Abstract
Background:
Visfatin, also known as nicotinamide phosphoribosyltransferase, has been suggested as a pro-inflammatory and immunomodulating marker for periodontitis. The aim of this study was an immunohistochemical analysis of visfatin in gingival tissues of patients with chronic periodontitis and aggressive periodontitis.
Materials and Methods:
In this cross-sectional study based on clinical evaluation and inclusion and exclusion criteria. Twenty patients with generalized chronic periodontitis, 13 patients with generalized aggressive periodontitis, and 20 periodontally healthy individuals enrolled. Gingival tissue samples were obtained during periodontal flap surgery and crown lengthening surgery in periodontal patients and healthy group, respectively. Tissue samples were transferred to a pathology laboratory to determine the degree of inflammatory infiltration by hematoxylin and eosin staining and the level of visfatin expression by immunohistochemistry. The data were analyzed using SPSS version 20 statistical software and paired t-test, Mann–Whitney test, and Spearman rank correlation coefficient. P < 0.05 was considered statistically significant.
Results:
Inflammation grading and visfatin expression were significantly higher in periodontally diseased gingiva compared to the control group (P < 0.05). However, no significant difference was found between two diseased groups. The relationship between inflammation grading and visfatin expression in aggressive periodontitis group was statistically significant, positive and relatively strong (P = 0.025, r = 0.617). However, no significant relationship has been found between visfatin expression and inflammation grading in the chronic periodontitits and control groups (P > 0.05).
Conclusion:
Visfatin expression was increased in gingival tissues of chronic periodontitis and aggressive periodontitis patients. Hence, visfatin may have a role in the etiopathogenesis of chronic periodontitis and aggressive periodontitis.
Key Words: Aggressive periodontitis, chronic periodontitis, immunohistochemistry, visfatin
INTRODUCTION
Periodontitis is an infectious inflammatory disease which is a pathological sign of the host response against periodontal pathogens and results in the destruction of tooth-supporting tissues, progressive attachment loss, and bone loss.[1]
Chronic periodontitis and aggressive periodontitis are the most significant forms of the all periodontal diseases. In contrast to chronic periodontitis, the quantity of plaque in the aggressive periodontitis may not be consistent with the level of periodontal destruction which may be due to inappropriate robust host response to rather little amounts of deposits.[2]
Although there are significant data about regarding the inflammatory and immune processes involved in periodontitis, the immunopathological differences between chronic periodontitis and aggressive periodontitis are not clearly understood.[2]
The periodontal pathogens and their components can evoke the inflammatory host response and the production of inflammatory mediators in periodontal tissues.[3] Evaluation of the similarities and differences between the pathogenic mechanism of aggressive periodontitis and chronic periodontitis is always an attractive field of research that is in need of further studies.
Visfatin, also known as nicotinamide phosphoribosyltransferase (NAMPT) and pre-B-cell colony-enhancing factor, is produced mainly by macrophages and adipocytes in adipose tissue.[4]
Moreover, visfatin has been found in other cell types such as lymphocytes, dendritic cells, and monocytes of peripheral blood and could induce the production of inflammatory mediators such as interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-alpha.[5,6]
Association of visfatin with a variety of inflammatory diseases, atherosclerosis, diabetes, obesity, metabolic syndromes, and cancers has been reported.[7,8,9] Moreover, several meta-analysis studies have indicated that periodontitis is associated with obesity, Type II diabetes, metabolic syndrome, cardiovascular diseases, bowel inflammatory disease, and rheumatoid arthritis.[10,11,12] Recently, it has been reported that visfatin level increases in serum, gingival crevicular fluid (GCF), saliva, and periodontal tissues in patients with chronic periodontitis and may have a role in pathogenesis of the disease.[13,14,15,16,17] Consequently, it has been suggested that visfatin could be the possible pathomechanistic link between periodontitis and some systemic diseases.[17]
It was also reported that synthesis of visfatin in the periodontal ligament and gingival fibroblast cells could be stimulated by periodontal pathogens (Porphyromonas gingivalis and Fusobacterium nucleatum) and some proinflammatory cytokines (IL-1β).[15,18]
The present study evaluates the visfatin expression and distribution in the gingival tissues of patients with chronic periodontitis and aggressive periodontitis and periodontally healthy individuals.
MATERIALS AND METHODS
Study population
This cross-sectional study was performed from March 2014 to April 2015 at Periodontics Department of Qazvin University of Medical Sciences, Qazvin, Iran. In this study, 20 patients with moderate-to-severe chronic periodontitis, 13 patients with generalized aggressive periodontitis, and 20 individuals with healthy periodontium, who visited in the Periodontics Department at Qazvin University of Medical Sciences, were enrolled.
The protocol was approved by the Ethical Committee of Qazvin University of Medical Sciences (# 28/20/10045).
Inclusion criteria of the study were good general health, a minimum of 18 teeth, and body mass index range 18.5–25. Exclusion criteria included history of alcoholism and smoking, infectious diseases, inflammatory bowel disease, rheumatoid arthritis, granulomatous diseases, hypertension, diabetes, atherosclerosis, organ transplantation, or cancer therapy. In addition, patients with the use of glucocorticoids, antibiotics, or immunosuppressant medication during the past 3 months, pregnancy or lactation and need for antibiotics for infective endocarditis prophylaxis during dental procedures were excluded from the study.
Clinical evaluation
Measurements of the clinical parameters including plaque index,[19] bleeding index (BI),[20] probing pocket depth (PPD), and clinical attachment level (CAL) were performed at six points around all teeth using UNC probe (Hu-Friedy, USA) by a periodontist. Disease groups had the diagnosis of generalized moderate-to-severe chronic periodontitis and generalized aggressive periodontitis established based on the classification of the American Academy of Periodontology and the following criteria:[21]
Generalized moderate-to-severe chronic periodontitis: age over 35 years, more than 30% of sites with CAL ≥3 mm, and PPD ≥5 mm with bleeding on probing (BOP)
Generalized aggressive periodontitis: aged between 18 and 35 years, at least 6 permanent incisors and first molars with at least one site of CAL ≥5 mm, and PPD ≥5 mm with BOP. At least three other permanent teeth had PPD ≥5 mm and CAL ≥5 mm in at least one site
Periodontally healthy individuals as control group had BI ≤1, with no evidence of radiographic bone loss, attachment loss, and PPD >3 mm.
Tissue collection
After selecting the subjects of the diseased and control groups, informed consents were obtained. Then, nonsurgical periodontal therapy was performed for diseased groups and control group (if needed). One month after completion of nonsurgical therapy, clinical parameters were measured again at six sites on each tooth.
Gingival tissue samples with approximate thickness of 2–3 mm and height of 1.5 mm were obtained during periodontal flap surgery and crown lengthening surgery in periodontal patients and healthy group, respectively. Diseased tissue samples were collected from sites with a probing depth of 5 mm or more, attachment loss of at least 3 mm, and presence of BOP. In the other hand, the healthy ones obtained from the sites with minimum pocket depth and the absence of BOP. In all groups, periodontal tissues were excised by internal bevel and sulcular incisions using 15c blade.
Histological evaluation
First, the tissue samples were fixed in 10% formalin, dehydrated in graded alcohol, and washed in xylene. Then, the samples were embedded in paraffin and cut into 3 μm sections. For histological evaluation, the sections were stained with hematoxyline and eosin (H and E) and examined under light microscope (Olympus BX41TF, Tokyo, Japan) in 10 field by a pathologist in a masked manner. The magnification ×400 was used. The score of inflammation grading was determined as follows: 1, <30 inflammatory cells; 2, 30–50 inflammatory cells; and 3, >50 inflammatory cells.[22] Unstained sections were used for immunohistochemical assessments.
Immunohistochemistry
In the standard envision immunohistochemical staining, sections were mounted on poly-L-lysine-coated slides. After deparaffinization and rehydration, the sections were incubated in 0.01M citrate buffer in a microwave oven for antigen retrieval. The slides were then washed in phosphate-buffered saline (PBS) and incubated in 0.5% H2O2 in methanol to block endogenous peroxidase activity. Visfatin primary antibody; anti-visfatin [EPR3392] ab109210 (Abcam, Cambridge, UK) was applied for 1 h at room temperature. After rinsing in PBS for 5 min, the sections were incubated with secondary antibody to enhance the sensitivity of the procedure; Goat Anti-Rabbit IgG H & L (HRP) ab6721 (Abcam, Cambridge, UK) for 30 min at room temperature.
After rinsing with PBS, the immunoreactivity was visualized by a diaminobenzidine hydrochloride (K5007, Dako REAL ™ Substrate Buffer and Dako REAL ™ DAB + Chromogen, Glostrup, Denmark) as the chromogen for 5 min. Sections were finally counterstained with hematoxylin, cleared, and mounted with PV mount. Negative controls consisted of PBS instead of primary antibody and samples of positive controls were human kidney tissue.
Then, the tissue sections were examined under the light microscope in 10 randomly selected high power (×400) fields by a pathologist who had no prior knowledge of patient's clinical status.
Visfatin immunohistochemical staining was scored based on the percentage and the intensity of positively cytoplasmic stained cells. The five score categories for positive staining percentage were as follows: 0, no positive cells; 1, 25% or fewer positive cells; 2, 26% to 50% positive cells; 3, 51% to 75% positive cells; and 4, 76% or more positive cells.[23] Scoring of intensity of staining was as follows: 0, no intensity; 1, weak intensity; 2, moderate intensity; and 3, strong intensity. Visfatin expression was determined by adding the positive staining percentage score and intensity score.[23]
Statistical analysis
Statistical analysis was performed using statistical software, SPSS version 20) IBM, Armonk, NY, United States of America). Clinical variables and visfatin expression were compared between groups using paired t-test. Inflammation grading was compared between groups using Mann–Whitney test.
The relationship between the visfatin expression and inflammation grading was analyzed using Spearman rank correlation coefficient. P < 0.05 was considered statistically significant.
RESULTS
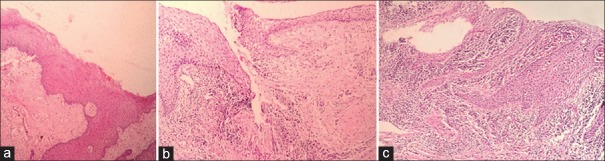
A total of 53 participants (34 females and 19 males) were enrolled in this study. They were twenty healthy individuals (12 females and 8 males/mean age: 33.5) and twenty patients with chronic periodontitis (14 females and 6 males/mean age: 42.58) and 13 aggressive periodontitis patients (8 females and 5 males/mean age: 29.3). Demographic and clinical characteristics of the groups are shown in Table 1. As expected, all periodontal indices were significantly higher in the periodontitis groups than in the healthy individuals (P< 0.05). However, no significant differences have been found between the periodontal indices of two test groups (P > 0.05). The inflammation grading and visfatin expression of gingival tissues in control and tests groups are indicated in Table 2. As analyzed by hematoxylin and eosin staining, healthy gingiva showed parakeratinized stratified squamous epithelium with rete pegs and connective tissue papillae. The connective tissue was fibrous with some fibroblasts and only a few inflammatory cells [Figure 1a]. In chronic and aggressive periodontitis, histologic evaluation exhibited an epithelium lining the pockets that vary in thickness. In the connective tissue, high-grade inflammatory cells were present mainly consist of lymphocytes and plasma cells in varying proportions [Figure 1b and c]. Inflammation grading was significantly higher in diseased tissues than the controls (P< 0.05) and no significant difference was found between chronic periodontitis and aggressive periodontitis groups (P > 0.05) [Table 2].
Table 1.
Clinical characteristics of the gingival biopsy sites
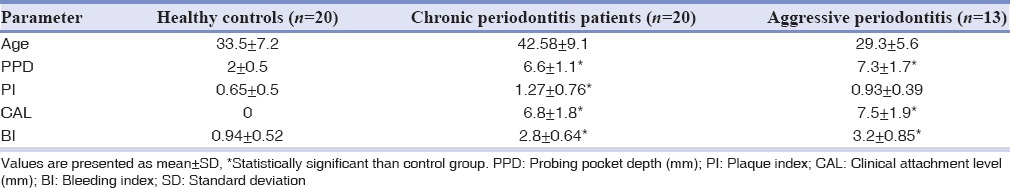
Table 2.
Inflammation grading and visfatin expression in the periodontally healthy individuals, chronic periodontitis and aggressive periodontitis patients
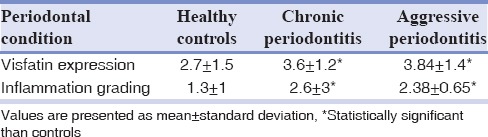
Figure 1.
Hematoxylin and Eosin staining of gingival tissues. (a) Healthy tissue, (b) chronic periodontitis tissue, (c) aggressive periodontitis tissue, (×100).
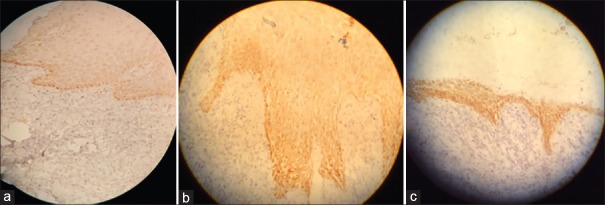
By immunohistochemistry (IHC), visfatin was mainly present in the epithelial layers in all three groups. In healthy gingiva, visfatin was mainly localized in the basal and parabasal layers of the epithelium and with low intensity in other layers of epithelium. Interestingly, no staining was found in the subepithelial connective tissue [Figure 2a]. More expression of visfatin with high intensity was observed in aggressive periodontitis and chronic periodontitis, compared with the healthy group [Figure 2b and c]. In these two lesions, visfatin was diffusely distributed in all layers of the epithelium and also observed in the subepithelial connective tissue, where staining was found in the cytoplasm of fibroblasts and endothelial cells. Visfatin expression was significantly higher in chronic periodontitis and aggressive periodontitis groups than the healthy group (P< 0.05), while no significant difference was observed between the diseased groups (P > 0.05) [Table 2].
Figure 2.
Immunohistochemical expression of visfatin in the gingival epithelium and connective tissue of (a) healthy gingiva, (b) chronic periodontitis tissue, and (c) aggressive periodontitis tissue, (×100).
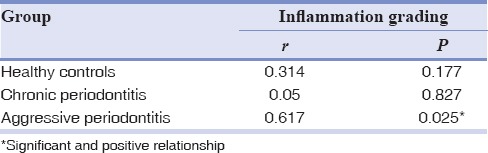
In contrast to healthy and chronic periodontitis groups, there was a significant and positive relationship between inflammation grading and visfatin expression in aggressive periodontitis tissue samples (P = 0.025, r = 0.617) [Table 3].
Table 3.
Correlation of visfatin expression with inflammation grading
DISCUSSION
Due to the variety of inflammatory and immunological functions of visfatin, recently many researches have been performed on the relationship between this cytokine and periodontitis. It has been shown that visfatin can be the diagnostic and therapeutic target for periodontal diseases.[13,14,24]
To our knowledge, no study has been performed to evaluate the relationship between visfatin and aggressive periodontitis in fields of pathogenesis, diagnosis, and therapy.
In this study, we examined immunohistochemical expression of visfatin in gingival tissues of subjects with chronic periodontitis, aggressive periodontitis, and healthy periodontium. In all three groups, visfatin has been found mainly in epithelial layer and to a lesser extent in connective tissue. However, visfatin levels were significantly higher in diseased tissues compared to healthy ones. In addition, inflammation grading was significantly higher in diseased gingival tissues than periodontally healthy ones.
Recently, increased visfatin levels have been found in serum, GCF, saliva, and gingival tissues of patients with chronic periodontitis in comparison to periodontally healthy subjects.[13,14,15]
Pradeep et al. reported that serum levels of visfatin were elevated in patients with chronic periodontitis. Furthermore, the levels of visfatin in GCF were increased significantly in inflamed sites that are suggestive of locally production of visfatin in the periodontium.[13]
Nokhbehsaim et al.[16] demonstrated that visfatin stimulates the production of C-C motif chemokine ligand 2 and matrix metalloproteinase-1 in the periodontal ligament cells and thus can lead to inflammation of periodontium and destruction of connective tissue.[16] It was also revealed that production of visfatin in the periodontal ligament cells could be induced by periodontal pathogens, P. gingivalis and F. nucleatum, and some pro-inflammatory cytokines, IL-1β. Hence, the microbial and inflammatory signals can use this adipocytokine for its destructive effects on periodontium.[18] In addition, visfatin can result in the production of pro-inflammatory and matrix-destructing cytokines, and therefore, interfere with the regenerative capacity of periodontal ligament cells.[25]
Compared to the periodontal ligament, gingival tissue is more exposed to periodontal pathogens. The gingival tissue has several cell types such as fibroblasts, epithelial, endothelial, and inflammatory cells and each of them can be the possible source of visfatin. In the present study, similar to the results of the study performed by Damanaki et al.,[15] visfatin was localized mainly in epithelium layers of healthy gingiva. Similarly, visfatin was observed in epithelial cells, fibroblast, and endothelial cells of inflamed gingival connective tissue.
In contrast, in a number of diseased tissue samples, no immunostaining was found in subepithelial connective tissue, whereas, in Damanaki et al. study,[15] in all samples, visfatin was present in connective tissue layers in addition to epithelium layers. This difference may be due to differences in methodology and laboratory kits that have been used. However, in Damanaki et al. study, it has not been explained specifically how to obtain the diseased gingival tissue. As a result, in this case, there is no possibility of comparison of our study with this research.
The study performed by Damanaki et al.,[15] the only research relatively similar to the present study, reported only the presence or absence of visfatin in the layers of the epithelium and sublayer connective tissue in gingival sulcus of the normal periodontium and the periodontal pocket wall. However, in our study, the extent of expression of visfatin in gingival tissues was determined based on the intensity and percentage of stained cells.
In the present study, in contrast to chronic periodontitis, a significant correlation has been found between the grade of inflammation and the level of visfatin expression in gingival tissues of aggressive periodontitis patients. It seems that microbial signals out of the epithelium layers have a significant role in the production of visfatin in gingival tissues of chronic periodontitis patients, whereas visfatin production in aggressive periodontitis is more affected by factors related to host immune responses. Gingival epithelium, in addition to be a physical barrier against bacterial infection and mechanical stimulus, serves an important role in the regulation of innate and acquired immune responses. Endogenous and bacterial mediators induce the production of pro-inflammatory cytokines and several chemokines by gingival epithelial cells through toll-like receptors and special receptors.[26] The absence of relationship between inflammation grading and visfatin expression in chronic periodontitis may be due to the direct effect of bacteria and their byproducts on epithelial cells.
According to the study performed by Eskan et al., the production of pro-inflammatory cytokines in epithelial cells is upregulated by IL-1β, and P. gingivalis increases the production of IL-1β by epithelial cells. This mechanism also can be involved in increasing the production of visfatin in epithelial cells of the diseased tissue.[27] Although this is actinobacillus actinomycetemcomitans is the main pathogenic bacteria of aggressive periodontitis, the prevalence of P. gingivalis is more in some Asian population.[28]
In this study, expression of visfatin in aggressive periodontitis tissue samples was more than the chronic periodontitis samples, but this difference was not statistically significant, which may be due to a small number of samples in aggressive periodontitis group.
However, it was reported that in both chronic periodontitis and aggressive periodontitis, the cytokine profiles are similar in diseased tissues which are accordance with the results of the present study.[29] Until now, it is not possible to establish exact differences in the immunopathology of the two diseases. This may be due to no differences or only because of variations in level of severity or susceptibility except real different immunopathologies.[30]
The limitations of this study were few cases with aggressive periodontitis, expensive IHC kit, and uncooperative patients for the second phase of treatment (surgery).
This study suggested to other that evaluate visfatin expression in patients with chronic periodontitis and systemic condition such as obesity and Type II diabetes.
CONCLUSION
Visfatin expression was increased in gingival tissues of chronic periodontitis and aggressive periodontitis patients. Mainly, epithelial cells, lining the periodontal pocket wall, are capable of producing visfatin. Therefore, visfatin may have a role in the etiopathogenesis of chronic periodontitis and aggressive periodontitis. However, more studies with larger sample size are needed to confirm these results and identify the exact mechanism of visfatin in inflammatory processes associated with periodontitis.
Financial support and sponsorship
This study has been supported by a grant from Qazvin University of Medical Sciences, Qazvin, Iran.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
Acknowledgment
This study has been supported by a grant from Qazvin University of Medical Sciences, Qazvin, Iran.
REFERENCES
- 1.Shojaee M, Fereydooni Golpasha M, Maliji G, Bijani A, Aghajanpour Mir SM, Mousavi Kani SN, et al. C-reactive protein levels in patients with periodontal disease and normal subjects. Int J Mol Cell Med. 2013;2:151–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni C, Kinane DF. Host response in aggressive periodontitis. Periodontol 2000. 2014;65:79–91. doi: 10.1111/prd.12017. [DOI] [PubMed] [Google Scholar]
- 3.Poorsattar Bejeh-Mir A, Parsian H, Akbari Khoram M, Ghasemi N, Bijani A, Khosravi-Samani M, et al. Diagnostic role of salivary and GCF nitrite, nitrate and nitric oxide to distinguish healthy periodontium from gingivitis and periodontitis. Int J Mol Cell Med. 2014;3:138–45. [PMC free article] [PubMed] [Google Scholar]
- 4.Moschen AR, Gerner RR, Tilg H. Pre-B cell colony enhancing factor/NAMPT/visfatin in inflammation and obesity-related disorders. Curr Pharm Des. 2010;16:1913–20. doi: 10.2174/138161210791208947. [DOI] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen AR. Role of adiponectin and PBEF/visfatin as regulators of inflammation: Involvement in obesity-associated diseases. Clin Sci (Lond) 2008;114:275–88. doi: 10.1042/CS20070196. [DOI] [PubMed] [Google Scholar]
- 6.Robati M, Ranjbari A, Ghafourian Boroujerdnia M, Chinipardaz Z. Detection of IL-4, IL-6 and IL-12 serum levels in generalized aggressive periodontitis. Iran J Immunol. 2011;8:170–5. [PubMed] [Google Scholar]
- 7.Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–24. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 8.Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: A meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27:515–27. doi: 10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- 9.Booth A, Magnuson A, Fouts J, Foster M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm Mol Biol Clin Investig. 2015;21:57–74. doi: 10.1515/hmbci-2014-0037. [DOI] [PubMed] [Google Scholar]
- 10.Suvan J, D'Aiuto F, Moles DR, Petrie A, Donos N. Association between overweight/obesity and periodontitis in adults. A systematic review. Obes Rev. 2011;12:e381–404. doi: 10.1111/j.1467-789X.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- 11.Chávarry NG, Vettore MV, Sansone C, Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: A meta-analysis. Oral Health Prev Dent. 2009;7:107–27. [PubMed] [Google Scholar]
- 12.Linden GJ, Herzberg MC. Working group 4 of Joint EFP/AAP Workshop Periodontitis and systemic diseases: A record of discussions of working group 4 of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Clin Periodontol. 2013;40(Suppl 14):S20–3. doi: 10.1111/jcpe.12091. [DOI] [PubMed] [Google Scholar]
- 13.Pradeep AR, Raghavendra NM, Prasad MV, Kathariya R, Patel SP, Sharma A, et al. Gingival crevicular fluid and serum visfatin concentration: Their relationship in periodontal health and disease. J Periodontol. 2011;82:1314–9. doi: 10.1902/jop.2011.100690. [DOI] [PubMed] [Google Scholar]
- 14.Tabari ZA, Azadmehr A, Nohekhan A, Naddafpour N, Ghaedi FB. Salivary visfatin concentrations in patients with chronic periodontitis. J Periodontol. 2014;85:1081–5. doi: 10.1902/jop.2013.130388. [DOI] [PubMed] [Google Scholar]
- 15.Damanaki A, Nokhbehsaim M, Eick S, Götz W, Winter J, Wahl G, et al. Regulation of NAMPT in human gingival fibroblasts and biopsies. Mediators Inflamm. 2014;2014:912821. doi: 10.1155/2014/912821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nokhbehsaim M, Eick S, Nogueira AV, Hoffmann P, Herms S, Fröhlich H, et al. Stimulation of MMP-1 and CCL2 by NAMPT in PDL cells. Mediators Inflamm. 2013;2013:437123. doi: 10.1155/2013/437123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghallab NA, Amr EM, Shaker OG. Expression of leptin and visfatin in gingival tissues of chronic periodontitis with and without type 2 diabetes mellitus: A Study using enzyme-linked immunosorbent assay and real-time polymerase chain reaction. J Periodontol. 2015;86:882–9. doi: 10.1902/jop.2015.140434. [DOI] [PubMed] [Google Scholar]
- 18.Nogueira AV, Nokhbehsaim M, Eick S, Bourauel C, Jäger A, Jepsen S, et al. Regulation of visfatin by microbial and biomechanical signals in PDL cells. Clin Oral Investig. 2014;18:171–8. doi: 10.1007/s00784-013-0935-1. [DOI] [PubMed] [Google Scholar]
- 19.Silness J, Loe H. Periodontal disease in pregnancy. II. correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 20.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35. [PubMed] [Google Scholar]
- 21.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Hasheminia SM, Feizi G, Razavi SM, Feizianfard M, Gutknecht N, Mir M, et al. Acomparative study of three treatment methods of direct pulp capping in canine teeth of cats: A histologic evaluation. Lasers Med Sci. 2010;25:9–15. doi: 10.1007/s10103-008-0584-9. [DOI] [PubMed] [Google Scholar]
- 23.Tian W, Zhu Y, Wang Y, Teng F, Zhang H, Liu G, et al. Visfatin, a potential biomarker and prognostic factor for endometrial cancer. Gynecol Oncol. 2013;129:505–12. doi: 10.1016/j.ygyno.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Tabari ZA, Ghaedi FB, Azadmehr A, Nohekhan A, Tabrizi MA, Ardakani MR, et al. Salivary visfatin concentration in response to non-surgical periodontal therapy. J Clin Diagn Res. 2015;9:ZC05–8. doi: 10.7860/JCDR/2015/11537.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nokhbehsaim M, Keser S, Jäger A, Jepsen S, Deschner J. Regulation of regenerative periodontal healing by NAMPT. Mediators Inflamm. 2013;2013:202530. doi: 10.1155/2013/202530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi N, Okui T, Tabeta K, Yamazaki K. Effect of interleukin-17 on the expression of chemokines in gingival epithelial cells. Eur J Oral Sci. 2011;119:339–44. doi: 10.1111/j.1600-0722.2011.00842.x. [DOI] [PubMed] [Google Scholar]
- 27.Eskan MA, Benakanakere MR, Rose BG, Zhang P, Zhao J, Stathopoulou P, et al. Interleukin-1beta modulates proinflammatory cytokine production in human epithelial cells. Infect Immun. 2008;76:2080–9. doi: 10.1128/IAI.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng X, Zhu L, Xu L, Meng H, Zhang L, Ren X, et al. Distribution of 8 periodontal microorganisms in family members of chinese patients with aggressive periodontitis. Arch Oral Biol. 2015;60:400–7. doi: 10.1016/j.archoralbio.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Salvi GE, Lawrence HP, Offenbacher S, Beck JD. Influence of risk factors on the pathogenesis of periodontitis. Periodontol 2000. 1997;14:173–201. doi: 10.1111/j.1600-0757.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 30.Ford PJ, Gamonal J, Seymour GJ. Immunological differences and similarities between chronic periodontitis and aggressive periodontitis. Periodontol 2000. 2010;53:111–23. doi: 10.1111/j.1600-0757.2010.00349.x. [DOI] [PubMed] [Google Scholar]