Abstract
Background:
An ideal root canal irrigant should be able to enhance the wettability of sealer to dentin walls. The aim of this study was to survey the contact angle between AH 26 sealer and dentin surface irrigated by four herbal essential oils, compared with normal saline and 5.25% sodium hypochlorite (NaOCl).
Materials and Methods:
In this in vitro study, 60 longitudinal dentin slices were obtained from thirty extracted single-rooted human mandibular first premolars. After smear layer removal, the samples were divided into six groups of 10 samples and irrigated for 1 min with the experimental solutions followed by distilled water: G1: Myrtus communis, G2: Cinnamomum zeylanicum, G3: Zataria multiflora (ZM), G4: Cuminum cyminum (CC), G5: normal saline, and G6: NaOCl 5.25%. The contact angle between AH 26 and the samples was measured using Motic Images Plus 2.0 software. Data were analyzed statistically using one-way analysis of variance (P < 0.05 consider significant).
Results:
All the herbal irrigants, except CC, increased the contact angle of AH 26 sealer (P ≤ 0.05). The least value of contact angle was seen in the samples treated with CC, and the maximum value was observed in samples treated with ZM. 5.25% NaOCl showed a slightly increased contact angle compared to normal saline; however, it was not statistically significant (P > 0.05).
Conclusion:
Under the experimental condition of this study, CC was able to increase dentin wettability and therefore may be regarded as a promising irrigant after careful evaluation of other properties of this irrigant.
Key Words: Dentin, epoxy resin AH-26, essential oil, root canal irrigants, wettability
INTRODUCTION
The aim of endodontic treatment is to eliminate or reduce the number of microorganisms and their byproducts and to achieve a three-dimensional seal.[1]
The mechanical and chemical effects of irrigants during irrigation to remove necrotic tissues, microbes, biofilms, and other debris from root canal space are crucial for endodontic success.[2]
Among the solutions used in root canal therapy, sodium hypochlorite (NaOCl) is the most widely used irrigant and appears to fulfill most of the requirements of an ideal root canal irrigant.[3] It is a powerful solvent of pulp remnants and has a marked bactericidal activity.[4] Unpleasant odor and taste, possibility of allergic reaction, cytotoxicity,[5] and negative effects on the mechanical properties, and cutting efficiency of nickel-titanium instruments [6] are some deficits of NaOCl.
Side effects of synthetic drugs have drawn the attention of researchers to use herbal essential oils as irrigation solutions or intracanal medications in recent years. Antibacterial effects of several herbal essential oils such as Zataria multiflora (ZM), Cuminum cyminum (CC), and Myrtus communis (MC) against the endodontic microorganisms have been demonstrated.[7,8,9,10,11,12,13,14] An ideal root canal irrigant should also provide proper contact between sealer and dentin walls. Contact angle shares an inverse relationship with surface free energy, i.e., the lower the contact angle, the higher the wettability and surface-free energy.[15,16]
Surface treatment of dentin might change its structural and chemical characteristics, thus affecting the wettability of various substances to dentin.[15] Previous studies evaluated the contact angle between different root canal sealers and root canal dentin treated with different synthetic solutions,[17,18,19,20,21,22] but there is no study on the effect of herbal irrigants on dentin surfaces. Kaushik et al. evaluated the contact angle between AH Plus and the treated dentin surface. They revealed that the most wettability was seen in the samples treated with QMix followed by 0.1% octenidine hydrochloride and the maximum value of contact angle was observed in samples treated with 3% NaOCl.[22] In another study, de Assis et al. showed that after removal of smear layer and final flush with chlorhexidine, wettability of dentin by AH Plus and Real Seal SE was increased.[23] One of the important features of essential oils and their components is their hydrophobicity, which may affect the dentin surface.[24]
To the best of our knowledge, there is no study in literature to assess the effect of herbal essential oils on contact angle between endodontic sealers and dentin. Therefore, this study was designed to investigate the influence of some plant essential oils on the contact angle between AH 26 sealer and dentin surfaces.
MATERIALS AND METHODS
Thirty extracted single-rooted human mandibular first premolars were used in this study.
This in vitro study was approved by the Ethics Committee of Shiraz University of Medical Sciences (100/40968/95.10.11). Teeth were scaled with ultrasonic (Varios 970, iPiezo engine, NSK, Japan) and washed with distilled water (DW) to remove the calculus and soft tissue debris upon extraction.
Preparation of samples
The sample size was in the range of previous studies.[17,23]
The teeth with a single root canal, no visible root caries, fractures or cracks on examination, no signs of internal or external resorption or calcification, and a fully formed apex were selected. Radiographs were taken for all the samples to confirm above criteria. After decoronation at the cementoenamel junction with low-speed diamond disc (Buehler, Lake Buff, IL, USA) under water coolant, the roots were split longitudinally to yield 60 sections.
Sixty dentin slices were ground smooth through 400–600 grit polishing papers (CarbiMet; Buehler, Lake Bluff, IL, USA) under DW to remove any surface scratches. A diamond disk (Buehler, Lake Buff, IL, USA) was used to achieve 10-mm standard segments. At each step, dentin samples were rinsed and ultrasonicated in purified water for 5 min.
Irrigant preparation
Briefly, the plant specimens of ZM (leaves), MC (leaves), CC (seeds), and Cinnamomum zeylanicum (barks) were collected from the same geographic region of Iran based on the previous studies [14,25,26] and identified by an expert plant taxonomist, according to the morphological description and previously known collected samples in the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran. The essential oil of each plant was obtained by steam distillation using a Clevenger-type apparatus, which was designed in the Department of Pharmacognosy, Faculty of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran, and then diluted by dimethyl sulfoxide to the level of their measured minimum inhibitory concentrations according to the previous studies.[14,25,26]
Irrigation protocol
Each sample was treated with 10 ml of 17% ethylenediaminetetraacetic acid for 1 min followed by a flush of 5.25% NaOCl to remove smear layer and then final washed with 10 ml of DW for 1 min. The roots were then divided into six groups of 10 samples each and then irrigated as indicated in Table 1. The dentin specimens were then placed in an incubator at 37°C to dry for the same period for all groups (15 min) and attached to a glass base.
Table 1.
Irrigation protocol for each group
Contact angle measurement
The AH 26 sealer (Dentsply De Trey GmbH, Konstanz, Germany) was prepared according to the manufacturer's instructions. A 0.5 mL ultrafine syringe was used to place one drop (0.1 ml) of sealer on the dentin surface under standard environmental condition in each sample. Immediately after drop placement, a Stereo Microscope (SMP-200, HP, USA) supplied with Moticam 480 Digital Camera (SP10.0224, Motic Instruments Inc., CA, USA) located perpendicular to dentin surface was used to take pictures from the samples at magnification of ×24. Images of the drop were immediately analyzed using Motic Images Plus 2.0 software (Motic China Group Co., Ltd.) to provide the values of contact angle. Because the measurement of the supplementary angles in most images was easier, the contact angles were calculated by this formula: contact angle = supplementary angle − 180° [Figure 1]. Ten measurements were taken in each group.
Figure 1.
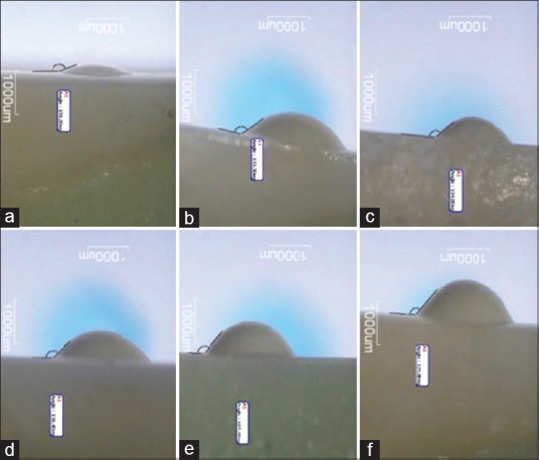
Supplementary angles between AH 26 sealer drop and dentin samples treated with different irrigant solutions. Contact angles were calculated by this formula: Contact angle = supplementary angle – 180° (a) Cuminum cyminum, (b) normal saline, (c) sodium hypochlorite, (d) Cinnamomum zeylanicum, (e) Myrtus communis, (f) Zataria multiflora.
Statistical analysis
The data were analyzed statistically using one-way analysis of variance followed by a post hoc Tukey's test using SPSS 11.0 software (SPSS, Chicago, IL). The significance level was established at 0.05.
RESULTS
The least and the most values of contact angle between AH 26 and the treated dentin surface were seen in the samples treated with CC (30.01 ± 4.82) and ZM (52.8 ± 3.15). The mean and standard deviation values of contact angles between sealer and treated dentin surface are summarized in Table 2.
Table 2.
Mean and standard deviation values of contact angle between AH-26 and treated dentin surface

CC group showed significantly lower contact angle than other groups (P< 0.001). MC and ZM groups showed significantly higher contact angles than normal saline group (P = 0.003, P = 0.0001, respectively). NaOCl increased the contact angle compared with normal saline; however, this increase was not significant (P = 0.713).
DISCUSSION
The purpose of obturation of the prepared root canal space is twofold: (i) to eliminate all avenues of leakage from the oral cavity and the periapical tissues and (ii) to embed any irritants which cannot be fully removed during the cleaning and shaping procedures.[27] Achievement of fluid-tight seal is dependent on the flow and wetting ability of sealers to play their role as binding agents between root canal walls and the main root filling material.[21,28] To have sealer with excellent wettability of root canal, the irrigants should not increase the contact angle between dentin and sealer.[18]
Contact angle measurement is a way to determine the wettability of the substratum. When the contact angle is <90°, the liquid wets the surface; however, when it is >90°, it is said to be nonwetting. A zero contact angle represents complete wetting. Low contact angles cause a better interaction between a solid surface and a liquid.[20]
In the present study, 5.25% NaOCl showed a slightly increased contact angle compared to normal saline, but this increase was not significant. Hu et al.[29] demonstrated that 5.25% NaOCl in 10 min increased the wettability of the dentin surface. This result was not in agreement with the present study. This can be attributed to the different methodology employed and time intervals used in these studies as they did not remove the smear layer before irrigation with NaOCl and also 10 min of irrigation used in their study. Smear layer with different shape and thickness can occlude the dentinal tubules and affect the wettability.[30]
In Dogan Buzoglu et al.'s study, NaOCl alone decreased the wettability of dentin surfaces which was in agreement with the result of our study.[17] A possible explanation for this might be the ability of NaOCl in dissolving the organic component of dentin.[31]
The results of the present investigation demonstrated that all the herbal irrigants except CC increased the contact angle of AH 26 sealer when compared to the other tested irrigants and normal saline as control group. Hydrophobic features of these herbal essential oils help them to destroy the lipids of the bacterial cell wall and the mitochondria.[24] This hydrophobicity may also explain the reduction of the wettability or spreading of AH 26 sealer on root canal dentin. It has also been demonstrated that the aldehyde and ketone groups of the essential oils may explain their antimicrobial activity.[32] The contradictory result of CC may be due to the carbonyl groups. These chemical groups can provide a great diversity of possible modification for surfaces and prepare switchable hydrophobic/hydrophilic surfaces.[33]
In the present study, a standard volume (0.1 ml) of AH 26 under standard environmental condition was used because the volume of sealer and temperature and humidity of environment may affect the contact angle.[34,35,36] Comparative evaluation of contact angle was done after removal of smear layer to compare standard smear layer-free dentin samples because growing evidence suggests this procedure in routine root canal therapy.[37,38] The heterogeneous nature of dentin, its ability to react with irrigants, and the existence of dentinal tubules are factors which make dentin not comparable with ideal surfaces used in contact angle measurement experiments. In addition, the prepared surfaces are not completely the same as the dentin surfaces found in clinical situation. According to the Wenzel equation, the roughness of surface can increase the wettability,[23] so polishing of dentin samples was done to standardize the dentin surface roughness as a factor that could affect the results.
CC from the Apiaceae family also known as cumin or Jeera (in Persian zeera) is a therapeutic herb found in the Mediterranean region. Its fruit is yellow to gray and has long oval shape with nine projections.[39] It has established antioxidant,[40] antibacterial,[8,9] antifungal,[41] and analgesic activities.[42] Considering the strong antimicrobial efficiency of this essential oil against the Enterococcus faecalis and its biocompatibility for L929 mouse fibroblasts [25] and the result of the present study which demonstrated the low level of hydrophobicity of this plant essential oil, it is worth performing further evaluations on animal or human models before a conclusive comment on its application in root canal treatment.
CONCLUSION
Under the experimental condition of this study, the essential oil of CC was able to decrease the contact angle between AH 26 sealer and dentin surface. Hence, this essential oil as an irrigant might result in better binding of sealers into dentinal walls. However, further in vitro and in vivo studies should be performed before its use as an endodontic irrigant.
Financial support and sponsorship
The study was funded through the Vice-Chancellery of Shiraz University of Medical Sciences (Grant No. 100/40968/95.10.11).
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
Acknowledgment
We thank the Vice-Chancellery of Shiraz University of Medical Sciences for supporting this research (Grant No. 100/40968). This manuscript is based on a thesis by Dr. Hale Bahrami. We would also like to thank Dr. Mehrdad Vosooghi of the Research Development Center for the statistical analysis.
REFERENCES
- 1.Schilder H. Cleaning and shaping the root canal. Dent Clin North Am. 1974;18:269–96. [PubMed] [Google Scholar]
- 2.Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am. 2010;54:291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Garg P, Tyagi SP, Sinha DJ, Singh UP, Malik V, Maccune ER. Comparison of antimicrobial efficacy of propolis, Morinda citrifolia, Azadirachta indica, Triphala, green tea polyphenols and 5.25% sodium hypochlorite against Enterococcus fecalis biofilm. Saudi Endod J. 2014;4:122. [Google Scholar]
- 5.Becker TD, Woollard GW. Endodontic irrigation. Gen Dent. 2001;49:272–6. [PubMed] [Google Scholar]
- 6.Busslinger A, Sener B, Barbakow F. Effects of sodium hypochlorite on nickel-titanium lightspeed instruments. Int Endod J. 1998;31:290–4. doi: 10.1046/j.1365-2591.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 7.Abbaszadegan A, Sahebi S, Gholami A, Delroba A, Kiani A, Iraji A, et al. Time-dependent antibacterial effects of Aloe vera and Zataria multiflora plant essential oils compared to calcium hydroxide in teeth infected with Enterococcus faecalis. J Investig Clin Dent. 2016;7:93–101. doi: 10.1111/jicd.12123. [DOI] [PubMed] [Google Scholar]
- 8.Hajlaoui H, Mighri H, Noumi E, Snoussi M, Trabelsi N, Ksouri R, et al. Chemical composition and biological activities of Tunisian Cuminum cyminum L. essential oil: A high effectiveness against Vibrio spp. strains. 2010;48:2186–92. doi: 10.1016/j.fct.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 9.Wanner J, Bail S, Jirovetz L, Buchbauer G, Schmidt E, Gochev V, et al. Chemical composition and antimicrobial activity of cumin oil (Cuminum cyminum, Apiaceae) Nat Prod Commun. 2010;5:1355–8. [PubMed] [Google Scholar]
- 10.Zomorodian K, Moein M, Lori ZG, Ghasemi Y, Rahimi MJ, Bandegani A, et al. Chemical composition and antimicrobial activities of the essential oil from Myrtus communis leaves. J Essent Oil Bearing Plants. 2013;16:76–84. [Google Scholar]
- 11.Mohammadi R, Mir HE, Shadzi S, Moatar F. Antifungal activity of Myrtus communis L esssent oil against clin isolates of Aspergillus. JOURNAL OF ISFAHAN MEDICAL SCHOOL (I.U.M.S) 2008;26:105, 11. [Google Scholar]
- 12.Yadegarinia D, Gachkar L, Rezaei MB, Taghizadeh M, Astaneh SA, Rasooli I, et al. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L essential oils. Phytochemistry. 2006;67:1249–55. doi: 10.1016/j.phytochem.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Rasooli I, Moosavi M, Rezaee M, Jaimand K. Susceptibility of microorganisms to Myrtus communis L.essential oil and its chemical composition. J Agric Sci Technol. 2010;4:127–33. [Google Scholar]
- 14.Nabavizadeh M, Abbaszadegan A, Gholami A, Sheikhiani R, Shokouhi M, Shams MS, et al. Chemical constituent and antimicrobial effect of essential oil from Myrtus communis leaves on microorganisms involved in persistent endodontic infection compared to two common endodontic irrigants: An in vitro study. J Conserv Dent. 2014;17:449–53. doi: 10.4103/0972-0707.139836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Omari WM, Mitchell CA, Cunningham JL. Surface roughness and wettability of enamel and dentine surfaces prepared with different dental burs. J Oral Rehabil. 2001;28:645–50. doi: 10.1046/j.1365-2842.2001.00722.x. [DOI] [PubMed] [Google Scholar]
- 16.Pécora JD, Guimarães LF, Savioli RN. Surface tension of several drugs used in endodontics. Braz Dent J. 1991;2:123–7. [PubMed] [Google Scholar]
- 17.Dogan Buzoglu H, Calt S, Gümüsderelioglu M. Evaluation of the surface free energy on root canal dentine walls treated with chelating agents and NaOCl. Int Endod J. 2007;40:18–24. doi: 10.1111/j.1365-2591.2006.01169.x. [DOI] [PubMed] [Google Scholar]
- 18.Attal JP, Asmussen E, Degrange M. Effects of surface treatment on the free surface energy of dentin. Dent Mater. 1994;10:259–64. doi: 10.1016/0109-5641(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 19.Tani C, Manabe A, Itoh K, Hisamitsu H, Wakumoto S. Contact angle of dentin bonding agents on the dentin surface. Dent Mater J. 1996;15:39–44. doi: 10.4012/dmj.15.39. [DOI] [PubMed] [Google Scholar]
- 20.Kontakiotis EG, Tzanetakis GN, Loizides AL. A comparative study of contact angles of four different root canal sealers. J Endod. 2007;33:299–302. doi: 10.1016/j.joen.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Prado M, de Assis DF, Gomes BP, Simão RA. Effect of disinfectant solutions on the surface free energy and wettability of filling material. J Endod. 2011;37:980–2. doi: 10.1016/j.joen.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Kaushik M, Sheoran K, Reddy P, Narwal P. Comparison of the effect of three different irrigants on the contact angle of an epoxy resin sealer with intraradicular dentin. Saudi Endod J. 2015;5:166. [Google Scholar]
- 23.de Assis DF, Prado Md, Simão RA. Evaluation of the interaction between endodontic sealers and dentin treated with different irrigant solutions. J Endod. 2011;37:1550–2. doi: 10.1016/j.joen.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbaszadegan A, Gholami A, Ghahramani Y, Ghareghan R, Ghareghan M, Kazemi A, et al. Antimicrobial and cytotoxic activity of Cuminum cyminum as an intracanal medicament compared to chlorhexidine gel. Iran Endod J. 2016;11:44–50. doi: 10.7508/iej.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbaszadegan A, Dadolahi S, Gholami A, Moein MR, Hamedani S, Ghasemi Y, et al. Antimicrobial and cytotoxic activity of Cinnamomum zeylanicum, calcium hydroxide, and triple antibiotic paste as root canal dressing materials. J Contemp Dent Pract. 2016;17:105–13. doi: 10.5005/jp-journals-10024-1811. [DOI] [PubMed] [Google Scholar]
- 27.Gutmann JL. Pathways of the Pulp. 7th ed. St. Louis, MO, USA: Mosby; 1998. Obturation of the cleaned and shaped root canal system; pp. 258–361. [Google Scholar]
- 28.Extrand CW. Contact angles and their hysteresis as a measure of liquid-solid adhesion. Langmuir. 2004;20:4017–21. doi: 10.1021/la0354988. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Ling J, Gao Y. Effects of irrigation solutions on dentin wettability and roughness. J Endod. 2010;36:1064–7. doi: 10.1016/j.joen.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Pashley DH. The effects of acid etching on the pulpodentin complex. Oper Dent. 1992;17:229–42. [PubMed] [Google Scholar]
- 31.Driscoll CO, Dowker SE, Anderson P, Wilson RM, Gulabivala K. Effects of sodium hypochlorite solution on root dentine composition. J Mater Sci Mater Med. 2002;13:219–23. doi: 10.1023/a:1013894432622. [DOI] [PubMed] [Google Scholar]
- 32.Arora DS, Kaur J. Antimicrobial activity of spices. Int J Antimicrob Agents. 1999;12:257–62. doi: 10.1016/s0924-8579(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 33.Godeau G, Darmanin T, Guittard F. Switchable surfaces from highly hydrophobic to highly hydrophilic using covalent imine bonds. J Appl Polym Sci. 2016;133:1–6. [Google Scholar]
- 34.Good RJ, Koo M. The effect of drop size on contact angle. J Colloid Interface Sci. 1979;71:283–92. [Google Scholar]
- 35.Vafaei S, Podowski MZ. Analysis of the relationship between liquid droplet size and contact angle. Adv Colloid Interface Sci. 2005;113:133–46. doi: 10.1016/j.cis.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Newmann A. Contact angles and their temperature dependence. Adv Colloid Interface Sci. 1974;4:105–91. [Google Scholar]
- 37.Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: A systematic review and meta-analysis. J Endod. 2007;33:96–105. doi: 10.1016/j.joen.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Hülsmann M, Heckendorff M, Lennon A. Chelating agents in root canal treatment: Mode of action and indications for their use. Int Endod J. 2003;36:810–30. doi: 10.1111/j.1365-2591.2003.00754.x. [DOI] [PubMed] [Google Scholar]
- 39.Iacobellis NS, Lo Cantore P, Capasso F, Senatore F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J Agric Food Chem. 2005;53:57, 61. doi: 10.1021/jf0487351. [DOI] [PubMed] [Google Scholar]
- 40.Škrovánková S, Mišurcová L, Machů L. Antioxidant activity and protecting health effects of common medicinal plants. Adv Food Nutr Res. 2012;67:75–139. doi: 10.1016/B978-0-12-394598-3.00003-4. [DOI] [PubMed] [Google Scholar]
- 41.Rabadia AG, Kamat S, Kamat D. Antifungal activity of essential oils against fluconazole resistant fungi. Int J Phytomed. 2011;3:506. [Google Scholar]
- 42.Derakhshan S, Sattari M, Bigdeli M. Effect of cumin (Cuminum cyminum) seed essential oil on biofilm formation and plasmid integrity of Klebsiella pneumoniae. Pharmacogn Mag. 2010;6:57–61. doi: 10.4103/0973-1296.59967. [DOI] [PMC free article] [PubMed] [Google Scholar]


