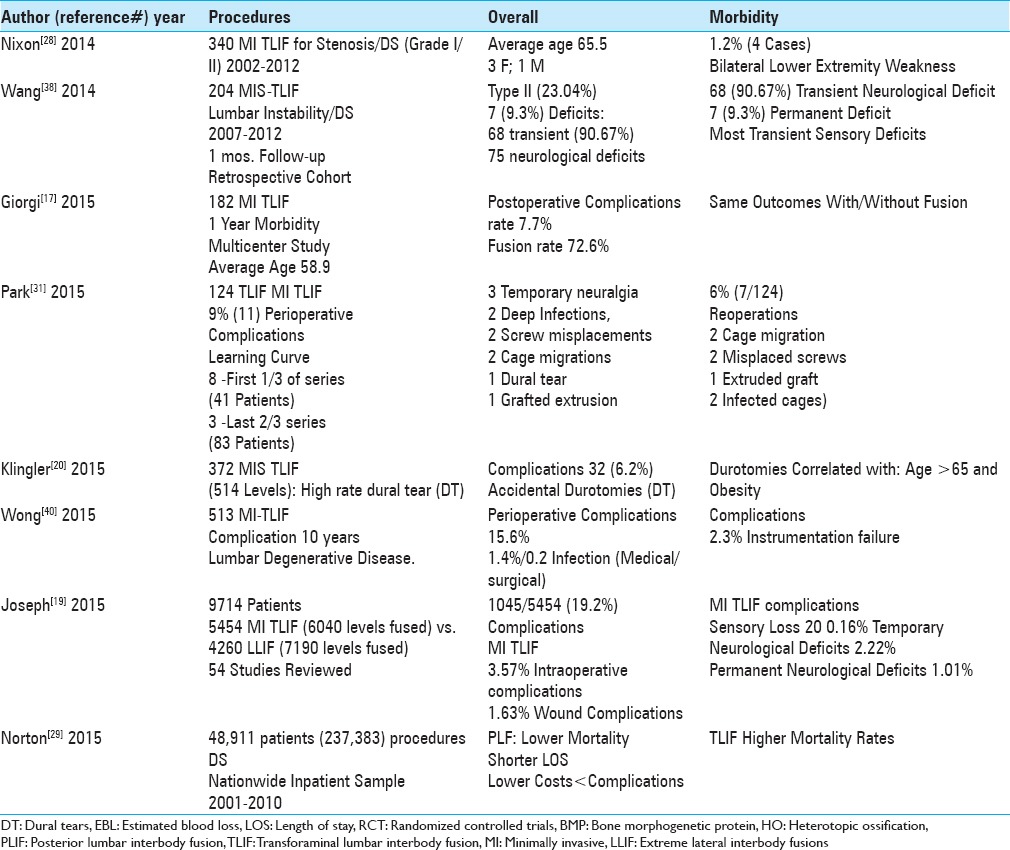
Complication rate for minimally invasive transforaminal interbody lumbar fusion (MI TLIF) For degenerative lumbar disease with degenerative spondylolisthesis (DS)
The overall complication rate for MI TLIF addressing degenerative lumbar disease with DS ranged from 13% to 23.04% [Tables 4 and 5].[23,38] Wang et al. (2014) found that from 2007 to 2012, the perioperative complication rate for degenerative lumbar disease/DS directly related to 204 MI TLIF up to 1 month postoperatively was 23% (64/204 patients); these included 1 complication (54 patients), 2 complications (9 patients), and 3 complications (1 patients); these included 68 transient (90.67% of 75 total neurological complications), and 7 (.93%) permanent complications [Table 4].[38] Liu et al. (2016) found a 13% perioperative complication rate for degenerative disease/DS treated with TLIF (101 patients) [Table 5].[23] Here, the overall range of complications for MI TLIF performed for degenerative lumbar disease with DS ranged up to 23%.
Minimally invasive transforminal interbody lumbar fusion (MI TLIF)-related new postoperative neurological deficits
Permanent neurological deficits following MI TLIF occurred in 0.2–9.7% of patients [Tables 3–5].[3,12,14,19,23,28,31,38] In a series of 204 MI TLIF, out of 75 reported neurological complications, Wang et al. (2014) found 68 (90.67%) transient deficits, while 7 were permanent (9.3%) [Table 4].[38] In a study by Nixon et al. (2014), out of 340 MI TLIF (2002–2012) performed for degenerative lumbar disease/DS (Grade I/II olisthesis), 4 (1.2%) patients averaging 65.5 years of age developed new bilateral lower extremity weakness [Table 4].[28] Joseph et al. (2015) observed that, following 5454 MI TLIF (6040 levels fused), there was a 20.2% incidence of new postoperative sensory deficits; postoperative motor deficits were transient in another 2.2% of patients and permanent motor deficits for 1.0% of patients [Table 4].[19] Liu et al. (2016) observed 2 (1.9%) patients with new permanent postoperative root dysfunction following 101 TLIF (101 patients) [Table 5].[23] Bakhsheshian et al. (2016) observed 2 (0.4%) new instances of neurological deterioration in a series of 513 MI-TLIF [Table 5].[3] In 2016, Epstein (2016) in two studies reviewed the quoted the incidence of nerve root injuries occurring utilizing conventional open decompressive techniques, some with fusions vs. minimally invasive lumbar surgery (e.g., decompressions with MIS TLIF, PLIF, ALIF, and XLIF) [Tables 3, 5].[12,14] For open discectomy, the frequency of nerve root injury ranged up to 0.25%, for open laminectomy for stenosis with/without fusion it was 0%; while for open laminectomy for stenosis/degenerative spondylolisthesis with/without fusion it was 2%.[14] Alternatively, root injuries were reported in 2% of MI TLIF (e.g., still 8 times higher than that with open discectomy/decompression), 7.8% of MIS PLIF, 15.8% of ALIFs, and a 23.8% frequency for extreme lateral interbody fusions (XLIFs).[14] As the incidence of nerve root injuries was so high for XLIFs, the inherent safety/efficacy of this procedure was questioned.
Frequency of dural tears with minimally invasive transforminal interbody lumbar fusion
Dural tears occurred in 3.9–6.1% of the patients undergoing MI-TLIF [Tables 3–5].[20,23,40] Wong et al. (2015) found a 5.1% durotomy rate for 513 MI-TLIF.[40] Klingler et al. (2015) noted that for 372 MIS TLIF (514 levels) there were 32 durotomies (6.2%) that highly correlated with more advanced age (e.g., over 65) and obesity.[20] Liu et al. (2016) found 4 (3.9%) instances of dural tears out of 101 TLIF (101 patients).[23] In Epstein's series, of 137 laminectomies without attendant fusions, the frequency of dural tears was lower e.g. 4 (2.9%).[16] Therefore, the incidence of dural tears was lower in standard open laminectomies vs. TLIF/MI/MIS TLIF fusions.
Reoperation rates for minimally invasive transforminal interbody lumbar fusion (MI TLIF)
Reoperations, performed in between 1.6% of the patients following MI TLIF, were largely attributed to instrumentation failures [Tables 3–5].[3,23,31,40] In Park et al. (2015), for 124 MI TLIF, 6% (7/124) required additional surgery; these included 2 (1.6%) for cage migrations, 2 (1.6%) for misplaced screws, and 1 (0.8%) for an extruded graft bone fragment.[31] In a study by Wong et al. (2015), out of 513 MI TLIF, there was a 2.3% instrumentation failure rate.[40] Liu et al. (2016) found that 2 (1.9 %) of 101 TLIF (101 patients) required additional surgery for instrument failure.[23] Bakhsheshian et al. (2016) noted that for 513 MI TLIF, 8 (1.6%) required reoperations; 7 (1.4%) for cage extrusions, and 1 (0.2%) for an epidural hematoma.[3] Of interest, the average cost of a reoperation was an additional $17,271.
Up to 8.3% infection rates for minimally invasive transforaminal interbody lumbar fusion (MI TLIF)
Spinal infections occurred in from 0.2% up to 8.3% of patients undergoing predominantly MI TLIF [Tables 3–5].[19,23,31,40] Out of the 513 MI-TLIF, Wong et al. (2015) found a 1.4% incidence of medical, and a 0.2% frequency of surgical infections.[40] Joseph et al. (2015) observed a 1.6% risk of infection for MI-TLIF (5454 patients; 6040 levels fused; 4 studies) [Table 4].[19] In Park et al. (2015), there were 2 infected cages, and additional operations were required in 6% of patients [Table 4].[31] Liu et al. (2016) found 5 (5%) cases of infections out of a series of 101 TLIF [Table 5].[23] In comparison, there were no reopertions for infections in Epstein's two series: (1) no infections out of 137 patients undergoing 2-3 vs. 4-6 level laminectomies without fusions, and no infections out of 336 lumbar laminectomies with accompanying noninstrumented fusions.[15,16]
Mortality rates reported higher for transforminal interbody lumbar fusion (TLIF) vs. posterolateral lumbar fusion (PLF)
When Norton et al. (2015) reviewed 48,911 patients (237,383 procedures) from the Nationwide Inpatient Sample Database (2001 to 2010) with degenerative lumbar disease/DS, those undergoing TLIF had higher morality rates vs. those having posterolateral lumbar fusions (PLF). Indeed, performance of PLF correlated with lower mortality rates, reduced hospital charges, LOS, and complication rates) [Table 4].
Restricted field of vision for minimally invasive transforaminal interbody lumbar fusion (MI TLIF)
Although Saetia et al. (2013) found similar complication rates of 8.3% for both open TLIF vs. MI TLIF for lumbar degenerative spondylolisthesis, the authors noted shortcomings of MI TLIF, including a restricted filed of vision (e.g. more limited exposure) that “required a very thorough knowledge of anatomy” [Table 3].[34] Although all surgeons need to have a thorough knowledge of the spinal anatomy whether performing open or MI procedures, surgeons performing MI surgery may lose perspective regarding the anatomy which can lead to higher complication rates.
Comparable outcomes for minimally invasive transforaminal lumbar fusion (MI TLIF) vs. other instrumented fusions including posterolateral lumbar fusion (PLF)
Multiple studies documented similar outcomes for MI TLIF/TLIF vs. other types of instrumented lumbar fusions (e.g., particularly PLF) [Tables 3–5].[17,18,41] Hoy et al. (2013) found comparable outcomes for TLIF (51 patients) vs. instrumented posterolateral fusions (PLF: 47 patients) [Table 3].[18] Giorgi et al. (2015) evaluated MI TLIF; although the fusion rate was just 72.6% at 1 postoperative year, the quality of outcomes were similar whether or not the patient successfully fused [Table 4].[17] In a meta-analysis involving 2 randomized controlled studies (RCTs) and 5 other studies (total 630 patients), Zhang et al. (2016) confirmed the comparable efficacy of 325 TLIF vs. PLF fusions for degenerative lumbar spondylosis [Table 5].[41] Specifically, TLIF did not increase the fusion rate compared with instrumented PLF, and there were no significant differences in outcomes (VAS, Oswestry Disability Index), reoperation rates, complications, duration of surgical procedures, blood loss, and duration of hospitalization.
BMP/INFUSE (Medtronic, Memphis, TN, USA) increased risks and complications for lumbar fusions including posterior lumbar interbody fusion (PLIF)/transforaminal interbody fusion/minimally invasive transforaminal interbody fusion (TLIF/MI TLIF)
BMP (bone morphogenetic protein)/INFUSE (Medtronic, Memphis, TN, USA) was and is still frequently applied “off-label” (e.g. posteriorly in the lumbar spine) for performing posterior lumbar spinal fusions including PLIF/TLIF/MI TLIF, thus increasing the risks and complication rates [Tables 3–5.[4,7,8,9,10,24,26,30,39] Wong et al. (2008) showed BMP/INFUSE used for PLIF and TLIF contributed to new neurological deficits due to significant ectopic bone formation (heterotopic ossification: HO) within the spinal canal [Table 3].[39] Mroz et al. (2010) noted the “off-label” use of BMP for TLIF was responsible for the following cage-related complications: a 44% resorption rate, a 25% subsidence rate, and 27% incidence of cage migration [Table 3].[26] Balseiro et al. (2010) additionally observed vertebral osteolysis (bone resorption) occurring following TLIF procedures, largely attributed to extremely high concentrations of BMP/INFUSE, and/or violation of end plates, resulting in subchondral cysts [Table 3].[4] Mannion et al. (2010) noted that, following TLIF performed with BMP/INFUSE, revision surgeries (4 PLIFs and 32 TLIFs) were much more “difficult” [Table 3].[24] Of the 30 complications noted in 240 TLIF using BMP/INFUSE in Owens et al. series (2010), 5 complications were directly attributed to BMP/INFUSE – 1 nonunion at 12 months (osteolysis), 2 with heterotopic ossification, and 2 exhibiting perineural cysts associated with migration of interbody cages.[30] In 2011, Epstein observed BMP/INFUSE was used off-label in the spine 96% of the time at one institution, and cited multiple other reports of BMP/INFUSE-related morbidity particularly associated with TLIF surgery [Table 3].[8,9] In 2013, both Epstein and Chrastil separately summarized multiple adverse events attributed to the off-label use of BMP/INFUSE in spine surgery; these included heterotopic ossification (HO), osteolysis, infection, adhesive arachnoiditis, increased postoperative neurological deficits/radiculitis, endplate osteolysis/interbody device subsidence, retrograde ejaculation/sterility, and cancer.[7,10]
Obviously, the use of BMP/INFUSE for TLIF/MI TLIF, PLIF and other instrumented fusions introduces additional complications not seen with the laminectomy alone/laminectomy with non instrumented fusion where BMP is not utilized.
Learning curve for minimally invasive surgery/transforaminal interbody lumbar fusion (MI TLIF)
In 2011, Payer utilized the PubMed and Medline databases and found only 9 RCT that adequately defined the pros and cons of MI lumbar decompressions/stabilization procedures.[33] Although the pros included smaller incisions, reduced perioperative pain, blood loss, and hospital stays, the cons included steep learning curves, they observed there was no relevant benefit from minimally invasive techniques (e.g., decompression/stabilization-fusion), and a tendency for more safety in open procedures for lumbar disc herniations, TLIF, and PLIF.
Learning curves: 33–44 cases for minimally invasive transforaminal interbody lumbar fusion (MI TLIF) vs. 0 for minimally invasive decompressions alone
The learning curve for MI TLIF, defined as how many MI TLIF a surgeon needs to perform before becoming technically proficient, was described as involving the first 33–44 cases (e.g. reported in 4 series) [Table 6].[1,22,27,31,37] In a study by Silva et al. (2013), for 110 patients undergoing 1-level and 18 patients undergoing 2-level MITLIF, a 90% learning milestone was achieved by the 39th case, with an overall complication rate of 12.67%.[37] Nandyala (2014) observed that 90% of the learning curve for 65 consecutive patients undergoing MI TLIF, addressing disk disease or lumbar spinal stenosis with grade I or II spondylolisthesis (2008–2011), was attained at about the 40th case.[27] Of interest, for the first 33 patients in Nandyala study (2014), the average operative time/duration of anesthesia for MIS TLIF was longer, there was more blood loss, and there were more complications (e.g., 2 radiographic pseudarthroses, 1 graft migration, 1 medial pedicle wall violation necessitating two operative revisions).[27] Alternatively, for the latter 32 patients, there were 2 pseudarthroses and 1 early surgical site infection; all 3 patients required revision surgery. For Lee et al. (2014), the learning curve for MI TLIF was 44 cases (e.g., total 90 cases 2005–2009 performed by a single surgeon); for the 44 initial cases, there were 3 complications – 1 incidental durotomy and 2 asymptomatic cage migrations, while for the latter 46 patients, there was just 1 asymptomatic cage migration.[22] Other observations for the latter 46 patients included reduced operative times, radiation dose, postoperative pain, and fewer new postoperative neurogenic symptoms. In 2015, Park et al. defined the learning curve for 124 MI TLIF as occurring after the first one-third of the cases (41 MI TLIF).[31] Notably, 8 of the total 11 complications occurred in the first 41 cases, with only 3 occurring in the remaining two-thirds of the patients (e.g., latter 83 patients). Total complications included 3 temporary postoperative neuralgias, 2 deep wound infections, 2 pedicle screw misplacements, 2 cage migrations, 1 dural tear, and 1 bone graft extrusion [Table 6].[31] In contrast, Ahn et al. documented no learning curve (e.g., no or 0 cases) required for learning how to safely and proficiently perform MI lumbar decompressions alone (LD).[1] They based this conclusion on an analysis of 50 open 1–2 level lumbar decompressions (LD) performed from 2005 to 2006 vs. 50 subsequent MI LD. They concluded “although surgical experience may improve perioperative parameters (operative time, length of hospitalization), a MIS LD may initially be performed safely without prior experience.” The results of Ahn et al. clearly indicate that there was no learning curve needed to perform MI lumbar decompressions. This does not, however, necessarily fit in with the experience of many surgeons learning any new operation including the simplest in which there is always some learning curve.
Increased relative frequency, costs, and reimbursement for instrumented lumbar fusions vs. lumbar laminectomy alone
Several studies documented the increased frequency, costs, and reimbursement rates for instrumented fusion vs. lumbar laminectomy alone addressing degenerative lumbar disease with/without DS.[5,25,42] Utilizing the Nationwide Inpatient Sample (NIS) plus US Census/other data, Bernstein et al. (2017) determined that from 2003 to 2012 the number of lumbar diskectomies decreased by 19.8% and laminectomies by 26.1%, while there was an increase of 56.4% in the incidence of lumbar spinal fusions.[5] Menger et al. (2015), evaluating Medicare data from 2012, found that for 206 spinal surgeons performing lumbar laminectomies (including add-on levels)/fusions), the average neurosurgeon was paid $142,075 for all procedures.[25] In 2017, Zygourakis et al. (2017), utilizing the 2001 to 2013 National Inpatient Sample database, evaluated the different costs for performing discectomy/laminectomy (181,267 patients) vs. instrumented lumbar fusions (433,364 patients) in different locations in the US.[42] The average cost increases from 2001 to 2013 were $8,316 to $11,405 for discectomy/laminectomy vs. $21,473 to $29,438 for instrumented fusions (e.g., a 2.6 fold increased cost for fusions). Notably, higher costs for fusions were also encountered at smaller hospitals in more rural locations. Thus, if a surgeon added a fusion to his or her procedure, the reimbursement would increase over 2.6 times.
DISCUSSION
In this review of the literature, for patients with lumbar degenerative disease/with or without DS, we found lower complication and reoperation rates utilizing laminectomy alone vs. instrumented fusions (e.g., predominantly MI TLIF). For 2 years following Epstein's 137 lumbar multilevel laminectomies without fusions, patients exhibited no new neurological deficits, no infections, 4 (2.9%) had intraoperative CSF fistulas, there were no readmissions, and just 1 patient (0.73%) required a reoperation.[16] Other literture showed a 4.8% complication rate for laminectomy alone vs. 8.3% for instrumented fusions; at 5 postoperative years, the reoperation rates were lower (10.6%) without than with instrumented procedures (18.4%). Furthermore, complication rates for predominantly MI TLIF ranged up to (23.04%); reoperations up to 6%, accompanied by a learning curve requiring 33–44 of the initial cases to attain proficiency compared to 0% necessitated for safely performing MI laminotomy.
Based on these data, the choice of procedures to address multilevel degenerative lumbar disease with/without DS should be obvious. Nevertheless, more fusions rather than decompressions alone are increasingly being performed. For example, Bae et al. (Nationwide Inpatient Sample Data) showed that, from 2004 to 2009, decompressions alone decreased from 58.5% to 49.2%, but “simple fusions” (1–2 disc levels/single approach) increased from 21.5% to 31.2%.[2] Using the National Inpatient Sample database, Zygourakis et al. (2017) showed the average cost for discectomy/laminectomy increased in 2001–2013 from $8,316 to $11,405, while for fusions it increased 2.6 fold ($21,473 to $29,438).[42] Furthermore, when Menger et al. (2015) utilized Medicare data from 2012 for 206 spinal surgeons, they documented neurosurgeon reimbursements for all lumbar fusions averaged $142,075 (e.g., lumbar laminectomies/add-on levels/fusions).[25] As both hospital charges and physician reimbursements are higher for more complicated fusions, one cannot dismiss financial gain as an added motivation for performing lumbar fusions. Certainly, our selection of operative alternatives for treating degenerative lumbar disease/DS must uphold the standard of care that “must be justified on a logical basis and must have considered the risks and benefits of competing options.”[35]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
REFERENCES
- 1.Ahn J, Iqbal A, Manning BT, Leblang S, Bohl DD, Mayo BC, et al. Minimally invasive lumbar decompression-the surgical learning curve. Spine J. 2016;16:909–16. doi: 10.1016/j.spinee.2015.07.455. [DOI] [PubMed] [Google Scholar]
- 2.Bae HW, Rajaee SS, Kanim LE. Nationwide trends in the surgical management of lumbar spinal stenosis. Spine (Phila Pa 1976) 2013;38:916–26. doi: 10.1097/BRS.0b013e3182833e7c. [DOI] [PubMed] [Google Scholar]
- 3.Bakhsheshian J, Khanna R, Choy W, Lawton CD, Nixon AT, Wong AP, et al. Incidence of graft extrusion following minimally invasive transforaminal lumbar interbody fusion. J Clin Neurosci. 2016;24:88–93. doi: 10.1016/j.jocn.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Balseiro S, Nottmeier EW. Literature review for infuse: Vertebral osteolysis originating from subchondral cyst end plate defects in transforaminal lumbar interbody fusion using rhBMP-2. Spine J. 2010;10:e6–10. doi: 10.1016/j.spinee.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein DN, Brodell D, Li Y, Rubery PT, Mesfin A. Impact of the Economic Downturn on Elective Lumbar Spine Surgery in the United States: A National Trend Analysis, 2003 to 2013. Global Spine J. 2017;7:213–9. doi: 10.1177/2192568217694151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bydon M, Macki M, De la Garza-Ramos R, McGovern K, Sciubba DM, Wolinsky JP, et al. Incidence of Adjacent Segment Disease Requiring Reoperation After Lumbar Laminectomy Without Fusion: A Study of 398 Patients. Neurosurgery. 2016;78:192–9. doi: 10.1227/NEU.0000000000001007. [DOI] [PubMed] [Google Scholar]
- 7.Chrastil J, Low JB, Whang PG, Patel AA. Complications associated with the use of the recombinant human bone morphogenetic proteins for posterior interbody fusions of the lumbar spine. Spine (Phila Pa 1976) 2013;38:E1020–7. doi: 10.1097/BRS.0b013e3182982f8e. [DOI] [PubMed] [Google Scholar]
- 8.Epstein NE. Pros, cons, and costs of INFUSE in spinal surgery. Surg Neurol Int. 2011;2:10. doi: 10.4103/2152-7806.76147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein NE, Schwall GS. Costs and frequency of “off-label” use of INFUSE for spinal fusions at one institution in 2010. Surg Neurol Int. 2011;2:115. doi: 10.4103/2152-7806.83929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein NE. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surg Neurol Int. 2013;4(Suppl 5):S343–52. doi: 10.4103/2152-7806.114813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein NE. Adjacent level disease following lumbar spine surgery: A review. Surg Neurol Int. 2015;6(Suppl 24):S591–9. doi: 10.4103/2152-7806.170432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein NE. More nerve root injuries occur with minimally invasive lumbar surgery: Let's tell someone. Surg Neurol Int. 2016;7(Suppl 3):S96–S101. doi: 10.4103/2152-7806.174896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein NE. Older literature review of increased risk of adjacent segment degeneration with instrumented lumbar fusions. Surg Neurol Int. 2016;7(Suppl 3):S70–6. doi: 10.4103/2152-7806.174892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein NE. More nerve root injuries occur with minimally invasive lumbar surgery, especially extreme lateral interbody fusion: A review. Surg Neurol Int. 2016;7(Suppl 3):S83–95. doi: 10.4103/2152-7806.174895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein NE. Low reoperation rate following 336 multilevel lumbar laminectomies with noninstrumented fusions. Surg Neurol Int. 2016;7(Suppl 13):S331–6. doi: 10.4103/2152-7806.182545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein NE. Tisseel's Impact On Hemostasis For 2-3 and 4-6 Level Lumbar Laminectomies. Surg Neurol Int. 2017;8:299. doi: 10.4103/sni.sni_302_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giorgi H, Prébet R, Delhaye M, Aurouer N, Mangione P, Blondel B, et al. Minimally invasive posterior transforaminal lumbar interbody fusion: One-year postoperative morbidity, clinical and radiological results of a prospective. Orthop Traumatol Surg Resm. 2015;101(6 Suppl):S241–5. doi: 10.1016/j.otsr.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Høy K, Bünger C, Niederman B, Helmig P, Hansen ES, Li H, Andersen T. Transforaminal lumbar interbody fusion (TLIF) versus posterolateral instrumented fusion (PLF) in degenerative lumbar disorders: A randomized clinical trial with 2-year follow-up. Eur Spine J. 2013;22:2022–9. doi: 10.1007/s00586-013-2760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph JR, Smith BW, La Marca F, Park P. Comparison of complication rates of minimally invasive transforaminal lumbar interbody fusion and lateral lumbar interbody fusion: A systematic review of the literature. Neurosurg Focus. 2015;39:E4. doi: 10.3171/2015.7.FOCUS15278. [DOI] [PubMed] [Google Scholar]
- 20.Klingler JH, Volz F, Krüger MT1Kogias E, Rölz R, Scholz C, et al. Accidental Durotomy in Minimally Invasive Transforaminal Lumbar Interbody Fusion: Frequency, Risk Factors, and Management. ScientificWorld Journal 2015. 2015:532628. doi: 10.1155/2015/532628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lad SP, Babu R, Baker AA, Ugiliweneza B, Kong M, Bagley CA, et al. Complications, reoperation rates, and health-care cost following surgical treatment of lumbar spondylolisthesis. J Bone Joint Surg Am. 2013;6(95):e162. doi: 10.2106/JBJS.L.00730. [DOI] [PubMed] [Google Scholar]
- 22.Lee KH, Yeo W, Soeharno H, Yue WM. Learning curve of a complex surgical technique: Minimally invasive transforaminal lumbar interbody fusion (MIS TLIF) JSDT. 2014;27:E234–40. doi: 10.1097/BSD.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Deng H, Long X, Chen X, Xu R, Liu Z. A comparative study of perioperative complications between transforaminal versus posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Eur Spine J. 2016;25:1575–80. doi: 10.1007/s00586-015-4086-8. [DOI] [PubMed] [Google Scholar]
- 24.Mannion RJ, Nowitzke AM, Wood MJ. Promoting fusion in minimally invasive lumbar interbody stabilization with low-dose bone morphogenic protein-2-but what is the cost? Spine J. 2010;11:527–33. doi: 10.1016/j.spinee.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Menger RP, Wolf ME, Kukreja S, Sin A, Nanda A. Medicare payment data for spine reimbursement; important but flawed data for evaluating utilization of resources. Surg Neurol Int. 2015;6(Suppl 14):S391–7. doi: 10.4103/2152-7806.163963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: A systematic review. Spine (Phila Pa 1976) 2010;35(9 Suppl):S86–104. doi: 10.1097/BRS.0b013e3181d81ef2. [DOI] [PubMed] [Google Scholar]
- 27.Nandyala SVFineberg SJ, Pelton M, Singh K. Minimally invasive transforaminal lumbar interbody fusion: One surgeon's learning curve. Spine J. 2014;14:1460–5. doi: 10.1016/j.spinee.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 28.Nixon AT, Smith ZA, Lawton CD, Wong AP, Dahdaleh NS, Koht A, et al. Bilateral neurological deficits following unilateral minimally invasive TLIF: A review of four patients. Surg Neurol Int. 2014;5(Suppl 7):S317–24. doi: 10.4103/2152-7806.139619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norton RP, Bianco K, Klifto C, Errico TJ, Bendo JA. Degenerative Spondylolisthesis: An Analysis of the Nationwide Inpatient Sample Database. Spine (Phila Pa 1976) 2015;40:1219–27. doi: 10.1097/BRS.0000000000000987. [DOI] [PubMed] [Google Scholar]
- 30.Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2011;20:612–7. doi: 10.1007/s00586-010-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park Y, Lee SB, Seok SO, Jo BW, Ha JW. Perioperative surgical complications and learning curve associated with minimally invasive transforaminal lumbar interbody fusion: A single-institute experience. Clin Orthop Surg. 2015;7:91–6. doi: 10.4055/cios.2015.7.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patil CG, Sarmiento JM, Ugiliweneza B, Mukherjee D, Nuño M, Liu JC, et al. Interspinous device versus laminectomy for lumbar spinal stenosis: A comparative effectiveness study. Spine J. 2014;14:1484–92. doi: 10.1016/j.spinee.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 33.Payer M. “Minimally invasive” lumbar spine surgery: A critical review. Acta Neurochir (Wien) 2011;153:1455–9. doi: 10.1007/s00701-011-1023-4. [DOI] [PubMed] [Google Scholar]
- 34.Saetia K, Phankhongsab A, Kuansongtham V, Paiboonsirijit S. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J Med Assoc Thai. 2013;96:41–6. [PubMed] [Google Scholar]
- 35.Samanta A, Samanta J. Legal standard of care: A shift from the traditional Bolam test. Clin Med (Lond) 2003;3:443–6. doi: 10.7861/clinmedicine.3-5-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schöller K, Alimi M, Cong GT, Christos P, Härtl R. Lumbar Spinal Stenosis Associated With Degenerative Lumbar Spondylolisthesis: A Systematic Review and Meta-analysis of Secondary Fusion Rates Following Open vs Minimally Invasive Decompression. Neurosurgery. 2017;80:355–67. doi: 10.1093/neuros/nyw091. [DOI] [PubMed] [Google Scholar]
- 37.Silva PS, Pereira P, Monteiro P, Silva PA, Vaz R. Learning curve and complications of minimally invasive transforaminal lumbar interbody fusion. Neurosurg Focus. 2013;35:E7. doi: 10.3171/2013.5.FOCUS13157. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Zhou Y. Perioperative complications related to minimally invasive transforaminal lumbar fusion: Evaluation of 204 operations on lumbar instability at single center. Spine J. 2014;14:2078–84. doi: 10.1016/j.spinee.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: A potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) Spine J. 2008;8:1011–8. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Wong AP, Smith ZA, Nixon AT, Lawton CD, Dahdaleh NS, Wong RH, et al. Intraoperative and perioperative complications in minimally invasive transforaminal lumbar interbody fusion: A review of 513 patients. J Neurosurg Spine. 2015;22:487–95. doi: 10.3171/2014.10.SPINE14129. [DOI] [PubMed] [Google Scholar]
- 41.Zhang BF, Ge CY, Zheng BL, Hao DJ. Transforaminal lumbar interbody fusion versus posterolateral fusion in degenerative lumbar spondylosis: A meta-analysis. Medicine (Baltimore) 2016;95:e4995. doi: 10.1097/MD.0000000000004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zygourakis CC, Liu CY, Wakam G, Moriates C, Boscardin C, Ames CP, et al. Geographic and Hospital Variation in Cost of Lumbar Laminectomy and Lumbar Fusion for Degenerative Conditions. Neurosurgery. 2017;81:331–40. doi: 10.1093/neuros/nyx047. [DOI] [PubMed] [Google Scholar]


