Abstract
Settings: All hospitals managing drug-resistant tuberculosis (DR-TB) according to national guidelines in Pakistan.
Objectives: To assess the effect of diabetes mellitus (DM) and factors associated with unfavourable outcomes in DR-TB.
Methods: A cross-sectional study based on a retrospective record review of patients enrolled on DR-TB treatment from 2010 to 2014 in Pakistan. DR-TB data reported to Pakistan's National TB Control Programme on a monthly basis were used for the study.
Result: Among 5811 patients enrolled on second-line drugs, 8.8% had DM. Overall, 68.9% had favourable outcomes. No association was found between DM and DR-TB treatment outcomes (risk ratio 0.90, 95%CI 0.74–1.05). Unfavourable outcomes were more frequent among DR-TB patients with human immunodeficiency virus (HIV) co-infection (OR 11.58, 95%CI 2.20–60.72), extensively drug-resistant TB patients (OR 5.36, 95%CI 1.00–28.72), patients with exposure to both first-line and second-line anti-tuberculosis drugs (OR 2.45, 95%CI 1.21–4.97) and those with a previous history of treatment in the private sector (OR 1.53, 95%CI 1.16–2.02).
Conclusion: Although there were limitations to correctly measuring DM and its management, DM appears not to be a risk factor for unfavourable outcomes in DR-TB patients in our study. DR-TB and HIV co-infection, second-line drug resistance and history of treatment in the private sector were nevertheless more frequently associated with adverse outcomes.
Keywords: DR-TB, DM, outcomes, Pakistan
Abstract
Contexte : Tous les hôpitaux prenant en charge la tuberculose pharmacorésistante (TB-DR) selon les directives nationales du Pakistan.
Objectif : Evaluer l'effet du diabète (DM) et les facteurs associés à un résultat défavorable du traitement de la TB-DR.
Méthode : Etude transversale basée sur une revue rétrospective de dossiers de patients enrôlés dans un traitement de TB-DR de 2010 à 2014 au Pakistan. Les registres de TB-DR envoyés au programme national de lutte contre la TB chaque mois ont été utilisés pour l'étude.
Résultats : Parmi 5811 patients enrôlés dans un traitement par médicaments de deuxième ligne, 8,8% avaient un DM. Dans l'ensemble, 68,9% ont eu des résultats favorables. Il n'a pas été trouvé d'association entre le DM et le résultat du traitement de la TB-DR (ratio de risque 0,90 ; IC95% 0,74–1,05). Les facteurs associés à des résultats défavorables sont la coinfection par TB-DR et le virus de l'immunodéficience humaine (VIH) (OR 11,58 ; IC95% 2,20–60,72), la TB ultrarésistante (OR 5,36 ; IC95% 1,00–28,72), l'exposition à la fois aux médicaments de première ligne et de deuxième ligne (OR 2,45 ; IC95% 1,21–4,97) et des antécédents de traitement dans le secteur privé (OR 1,53 ; IC95% 1,16–2,02).
Conclusion : Dans notre étude, avec ses limites en termes de mesures correctes du DM et de sa prise en charge, le DM ne semble pas être un facteur de risque de résultat défavorable pour les patients TB-DR. Par contre, la coinfection TB-DR et VIH, la résistance aux médicaments de deuxième ligne et les antécédents de traitement dans le secteur privé ont été associés à des résultats médiocres.
Abstract
Marco de referencia: Todos los hospitales que suministran tratamiento contra la tuberculosis farmacorresistente (TB-DR) en el marco de las directrices nacionales de Pakistán.
Objetivo: Evaluar el efecto de la diabetes (DM) sobre el desenlace de la TB-DR y los factores que se asocian con los desenlaces desfavorables.
Método: Un estudio transversal realizado a partir del examen retrospectivo de las historias clínicas de los pacientes que iniciaron tratamiento por TB-DR del 2010 al 2014 en el Pakistán.
Métodos: En el presente estudio se consultaron los registros de los casos de TB-DR que se notifican mensualmente al Programa Nacional de control de la Tuberculosis.
Resultados: De los 5811 pacientes que iniciaron tratamiento con medicamentos de segunda línea, el 8,8% sufría DM. En general, el 68,9% de los casos alcanzó desenlaces favorables. No se observó ninguna asociación entre la presencia de DM y el desenlace terapéutico de la TB-DR (riesgo relativo 0,90; IC95% 0,74–1,05). Los factores que se asociaron con desenlaces desfavorables fueron la coinfección por el virus de la inmunodeficiencia humana (VIH) y la TB-DR (OR 11,58; IC95% 2,20–60,72), la TB ultrarresistente (OR 5,36; IC95% 1,00–28,72), la exposición a los dos tipos de fármacos, de primera y de segunda línea (OR 2,45; IC95% 1,21–4,97) y el antecedente de tratamiento antituberculoso en el sector privado (OR 1,53; IC95% 1,16–2,02).
Conclusión: Según los resultados del presente estudio, pese a algunas limitaciones en la evaluación correcta de la DM y su tratamiento, no pareciera que la presencia de DM fuese un factor de riesgo de resultados desfavorables del tratamiento de pacientes con TB-DR. Sin embargo, la coinfección por el VIH y la TB-DR, la resistencia a fármacos de segunda línea y el antecedente de tratamiento antituberculoso en el sector privado se asociaron con desenlaces desfavorables.
Drug-resistant tuberculosis (DR-TB) is a major challenge to national TB control programmes (NTPs). In 2015, there were an estimated 580 000 incident DR-TB patients worldwide; the treatment success rate among patients enrolled in 2013 was reported to be 52%.1 Pakistan is among the world's high-burden countries for drug-susceptible (DS) and DR-TB, with an estimated 14 000 new DR-TB patients (respectively 4.2% and 16% of new and retreatment patients) in 2015. The treatment success rate for DR-TB in Pakistan was 69% in 2013, which is higher than the global treatment success rate.1 The World Health Organization's (WHO's) End TB Strategy has set a very ambitious target of 90% treatment success by 2020.2
Diabetes mellitus (DM), one of the leading non-communicable diseases, is on the rise. The 2016 Global Diabetes Report stated that 422 million people were affected by DM, with an increase from 4.7% in 1980 to 8.5% in 2014 among adults aged >18 years. It is also estimated that 1.5 million deaths were directly caused by DM in 2012.3 A recent study estimated that by 2030 Pakistan will have the fifth largest number of type 2 DM patients.4
DM patients with active TB have a greater bacillary load at presentation, which results in longer time to culture conversion and prolongs treatment. DM can also cause changes in oral absorption, decreased protein binding of drugs, and renal insufficiency or fatty liver with impaired drug clearance.5 Management of DR-TB is very difficult. The second-line drugs (SLDs) used to treat DR-TB are less potent and more costly than first-line drugs (FLDs). They are also associated with more adverse events and are less well tolerated. In addition, the duration of treatment is very long (⩾20 months).6 It may be hypothesised that the presence of both diseases increases the risk of an unfavourable outcome.
Various studies conducted in different countries have suggested that there is a significantly higher risk of adverse outcomes among patients with DR-TB and DM,7–10 although other studies reported finding no association between the two.11–14 Recent studies in Pakistan have shown more unfavourable outcomes among DS-TB patients with DM;15,16 however, we could not find any relevant study examining the effect of DM on treatment outcomes among DR-TB patients in Pakistan.
The aim of the present study was to assess the effect of DM and factors associated with unfavourable treatment outcomes among DR-TB patients enrolled on treatment in Pakistan from 2010 to 2014.
METHODOLOGY
Study design
This was a cross-sectional study based on a retrospective record review of all patients with DR-TB enrolled at 24 PMDT (Programmatic Management of DR-TB) sites in Pakistan from June 2010 to December 2014.
Setting
In Pakistan, the health care system and services are evolving. Although communicable diseases remain the leading causes of morbidity and mortality, non-communicable diseases are on the rise. The public sector is the main provider of preventive care and hospital care for both the urban and rural populations. Factors such as poverty, malnutrition, poor housing and sanitation, inadequate health care facilities, population migration and urbanisation, political instability and refugee influx have all continued to aggravate the problem of TB and DR-TB.
With the support of the Global Fund, Pakistan's NTP started piloting PMDT in hospital-based and ambulatory models in three hospitals in 2010.17 The intervention was scaled up to 24 hospitals in urban districts across the country by the end of 2014. Dedicated staff are posted at these PMDT sites for the management of DR-TB patients in line with national guidelines. Diagnostic services, treatment with SLDs and socio-psychological support are provided to all patients.
Presumptive DR-TB patients are diagnosed using the Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) and phenotypic drug susceptibility testing (DST). Patients diagnosed with DR-TB are then referred to the nearest PMDT site for enrolment on treatment. DR-TB physicians and their teams at the PMDT site assess the patients and review the referral documents and laboratory and radiological test results, a complete history and physical examination are performed, a treatment regimen selected, the record is entered in the treatment card and the patient is registered in the DR-TB register (Excel-based sheet with data from the DR-TB treatment card; MicroSoft, Redmond, WA, USA). All patients undergo fasting blood glucose testing using a glucose meter. The patients are then screened for HIV co-infection using a rapid test, and positive test results are confirmed. All of these variables, including history of DM, are recorded in the Excel-based register.
Study population
The DR-TB register data are reported to the NTP by the PMDT site on a monthly basis in a routine reporting cycle. For our study, exposure variables such as TB clinical features were extracted from the DR-TB registers, along with information on age, sex, history of care provider, previous anti-tuberculosis treatment, previous treatment outcome, site of disease and type of resistance, comorbidity status and treatment outcomes. Sociodemographic characteristics (address and occupation) were extracted from the patient files. The primary study exposure of interest was diagnosed DM (Figure). Treatment outcomes based on WHO-designated definitions were also extracted from the DR-TB registers. Data on patients enrolled in 2010–2014 were verified and validated on-site from April to June 2017 during routine monitoring visits.
FIGURE.
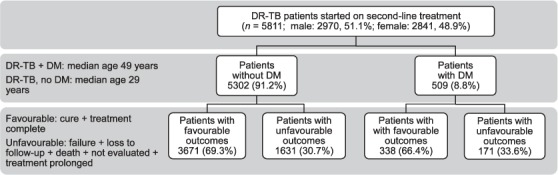
Study flow diagram of patients with DR-TB, Pakistan, 2010–2014. DR-TB = drug-resistant tuberculosis; DM = diabetes mellitus.
Data analysis
Validated Excel data were imported to SPSS v 21 (Statistical Package for the Social Sciences; IBM Corp, Armonk, NY, USA). A descriptive analysis was performed for patients with and those without DM. The treatment outcomes ‘cured’ and ‘treatment completed’ were classified as favourable, and the remainder of the outcomes were categorised as unfavourable. Outcomes among DR-TB patients with and those without DM were analysed using unadjusted relative risks (RRs) with 95% confidence intervals (CIs). Associations between all clinical and sociodemographic characteristics with adverse treatment outcomes were explored using binary logistic multivariate models.
Ethics approval
Permission to use the data and local ethics exemption were obtained from the Pakistan NTP. As the study involved a review of records with no patient interaction, informed consent was not required.
RESULTS
Of the 5811 patients who started second-line anti-tuberculosis treatment from June 2010 to December 2014, 5617 (96.6%) were adults (age ⩾15 years)and 51.1% were male; the median age was 29 years (inter-quartile range [IQR] 22–42). Among those DR-TB patients with a comorbidity, DM was the most frequent (52.4%). The prevalence of DM in patients with DR-TB starting treatment with SLDs was 8.8% (Table 1). All patients with DM had type 2 diabetes. DM was more prevalent in patients with DR-TB aged ⩾35 years (median age 49 years, IQR 41–56); 37.4% of the patients were unemployed.
TABLE 1.
WHO-defined treatment outcomes of drug-resistant tuberculosis patients with DM in Pakistan, 2010–2014
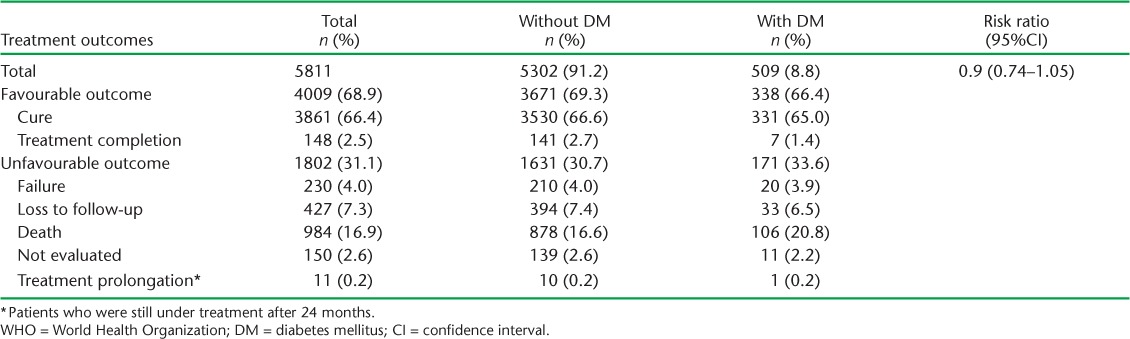
Overall, 4009 (68.9%) patients had a favourable treatment outcome. No significant association was observed between DM status and poor treatment outcome (RR 0.90, 95%CI 0.74–1.05). Although the death rate was higher in patients with DM than in patients without DM (20.8% vs. 16.6%), the difference was not statistically significant (Table 1).
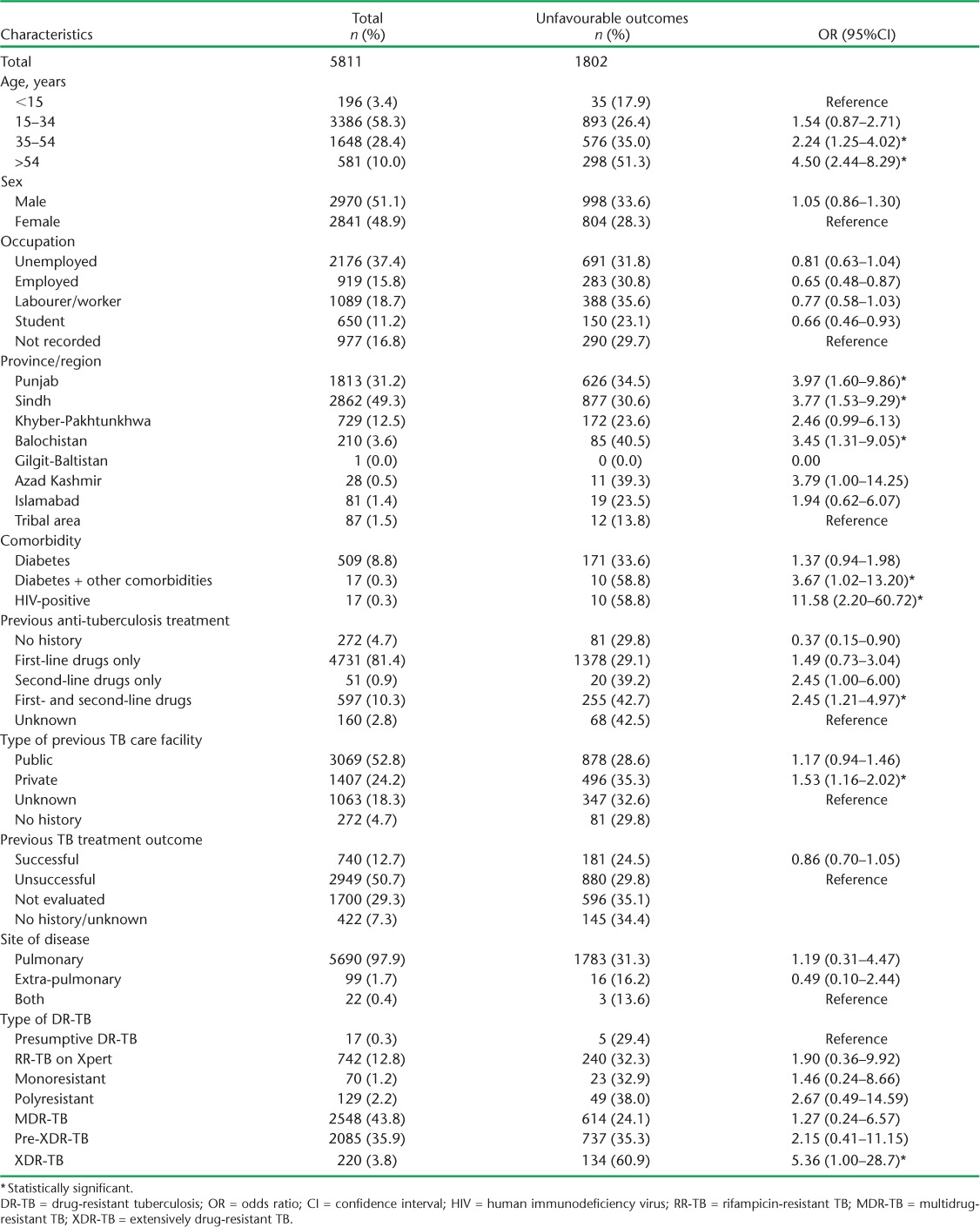
In the multivariable binary logistic regression model, characteristics significantly associated with unfavourable outcomes were DR-TB and HIV co-infection (OR 11.58, 95%CI 2.20–60.72), extensively drug-resistant TB (XDR-TB) (OR 5.36, 95%CI 1.00–28.72), age ⩾35 years (age 35–54 years, OR 2.24, 95%CI 1.25–4.02; age ⩾55 years, OR 4.50, 95%CI 2.44–8.29), other comorbidities in addition to DM (OR 3.67, 95%CI 1.02–13.20), exposure to both FLDs and SLDs (OR 2.45, 95%CI 1.21–4.97) and history of previous treatment in the private sector (OR 1.53, 95%CI 1.16–2.02) (Table 2).
TABLE 2.
Sociodemographic characteristics of DR-TB patients with unfavourable outcomes, Pakistan, 2010–2014
DISCUSSION
Treatment outcomes for DR-TB were similar in patients with and those without DM (success rate 66.4% vs. 69.3%). DM prevalence in this cohort of DR-TB patients was 8.8%. Older age, DR-TB and HIV co-infection, XDR-TB, a history of treatment with SLDs and previous anti-tuberculosis treatment in the private sector were found to be associated with unfavourable outcomes.
The lack of significant association observed between DM and DR-TB treatment outcome is consistent with findings from studies conducted in other countries, such as Georgia, Peru, Mexico and China.11–14 However, studies in South Korea, Taiwan and Brazil have reported an association of DM with unfavourable outcomes.7–9 These studies also report that unfavourable outcomes were significantly associated with poor glycaemic controls and other comorbidities caused by DM, such as chronic renal diseases, cardiovascular diseases and cerebrovascular diseases in patients with DM.9 Our results show a higher death rate in patients with DM than in those without DM (20.8% vs. 16.6%). As cause of death is not recorded in the surveillance system, it is unknown whether the high mortality is due to the increased severity of TB in patients with DM or the presence of other factors.
According to the WHO, DM prevalence in the general population in Pakistan in 2016 was 9.8%.18 Other studies have found a high prevalence of DM among TB patients, e.g., 26.7% in Malaysia,6 while a meta-analysis of DM and multidrug-resistant TB in various countries showed a prevalence of 10–23%.19 Our study finding of 8.8% DM in our patient population is similar to the DM prevalence in the adult general population. Given that the NTP has a low case detection rate for DR-TB compared to the estimated burden, it is possible that DM prevalence will increase if more cases are detected and enrolled for treatment. The national testing criteria for DR-TB should include patients with medical conditions such as DM to find missing DR-TB cases.
The factors identified in our study as being associated with unfavourable outcomes of DR-TB treatment are similar to findings from studies conducted in different countries such as the former Soviet Union, where older age and a previous history of SLD use were strongly associated with adverse outcomes.20,21 Age-specific treatment strategies are needed to improve outcomes in older age patients. We also observed that patients with a history of anti-tuberculosis treatment in the private sector were more likely to have unfavourable treatment outcomes than those treated in the public sector (OR 1.53, 95%CI 1.16–2.02).
It is well known that a history of the use of SLDs outside programmatic conditions leads to the amplification of resistance.20,21 Such patients are very difficult to manage and are more at risk of adverse outcomes. The NTP needs to control the irrational use of SLDs and take firm steps to ban over-the-counter sales of both FLDs and SLDs.
Pakistan has a huge private sector. About 75% of the general population use private facilities as their first point of contact.22 It is therefore very important for the NTP to engage the private sector in TB control.23 The costs of quality-assured SLDs and monthly culture are usually unaffordable for patients, resulting in treatment interruption and loss to follow-up. Regulating the private sector will be challenging but vital for TB control in the country.24 Private practitioners should be engaged, trained in NTP guidelines and encouraged to refer DR-TB patients to PMDT sites for treatment.23
The study had several strengths. It is the first study from Pakistan to examine treatment outcomes among DR-TB patients, and as the study had a large sample size and used routinely collected data, the findings reflect programme realities. Finally, we followed STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting the study.25
Limitations of the study include its retrospective nature and reliance on existing patient databases as a data source. Although all patients diagnosed with DR-TB were tested for fasting blood glucose, the DM measurement did not include a comprehensive assessment of glucose control (e.g., haemoglobin A1c) to determine the effect of DM management on DR-TB treatment. Data recorded at the PMDT site also did not mention whether or not patients with DM were insulin-dependent. We did not have any information on variables such as smoking, alcoholism, drug use and cavitation, etc., which might also have an association with adverse outcomes in DR-TB patients. Pharmacovigilance data were not properly recorded to determine whether there were more adverse effects of SLD treatment in patients with DM. Future studies should measure the impact of DM management on the clinical management of DR-TB by reviewing glycaemic controls during the course of treatment.
CONCLUSION
Although DM was not associated with unfavourable outcomes in DR-TB treatment, our study identified important factors associated with unfavourable treatment outcomes in DR-TB. The Pakistan NTP should adopt a patient-centred approach to address these factors. Glycaemic control in DR-TB patients should be monitored to assess the severity of DM and its impact on DR-TB management and outcomes. National guidelines should be revised to include the need for patients attending primary health clinics with medical conditions such as DM to be screened for TB. Glycated haemoglobin testing should be performed in patients with DM every third month to monitor DM management. Recording and reporting tools for DR-TB should be revised to include risk factors such as smoking, excessive alcohol and drug use, radiological findings and body mass index.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médecins Sans Frontières (MSF, Geneva, Switzerland).
The specific programme that resulted in this publication was conducted in 2017 by the National Tuberculosis Control Programme (NTP) of Pakistan, through the support of the Global Fund (Geneva, Switzerland). The authors wish to thank PMDT (Programmatic Management of DR-TB) staff for record keeping and their support in conducting the study; and A Safdar, M Tariq and Mr Waseemullah for their valuable input as participants of SORT IT 2017. The publication fee was covered by the NTP, Pakistan, through the support of the Global Fund.
Footnotes
Conflicts of interest: none declared.
References
- 1. World Health Organization. . Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016: p 211. [Google Scholar]
- 2. World Health Organization. . Implementing the End TB Strategy: the essentials. WHO/HTM/TB/2015.31 Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 3. World Health Organization. . Global report on diabetes. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 4. Zafar J, Nadeem D, Khan S A, Jawad Abbasi M M, Aziz F, Saeed S.. Prevalence of diabetes and its correlates in urban population of Pakistan: a cross-sectional survey. J Pak Med Assoc 2016; 66: 922– 927. [PubMed] [Google Scholar]
- 5. Dooley K E, Chaisson R E.. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2010; 9: 737– 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Azhar S, Sulaiman S, Khan A H, . et al. Impact of diabetes mellitus on treatment outcomes of tuberculosis patients in tertiary care setup. Am J Med Sci 2013; 345: 321– 325. [DOI] [PubMed] [Google Scholar]
- 7. Kang Y A, Kim S Y, Jo K-W, . et al. Impact of diabetes on treatment outcomes and long-term survival in multidrug-resistant tuberculosis. Respiration 2013; 86: 472– 478. [DOI] [PubMed] [Google Scholar]
- 8. Reis-Santos B, Gomes T, Locatelli R, . et al. Treatment outcomes in tuberculosis patients with diabetes: a polytomous analysis using Brazilian surveillance system. PLOS ONE 2014; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiang C-Y, Bai K-J, Lin H-H, . et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLOS ONE 2015; 10: e0121698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Odone A, Houben R M G J, White R G, Lönnroth K.. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol 2014; 2: 754– 764. [DOI] [PubMed] [Google Scholar]
- 11. Magee M J, Kempker R R, Kipiani M, . et al. Diabetes mellitus is associated with cavities, smear grade, and multidrug-resistant tuberculosis in Georgia. Int J Tuberc Lung Dis 2016; 19: 685– 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magee M J, Kempker R R, Kipiani M, . et al. Diabetes mellitus, smoking status, and rate of sputum culture conversion in patients with multidrug-resistant tuberculosis: a cohort study from the country of Georgia. PLOS ONE 2014; 9: e94890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munoz-Torrico M, Caminero-Luna J, Migliori G B, . et al. Diabetes is associated with severe adverse events in multidrug-resistant tuberculosis. Arch Bronconeumol 2017; 53: 245– 250. [DOI] [PubMed] [Google Scholar]
- 14. Leung C-C, Yew W-W, Mok T Y W, . et al. Effects of diabetes mellitus on the clinical presentation and treatment response in tuberculosis. Respirology 2017; 22: 1225– 1232. [DOI] [PubMed] [Google Scholar]
- 15. Mukhtar F, Butt Z A.. Cohort profile: the diabetes-tuberculosis treatment outcome (DITTO) study in Pakistan. BMJ Open 2016; 6: e012970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tahir Z, Ahmad M-U-D, Akhtar A M, Yaqub T, Mushtaq M H, Javed H.. Diabetes mellitus among tuberculosis patients: a cross sectional study from Pakistan. Afr Health Sci 2016; 16: 671– 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Tuberculosis Control Programme Pakistan. . National Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis (PMDT). Second revision, 2014. Lahore, Pakistan: NTCP, 2014. http://ntp.gov.pk/uploads/ntp_1368669324_National_Guidelines_PMDT.zip Accessed February 2018 [Google Scholar]
- 18. World Health Organization. . WHO diabetes country profile. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 19. Liu Q, Li W, Xue M, . et al. Diabetes mellitus and the risk of multidrug resistant tuberculosis : a meta-analysis. Sci Rep 2017; 7: 1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khaliaukin A, Kumar A M, Skrahina A.. Poor treatment outcomes among multidrug-resistant tuberculosis patients in Gomel Region, Republic of Belarus. Public Health Action 2014; 4 Suppl 2: S24– S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danilovitz M, Falzon D, Gelmanova I, Keshavjee S.. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis (Edinb) 2016; 92: 397– 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naqvi S A, Naseer M, Kazi A, . et al. Implementing a public-private mix model for tuberculosis treatment in urban Pakistan: lessons and experiences. Int J Tuberc Lung Dis 2012; 16: 817– 821. [DOI] [PubMed] [Google Scholar]
- 23. Udwadia Z F, Pinto L M, Uplekar M W.. Tuberculosis management by private practitioners in Mumbai, India: has anything changed in two decades ? PLOS ONE 2010; 5: e12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wells W A, Ge C F, Patel N, Oh T, Gardiner E, Kimerling M E.. Size and usage patterns of private TB drug markets in the high burden countries. PLOS ONE 2011; 6: e18964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Elm E, Altman D G, Egger M, . et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495– 1499. [DOI] [PubMed] [Google Scholar]


