Abstract
Ischemic stroke is a highly complex pathological process that is divided into acute, subacute and chronic phases. Paeonol is a biologically active natural product with a variety of pharmacological effects, including those on neuronal activity. However, the effects of paeonol on subacute/chronic ischemic stroke have remained to be elucidated. The present study was designed to investigate the effects of paeonol against subacute and chronic cerebral ischemic injury and to explore the possible underlying mechanisms. Male adult Sprague Dawley rats were randomly divided into a sham group (treated with saline), a model group [subjected to middle cerebral artery occlusion (MCAO) and treated with saline] and a paeonol-treated group (MCAO + paeonol at 25 mg/kg). Behavioral impairment, infarct volume and ischemic/contralateral hemispheric ratios were assessed at 72 h and at 28 days after MCAO, respectively. Immunofluorescence was employed to determine the neuronal damage and glial responses after MCAO. Compared with the model group, paeonol treatment significantly attenuated behavioral impairment, ischemic infarct volume and moderate cerebral edema in the ischemic brain at 72 h, as well as brain atrophy at 28 days after reperfusion. Furthermore, paeonol treatment ameliorated neuronal damage in the ischemic core and boundary zone regions at 72 h after reperfusion and in the boundary zone at 28 days after reperfusion. In addition, paeonol treatment reduced the proliferation of astrocytes in the boundary zone, and inhibited microglial activation in the ischemic core and boundary zone regions at 72 h and 28 days after reperfusion. These results demonstrated the protective effects of paeonol against subacute/chronic cerebral ischemia, and the mechanism of action may include subacute/chronic microglial activation and astrocyte proliferation.
Keywords: paeonol, subacute/chronic brain injury, cerebral ischemia, rat
Introduction
Ischemic stroke is the primary cause of cerebrovascular diseases and remains to be one of the leading causes of death and disability in patients worldwide (1,2). Despite tremendous research efforts leading to improvements in ischemic stroke treatment, the available therapeutic strategies are currently limited, and several promising agents evaluated in extensive preclinical trials failed to enter clinical trials (3,4). At present, thrombolytic therapy remains to be the gold standard for ischemic stroke treatment; however, it is only efficacious within a narrow therapeutic window (1,2,4,5). Hence, beyond the therapeutic window, management of stroke mainly depends on supportive therapy, secondary prevention and rehabilitation (1). Thus, an enhanced understanding regarding the pathological processes may provide novel therapeutic options to promote stroke recovery.
Ischemic stroke is a highly complex pathological process, which induces a series of cellular and molecular events and may be divided into three stages: The acute phase (hours), the subacute phase (hours to days) and the chronic phase (days to months) (6). In the acute stage, metabolic disturbances and excitotoxicity are involved in the progression of neuronal damage; in the subacute period, inflammation and cell death are the dominant events; in the chronic phase, brain repair is the major response and includes microgliosis, glial scar formation, angiogenesis, as well as neuronal regeneration (6–8). In addition, during the pathological process of ischemic stroke, the subacute and chronic phases are generally termed as late (subacute/chronic) phases (6,9). In the late phase, one of the dominant responses is reactive astrogliosis with subsequent glial scar formation, which not only protects the neurons against harmful substances by isolating the injury area but also obstructs neuronal regeneration by suppressing axonal sprouting (10,11). Another important event is the activation of microglia, which endangers neuronal survival by releasing numerous proinflammatory and neurotoxic mediators (8,12,13).
Recently, considerable attention has been paid to Traditional Chinese Medicine, as it includes sources of neuroprotective components (14). Natural products, particularly medicinal plants, may provide an ideal choice for the development of safe and effective drugs for stroke treatment (14). Paeonol, an active component isolated from the Chinese herbal medicine Cortex Moutan, which is the root bark of Paeonia suffruticosa Andr., has been demonstrated to possess diverse pharmacological activities, including anti-oxidant (15), anti-atherosclerotic (16), anti-tumor (17), anti-diabetic (18), and anti-inflammatory effects (19). Regarding its applicability for central nervous system diseases, previous studies have demonstrated that paeonol exerts neuroprotective actions against acute ischemic stroke, as well as Parkinson's and Alzheimer's disease in animal models, (19–22). These studies indicate that paeonol may be a promising drug for the treatment of neurological disorders. However, to the best of our knowledge, the potential effects of paeonol on subacute/chronic ischemic stroke have remained to be determined. The present study pursued to investigate the therapeutic potential of paeonol in middle cerebral artery occlusion (MCAO)-induced subacute/chronic cerebral ischemia. Furthermore, the present study focused on the post-ischemic microglial and astrocyte responses in the rat brain in an attempt to elucidate the underlying mechanisms.
Materials and methods
Experimental animals
A total of 125 adult male Sprague Dawley rats weighing 230–280 g (10–12 weeks old) were obtained from the Animal Science Center of Zhejiang Academy of Medical Sciences, [Hangzhou, China; certificate no. SCXK (Zhe) 2014-0001]. Animals were housed in an animal center at a constant temperature of 22±2°C, a relative humidity of 50±10% and a 12-h light/dark cycle. They were allowed free access to food and water. Behavioral experiments were arranged at 10:00 a.m.-05:00 p.m. during the day. All efforts were made to minimize their suffering. All experimental procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals and were approved by the Animal Care and Use Committee of Zhejiang University (Hangzhou, China).
Induction of transient focal cerebral ischemia
Transient focal cerebral ischemia was induced by MCAO according to previous methods (23) with certain modifications, such as using the nylon suture with a poly-L-lysine coated and without performing the ligation of the pterygopalatine artery (9,24). In brief, after animals were anesthetized by intraperitoneal (i.p.) injection chloral hydrate (400 mg/kg), and the left common carotid artery, the left external carotid artery (ECA) and the left internal carotid artery (ICA) were isolated. A nylon suture coated with poly-L-lysine was inserted into the ICA from the ECA until a slight resistance was felt, which indicated blockage of the MCA origin. Occlusion was performed for a period of 30 min, after which the suture was removed to allow for reperfusion. After the surgery, the animals were placed in a warm box and allowed to recover from anesthesia. The sham animals underwent the same surgical procedures without insertion of the suture. During the experiment, the change in regional cerebral blood flow (rCBF) was monitored as described previously with the steady-state baseline prior to the operation being regarded as 100% (9,25). In addition, the blood pressure, PaO2, PaCO2, glucose and pH were continuously monitored as described previously during the entire course of the operation (9,25). At the designated endpoint, all animals were euthanized by inhalation of CO2, and additional animals were allocated to ensure that a sufficient number of animals survived until the designated endpoint. Animals exhibiting signs including subarachnoid hemorrhage, or a moribund or comatose state with labored respiration, were excluded and euthanized with CO2 (26,27).
Drug administration and groups
Paeonol was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) with a purity of >99% and dissolved in saline at a concentration of 2 mg/ml. Based on a preliminary experiment, a dose of 25 mg/kg paeonol (i.p.) was used in the present study, which exerted the best neuroprotective effect in acute cerebral ischemia [2,3,5-triphenyltetrazolium chloride (TTC) staining and histopathological results; data not shown]. The regimen of drug administration applied in the present study was established in previous studies (9,24).
To evaluate the effects of paeonol on subacute ischemic injury, the animals were randomly divided into a sham group (saline, n=16), a model group (MCAO + saline, n=23) and a paeonol-treated group (MCAO + Pae at 25 mg/kg, n=21). For drug delivery, the same volume of saline or paeonol solution was injected i.p. at the onset of MCAO (when the nylon suture was inserted and the MCA origin was blocked), and then injected once a day for 3 days. During the experiment, one part of the animals (n=8) were used for determination of infarct volume, and the remaining animals were prepared to cut frozen sections for immunostaining. The 10-µm frozen sections at 2-mm intervals from the frontal to the occipital poles were cut by cryomicrotomy (CM1900, Leica, Wezlar, Germany).
To evaluate the effects of paeonol on chronic ischemic injury, the animals were randomly divided into a sham group (saline, n=16), a model group (MCAO + saline, n=26) and a paeonol-treated group (MCAO + Pae at 25 mg/kg, n=23) in another separate experiment. For drug delivery, the same volume of saline or paeonol was injected i.p. at the onset of MCAO, then injected once a day from days 2–7, and then once every 2 days from days 8–28.
Behavioral assessment
The neurological deficit score was determined at the indicated time-points according to the established scoring system: In the absence of neurological deficits, the score of 0 was given, upon failure to extend right paw fully, the score of 1 was given, animals circling to the right received the score of 2, falling to the right was scored as 3, and no spontaneous walking and depressed levels of consciousness was scored as 4 (23). The test was repeated for three times and the average value was recorded.
The inclined board test was performed to evaluate the balance and coordination of animals according to previous research (28) with certain modifications, including the material and size of the board, and the rotation angle (24). Animals were placed on the board (50×30 cm), and once they were stable, the board was inclined from horizontal to vertical at a rate of 2°/sec. The holding angle was defined as the angle at which the animal fell off the board. The test was repeated for three times and the average value was calculated.
One observer who was blinded to the experimental groups performed all of the assessments.
Determination of infarct volume
After the neurological assessment, one part of the animals was re-anesthetized, and the brains were quickly removed and coronally sliced into 6 sections of 2 mm in thickness. The slices were then incubated in 0.5% 2,3,5-triphenyltetrazolium chloride (TTC) at 37°C for 20 min, followed by fixation in 4% paraformaldehyde for 2 h. Subsequently, images of the fixed slices were captured. The infarction area was identified as the unstained area in the brain sections. The infarct volume was calculated by summing up the volumes of the 6 slices, and represented as the percentage of infarction in the total brain hemisphere.
Histological analysis
Following the behavioral tests, the remaining animals were re-anesthetized and then transcardially perfused with 4% paraformaldehyde after a pre-flush with saline. The brains were quickly removed, and were immediately immersed in 4% paraformaldehyde for 24 h, followed by dehydration in 30% sucrose for 3 days, then images were captured with a digital camera. Finally, a series of sections (10 µm) from the frontal to the occipital poles were cut by cryomicrotomy (CM1900; Leica Microsystems, Wetzlar, Germany). The sections were prepared for immunostaining.
To determine the changes in the number of different cell types, immunofluorescence staining was performed. The 10-µm slides were rinsed with PBS and then incubated with 10% normal goat serum (Zhongshan Belling Biotechnology Co., Ltd., Beijing, China) for 2 h at room temperature. Subsequently, the sections were incubated at 4°C overnight with the following primary antibodies: Mouse anti-glial fibrillary acidic protein (GFAP; cat. no. MAB3402; 1:800 dilution; EMD Millipore, Billerica, MA, USA), mouse anti-neuronal nuclei (NeuN; cat. no. MAB377; 1:200 dilution; EMD Millipore) and rabbit anti-ionized calcium binding adaptor molecule 1 (Iba1; cat. no. 019-19741; 1:1,000 dilution; Wako Pure Chemical Industries, Ltd., Wako, Japan). Thereafter, sections were washed three times in PBS and incubated with fluorescein isothiocyanate-conjugated secondary antibody (1:200 diluton; cat. nos. AP132F or AP124F; EMD Millipore) for 2 h at room temperature. For the negative control, PBS was applied instead of the primary antibodies. Images of the stained sections were captured using a fluorescence microscope (Olympus BX51: Olympus, Tokyo, Japan). Eight non-overlapping images for each site of the same rats were randomly selected, and the average value was determined. The neurons and the microglia were calculated according to the average number of stained cells and the astrocytes were calculated according to the average fluorescence intensity.
Statistical analysis
Values are expressed as the mean ± standard error of the mean. Significance of differences was assessed by one-way analysis of variance, followed by Dunnett's post-hoc test (SPSS 10.0 for Windows; SPSS, Inc., Chicago, IL, USA). The results of the behavioral assessments were analyzed using the Kruskal-Wallis test. P<0.05 was considered to indicate a statistically significant difference.
Results
Physiological changes after the operation
No significant differences in the physiological parameters, including mean arterial blood pressure, partial pressure of CO2 (PaCO2), PaO2, blood glucose, pH and weight were identified between 30 min prior to and after the operation. After cerebral ischemia, the rCBF was ~70% decreased at 30 min after occlusion, and then returned to baseline levels after reperfusion (P<0.01; Table I). Paeonol did not affect these physiological variables, including the reduction of rCBF (Table I). During the 28 days, all of the sham animals were survived. However, no significant difference in the survival rate was identified between the model group (61.54%) and the paeonol-treated group (69.57%), and these rates were consistent with those reported by previous studies (29).
Table I.
Physiological variables prior to and after operation.
| Ischemia | |||
|---|---|---|---|
| Variable | Sham | Model | Pae |
| Body weight (g) | 262.3±11.4 | 272.8±14.3 | 264.4±11.6 |
| MABP (mmHg) | |||
| Baseline | 105.2±10.1 | 102.3±8.9 | 110.0±14.7 |
| 30 min after reperfusion | 111.8±11.2 | 110.1±14.3 | 118.5±10.2 |
| PaO2 (mmHg) | |||
| Baseline | 109.2±4.1 | 105.2±4.5 | 103.8±5.3 |
| 30 min after reperfusion | 104.7±3.0 | 99.8±4.1 | 101.2±5.6 |
| PaCO2 (mmHg) | |||
| Baseline | 38.3±5.1 | 40.4±6.2 | 39.4±4.1 |
| 30 min after reperfusion | 41.2±3.9 | 42.8±4.3 | 44.3±4.9 |
| pH | |||
| Baseline | 7.45±0.38 | 7.38±0.41 | 7.39±0.43 |
| 30 min after reperfusion | 7.40±0.42 | 7.48±0.39 | 7.46±0.36 |
| Glucose (g/l) | |||
| Baseline | 6.58±0.81 | 6.85±0.64 | 6.12±0.53 |
| 30 min after reperfusion | 7.10±0.75 | 6.99±0.68 | 7.08±0.65 |
| rCBF (%) | |||
| Baseline | 100 | 100 | 100 |
| 30 min after ischemia | 98.5±6.2 | 35.8±7.5a | 34.6±5.7a |
| 30 min after reperfusion | 98.9±5.8 | 93.6±8.3 | 95.1±7.9 |
P<0.01 compared with sham group, analyzed by one-way analysis of variance. The variables were measured 30 min prior to operation (baseline) and 30 min after reperfusion. Values are expressed as the mean ± standard error of the mean for six animals per group. MABP, mean arterial blood pressure; Pa, partial pressure; rCBF, regional cerebral blood flow; Pae, paeonol.
Effect of paeonol on behavioral impairment
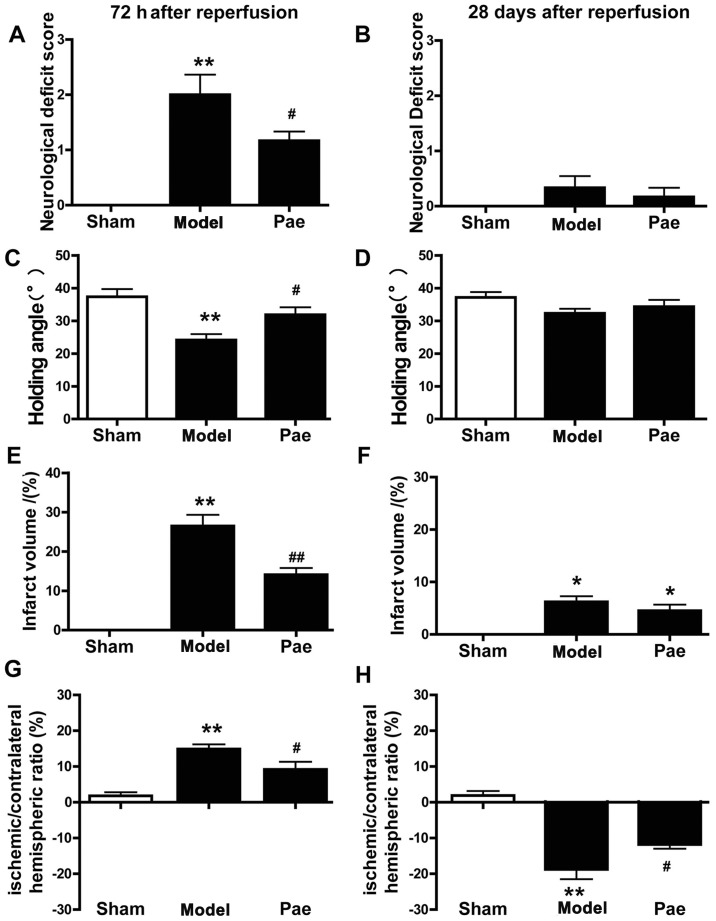
In the subacute experiment, behavioral impairments were evaluated at 24 (data not shown) and 72 h after reperfusion (Fig. 1). It was demonstrated that the neurological deficit scores and the holding angle were aggravated at 72 h after reperfusion. Compared with those in the sham group, the neurological deficit scores were significantly increased in the MCAO rats, whereas the holding angle in the inclined board test was significantly decreased (P<0.01; Fig. 1A and C). By contrast, a significant alleviation in the behavioral impairment was observed in the paeonol-treated group compared with that in the model group (P<0.05; Fig. 1A and C).
Figure 1.
Effects of paeonol on neurological dysfunction, infarct volume and the ischemic/contralateral hemispheric ratio after focal cerebral ischemia. (A-D) Paeonol treatment significantly ameliorated the neurological deficit score and holding angle at 72 h after reperfusion compared with those in the model group; however, it did not affect the already recovered neurological deficit score and holding angle at 28 days after reperfusion. (E and F) Paeonol treatment significantly decreased the infarct volume at 72 h after reperfusion compared with that in the model group; however, it did not affect the already decreased infarct volume at 28 days after reperfusion. (G and H) The ischemic/contralateral hemispheric ratio was increased (brain edema) at 72 h and decreased (brain atrophy) at 28 days after reperfusion, and these changes were significantly ameliorated by paeonol treatment. Values are expressed as the mean ± standard error of the mean from eight rats per group. *P<0.05 and **P<0.01 compared with sham group, #P<0.05 and ##P<0.01 compared with model group. Pae, paeonol.
In the chronic experiment, the measures of behavioral impairment, including the neurological deficit score and the holding angle, were aggravated at 72 h after reperfusion, and then gradually recovered at 7, 14 and 28 days after reperfusion (data not shown). Compared with the model group, paeonol administration led to a more efficient improvement in neurological scores and the holding angle in rats at 72 h after reperfusion. However, no significant difference in the behavioral impairments was identified between these groups from days 28 after reperfusion due to the chronic recovery (P>0.05; Fig. 1B and D).
Effect of paeonol on infarct volumes and ischemic/contralateral hemispheric ratio
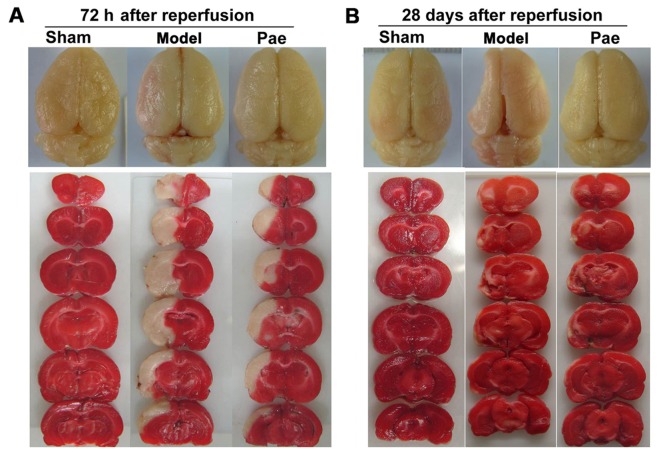
The representative images of total brains and the TTC-stained coronal slices indicated swelling/atrophy on the surface and infarctions in the hemispheres after cerebral ischemia, respectively (Fig. 2A-B).
Figure 2.
Effects of paeonol on ischemic brain injury. Representative images of total brains indicated brain lesions at (A) 72 h and (B) 28 days after reperfusion (upper panel). Representative images of TTC-stained coronal slices with 2 mm-thickness indicated ischemic lesions at (A) 72 h and (B) 28 days after reperfusion (lower panel). After TTC staining, the viable tissue stained deep red, whereas the infarct area was white in color. TTC, 2,3,5-triphenyltetrazolium chloride; Pae, paeonol.
The effects of paeonol on subacute brain injury at 72 h after reperfusion were assessed. Compared with those in the sham group, the infarct volume and the ischemic/contralateral hemispheric ratio, an index of brain edema, were significantly increased at 72 h after reperfusion (P<0.01; Fig. 1E and G, respectively, and Fig. 2A). However, compared with that in the model group, paeonol treatment significantly alleviated the increase of infarct volume and ischemic/contralateral hemispheric ratio at 72 h after reperfusion (P<0.05 or P<0.01; Fig. 1E and G, respectively, and Fig. 2A). Furthermore, the effects of paeonol on chronic brain injury were assessed at 28 days after reperfusion. Compared with that in the sham group, the ischemic/contralateral hemispheric ratio was markedly decreased at 28 days after reperfusion (P<0.01; Figs. 1H and 2B), indicating brain atrophy, which was consistent with the results of previous studies (9,29,30). By contrast, administration of paeonol markedly alleviated chronic brain atrophy in comparison with that in the model group (P<0.05; Figs. 1H and 2B). However, no significant differences were identified in the infarct volumes between the paeonol-treated and model groups (P>0.05; Figs. 1F and 2B). This may have been due to the apparent atrophy, which hindered the observation of further changes, and the chronic functional recovery. Collectively, these results indicated that paeonol exerted neuroprotective effects on subacute and chronic cerebral ischemic injury.
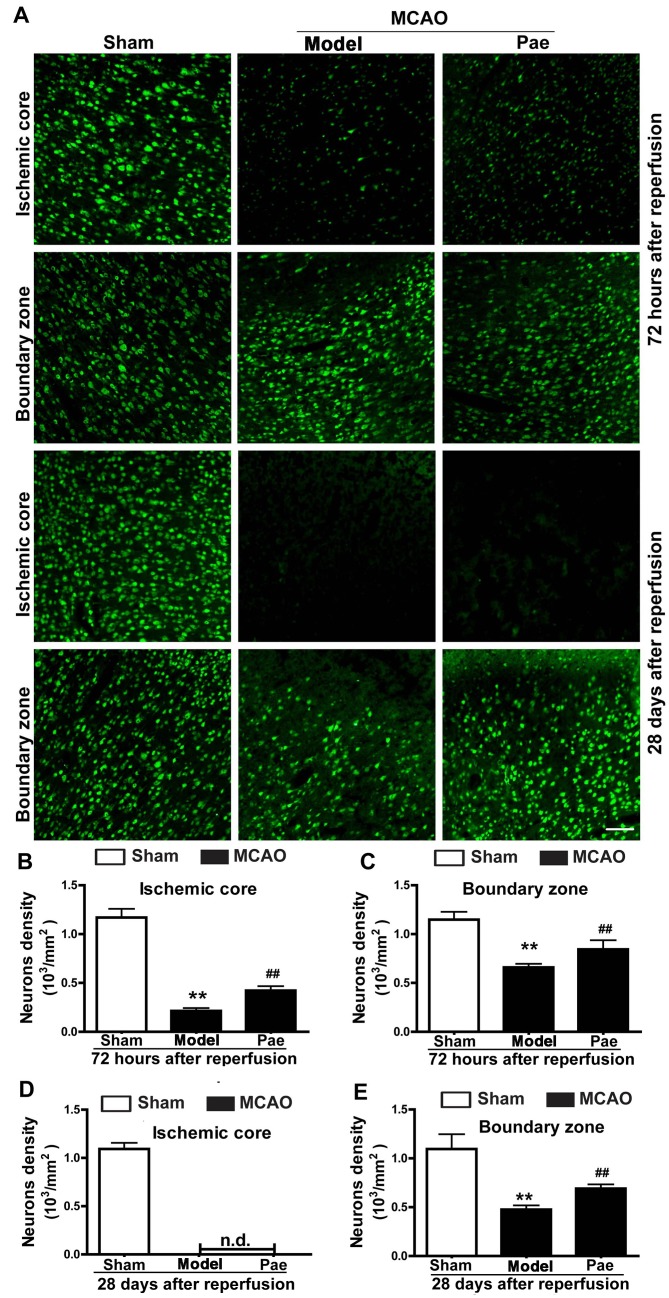
Effect of paeonol on neuronal damage
To assess the effect of paeonol on ischemic neuronal injury, the changes of neuronal damage in the ischemic brains after paeonol treatment were observed (Fig. 3). Cerebral ischemia induced evident ischemic lesions exhibiting neuronal injury, which included the shrinkage of cell bodies that were deeply stained using Nisssl staining, and the disappearance of Nissl bodies (data not shown). In the ischemic core, compared with that in the sham group, the density of neurons identified by immunofluorescent staining for NeuN was markedly decreased at 72 h after reperfusion, and disappeared at 28 days after reperfusion (P<0.01; Fig. 3A, B and D). In the boundary zone, neuronal density was markedly decreased at 72 h and 28 days after reperfusion (P<0.01; Fig. 3A, C and E). Paeonol treatment significantly ameliorated neuronal loss in the ischemic core, as well as in the boundary zone at 72 h after reperfusion in comparison with that in the model group (P<0.01; Fig. 3A-C). In addition, paeonol treatment markedly inhibited neuronal loss in the boundary zone at 28 days after reperfusion (P<0.01; Fig. 3A and E). However, no significant differences in the neuronal density in the ischemic core area were observed between the paeonol-treated and model groups, as neurons disappeared at 28 days after reperfusion in the ischemic core area (Fig. 3A and D). However, a limited number of degenerated neurons or background staining may be observed (Fig. 3A and D).
Figure 3.
Effects of paeonol on neuronal injury at 72 h and 28 days after reperfusion in rats. (A) Representative photomicrographs indicated neurons by immunostaining for NeuN at 72 h and at 28 days after reperfusion (scale bar, 100 µm) and (B-E) quantification results. In the ischemic core, the reduction of neuronal density was significantly attenuated by paeonol treatment at 72 h after reperfusion, whereas NeuN-expressing cells completely disappeared at 28 days after reperfusion. In the boundary zone, the decrease of neuronal density was significantly attenuated by paeonol treatment at 72 h and 28 days after reperfusion. Values are expressed as the mean ± standard error of the mean from eight rats per group. **P<0.01 compared with sham group, ##P<0.01 compared with model group. Pae, paeonol; MCAO, middle cerebral artery occlusion; n.d., not detectable; NeuN, neuronal nuclei.
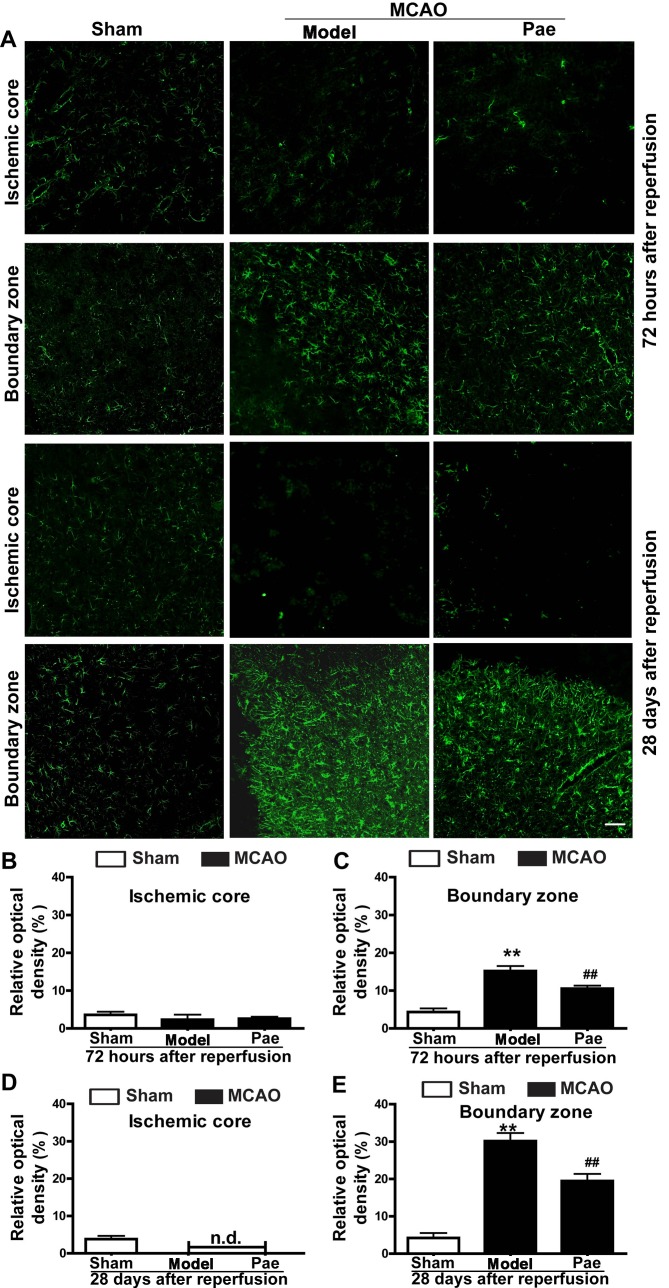
Effect of paeonol on astrocyte proliferation
To determine the underlying mechanisms involved in the neuroprotective effects of paeonol on subacute and chronic ischemic injury, the present study further assessed the astrocyte responses in the ischemic hemisphere after paeonol administration (Fig. 4). The results indicated that the density of GFAP-positive astrocytes was not significantly changed at 24 h after reperfusion (data not shown). However, at 72 h after reperfusion, astrocytes in the boundary zone were significantly increased and hypertrophied, and a glial scar surrounding the ischemic core area had formed at 28 days after reperfusion (P<0.01; Fig. 4A, C and E). By contrast, in the ischemic core area, astrocytes were initially decreased at 72 h after reperfusion, and eventually disappeared at 28 days after reperfusion (Fig. 4A, B and D). Paeonol treatment significantly reduced the density of GFAP-positive astrocytes in the boundary zone at 72 h and 28 days after reperfusion compared with that in the model group (P<0.01; Fig. 4A, C and E). However, no significant differences were observed in the density of GFAP-positive astrocytes in the ischemic core area between the paeonol-treated and model group at 72 h and 28 days after reperfusion, and the astrocytes gradually disappeared from this area (Fig. 4A, B and D). Overall, the results indicated that paeonol exerted its beneficial effects by reducing post-ischemic astrocyte proliferation.
Figure 4.
Effects of paeonol on astrocyte proliferation at 72 h and 28 days after reperfusion in rats. (A) Representative photomicrographs indicating glial fibrillary acidic protein-immunopositive astrocytes at 72 h and 28 days after reperfusion (scale bar, 100 µm) and (B-E) quantification results. At 72 h after reperfusion, astrocytes were initially increased in the boundary zone, and paeonol treatment significantly suppressed the changes in the boundary zone, but not those in the ischemic core. At 28 days after reperfusion, astrocytes were significantly increased in the boundary zone, and paeonol treatment significantly attenuated the astrogliosis in the boundary zone, but not that in the ischemic core. Values are expressed as the mean ± standard error of the mean from eight rats per group. **P<0.01 compared with sham group, ##P<0.01 compared with model group. Pae, paeonol; MCAO, middle cerebral artery occlusion; n.d., not detectable.
Effect of paeonol on microglial activation
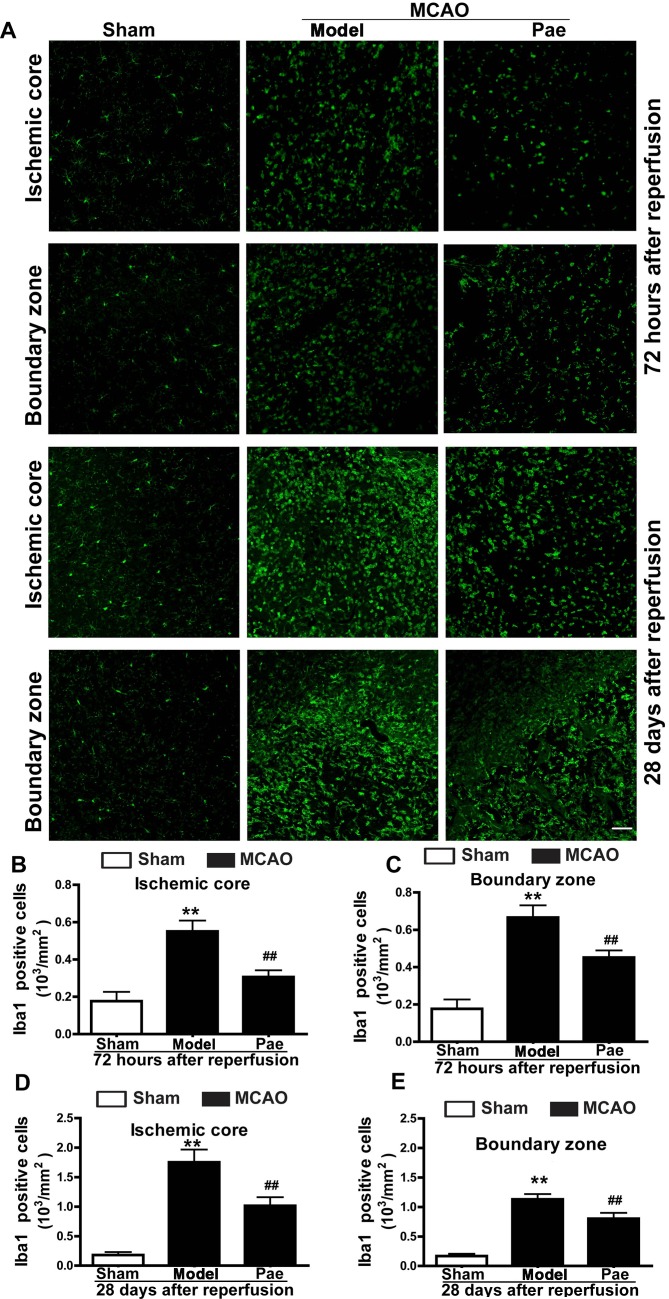
To evaluate whether microglial activation was associated with the neuroprotective effects of paeonol on post-ischemic injury, immunostaining analysis for microglia was performed. Microglia are inflammatory cells that are rapidly activated after brain injury. Activation of microglia involves their proliferation, migration into the injured area, upregulation of various immunomodulators and phagocytosis of the damaged cells and debris. In the cerebral cortex of sham rats, ramified Iba-1-positive microglial cells were diffusely distributed (Fig. 5A). At 24 h after reperfusion, the number of Iba-1-positive microglia was not particularly changed, but the of Iba-1-positive microglia morphology became irregular (data not shown). However, the number of Iba-1-positive cells, including that of the ramified microglia and activated (ameboid/round) microglia/macrophages, was gradually increased at 72 h after reperfusion compared with that in the sham group (P<0.01; Fig. 5A-C). In addition, at 28 days after reperfusion, the number of Iba-1-positive cells, particularly that of activated (ameboid/round) microglia/macrophages, was markedly increased in the ischemic core and boundary zone regions compared with that in the sham group (P<0.01; Fig. 5A, D and E). By contrast, compared with that in the model group, paeonol treatment significantly inhibited the increase in Iba-1-positive microglia and ameliorated the morphological changes in the ischemic core, as well as the boundary zone at 72 h and 28 days after reperfusion (P<0.01; Fig. 5A-E). These results indicated the protective effects of paeonol, which were associated with post-ischemic microglial activation.
Figure 5.
Effects of paeonol on microglial activation at 72 h and 28 days after reperfusion in rats. (A) Representative photomicrographs displaying Iba-1-immunopositive microglia in the ischemic core and boundary zone regions at 72 h and 28 days after reperfusion (scale bar, 100 µm) and (B-E) quantification results. At 72 h after reperfusion, Iba-1-positive microglia initially increased in the ischemic core and the boundary zone regions, and these changes were significantly inhibited by paeonol treatment. At 28 days after reperfusion, microglial cells in the ischemic core and boundary zone regions were significantly increased, which was attenuated by paeonol treatment. Values are expressed as the mean ± standard error of the mean from eight rats per group. **P<0.01 compared with sham group, ##P<0.01 compared with model group. Pae, paeonol; MCAO, middle cerebral artery occlusion; Iba-1, ionized calcium binding adaptor molecule 1.
Discussion
Traditional Chinese Medicine has been increasingly recognized and has demonstrated a therapeutic significance in the treatment of ischemic stroke (14). Paeonol, an active phenolic component of Cortex Moutan, has been demonstrated to possess diverse biological properties (16,17,19–22). Paeonol has been proved to be effective in treating acute experimental ischemic stroke by inhibiting the excitotoxicity, calcium overload and oxidative stress (19,20). However, to date, the effects of paeonol against subacute/chronic cerebral ischemic injury have remained elusive. Therefore, the present study investigated the effects of paeonol on subacute/chronic cerebral ischemic injury and further determined the underlying mechanisms.
The amphiphilic structure and low molecular weight of paeonol facilitate the easy penetration of the blood brain barrier (19). Thus, in the present study, paeonol was administered to rats by intraperitoneal injection, and its effect against subacute/chronic cerebral ischemic injury was explored. Numerous animal studies on ischemia have established a model where the injury at 24 h following ischemia/reperfusion is regarded as acute injury of cerebral ischemia, that at 72 h to 7 days after reperfusion is regarded as subacute injury and that at 14–35 days following ischemia/reperfusion is regarded as chronic injury (6,9,29,31). Thus, in the present study, the ischemic injury in rats was observed at 72 h and 28 days after cerebral ischemia/reperfusion. The behavioral impairments in these rats were also evaluated in 24 h after reperfusion (data not shown). Consistent with the previous studies, the behavioral impairments were aggravated at 24 h after reperfusion, and paeonol treatment improved their neurological deficits. The major results of the present study demonstrated that the increase in the infarct volume and ischemic/contralateral hemispheric ratio (edema) in the ischemic hemisphere at 72 h after reperfusion and the decrease in the ischemic/contralateral hemispheric ratio (atrophy) at 28 days after reperfusion were significantly attenuated by paeonol treatment. Furthermore, paeonol treatment greatly ameliorated the behavioral impairment at 72 h after reperfusion compared with that in the model group. In addition, paeonol treatment significantly ameliorated neuronal damage in the ischemic core and the boundary zone regions at 72 h after reperfusion, and in the boundary zone at 28 days after reperfusion. Taken together, it was indicated that paeonol had protective effects on subacute and chronic cerebral ischemic injury.
In addition to the above changes, the present study further investigated the potential mechanisms underlying the protective effects of paeonol on subacute and chronic ischemic stroke. As is known, astrocytes and microglia have pivotal roles in the progression of ischemic stroke (10,32). Accumulating evidence suggests that glial responses require hours to days to fully develop, and may in turn provide potential targets for stroke recovery with longer therapeutic windows compared with those of other treatments (3,13). In addition, post-ischemic inflammation is a crucial step during the process of ischemic stroke (13,33). Thus, the present study subsequently focused on the effects of paeonol on inflammation-associated glial cells.
During the post-ischemic phase, one important event in subacute/chronic ischemic brain injury is reactive astrogliosis and glial scar formation (10,11). As a vital part of the neurovascular unit, astrocytes have an important role in the physiology of the normal brain, which includes blood circulation, extracellular ionic homeostasis, release of energy substrates and growth factors, as well as neurotransmission (3,11). Numerous studies have demonstrated that astrocytes protect the neurons from glutamate excitotoxicity during ischemic stroke (10,11). However, increasing evidence has indicated that astrocytes paradoxically exacerbate ischemic injury with morphological and phenotypic changes; this process is termed reactive gliosis or astrogliosis (7,10). Following focal ischemic injury, reactive astrocytes migrate towards the boundary zone and then eventually organize into the glial scar that separates the ischemic core region from healthy tissue (3,7,10). The formation of a glial scar is a critical event in the brain repair responses after ischemic injury, which act as a physical and biochemical barrier that separates the viable and dead tissues, but also obstructs neuronal regeneration by suppressing axonal sprouting (3,10,11). Growing evidence suggests that modulation of reactive astrocytes may be a promising strategy for the management of stroke (11). In the present study, paeonol treatment significantly reduced GFAP-positive astrocytes in the boundary zone at 72 h and 28 days after reperfusion compared with those in the model group, but not in the ischemic core, which may be due to the gradual disappearance of astrocytes in the area. Different from the mechanisms observed during acute cerebral ischemic injury, paeonol treatment may regulate the astrocyte responses and inhibit undesired outcomes, including glial scar formation, because of its association with the obstruction neuronal regeneration by suppressing axonal sprouting. Thus, the present study provided valuable details regarding the role of paeonol in modulating post-ischemic astrocyte proliferation.
With regard to the changes in the late phase, another important event in post-ischemic brain injury is microgliosis and the associated inflammatory responses (13,32,34). Microglial cells are the resident immune cells and are the first and major line of active immune defense in the intact or injured brain (12,13). During neuropathological conditions, microglial cells are rapidly activated to restore homeostasis of the central nervous system (12,35). The activation of microglia involves their proliferation and migration into the injured area, upregulation of various immunomodulators, phagocytose of the damaged cell debris and antigenic substances (8,33,34). However, microglial activation has dual effects, and uncontrolled or overactivated microglia are detrimental for the pathological conditions (35). Activated microglia may aggravate neuronal damage through the release of nitric oxide, reactive oxygen species, protease and proinflammatory cytokines such as tumor necrosis factor-α, as well as interleukin-1β and −6 (35,36). Chronic microglial activation has been considered to be the possible underlying mechanism in the neuronal damage and is associated with numerous neurodegenerative diseases, including ischemic stroke (12,13). Therefore, appropriate identification of agents that inhibit microglial activation may act as effective treatment strategies for providing neuroprotection (12,35). In the present study, paeonol treatment significantly inhibited the increase in Iba-1-positive microglia and ameliorated the morphological changes in the ischemic core and boundary zone areas at 72 h and 28 days after reperfusion. Among the various biological properties, the anti-inflammatory activities of paeonol have been demonstrated in macrophages, microglia and in the BV2 cell line in vitro, which was consistent with the present in vivo results (37,38). It was indicated that paeonol treatment may regulate microglial activation, suggesting the potential use of paeonol in the treatment of post-ischemic microgliosis.
In summary, the present study indicated the protective effects of paeonol and its involvement in the treatment of subacute/chronic cerebral ischemia, particularly in the late phase of microglial activation and astrocyte proliferation. During subacute cerebral ischemia, paeonol effectively alleviated neurological impairment, reduced the infarct volume, cerebral edema and neuronal loss, and suppressed microglial activation and astrocyte proliferation. During chronic cerebral ischemia, paeonol markedly ameliorated brain atrophy and neuronal loss, and inhibited microglial proliferation and astrocyte proliferation. Based on the above results obtained in rats, understanding the long-term neuroprotective effects of paeonol in the process of chronic cerebral ischemia may lead to the development of a novel clinical treatment for ischemic stroke. Overall, the present study broadened the current knowledge on the range of pharmacological properties of paeonol for the treatment of ischemic stroke. However, the precise molecular mechanisms of the neuroprotective effects of paeonol require elucidation in future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National Natural Science Foundation (grant nos. 81401566, 31301933, 81301122 and 81400594), the Zhejiang Provincial Natural Science Foundation (grant no. LQ15H090005) and the Science and Technology Planning Project of Zhejiang Province (grant nos. 2015KYB076 and 2015ZB012).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BZ, QJS and HW designed the research. BZ, QJS, ZZZ, HW performed the experiments and data collection. BZ, SYW and XW analyzed the data. BZ, QJS and HW wrote the manuscript. All authors have read and approved this version of the article, and ensure the integrity of the work.
Ethics approval and consent to participate
All experimental procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals and were approved by the Animal Care and Use Committee of Zhejiang University (Hangzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hankey GJ. Stroke. Lancet. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 2.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Stary CM. Targeting glial mitochondrial function for protection from cerebral ischemia: Relevance, mechanisms, and the role of MicroRNAs. Oxid Med Cell Longev. 2016;2016:6032306. doi: 10.1155/2016/6032306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 5.Shichita T, Ago T, Kamouchi M, Kitazono T, Yoshimura A, Ooboshi H. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J Neurochem. 2012;123(Suppl 2):S29–S38. doi: 10.1111/j.1471-4159.2012.07941.x. [DOI] [PubMed] [Google Scholar]
- 6.Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 7.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanisch UK, Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, Zhao CZ, Zhang XY, Huang XQ, Shi WZ, Fang SH, Lu YB, Zhang WP, Xia Q, Wei EQ. The new P2Y-like receptor G protein-coupled receptor 17 mediates acute neuronal injury and late microgliosis after focal cerebral ischemia in rats. Neuroscience. 2012;202:42–57. doi: 10.1016/j.neuroscience.2011.11.066. [DOI] [PubMed] [Google Scholar]
- 10.Choudhury GR, Ding S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol Dis. 2016;85:234–244. doi: 10.1016/j.nbd.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol. 2016;144:103–120. doi: 10.1016/j.pneurobio.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JY, Kim N, Yenari MA. Mechanisms and potential therapeutic applications of microglial activation after brain injury. CNS Neurosci Ther. 2015;21:309–319. doi: 10.1111/cns.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin X, Yamashita T. Microglia in central nervous system repair after injury. J Biochem. 2016;159:491–496. doi: 10.1093/jb/mvw009. [DOI] [PubMed] [Google Scholar]
- 14.Bu Y, Lee K, Jung HS, Moon SK. Therapeutic effects of traditional herbal medicine on cerebral ischemia: A perspective of vascular protection. Chin J Integr Med. 2013;19:804–814. doi: 10.1007/s11655-013-1341-2. [DOI] [PubMed] [Google Scholar]
- 15.Ding Y, Li Q, Xu Y, Chen Y, Deng Y, Zhi F, Qian K. Attenuating oxidative stress by paeonol protected against Acetaminophen-induced hepatotoxicity in mice. PLoS One. 2016;11:e0154375. doi: 10.1371/journal.pone.0154375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YR, Chen JJ, Dai M. Paeonol protects rat vascular endothelial cells from ox-LDL-induced injury in vitro via downregulating microRNA-21 expression and TNF-α release. Acta Pharmacol Sin. 2014;35:483–488. doi: 10.1038/aps.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Tan SY, Wang XF. Paeonol exerts an anticancer effect on human colorectal cancer cells through inhibition of PGE, synthesis and COX-2 expression. Oncol Rep. 2014;32:2845–2853. doi: 10.3892/or.2014.3543. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Feng L, Ma D, Zhang M, Gu J, Wang S, Fu Q, Song Y, Lan Z, Qu R, Ma S. Neuroprotective effect of paeonol on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. Neurosci Lett. 2013;549:63–68. doi: 10.1016/j.neulet.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Liao WY, Tsai TH, Ho TY, Lin YW, Cheng CY, Hsieh CL. Neuroprotective effect of paeonol mediates anti-inflammation via suppressing Toll-like receptor 2 and Toll-like receptor 4 signaling pathways in cerebral ischemia-reperfusion injured rats. Evid Based Complement Alternat Med. 2016;2016:3704647. doi: 10.1155/2016/3704647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Fu B, Zhang X, Zhao T, Chen L, Zhang J, Wang X. Paeonol pretreatment attenuates cerebral ischemic injury via upregulating expression of pAkt, Nrf2, HO-1 and ameliorating BBB permeability in mice. Brain Res Bull. 2014;109:61–67. doi: 10.1016/j.brainresbull.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Shi X, Chen YH, Liu H, Qu HD. Therapeutic effects of paeonol on methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid-induced Parkinson's disease in mice. Mol Med Rep. 2016;14:2397–2404. doi: 10.3892/mmr.2016.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou A, Wu H, Pan J, Wang X, Li J, Wu Z, Hui A. Synthesis and evaluation of paeonol derivatives as potential multifunctional agents for the treatment of Alzheimer's disease. Molecules. 2015;20:1304–1318. doi: 10.3390/molecules20011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Shi QJ, Wang H, Liu ZX, Fang SH, Song XM, Lu YB, Zhang WP, Sa XY, Ying HZ, Wei EQ. HAMI 3379, a CysLT2R antagonist, dose- and time-dependently attenuates brain injury and inhibits microglial inflammation after focal cerebral ischemia in rats. Neuroscience. 2015;291:53–69. doi: 10.1016/j.neuroscience.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003;98:465–473. doi: 10.1097/00000542-200302000-00028. [DOI] [PubMed] [Google Scholar]
- 26.Graham SM, McCullough LD, Murphy SJ. Animal models of ischemic stroke: Balancing experimental aims and animal care. Comp Med. 2004;54:486–496. [PubMed] [Google Scholar]
- 27.Rewell SS, Churilov L, Sidon TK, Aleksoska E, Cox SF, Macleod MR, Howells DW. Evolution of ischemic damage and behavioural deficit over 6 months after MCAo in the rat: Selecting the optimal outcomes and statistical power for multi-centre preclinical trials. PLoS One. 2017;12:e0171688. doi: 10.1371/journal.pone.0171688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yonemori F, Yamaguchi T, Yamada H, Tamura A. Evaluation of a motor deficit after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1998;18:1099–1106. doi: 10.1097/00004647-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Lu LQ, Lou Q, Guo HG, Zhou WW, Ying HZ, Wei EQ, Shi QJ. Neuroprotective effect of HAMI 3379, a CysLT2R receptor antagonist, on chronic brain injury after focal cerebral ischemia in rats. Int J Clin Exp Pathol. 2017;10:4123–4136. [Google Scholar]
- 30.Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38:2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- 31.Garbuzova-Davis S, Haller E, Tajiri N, Thomson A, Barretta J, Williams SN, Haim ED, Qin H, Frisina-Deyo A, Abraham JV, et al. Blood-spinal cord barrier alterations in subacute and chronic Stages of a rat model of focal cerebral ischemia. J Neuropathol Exp Neurol. 2016;75:673–688. doi: 10.1093/jnen/nlw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2017;157:247–272. doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Xu L, He D, Bai Y. Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol. 2016;53:6709–6715. doi: 10.1007/s12035-015-9593-4. [DOI] [PubMed] [Google Scholar]
- 34.Benarroch EE. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology. 2013;81:1079–1088. doi: 10.1212/WNL.0b013e3182a4a577. [DOI] [PubMed] [Google Scholar]
- 35.Lee Y, Lee SR, Choi SS, Yeo HG, Chang KT, Lee HJ. Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed Res Int. 2014;2014:297241. doi: 10.1155/2014/297241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawabori M, Yenari MA. The role of the microglia in acute CNS injury. Metab Brain Dis. 2015;30:381–392. doi: 10.1007/s11011-014-9531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam KN, Woo BC, Moon SK, Park SU, Park JY, Hwang JW, Bae HS, Ko CN, Lee EH. Paeonol attenuates inflammation-mediated neurotoxicity and microglial activation. Neural Regen Res. 2013;8:1637–1643. doi: 10.3969/j.issn.1673-5374.2013.18.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Himaya SW, Ryu B, Qian ZJ, Kim SK. Paeonol from Hippocampus kuda Bleeler suppressed the neuro-inflammatory responses in vitro via NF-kB and MAPK signaling pathways. Toxicol In Vitro. 2012;26:878–887. doi: 10.1016/j.tiv.2012.04.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.