Abstract
Endometrial cancer (EC) is the most common malignant gynecological disease. Cancer-associated fibroblasts (CAFs) serve an important role in the development and progression of EC through epithelial-mesenchymal transition (EMT). The aim of the present study was to examine the association between CAFs and EMT, and the possible mechanisms of action. Firstly, the CAFs and normal fibroblasts (NFs) were isolated and cultured, then an immunofluorescence assay was performed to analyze the purity and level of activation of CAFs and NFs, and then the conditional medium (CM) of CAFs and NFs was prepared. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) and western blotting examined the expression levels of epithelial (E)-cadherin, neural (N)-cadherin and vimentin. A Matrigel® invasion assay and wound healing assay were used to analyze the effect of the CM on invasion and migration. An ELISA assay also measured the levels of various cytokines in the CM. In addition, EMT-associated proteins in metastatic lung tissues were detected by immunohistochemical assay. The results indicated that the CM of CAFs may decrease the level of E-cadherin, and increase the levels of N-cadherin and vimentin, while increasing the levels of invasion and metastasis in EC cells. The concentration of epidermal growth factor, transforming growth factor-β, hepatic growth factor and fibroblast growth factor in the CM of CAFs increased significantly, in comparison with the NFs group (P<0.05). The exogenous growth factors induced migration and invasion of EC cells. CAFs induced lung metastasis and the EMT process in vivo. These data suggested that cancer-associated fibroblasts may induce EMT through the secreted cytokines in endometrial cancer cells.
Keywords: endometrial cancer, cancer-associated fibroblasts, epithelial-mesenchymal transition, cytokines, co-culture
Introduction
Endometrial cancer (EC) is the 5th most common malignancy among females globally with a rising morbidity number, and >90% of cases occur in females >50 years of age (1). The prognosis of patients with advanced endometrial carcinoma and who are at least 60-years old is poor, the mortality rate is high, and it was demonstrated that invasion and metastasis are the primary factors leading to the mortality of patients with EC (2). Therefore, the key of the treatment and improvement of prognosis of patients with EC is to study the mechanisms of invasion and metastasis, and design appropriate intervention strategies.
Tumor tissue is composed of the tumor parenchyma cells and stromal cells. Various studies have indicated that tumor microenvironment serves an important role in the occurrence and development of tumors (3,4), and stromal cell are the major component of the tumor microenvironment. Cancer-associated fibroblasts (CAFs), as the most abundant cellular components in the tumor microenvironment, serve an important role in tumor growth, angiogenesis, invasion, metastasis and clinical prognosis through the remodeling of the extracellular matrix and secretion of growth factors that regulate tumor cell proliferation, survival and dissemination (5).
Epithelial-mesenchymal transition (EMT) is a differentiation process that directs polarized epithelial cells to differentiate into mesenchymal cells. EMT, as an important process of invasion and metastasis of the malignant tumors, has been widely studied (6,7). There is increasing evidence indicating that EMT is stimulated by signals from the tumor microenvironment, including a variety of growth factors and cytokines (8,9). These studies have demonstrated that CAFs may regulate EMT; however, the underlying mechanisms remain incompletely understood. In the present study, CAFs were isolated and cultured, the association between CAFs and invasion and metastasis was analyzed and the possible association between the growth factors and EMT was studied.
Materials and methods
Ethics statement
The present study was approved by the Ethical Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China). Written informed consent was obtained from the participant prior to enrolment in the present study. The use of endometrial adenocarcinoma tissue was also approved by the aforementioned ethical committee.
Isolation and purification of CAFs and normal fibroblasts (NFs)
EC tumor tissues and adjacent normal tissues (at least 3 cm away from the EC tumor margin) were isolated during surgical resection from one female patient (40 years-old), with inclusion/exclusion criteria being that the sample be diagnosed (January 2016) as EC prior to or during surgery, without the presence of any other tumor type, and with cell isolation from the tumor tissues or adjacent tissues being obtained 2 h after the operation. The tumor or normal tissue from one patient was removed and digested in 1 mg/ml collagenase I (cat. no. C0130; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) solution overnight at 37°C. Following centrifugation (300 × g at room temperature for 10 min), cells (sourced from the aforementioned patient) were passed through 100 and 40 mm filters in turn, and then the cell suspension was cultured in Dulbecco's modified Eagle's medium supplemented (DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) with 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences) for several days at 37°C. As CAFs or NFs that are more sensitive to trypsin were detached from the flask, while epithelial cells remained attached, differential trypsinization was utilized to separate and purify the CAFs or NFs. Following several rounds of differential trypsinization, the CAFs or NFs were purified, and then cultured.
Immunofluorescence assay analysis of cell purity and activated fibroblasts
The purity of the CAFs and NFs was determined by analyzing the fibroblast-specific protein vimentin, and activated fibroblast marker fibroblast activation protein (FAP) and α-smooth muscle actin (α-SMA). Briefly, cells were fixed in 4% paraformaldehyde in PBS solution at room temperature for 30 min. Following washing with PBS three times, cells were treated in a permeabilization (0.1% saponin in PBS) solution. Subsequently, the cells were incubated with anti-vimentin (cat. no. ab92547; dilution, 1:500), anti-FAP (cat. no. ab53066; dilution, 1:100) and anti-α-SMA (cat. no. ab5694; dilution, 1:100; all from Abcam, Cambridge, MA, USA) at 4°C overnight. Anti-Rabbit IgG-fluorescein isothiocyanate (cat. no. ab97050; Abcam; dilution, 1:200) was used and counterstaining was performed with DAPI (cat. no. C1006; Beyotime Institute of Biotechnology, Shanghai, China) at room temperature for 15 min. Visual analysis was performed with an Olympus fluorescence microscope (CX71; Olympus Corporation, Tokyo, Japan) at magnification, ×400.
Preparation of conditioned medium
When CAFs and NFs had reached 70–80% confluence, the medium was replaced with fresh serum-free DMEM and cultured at 37°C in a 5% CO2 atmosphere for 48 h. Then, the culture medium was collected, centrifuged at 300 × g at room temperature for 5 min and the cell debris was removed. The supernatant was designated CAFs/NFs conditional medium (CM).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
HEC-1A and RL95-2 cells (Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China) were cultured in the CM supplemented with 10% FBS at 37°C in a 5% CO2 humidified atmosphere for 48 h. Subsequent to culture, total RNA was extracted using RNA Isolation kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Concentration of total RNA was measured using light densitometry, and 1.5 µg of total RNA was reverse transcribed to cDNA using the PrimeScript™ II 1st strand cDNA synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China) and subsequently diluted with nuclease-free water to 10 ng/µl cDNA. qPCR was performed using the VeriQuest SYBR-Green qPCR Master Mix kit (Thermo Fisher Scientific, Inc.) in a 25 µl volume. DNA was amplified with an initial denaturation at 95°C for 3 min, followed by 45 cycles of 95°C for 15 sec (denaturation), 60°C for 30 sec (annealing) and 72°C for 3 mins (elongation), then 72°C for 10 mins (final extension). The primers used in the present study are summarized in Table I. Average threshold cycle (Cq) values for the triplicate PCR reactions were normalized against the average Cq values of β-actin from the same cDNA sample (10).
Table I.
Primers used in the reverse transcription quantitative polymerase chain reaction.
| Genes | Sequence (5′-3′) |
|---|---|
| Epithelial | F: AAGCGTGAGTCGCAAGAATG |
| cadherin | R: TCTCCAGGTTTTCGCCAGTG |
| Neural | F: CAGAAAATAACGTTCTCCAGTTGCT |
| cadherin | R: CCCCGTGTGTTAGTTCTGCT |
| Vimentin | F: GACGCCATCAACACCGAGTT |
| R: CTTTGTCGTTGGTTAGCTGGT | |
| β-actin | F: CCTGTACGCCAACACAGTGC |
| R: ATACTCCTGCTTGCTGATCC |
F, forward; R, reverse.
Western blotting
HEC-1A and RL-952 cells were cultured in the CM at 37°C in a 5% CO2 humidified atmosphere for 48 h. Subsequent to culture, the cells were collected and lysed with ice-cold radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology) with 1 mmol/l phenylmethanesulfonyl fluoride (Beyotime Institute of Biotechnology), and the concentrations were measured using a micro BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total of 50 µg per lane of nucleoproteins or cytoplasmic proteins was resolved on 12% PAGE (Invitrogen; Thermo Fisher Scientific, Inc.), transferred to polyvinylidene fluoride membranes and visualized with enhanced chemiluminescence western blot detection reagents (Pierce, USA). Immunoblotting was performed using mouse anti-E-cadherin (cat. no. ab1416; Abcam; dilution, 1:50), mouse anti-N-cadherin (cat. no. ab98952; Abcam; dilution, 1:1,000) and mouse anti-vimentin antibodies (cat. no. ab8978; Abcam; dilution, 1:500) at 37°C for 2 h, followed by incubation with the goat anti-mouse appropriate horseradish-peroxidase-conjugated IgG secondary antibodies (cat. no. ab97023; Abcam; dilution, 1:5,000) for 1 h at room temperature. GAPDH (cat no. ab8245; Abcam; dilution, 1:1,000) levels were used for normalization. Protein bands were scanned and quantified using a ChemiDoc MP image analysis system (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and also analyzing using Image J2X software (Rawak Software, Inc., Dresden, Germany).
Quantitative detection of various growth factors by ELISA
The secretion of epidermal growth factor (EGF), transforming growth factor-β, (TGF-β), hepatocyte growth factor (HGF) and fibroblast growth factor-2 (FGF-2-2) was assessed by ELISA. The Human EGF ELISA (cat. no. ab179888; Abcam), Human TGF-β ELISA (cat. no. ab100647; Abcam), Human HGF ELISA (cat. no. ab100534; Abcam) and Human FGF-2 basic Quantikine ELISA (cat. no. DFB50; BD Biosciences, Franklin Lakes, NY, USA) kits were used to determine the concentrations in the CM. The absorbance (OD) was measured at 450 nm wavelength.
Matrigel® invasion assay
Firstly, 40 µl Matrigel® (cat. no. 353097; BD Biosciences) was added into the polycarbonate membrane filters and incubated for 24 h at 37°C. Following this incubation, EC lines HEC1-A and RL-952 were trypsinized using a trypsin solution (cat no. C0201; Beyotime Institute of Biotechnology) and resuspended to a density of 5×105/ml in the CM. A total of ~1×105 CAFs or NFs resuspended in CM were added into the upper Transwell chamber. A total of 600 µl culture medium with 10% FBS was added into the lower well. Following incubation at 37°C for 60 h, cells that remained on the upper surface of the filters were removed using a cotton bud, and cells that migrated into the lower surface of the filters were fixed at room temperature for 20 min in 4% paraformaldehyde followed by staining for 30 min with 0.1% crystal violet dye at room temperature. A total of 5 fields of view were counted randomly to calculate the number of cells invading through the polycarbonate membrane at magnification, ×100 using an inverted/light microscope (CX71; Olympus Corporation). Each experiment was performed in triplicate.
Wound healing assay
HEC1-A and RL-952 cells were trypsinized using a trypsin solution (cat no. C0201; Beyotime Institute of Biotechnology), and 2×106 cells/well were seeded into 6-well plates cultured for 36 h until they reached 100% confluence. Subsequently, each well was divided into 3–5 grids. An artificial homogenous wound was created by scratching the cell monolayer with the 200 µl pipette tip, and then the cells were washed 2 times with free-serum medium. The cells were cultured in CM for 48 h. Microscopic images of the same area were captured at 0, 24 and 48 h time points using an inverted/light microscope (CX71; Olympus Corporation) at magnification, ×100. The cell migration distance was calculated using the equation: Initial distance-final distance.
Effect of exogenous growth factors on the migration and invasion of EC cells
Exogenous growth factors EGF (cat. no. E5036; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), TGF-β (cat. no. 155613; Abcam) HGF (cat. no. 105061; Abcam) and FGF-2-2 (cat. no. 61845; Abcam) were added into the conditional medium of NFs [as the fortified CM (FCM) of NFs], and their concentrations were 40, 200, 50 and 95 pg/ml, respectively, so that the final concentrations of these four growth factors was close to their concentration in the conditional medium (CM) of CAFs. Then, Matrigel® invasion and wound healing assays were performed using the FCM and CM, according to the aforementioned protocols.
In vivo xenograft experiments
Female non-obese diabetes-severe combined immune deficiency mice (n=20) at the age of 6–8 weeks (18–20 g) were obtained from the Laboratory Animal Centre of Zhejiang University (Hangzhou, China). The mice were freely fed with standard forage and clean water, and maintained on a 12-h light/dark cycle under room temperature (25±1°C) and humidity of 55±10%. They were randomly divided into two groups (10 mice per group): The NFs group (0.5×106 RL-952 + 0.5×106 NFs) and CAFs group (0.5×106 RL-952 + 0.5×106 CAFs). Cell suspensions (RL-952 cells) in 200 µl serum-free medium were subcutaneously injected into the 2 flanks of each mouse. Following 4 weeks, the tumor and lung samples were carefully isolated, and tumor weight and volume of each tumor samples were measured. Tumor tissues were monitored by caliper measurements of the length and width. Tumor volumes were calculated according to the formula: Volume=width × length × (width + length)/2.
Immunohistochemical analysis
The lung tissue was fixed in 4% paraformaldehyde-PBS solution at room temperature for 30 min, and sliced into 3–5 µm sections. Following deparaffinization at room temperature (dipped successively in xylene twice, 10 min/time) and hydration (100% ethanol for 5 min, 95% ethanol for 5 min, 90% ethanol for 5 min, 85% ethanol for 5 min, 75% ethanol for 5 min, and then washed using PBS twice, 5 min/time), the slides were treated with peroxide after blocking with serum (Invitrogen, USA). The anti-rabbit primary antibodies against vimentin (cat. no. ab92547; Abcam; dilution, 1:500), Zinc finger protein SNAI1 (Snail; cat. no. ab180714; Abcam; dilution, 1:100), cluster of differentiation (CD)44 (cat. no. ab157107; Abcam; dilution, 1:1,000) and CD24 (cat. no. ab214231; Abcam; dilution, 1:200) were incubated at 4°C overnight. Subsequent to washing with PBS three times and incubating with goat anti-rabbit horseradish-peroxidase-conjugated IgG secondary antibody (cat. no. ab6721; Abcam; dilution, 1:1,000) at 37°C for 30 min, the slides were treated with DAB kit (ZLI-9017; OriGene Technologies, Inc., Rockville, MD, USA) at room temperature for 10 min. Finally, the slides were lightly counterstained at room temperature with hematoxylin (cat no. C0107; Beyotime Institute of Biotechnology) for 3 min, washed five times with PBS solution, dehydrated (two times successively using 70% ethanol for 2 sec, 80% ethanol for 2 sec, 90% ethanol for 5 sec, 95% ethanol for 10 sec, 100% ethanol for 30 sec, then washed in xylene twice until transparent) and mounted at room temperature and finally subjected to neutral resin sealing. Vimentin, Snail, CD44 and CD24 expression in the lung tissues were determined by counting 5 random visual fields with an inverted/light microscope (CX71; Olympus Corporation) at magnification, ×400.
Statistical analyses
The data were analyzed by one-way analysis of variance and an unpaired Student's t-test to determine statistical significance using SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL, USA). Furthermore, a least significant difference post-hoc test was employed where equal variances were assumed, while Dunnett's T3 test was used when equal variances were not assumed. Each experiment was repeated at least three times. The results are presented as mean ± standard error of the mean. A two-tailed P<0.05 was considered to indicate a statistically significant difference.
Results
Isolation and level of activation of isolated CAFs and NFs
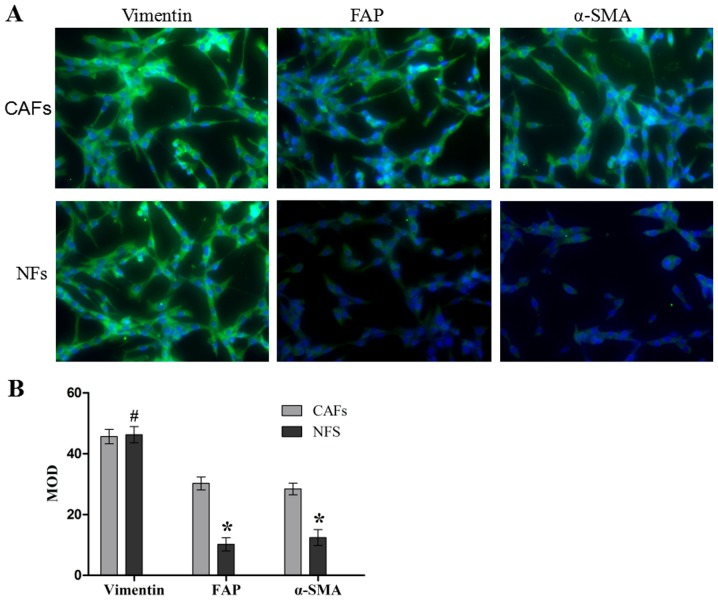
The purity and activation degree of the isolated cells was assessed by immunofluorescence (Fig. 1). The CAFs and NFs demonstrated a positive expression of vimentin (a fibroblast-specific protein), which meant that the CAFs and NFs were successfully isolated and cultured. In addition, CAFs exhibited positive expression of FAP and α-SMA through a marked green fluorescent signal, and the expression of FAP and α-SMA in NFs was identified to be weakly positive by a weak green fluorescent signal, and the differences between these expression levels were significant compared with the NFs group (both P<0.05). These results suggest that the CAFs and NFs were successfully isolated and cultured, and that the activation degree of CAFs was increased compared with that of NFs.
Figure 1.
Immunofluorescence assay analysis of vimentin, FAP and α-SMA in the CAFs and NFs. (A) Macroscopic immunofluorescence images of vimentin, FAP and α-SMA. (B) The MOD statistical results of vimentin, FAP and α-SMA. Data are presented as the mean ± the standard error of the mean of three independent experiments. #P>0.05 vs. CAFs group. *P<0.05 vs. CAFs group. MOD, medium optical density; CAFs, cancer-associated fibroblasts; NFs, normal fibroblasts; FAP, fibroblast activation protein; α-SMA, α-smooth muscle actin.
CAFs induce the progress of EMT
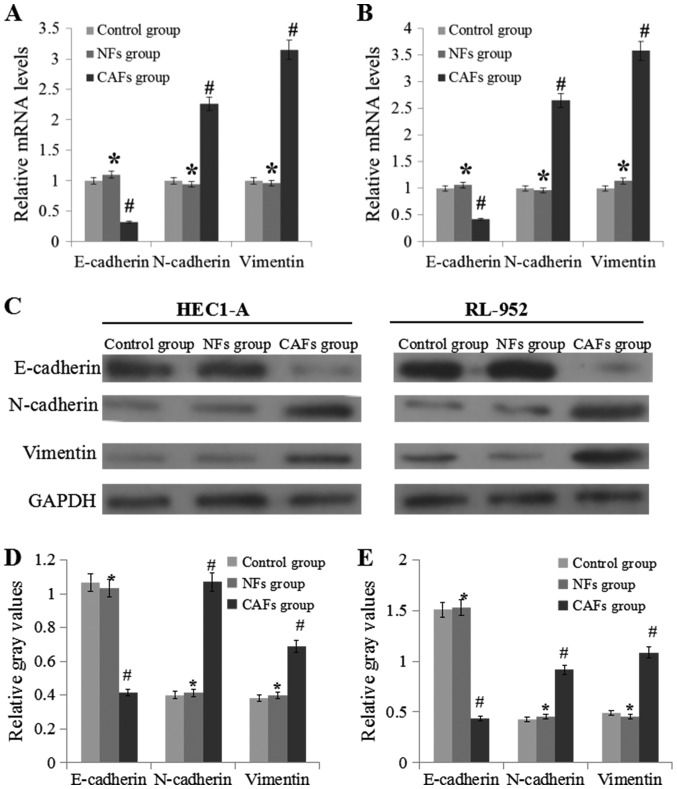
RT-qPCR and western blotting were used to analyze E-cadherin, N-cadherin and vimentin expression levels (Fig. 2). Compared with the NFs groups, the expression levels of N-cadherin and vimentin in the CAFs group was significantly upregulated (P<0.05), while the expression level of E-cadherin was markedly downregulated (P<0.05).
Figure 2.
CAF regulate the mRNA and protein expression levels of E-cadherin, N-cadherin and vimentin. (A) mRNA expression levels of E-cadherin, N-cadherin and vimentin in HEC1-A cells. (B) mRNA expression levels of E-cadherin, N-cadherin and vimentin in RL-952 cells. (C) Experimental schematic image of western blotting experiments performed in the HEC1-A and RL-952 cells. (D) Relative levels of E-cadherin, N-cadherin and vimentin to GAPDH in HEC1-A cell samples. (E) Relative levels of E-cadherin, N-cadherin and vimentin to GAPDH in RL-952 cell samples. Data are presented as the mean ± the standard error of the mean of three independent experiments. *P>0.05 vs. control group. #P<0.05 vs. NFs group. E-cadherin, epithelial cadherin; N-cadherin, neural cadherin; CM, conditional medium; CAFs, cancer-associated fibroblasts; NFs, normal fibroblasts.
CAFs increase EC cell invasion and migration
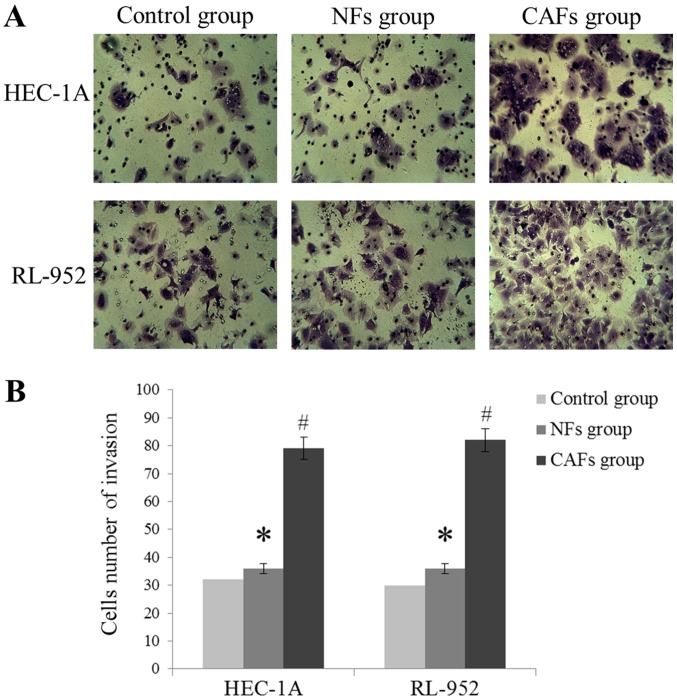
As demonstrated in Fig. 3, HEC-1A and RL-952 cell invasion levels were markedly increased in the CAFs group, compared with the NFs group (P<0.05), exhibited by the increase of the number of invading cells. In addition, compared with the NFs group, the wound healing assay indicated that the migration distance was significantly increased in the CAFs group (P<0.05; Fig. 4). These results suggest that the CM of CAFs may induce migration and invasion of EC cells.
Figure 3.
CAF induces endometrial cancer cells invasion in vitro. (A) Macroscopic images of invasion in each group (magnification, ×400). (B) The statistical results of the cell invasion number in each group. Data are presented as the mean ± the standard error of the mean of three independent experiments. *P>0.05 vs. control group. #P<0.05 vs. NFs group. CAFs, cancer-associated fibroblasts.
Figure 4.
CAF induces endometrial cancer cells migration in vitro. (A) Macroscopic images of migration in each group (magnification, ×100). (B) The statistical results of cell migration distance in each group. Data are presented as the mean ± the standard error of the mean of three independent experiments. *P>0.05 vs. control group. #P<0.05 vs. NFs group. CM, conditional medium; CAFs, cancer-associated fibroblasts.
CAFs induce the secretion of EGF, TGF-β, HGF and FGF-2
In comparison between the CAFs and NFs, the concentration of EGF, TGF-β, HGF and FGF-2 were increased in CM of CAFs, and the difference was significant (P<0.05). The results are summarized in Table II.
Table II.
Various cytokine levels in the conditional medium by ELISA.
| Cytokines, pg/ml | Control medium | NFs | CAFs |
|---|---|---|---|
| EGF | 0.26±0.12 | 1.23±0.85 | 43.52±4.26a |
| TGF-β | 0.34±0.11 | 2.12±1.02 | 212.58±10.24a |
| HGF | 0.12±0.09 | 1.45±0.75 | 52.37±3.26a |
| FGF-2 | 0.97±0.55 | 3.12±1.46 | 95.64±8.97a |
P<0.05 vs. NFs group. EGF, epithelial growth factor; TGF-β, transforming growth factor-β; HGF, hepatic growth factor; FGF-2, fibroblast growth factor-2; NFs, normal fibroblasts; CAFs, cancer-associated fibroblasts.
Exogenous growth factors induce invasion and migration of EC cells
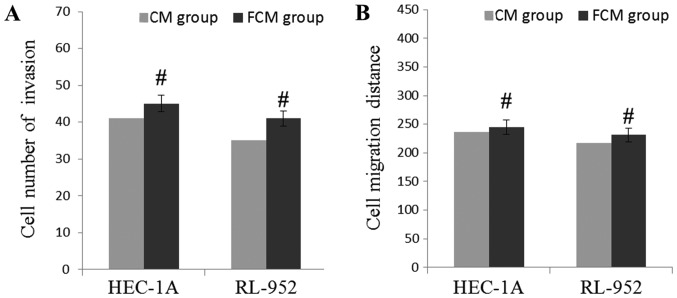
In order to confirm whether these growth factors affected the levels of invasion and migration, EGF, TGF-β, HGF and FGF-2 were artificially added into CM of NFs, and the levels of cell invasion and migration were observed. The results are demonstrated in Fig. 5. Compared with the CM of CAFs, the number of invading HEC-1A and RL-952 cells were markedly increased in the FCM of the NFs group, but the difference was not significant (P>0.05). In addition, compared with the CM of CAFs, the wound healing assay indicated that the migration distance was significantly increased in the FCM of NFs group, but the difference between the CAFs and NFs group was not significant (P>0.05). Compared with Figs. 3 and 4, these results suggest that these growth factors may induce the migratory and invasive capabilities of EC cells.
Figure 5.
Cytokines in the CM regulate the invasion and migration of EC cells in vitro. (A) FCM of NFs induces EC cells invasion. (B) FCM of NFs induces EC cells migration. Data are presented as the mean ± the standard error of the mean of three independent experiments. #P>0.05 vs. CM group. CM, conditional medium; FCM, fortified conditional medium; NFs, normal fibroblasts; EC, endometrial cancer.
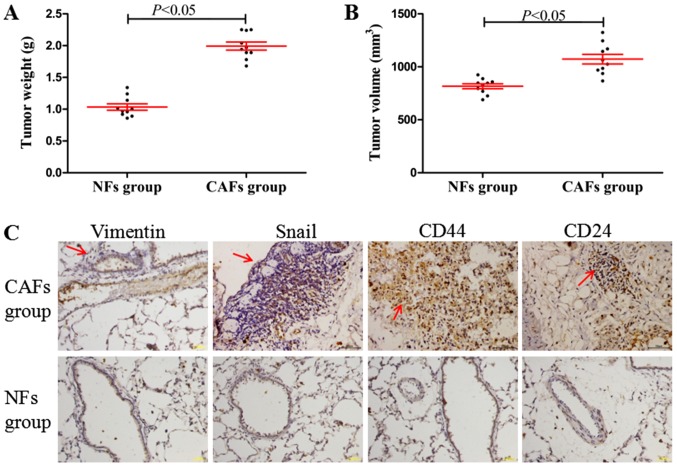
CAFs induce lung metastasis and the EMT process in vivo
In the in vivo xenograft experiments, it was identified that the tumor weights and volumes (maximum tumor volume obtained: ~9.2×7.9 mm) in CAFs group were significantly increased compared with those in the NFs group (Fig. 6A and B; P<0.05). Furthermore, compared with the NFs group (Fig. 6C; no marked lung metastasis, observed under a microscope, and positive expression determined using immunohistochemistry), the CAFs group demonstrated significant lung metastasis, while the EMT-associated proteins (vimentin and Snail) and the cancer stem cell molecular markers CD44 and CD24 demonstrated marked positive staining in areas of lung metastasis (Fig. 6C). These results indicated that CAFs may induce tumorigenesis, tumor metastasis and the EMT process in vivo.
Figure 6.
CAFs induce tumorigenesis, lung metastasis and the epithelial-mesenchymal transition process in vivo. (A) The tumor weight in the CAFs and NFs group. (B) The tumor volume in the CAFs and NFs group. (C) Vimentin, Snail, CD44 and CD24 macroscopic images of immunohistochemistry in the CAFs and NFs group at magnification, ×400. The arrows indicated the areas of lung metastasis. Data are presented the mean ± standard error of the mean (n=10). CAFs, cancer-associated fibroblasts; NFs, normal fibroblasts; Snail, zinc finger protein SNAI1; CD, cluster of differentiation.
Discussion
It has been demonstrated that the progress of EMT was associated with a downregulation of the apical and basolateral epithelial cells specific tight and adherens junction proteins such as E-cadherin, and increased expression of mesenchymal molecules such as vimentin and N-cadherin (8,9). E-cadherin and N-cadherin, functioning as adhesion molecules, and vimentin, are considered the markers of EMT (11). In pancreatic cancer and human squamous carcinoma cells, overexpression of N-cadherin is involved in EMT, and is associated with a reduction in E-cadherin level (12,13). Various studied have suggested that CAFs may reduce EMT (14). Downregulation of E-cadherin expression and upregulation of N-cadherin reduces the adhesion ability of cells and simultaneously enhances cell mobility, so that the cells may migrate and invade, which is a typical feature of EMT (15). Kim et al (16) indicated that CAFs affected the motility of cancer cells through inducing EMT. In the present study, CM induced N-cadherin and vimentin expression, and inhibited the expression of E-cadherin, indicating that CM of CAFs may induce the EMT progress.
Although CAFs are key determinants in the malignant progression of cancer, their functional contributions to this process remain unclear (17). Previous data demonstrated that CAFs may secrete a variety of growth factors through autocrine and paracrine pathways (18). Under certain conditions, fibroblasts changed the tumor progression and fibroblasts contribute to tumor development through secreting certain cytokine factors (19–21). In a mouse model, Tyan et al (22) identified that the HGF level in CAFs was positively correlated with their ability to enhance breast tumorigenesis. TGF-β is a multifunctional cytokine that serves important roles in tumor formation, progression and metastasis (23). In the CM of CAFs in the present study, the concentration of EGF, TGF-β, HGF and FGF-2 were increased compared with the CM of NFs, which indicated that CAFs may secrete these various cytokines.
Based on the effect of cytokines on the tumor progression, artificial adjustment the concentration of cytokines may affect the progression of the tumor (24). In breast cancer cells, the reduction of HGF concentration using a neutralizing antibody reduced CAF-mediated colony formation (22). Using comparisons of the gene expression profiles between 6 pairs of tumor fibroblasts (TFs) and NFs from esophageal squamous cell carcinoma (ESCC) using Affymetrix expression microarray, Zhang et al (25) indicated that the CM from TFs (of which the most significant result was the upregulation of FGF receptor 2) was identified to be able to promote ESCC tumor cell growth, migration and invasion in vitro. In gastrointestinal cancer, increased TGF-β regulated the tumor microenvironment and metastasis (26). In the present study, it was first identified that the levels of EGF, TGF-β, HGF and FGF-2 in the CAFs CM were increased compared with that in the NFs CM. In addition, the CM of CAFs exhibited an increased ability to promote migration and invasion compared with the conditioned medium of NFs. Notably, in comparison with the regular CM of NFs, the addition of exogenous growth factors to the CM of NFs increased EC cells migratory and invasive capabilities. From these results, it was inferred that EGF, TGF-β, HGF and FGF-2 were important for inducing migration and invasion in EC cells. However, the migratory and invasive capabilities in the FCM and CM groups were not completely similar. This suggests that there are a number of additional cytokines, not assessed within the present study, which may affect EC cell migration and invasion, for example interleukin (IL)-6 receptor and glycoprotein 130 (27). Treatment with Janus kinase- and Signal transducer and activator of transcription 3-specific inhibitors, AD412 and STATTIC, respectively, significantly abrogated CAF-mediated cell proliferation, indicating the role of IL-6 activation in EC cell proliferation (27); also, aberrant S100A4 expression may predict EC progression and serve an important role in regulating EC cell invasion through EMT regulation. Therefore, S100A4 is a promising therapeutic target (28). In the present study, only four growth factors were studied; a more comprehensive analysis will be included in our future studies.
Tumor growth depends on interactions between multiple inter-dependent cell types, among these different cell types, CAFs are becoming a topic of study as recipients and as producers of pro-tumorigenic signals (29). There are a number of previous studies concerning the role of CAFs and their mechanisms of action in various tumors (27,28,30,31). Hwang et al (32) examined pancreatic cancer, and identified that cancer-associated stromal fibroblasts exhibited an important role in supporting and promoting growth and metastasis in pancreatic cancer. In human prostatic cancer, Olumi et al (33) suggested that human prostatic CAFs grown with initiated human prostatic epithelial cells markedly stimulated growth and altered the histology of the epithelial cell population. In the present study, it was only identified that CAFs had a significant effect on lung metastasis. Immunohistochemistry was utilized to detect the markedly positive expressions of vimentin, Snail, CD44 and CD24 in areas of lung metastasis; in the NFs group there was no marked protein (vimentin, snail, CD44 and CD24) expression identified. The number of metastasized tumors cannot be calculated using the naked eye or immunohistochemistry assays; computed tomography (CT) and magnetic resonance imaging (MRI) technology is required. As CT and MRI were not used during the xenograft experiments; the number of metastasized tumors was not calculated. However, by comparing the results visually and with immunohistochemistry, it was concluded that CAFs induced lung metastasis and the EMT process in vivo. The present study again demonstrated the role of CAFs in promoting tumorigenesis.
In conclusion, the present study focused on identifying the role of CAFs on the EMT process from the perspective of the cytokines secreted. The results provide a novel perspective for the treatment of endometrial cancer through cytokines. Previous articles (28–30) have not examined CAFs by describing and analyzing their cytokine expression profiles, which is the primary focus of the present study. The data suggest that CAFs may induce EMT through secreted cytokines in endometrial cancer cells. Therapeutic drugs could be designed to perform as regulators of these cytokines, and may be useful for EC therapeutic intervention.
Acknowledgements
The present study was supported by the National Natural Science Fund of China (grant no. 81301808).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Aune D, Navarro Rosenblatt DA, Chan DS, Vingeliene S, Abar L, Vieira AR, Greenwood DC, Bandera EV, Norat T. Anthropometric factors and endometrial cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Ann Oncol. 2015;26:1635–1648. doi: 10.1093/annonc/mdv142. [DOI] [PubMed] [Google Scholar]
- 2.Marnitz S, Köhler C. Current therapy of patients with endometrial carcinoma. A critical review. Strahlenther Onkol. 2012;188:12–20. doi: 10.1007/s00066-011-0004-0. [DOI] [PubMed] [Google Scholar]
- 3.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 4.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumors. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: A diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31:195–208. doi: 10.1007/s10555-011-9340-x. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari N, Gheldof A, Tatari M, Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol. 2012;22:194–207. doi: 10.1016/j.semcancer.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Chaw SY, Majeed AA, Dalley AJ, Chan A, Stein S, Farah CS. Epithelial to mesenchymal transition (EMT) biomarkers-E-cadherin, beta-catenin, APC and Vimentin-in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012;48:997–1006. doi: 10.1016/j.oraloncology.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res. 2014;10:4125–4133. doi: 10.1158/1078-0432.CCR-0578-03. [DOI] [PubMed] [Google Scholar]
- 13.Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol. 1996;135:1643–1654. doi: 10.1083/jcb.135.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 15.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Choe C, Shin YS, Jeon MJ, Choi SJ, Lee J, Bae GY, Cha HJ, Kim J. Human lung cancer-associated fibroblasts enhance motility of non-small cell lung cancer cells in co-culture. Anticancer Res. 2013;33:2001–2009. [PubMed] [Google Scholar]
- 17.Du Y, Long Q, Zhang L, Shi Y, Liu X, Li X, Guan B, Tian Y, Wang X, Li L, He D. Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAOA/mTOR/HIF-1α signaling. Int J Oncol. 2015;47:2064–2072. doi: 10.3892/ijo.2015.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Räsänen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer. 2006;119:477–483. doi: 10.1002/ijc.21808. [DOI] [PubMed] [Google Scholar]
- 21.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 22.Tyan SW, Kuo WH, Huang CK, Pan CC, Shew JY, Chang KJ, Lee EY, Lee WH. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast Tumorigenesis. PLoS One. 2011;6:e15313. doi: 10.1371/journal.pone.0015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massagué J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang W, Hiscox S, Matsumoto K, Nakamura T. Hepatocyte growth factor/scatter factor, its molecular, cellular and clinical implications in cancer. Crit Rev Oncol Hematol. 1999;29:209–248. doi: 10.1016/S1040-8428(98)00019-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Fu L, Fu J, Hu L, Yang H, Rong TH, Li Y, Liu H, Fu SB, Zeng YX, Guan XY. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clin Cancer Res. 2009;15:4017–4027. doi: 10.1158/1078-0432.CCR-08-2824. [DOI] [PubMed] [Google Scholar]
- 26.Achyut BR, Yang L. Transforming growth factor-β in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141:1167–1178. doi: 10.1053/j.gastro.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramaniam KS, Omar IS, Kwong SC, Mohamed Z, Woo YL, Mat Adenan NA, Chung I. Cancer-associated fibroblasts promote endometrial cancer growth via activation of interleukin-6/STAT-3/c-Myc pathway. Am J Cancer Res. 2016;6:200–213. [PMC free article] [PubMed] [Google Scholar]
- 28.Hua T, Liu S, Xin X, Cai L, Shi R, Chi S, Feng D, Wang H. S100A4 promotes endometrial cancer progress through epithelial-mesenchymal transition regulation. Oncol Rep. 2016;35:3419–3426. doi: 10.3892/or.2016.4760. [DOI] [PubMed] [Google Scholar]
- 29.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth-bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Teng F, Tian WY, Wang YM, Zhang YF, Guo F, Zhao J, Gao C, Xue FX. Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis. J Hematol Oncol. 2016;9:8–22. doi: 10.1186/s13045-015-0231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramaniam KS, Tham ST, Mohamed Z, Woo YL, Mat Adenan NA, Chung I. Cancer-associated fibroblasts promote proliferation of endometrial cancer cells. PLoS One. 2013;8:e68923. doi: 10.1371/journal.pone.0068923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]