Abstract
Setting: A southern Myanmar district providing isoniazid preventive therapy (IPT) in one of the last countries to formally recommend it as part of human immunodeficiency virus (HIV) care.
Objective: To assess coverage and adherence and the feasibility of IPT scale-up in a routine care setting in Myanmar.
Design: A retrospective analysis of people living with HIV (PLHIV) screened for tuberculosis (TB) and enrolled in IPT over a 3-year period (July 2011–June 2014) using clinical databases.
Results: Among 3377 patients under HIV care and screened for TB, 2740 (81.1%) initiated IPT, with 2651 (96.8%) completing a 6- or 9-month course of IPT; 83 (3.1%) interrupted treatment for different reasons, including loss to follow-up (n = 41), side effects (n = 15) or drug adherence issues (n = 9); 6 (0.2%) died. Among the IPT patients, 33 (1.2%) were diagnosed with TB, including 9 (0.3%) while on IPT and 24 (0.9%) within 1 year of completion of therapy. Among the PLHIV who completed IPT, one case of isoniazid resistance was detected.
Conclusion: Scaling up IPT in Myanmar HIV settings is feasible with high rates of drug adherence and completion, and a low rate of discontinuation due to side effects. IPT scale-up should be prioritised in HIV clinical settings in Myanmar.
Keywords: tuberculosis, TB, INH resistance, isoniazid preventive therapy, scale-up, South-East Asia
Abstract
Contexte : Un district du sud du Myanmar fournissant le traitement préventif par isoniazide (IPT) dans l'un des derniers pays à le recommander formellement comme élément de la prise en charge de l'infection par le virus de l'immunodéficience humaine (VIH).
Objectif : Evaluer la couverture, l'adhérence et la faisabilité d'une accélération de l'IPT dans un contexte de soins de routine au Myanmar.
Schéma : Analyse rétrospective de personnes vivant avec le VIH (PVVIH) dépistés pour la tuberculose (TB) et enrôlés dans l'IPT sur une période de 3 ans, de juillet 2011 à juin 2014, grâce à des bases de données cliniques.
Résultats : Sur 3377 patients pris en charge pour le VIH et dépistés pour la TB, 2740 (81,1%) ont mis en route le TPI, dont 2651 (96,8%) ont achevé un traitement préventif de 6 ou 9 mois ; 83 (3,1%) ont interrompu leur traitement pour différentes raisons incluant les pertes de vue (n = 41), les effets secondaires (n = 15) ou des problèmes d'adhérence au médicament (n = 9), et six (0,2%) sont décédés. Parmi les patients IPT, 33 (1,2%) ont eu un diagnostic de TB, dont 9 (0,3%) pendant la prophylaxie et 24 (0,9%) dans l'année qui a suivi la fin de l'IPT. Un cas de résistance à l'isoniazide a été détecté parmi les PVVIH qui ont achevé l'IPT.
Conclusion: L'accélération de l'IPT dans les structures VIH du Myanmar est faisable, avec un taux élevé d'adhérence au médicament et d'achèvement et un taux faible d'arrêt du traitement dû à des effets secondaires. L'accélération de l'IPT devrait être considérée comme une priorité dans les structures cliniques VIH du Myanmar.
Abstract
Marco de referencia: Un distrito del sur de Birmania que provee el tratamiento preventivo con isoniazida (IPT). Birmania es uno de los últimos países que incluyó esta profilaxis en las recomendaciones formales de atención de la infección por el virus de la inmunodeficiencia humana (VIH).
Objetivo: Evaluar la cobertura, el cumplimiento terapéutico y la factibilidad de ampliar la escala de aplicación del IPT en un entorno de tratamiento corriente en Birmania.
Método: Fue este un análisis retrospectivo de personas con infección por el VIH, en quienes se practicó la detección sistemática de la tuberculosis (TB) y se registraron para recibir el IPT. Se obtuvo la información a partir de las bases de datos clínicos durante un período de 3 años, de julio del 2011 hasta junio del 2014.
Resultados: De los 3377 pacientes que recibían atención por infección por el VIH, con investigación sistemática de la TB, 2740 iniciaron el TPI (81,1%) y 2651 completaron un esquema de 6 o 9 meses de profilaxis (96,8%). Ochenta y tres pacientes interrumpieron por razones diversas el tratamiento (3,1%), entre ellas, la pérdida durante el seguimiento (n = 41), los efectos secundarios (n = 15) o los problemas de cumplimiento terapéutico (n = 9) y seis pacientes fallecieron (0,2%). De los pacientes que recibieron IPT, en 33 se diagnosticó TB (1,2%), en 9 de ellos durante la profilaxis (0,3%) y en 24 casos durante el primer año después de haber completado el esquema (0,9%). Se detectó un caso de resistencia a isoniazida en las personas infectadas por el VIH que completaron el IPT.
Conclusiôn: La ampliación de escala del IPT en los entornos de atención de la infección por el VIH es factible en Birmania y se pueden alcanzar altas tasas de cumplimiento terapéutico y compleción del esquema, con una baja tasa de interrupción debida a efectos colaterales. Es importante dar prioridad a la ampliación de escala del IPT en los medios de atención de la infección por el VIH en el país.
Tuberculosis (TB) is the leading cause of death among people living with human immunodeficiency virus (PLHIV). This is particularly true in Asia, where 13% of the world's PLHIV are found, along with 60% of the global TB burden and high rates of TB-HIV coinfection.1,2 Preventive approaches such as isoniazid (INH) preventive therapy (IPT) are increasingly important to reduce TB disease incidence in HIV programmes worldwide.3 The World Health Organization's (WHO's) ‘Three Is’ approach to TB management (intensified case finding, infection prevention and control and IPT) included 6 months of treatment with INH in PLHIV to reduce TB incidence and increase overall life expectancy, while remaining cost-effective.4–7
In December 2014, the Myanmar Ministry of Health's (MoH's) National HIV/AIDS Programme (NAP) officially included IPT in its guidelines for HIV care. At the time, it was one of only six other countries without formal IPT guidance for PLHIV (including China, Cote d'Ivoire, Democratic Republic of Congo, India, Indonesia and Zimbabwe).8,9 Nevertheless, even countries that have recommended IPT for years may not achieve adequate coverage, and policy changes may not always reach the clinical level due to implementation delays on the ground, lack of training in IPT administration among physicians and other HIV actors, or because reluctant clinicians may fear the creation of INH-resistant strains of TB despite global validation of the approach.10–12
Limited research is available on IPT implementation in South-East Asia, and none has been published to date from Myanmar. The few studies from the region that do exist show widely varying results, from relatively high discontinuation rates in a Cambodian IPT cohort (n = 445, 21.7%), to a 50% treatment completion rate and a further 12% dying in a Thai cohort (n = 412).13,14 A cohort in Viet Nam, a wealthier country with longer experience with IPT, has shown high completion rates (91.8%), with few cases of loss to follow-up (LTFU) or death.13,15,16
Médecins Sans Frontières (MSF) has been providing HIV-TB treatment and care in southern Myanmar's Tanintharyi Region since 2004, and was one of the first actors in the country to incorporate IPT into routine HIV care. In Asia, there are some descriptions of IPT in clinical care settings, but there remains a dearth of IPT-related information from Myanmar, and recent IPT policy changes in the country require evidence surrounding the feasibility of IPT integration.17 In an effort to identify barriers and facilitators of successful implementation in varied programmatic contexts, we describe the experience of introducing IPT in an MSF-supported clinic serving PLHIV. This is the first report of its kind from Myanmar.
MATERIALS AND METHODS
This was a retrospective cohort analysis of patients who received IPT over a nearly 3-year period of prophylaxis provision (July 2011–June 2014) at an MSF clinic in the south-eastern Tanintharyi Region of Myanmar. Routine medical data were collected from patients diagnosed since May 2004. Once diagnosed, those PLHIV considered stable (i.e., those on antiretroviral therapy [ART] for >3 months with no complications or active opportunistic infections [OIs]) accessed the clinic every 3 months for follow-up HIV care (Figure 1). Those who were not yet stable, especially those in the first 3 months of ART, returned to the clinic each month. Patients were evaluated for TB at each clinical interaction, first with a routine medical evaluation for TB symptoms or risk factors (cough ≥ 14 days, cough with blood, fever, weight loss, night sweats or contact with another TB patient). If one of the TB symptoms or risk factors was present, they then underwent laboratory investigation using sputum smear acid-fast bacilli (AFB) and/or Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) and chest radiography as needed.
FIGURE 1.
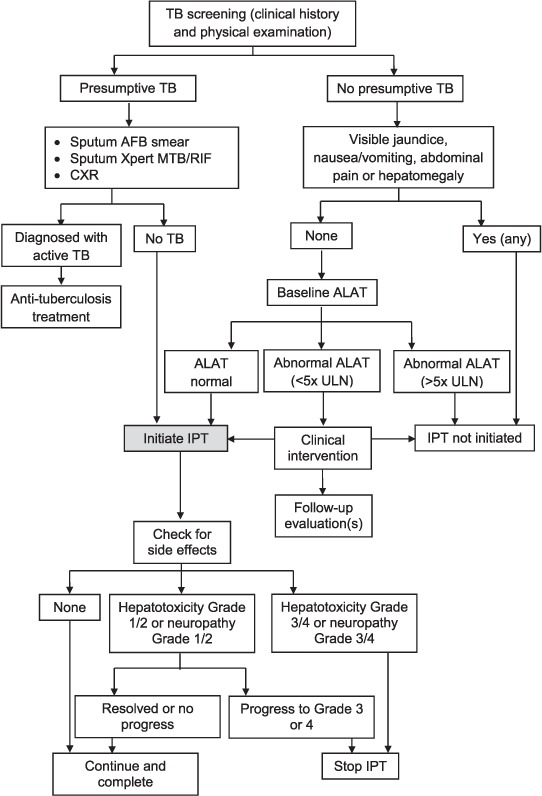
Clinical screening algorithm for provision and follow-up of IPT in HIV patients enrolled in MSF care in southern Myanmar, 2011–2014. TB = tuberculosis; AFB = acid-fast bacilli; CXR = chest radiography; ALAT = alanine transaminase; ULN = upper limit of normal; IPT = isoniazid preventive therapy; HIV = human immunodeficiency virus; MSF = Médecins Sans Frontières.
Patients were treated for TB and HIV in the same location using an integrated care approach, often even seeing the same clinician for both conditions over time, increasing the likelihood of identifying patients who developed TB in the first year after completing IPT. INH resistance was detected using sputum culture and drug susceptibility testing (DST) using a line-probe assay (GenoType MTBDRplus; Hain Lifesciences, Nehren, Germany) at the Myanmar National TB Reference Laboratory in Yangon, Myanmar. All patients who developed pulmonary TB during and after IPT underwent both culture and DST, except for paediatric patients or those with extra-pulmonary TB (n = 6) because of the challenges of obtaining samples in a resource-limited context.
All IPT-related data were collected from patients as part of routine clinical care. Information was handwritten in standardised patient files and registers and entered into MSF's electronic medical record database (FUCHIA v1.7.1). Data collected from the patients included demographic, clinical and treatment characteristics, including drug regimen, co-infection, immunological and virological information, prophylactic medications, drug adherence and intolerance information (side effects such as peripheral neuropathy and/or hepatotoxicity), diagnosed OIs and other conditions, laboratory results and appointment dates. Data were extracted from the FUCHIA database or the individual patient files and entered into an Excel database (MicroSoft, Redmond, WA, USA) customised for this analysis.
Approval from the Myanmar Ethical Review Board was not sought for this retrospective analysis of routinely collected, de-identified programme data, as Myanmar regulations currently do not require formal review for programme implementation analysis. The research also fulfilled the exemption criteria set by the MSF Ethics Review Board for a posteriori analyses of routinely collected clinical data. The study was conducted with permission from the Medical Director at MSF's Operational Centre in Geneva, Switzerland. All clinical care received by participants during the course of their treatment and reviewed in this analysis followed established medical ethical practice, and all research procedures conform to standards defined in the Helsinki Declaration. All data are freely available from the corresponding author upon request.
Isoniazid preventive therapy
MSF collaborated with the Myanmar National TB Programme (NTP) to provide IPT as one of the first clinical actors to integrate IPT into routine HIV care on a large scale in the country. Although IPT administration did not differ between MoH and MSF sites at this time, it was piloted in only a few sites across the country. From July to August 2011, 15 PLHIV per month initiated prophylaxis during a ‘pilot’ period, when all PLHIV patients were screened for TB symptoms (described above), and those with no current or presumptive TB (diagnosed using sputum AFB and/or Xpert) and no hepatitis (alanine transaminase [ALAT] level > 5 times the upper limit of normal or symptoms, including visible jaundice, nausea/vomiting, abdominal pain or hepatomegaly) qualified for IPT. During the initial 15 patients/month pilot period, eligible patients had not yet begun ART, had a CD4 count > 350 cells/mm3, and had no symptoms of TB or hepatitis. From September 2011 to May 2013, a further 70 patients were enrolled per month. All patients under HIV care were eligible, including both pre-ART patients and those on treatment, except for those with TB symptoms, hepatitis, and those who had a history of drug adherence problems. From May 2013 to June 2014, all eligible paediatric and adult patients were enrolled, except during several brief periods of drug stock-outs. In 2014, MSF had been collaborating with the NAP for IPT provision when IPT provision-related responsibilities were handed over from the NTP to the NAP.
Adult and paediatric (age < 15 years) IPT recipients were given on-site counselling about the prophylaxis. The 6–9 month INH regimen was self-administered at a daily oral dose of 300 mg for adults (10 mg/kg for children weighing <25 kg) according to WHO recommendations. In 2011–2012, patients received a 6-month regimen in accordance with national guidelines, while in 2013–2014 patients received the WHO-recommended 9-month regimen.18 There was no difference in the inclusion/exclusion criteria for children and adults. Due to contextual limitations, screening for latent tuberculous infection (LTBI) was unavailable. Baseline ALAT was measured for each patient, and was investigated further if clinical signs or symptoms of hepatotoxicity were seen. To prevent peripheral neuropathy, pyridoxine was given at a dose of 10 mg daily.
Patients initially followed a standard follow-up schedule after initiating IPT, returning at months 1, 3 and 6 to the clinic. During this period, those in whom side effects were suspected or detected were asked to return for monthly follow-up. At each follow-up visit, patients underwent screening for INH side effects (i.e., peripheral neuropathy and hepatotoxicity) using established clinical protocols (Figure 1). Patients with presumptive TB were then screened for active TB using both clinical criteria and available diagnostic modalities: chest X-ray, AFB and Xpert (since 2013). Confirmed cases were switched to standardised anti-tuberculosis treatment. IPT drug adherence was assessed by pill count conducted during follow-up counselling sessions at each follow-up visit (at months 1, 3 and 6, and quarterly thereafter). No other IPT adherence incentives, education and community follow-up outside of these clinical interactions were provided in this context, beyond the travel incentives and community follow-up that MSF routinely provided only for extremely sick patients. A patient who presented for his/her 6-month visit with good adherence was considered to have completed IPT. Other IPT outcomes were defined as 1) death or 2) interruption. Reasons for IPT interruption were due to side effects (such as hepatitis and peripheral neuropathy), drug adherence-related (defined as not taking medication for any reason except for drug toxicity issues) or because the patient developed TB, was lost to follow-up, or was transferred to another care facility. LTFU was defined as failure to attend for a scheduled medication refill for 2 months.
RESULTS
A total of 3377 patients were under HIV care and screened for TB during the study period. Of these, 3234 patients initiated and remained on ART, with another 143 in a pre-ART phase of HIV care. A total of 2740 (81.1%) patients initiated IPT during the roll-out period, 1421 (51.2%) of whom were male. The 637 (18.8%) eligible patients who did not initiate IPT did not do so because of the gradual roll-out period, because of periodic drug stock-outs or because they had active TB, although a detailed subanalysis of this group was not possible due to the limitations of the clinical records. The majority of those initiating IPT were adults (n = 2500, 91.2%), with 144 (5.3%) adolescents aged 10–19 years and 82 (3.0%) children aged <10 years. Patients on IPT came from a range of professions: economic migrants (n = 719, 26.2%), manual labourers (n = 580, 21.2%), fishermen (n = 553, 20.2%), and unemployed or otherwise ‘dependent’, including female homemakers (n = 397, 14.5%); 423 (15.4%) lived >3 h by car outside the district.
Among 2740 individuals initiating IPT, 83 (3.0%) interrupted their treatment (Figure 2): 41 (49.4%) were lost to follow-up, 15 (18.1%) interrupted IPT due to side effects, 9 (10.8%) had drug adherence issues, 2 (2.4%) asked to be taken off therapy for reasons unrelated to drug toxicity (citing a ‘daily pill burden’ in both cases), and 9 (10.8%) developed TB while on IPT. Seven (8.5%) of those who interrupted treatment were transferred to a different facility during IPT, although these patients were given enough INH medication to complete their 6- or 9-month course of IPT treatment at the new site and may therefore still have completed treatment.
FIGURE 2.
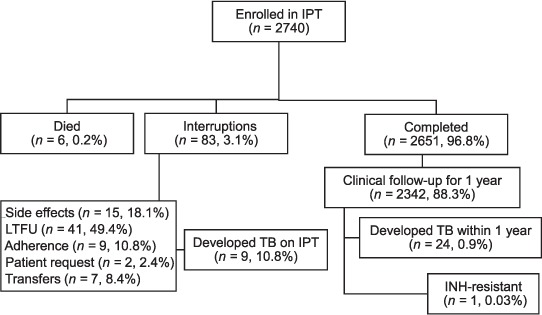
IPT among HIV-positive patients at an MSF clinic in southern Myanmar. IPT = isoniazid preventive therapy; LTFU = loss to follow-up; TB = tuberculosis; INH = isoniazid; IPT = INH preventive therapy; HIV = human immunodeficiency virus; MSF = Médecins Sans Frontières.
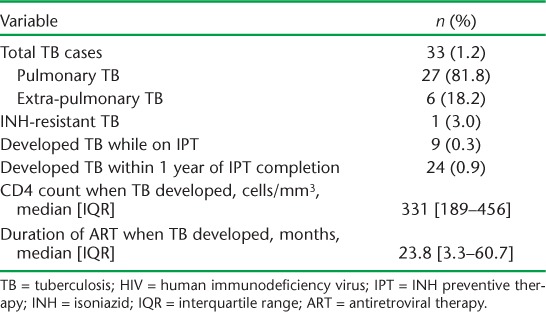
Of those initiating IPT, 2651 (96.5%) completed the 6- or 9-month course of prophylaxis, 2342 (88.5%) of whom had 1 year of clinical follow-up available for review after IPT completion. Among these patients, 33 (1.4%) developed TB: 9 (0.3%) during IPT and 24 (0.9%) within 1 year of completion (Table). Six (18.2%) of these TB cases were extra-pulmonary (4 TB lymphadenopathies, 1 pleural effusion and 1 case of ovarian TB). In the 3 years of IPT roll-out in which all patients who developed TB underwent both culture and DST, one case of INH resistance was detected in a patient who had taken IPT. Six (0.2%) patients who were enrolled in IPT died during the study period; four of these deaths were not HIV- or IPT-related, including deaths related to complications of diabetes and hypertension, gastric erosion, severe diarrhoea and suspected lactic acidosis in a patient taking stavudine; two were due to unspecified HIV-related conditions, although MSF clinicians report unconfirmed suspicions of side effects from prolonged exposure to stavudine in both cases.
TABLE.
TB cases in cohort of HIV-positive patients enrolled for IPT (n = 2740)
DISCUSSION
This is the first study to look at the feasibility of IPT scale-up in a routine care setting in Myanmar, where national HIV guidelines recently changed to formally recommend IPT prophylaxis as part of HIV care. We show that when drug supplies are consistent, IPT can be implemented safely and effectively, with a low rate of discontinuation due to side effects and high levels of completion.
In less than 5 years, despite inconsistencies in supply and little history of IPT provision in Myanmar, >80% of MSF's HIV cohort was able to initiate IPT, with >90% able to complete therapy, a rate that is nearly as high as or higher than in most neighbouring countries across South and South-East Asia.13,14,16,19,20 Myanmar thus compares well across indicators. Only one case of INH resistance was documented among 2740 patients initiating IPT. Although DST could not be performed in those who developed extra-pulmonary TB in this cohort, all such patients were subsequently treated successfully at the MSF clinic, making resistance in this group highly unlikely. The single case of INH resistance may have been the result of infection with an INH-resistant strain as opposed to acquired resistance in response to IPT, particularly in the light of a 2005 study showing that 22% of a Myanmar TB cohort had INH-resistant disease.19 The nine cases who developed TB during IPT were likely cases of activated LTBI, missed diagnosis before IPT initiation or TB reinfection.
These results from Myanmar are encouraging signals, and are reaffirmations of the importance of screening PLHIV for TB at each clinical visit (even those on IPT) and providing strong counselling services. These findings should encourage other HIV care providers in Myanmar to integrate IPT into their treatment protocols more aggressively, in line with NAP and WHO guidelines.
Less than 1% of individuals who initiated IPT interrupted their treatment due to drug adherence problems, side effects, or upon their own request because of treatment fatigue and reference to a ‘daily pill burden’. This last problem may be related to the fact that, for much of the study period, only paediatric INH formulations were available due to supply issues, and some adult patients therefore had a higher daily pill count (three 100 mg INH tablets rather than a 300 mg single-dose pill). Overall, however, the low rate of drug adherence challenges seen in this context is reassuring and may have contributed to low LTFU rates and a reduced risk of INH resistance.14 Strong adherence may also have been due to MSF's flexible medication refill policy, where up to 3 months of IPT was provided upon request, making the patient's physical distance from the clinic less of a barrier to care; 15% of the IPT cohort lived >3 h from care, and nearly half had highly mobile professions as economic migrants or in the fishing industry. The importance of strong drug adherence counselling cannot be overstated. Because this was a new prophylaxis approach that had never before been used, extra care was taken to make sure that patients were well informed of its benefits and potential side effects, especially patients who had had previous issues with ART adherence.
There were some limitations to this study. The operational data available were significantly limited in the patient records and registers. We were not able to retrospectively identify reasons for non-inclusion under IPT, and some variables that would have been useful to analyse were unavailable, including demographic information, detailed drug regimen data, or data on OIs or other co-infections. Patients' experience of side effects, and their balancing the risks and benefits of continuing medication in the presence of side effects, may not always have been uniform. Their willingness or ability to tell their doctor about the side effects may have also varied. Detailed information on drug side effects was unavailable in the electronic medical record, except for binary data about treatment discontinuation due to drug toxicity.
The strengths of this study are that all data came from a routine HIV care setting outside of a capital city or other large urban centre with large proportions of migrant workers. The study also had a relatively large sample size compared to other IPT studies conducted in Asia.19
CONCLUSION
The MSF experience shows that high IPT coverage, adherence and completion rates are achievable when supplies are available, and that scaling up IPT at HIV care sites is possible in these and similar contexts. The relatively rapid scale-up by clinical actors after formal NAP/MoH endorsement is encouraging for those countries that are yet to recommend IPT as part of HIV care.
Acknowledgments
The authors thank the staff of Médecins Sans Frontières, Yangon, Myanmar, particularly N T Antabak and E Bermudez, for their careful review and support.
Footnotes
Conflicts of interest: none declared.
References
- 1. World Health Organization Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS. . UNAIDS report on the global AIDS epidemic. Geneva, Switzerland: UNAIDS, 2015. [Google Scholar]
- 3. Akolo C, Adetifa I, Shepperd S, Volmink J.. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; 1: CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dowdy D W, Golub J E, Saraceni V, . et al. Impact of isoniazid preventive therapy for HIV-infected adults in Rio de Janeiro, Brazil: an epidemiologic model. J AIDS 2014; 66: 552– 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pho M T, Soumya S, Nagalingeswaran K, . et al. The cost-effectiveness of tuberculosis preventive therapy for HIV-infected individuals in southern India: a trial based analysis. PLOS ONE 2012; 7: e36001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Azadi M, Bishai D M, Dowdy D W, . et al. Cost-effectiveness of tuberculosis screening and isoniazid treatment in the TB/HIV in Rio de Janeiro (THRio) Study. Int J Tuberc Lung Dis 2014; 18: 1443– 1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization, HIV/AIDS Department. . WHO three I's meeting: intensified case finding (ICF), isoniazid preventive therapy (IPT), and TB infection control (IC) for people living with HIV. Geneva, Switzerland: WHO, 2008. [Google Scholar]
- 8. Myanmar Ministry of Health. . National AIDS/STD Programme. National Tuberculosis Program. Guidelines for the clinical management of TB/HIV in Myanmar. Yangon, Myanmar: MoH, 2014. [Google Scholar]
- 9. Gupta S, Granich R, Date A, . et al. Review of policy and status of implementation of HIV/TB activities in 23 high burden countries. Int J Tuberc Lung Dis 2014; 18: 1149– 1158. [DOI] [PubMed] [Google Scholar]
- 10. Date A, Vitoria M, Reuben G, Mazuwa B, Mayada Y F, Gilks C.. Implementation of co-trimoxozole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ 2010; 88: 253– 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hiransuthikul N, Hiranshikul P, Nelson K E, . et al. Physician adherence to isoniazid preventive therapy guidelines for HIV-infected patients in Thailand. Southeast Asian J Trop Med Pub Health 2005; 36: 1208– 1215. [PubMed] [Google Scholar]
- 12. Akolo C, Bada F, Okpokoro E, . et al. Debunking the myths perpetuating low implementation of isoniazid preventive therapy amongst human immunodeficiency virus-infected patients. World J Virol 2015; 4: 105– 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piyaworawong S, Yanai H, Nedsuwan S, . et al. Tuberculosis preventive therapy as part of a care package for people living with HIV in a district of Thailand. AIDS 2001; 15: 1739– 1741. [DOI] [PubMed] [Google Scholar]
- 14. van Griensven J, Choun K, Chim B, Thai S, Lorent N, Lynen L.. Implementation of isoniazid preventive therapy in an HIV clinic in Cambodia: high rates of discontinuation when combined with antiretroviral therapy. Trop Med Intl Health 2015; 20: 1823– 1831. [DOI] [PubMed] [Google Scholar]
- 15. Namuwenge P M, Mukonzo J K, Kiwanuka N, . et al. Loss to follow-up from isoniazid preventive therapy among adults attending HIV voluntary counselling and testing sites in Uganda. Trans R Soc Trop Med Hyg 2012; 106: 84– 89. [DOI] [PubMed] [Google Scholar]
- 16. Trinh T T, Han D T, BLoss E, . et al. Implementation and evaluation of an isoniazid preventive therapy pilot program among HIV-infected patients in Vietnam, 2008–2010. Trans R Soc Trop Med Hyg 2015; 109: 653– 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swaminarthan S, Menon P A, Gopalan N, . et al. Efficacy of a six-month versus a 36-month regimen for prevention of tuberculosis in HIV-infected persons in India: a randomized control trial. PLOS ONE 2012; 7: e47400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Getahun H, Granich R, Sculier D, . et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS 2010; 24 Suppl 5: S57– S65. [DOI] [PubMed] [Google Scholar]
- 19. Phyu S, Lwin T, Ti T, . et al. Drug-resistant tuberculosis in Yangon, Myanmar. Scand J Infect Dis 2005; 37: 846– 851. [DOI] [PubMed] [Google Scholar]
- 20. Hall C, Sukijthemapan P, dos Santos R, . et al. Challenges to delivery of isoniazid preventive therapy in a cohort of children exposed to tuberculosis in Timor-Leste. Trop Med Int Health 2015; 20: 730– 736. [DOI] [PubMed] [Google Scholar]


