Abstract
As men grow older, circulating testosterone declines while the incidence of cardiovascular disease increases. Thus, the role of sex hormones as biomarkers, and possibly contributing factors to clinical manifestations of cardiovascular disease in the increasing demographic of aging men, has attracted considerable interest. This review focuses on observational studies of endogenous androgens, namely circulating testosterone and dihydrotestosterone, which have examined their associations with cardiovascular events such as myocardial infarction and stroke. Studies which have examined the associations of endogenous estrogens, namely circulating estradiol, with these outcomes are also discussed. In large prospective cohort studies of predominantly middle-aged and older men, lower circulating testosterone consistently predicts higher incidence of cardiovascular events. Of note, both lower circulating testosterone and lower dihydrotestosterone are associated with higher incidence of stroke. These associations are less apparent when myocardial infarction is considered as the outcome. Results for estradiol are inconsistent. Lower circulating testosterone has been shown to predict higher cardiovascular disease-related mortality, as has lower circulating dihydrotestosterone. It is possible that the relationship of circulating androgens to cardiovascular events or mortality outcomes may be U-shaped rather than linear, with an optimal range defining men at lowest risk. Epidemiological studies are observational in nature and do not prove causality. Associations observed in studies of endogenous androgens need not necessarily translate into similar effects of exogenous androgens. Rigorous randomized controlled trials are needed to clarify the effects of testosterone treatment on cardiovascular risk in men.
Keywords: cardiovascular disease, cardiovascular-related mortality, dihydrotestosterone, estradiol, male aging, myocardial infarction, stroke, testosterone
INTRODUCTION
The decline in circulating testosterone (T) that occurs as men grow older is a recognized phenomenon.1,2 Part of this change may relate to the presence of obesity and medical comorbidities.3,4 However, there is growing recognition that a reference range for T may be lower when defined in healthy older men5 compared with a reference range reported in reproductively normal younger men.6 Older men are also most at risk for cardiovascular disease (CVD) presenting as myocardial infarction, stroke, or CVD-related deaths.7 Thus, there is a considerable interest in whether reduced circulating androgens are risk predictors for higher incidence of CVD events, and if so, whether an opportunity exists to develop novel interventions.8 Epidemiological analyses conducted using prospective cohort studies in which large numbers of men are assessed and undergo longitudinal follow-up are a vital step in addressing this question, providing an understanding of the underlying associations between sex hormones and outcomes of interest. This article reviews prospective cohort studies of men in which baseline sex hormone profiles are available and CVD-related outcomes have been ascertained during follow-up. Unless otherwise specified, T and its bioactive metabolites, dihydrotestosterone (DHT) and estradiol (E2), refer to circulating concentrations of the total hormone. In some studies, free T has been calculated using mass action or empirical equations albeit there are limitations to this approach.9,10
Immunoassays are commonly used for the measurement of sex steroid concentrations, but they can exhibit nonspecificity and assay-dependent bias, meaning that particular immunoassays can under- or over-estimate hormone concentrations when compared with the reference method which is mass spectrometry.6 Therefore, in this review, particular attention is given to recent studies that have used mass spectrometry to assay sex hormones, making a distinction between these studies and other studies based on immunoassay for sex steroids. For longitudinal analyses of this type, the power to detect an association is related to the number of outcome events,11 thus studies that have analyzed larger numbers of incident CVD events or CVD-related deaths are likely to be more informative. These outcome events are categorized as a composite outcome of CVD events, specific outcomes of incident myocardial infarction or stroke, and occurrence of deaths due to CVD.
CARDIOVASCULAR EVENTS AS A COMPOSITE OUTCOME
Cohort studies using immunoassays for T and E2
Prospective cohort studies in predominantly middle-aged and older men, which have examined associations between sex hormones and incident cardiovascular events, are summarized in chronological order in Table 1. These include studies in which, in addition to CVD events, CVD-related mortality has also been reported. Of studies using immunoassay for sex steroids, Arnlov et al.12 found no association of T with a composite measure of CVD events (including myocardial infarction, angina, coronary insufficiency, coronary heart disease death, stroke, transient ischemic attack, congestive heart failure, and claudication). In that study, higher E2 was associated with lower risk of CVD events.12 Haring et al.13 found no associations of T or E2 with the composite outcome of CVD events (myocardial infarction, angina, coronary insufficiency, coronary heart disease death, stroke, transient ischemic attack, and congestive heart failure). Soisson et al.14 described a J-shaped association of T with the composite outcome of incident ischemic arterial disease (encompassing angina, coronary dilatation or bypass, myocardial infarction, and ischemic stroke).
Table 1.
Cohort studies examining associations between sex hormones with cardiovascular events in middle-aged and older men
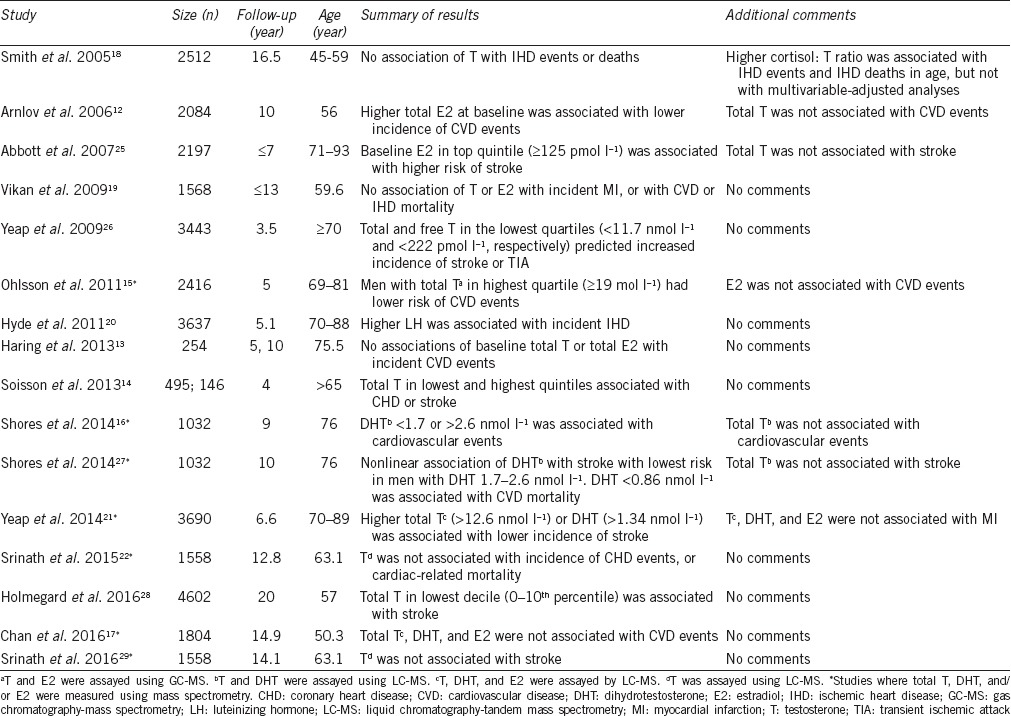
Cohort studies using mass spectrometry for T, DHT, and E2
Of studies using mass spectrometry to assay sex steroids, Ohlsson et al.15 studied 2416 men aged 69–81 years at baseline, followed up for 5 years during which time 485 CVD events occurred which are defined as a composite of coronary heart disease (hospitalizations due to myocardial infarction, unstable angina, revascularization, and deaths due to coronary heart disease) and stroke (hospitalizations due to stroke or transient ischemic attack, and deaths due to stroke). After adjusting for conventional cardiovascular risk factors, men with T in the highest quartile of values (≥19 nmol l−1) had lower risk of CVD events compared with men in the lower 3 quartiles (hazard ratio [HR]: 0.71, 95% confidence interval [CI]: 0.53–0.95), while E2 was not associated with CVD events.15 Shores et al.16 studied 1032 men from the Cardiovascular Health Study (CHS) with mean age 76 years for 9 years, during when 436 incident CVD events occurred (incident myocardial infarction, incident stroke, and mortality from CVD). Of interest, this analysis from the CHS found no association of T, and a U-shaped association of DHT with incident CVD events.16 Chan et al.17 studied 1804 men from the Busselton Health Survey (BHS) with a mean age of 50.3 years followed up for 14.9 years during when 399 CVD events occurred (hospital admissions from coronary heart disease, stroke, congestive heart failure, or peripheral arterial disease, and death due to diseases of the circulatory system). There were no associations of T, DHT, or E2 with incident CVD events.17
Summary: sex steroids and CVD events
Overall, the study by Ohlsson et al.15 provides evidence of a linear association between T and risk of CVD events in middle-aged and older men, when composite outcomes are considered. However, other studies support the concept that U-shaped associations are present for both T14 and DHT16 when CVD events are considered as a composite outcome of both coronary and cerebrovascular events. Therefore, the overall interpretation of these studies would be that men with lower circulating T are at the higher risk for CVD events, but having a T concentration at the higher end of the range possibly might not be beneficial. Additional studies would be needed to confirm whether the optimal concentration of T, which best predicts CVD outcome, lies in the middle of the range or higher. The evidence for E2 being associated with CVD events is limited12 and inconsistent.13,15 It is possible that associations of circulating androgens may be more prominent in older men, rather than in younger or predominantly middle-aged men, which would explain the lack of associations seen in the study by Chan et al.17
MYOCARDIAL INFARCTION
Cohort studies using immunoassays for T and E2
Several studies examined the associations of sex steroids measured using immunoassay with the outcome of myocardial infarction, coronary heart disease, or ischemic heart disease. Smith et al.18 studied 2512 men aged 45–59 years followed up for 16.5 years during which time 320 fatal and nonfatal ischemic heart disease events occurred. There was no association of T with ischemic heart disease events.18 Vikan et al.19 studied 1318 men with a mean age of 59.6 years followed up for 9.1 years, during which time 146 men had a first ever myocardial infarction. Neither T nor E2 was associated with incident myocardial infarction.19 Of note, Hyde et al.20 studied 3637 men aged 70–88 years from the Western Australian Health In Men Study (HIMS), who were followed up for 5.1 years during which time 618 men experienced an ischemic heart disease event (hospitalizations for angina, myocardial infarction or related complications, other ischemic heart disease events and revascularization, and deaths from ischemic heart disease including myocardial infarction and atherosclerotic heart disease). In that analysis, higher T was associated with fewer ischemic heart disease events in univariate and age-adjusted analysis but not after adjustment for conventional cardiovascular risk factors.20 However, in the fully-adjusted analysis, men with higher luteinizing hormone (LH) concentrations had reduced event-free survival.20
Cohort studies using mass spectrometry for T, DHT, and E2
Two studies using mass spectrometry for assay of sex steroids are of interest. An updated analysis was reported from HIMS in which plasma T, DHT, and E2 at baseline were assayed using mass spectrometry in 3690 men aged 70–89 years with follow-up extended to 6.6 years.21 During this time, incident myocardial infarction (hospital admission or death due to myocardial infarction) occurred in 344 men. T, DHT, and E2 were not associated with incident myocardial infarction.21 Srinath et al.22 analyzed plasma T assayed by mass spectrometry in 1558 men from the Atherosclerosis Risk in Communities (ARIC) study with a mean age of 63.1 years followed up for 12.8 years during which time 287 had a coronary heart disease event (myocardial infarction, coronary death, or coronary revascularization). T was not associated with incident CHD.22 Neither of these studies support an association of lower T with the incidence of myocardial infarction or ischemia, and in HIMS, neutral associations were also observed for DHT and E2.
Summary: sex steroids and myocardial infarction
In aggregate, these studies fail to demonstrate a convincing relationship between endogenous T concentrations and the incidence of myocardial infarction. The finding that higher LH, which is consistent with the presence of impaired testicular endocrine function and a relative deficiency of T, predicts the incidence of ischemic heart disease events,20 suggests that LH may provide an added insight for sex steroid exposure and its prognostic implications beyond that of circulating T. Given the association of lower T or DHT with the incidence of CVD events,14,15,16 yet the lack of association or neutral associations is seen for T or DHT with the incidence of myocardial infarction or ischemia,18,19,20,21,22 the question arises whether a distinction can be made between CVD events in coronary compared with other vascular territories.
STROKE
Cohort studies using immunoassays for T and E2
A logical method of analyzing specific CVD-related outcome events is to consider stroke distinct from myocardial infarction, i.e., events in the cerebrovascular territory separately from the coronary circulation. Myocardial infarction (MI) typically arises due to unstable atherosclerotic plaques and thrombosis in coronary arteries resulting in ischemia, while the etiology of stroke encompasses both ischemic and hemorrhagic events, and distal embolism as well as thrombosis, involving extra- and intra-cranial arteries that may respond to risk factors differently.23,24 Abbott et al.25 analyzed 2197 men aged 71–93 years, followed up for up to 7 years during which time 124 men had a first stroke. Measured using immunoassay, T was not associated with stroke risk but men who had higher E2 had a higher risk of stroke.25 In an analysis from HIMS using immunoassay for T in 3443 men aged ≥70 years followed up for 3.5 years, a first stroke or transient ischemic attack occurred in 119 men.26 Men with T in the lowest quartile of values had a 2-fold increase in the risk of stroke or transient ischemic attack after adjusting for age and other conventional cardiovascular risk factors.26 This study identified lower circulating T as a novel biomarker or risk predictor of cerebrovascular events in men.
Cohort studies using mass spectrometry for T, DHT, and E2
Subsequent studies using mass spectrometry have provided additional information. Shores et al.27 analyzed 1032 men aged 76 years from the CHS followed up for 10 years during which time 114 men had an incident fatal or nonfatal ischemic stroke. In that analysis, T measured using mass spectrometry was not associated with stroke, but there was a nonlinear association of DHT with the risk of ischemic stroke with the lowest risk seen in men with DHT in the range of 1.7–2.6 nmol l−1.27 An updated analysis from HIMS used mass spectrometry for assay of T, DHT, and E2 in 3690 men aged 70–89 years followed up for an extended period of 6.6 years during which time 300 men experienced an incident stroke (hospitalization or death due to stroke).21 After adjustment for age and other risk factors, higher T was associated with lower incidence of stroke with a fully adjusted HR of 0.56 (95% CI: 0.39–0.81) for men with T in the highest versus lowest quartile.21 A similar association was seen with higher DHT and lower incidence of stroke with a HR of 0.57 (95% CI: 0.40–0.81), but E2 was not associated with stroke (Figure 1).21 This updated analysis from HIMS confirms the earlier finding of lower T as a risk predictor for incident stroke and supports the CHS analysis in identifying lower DHT as a risk predictor for incident stroke.
Figure 1.
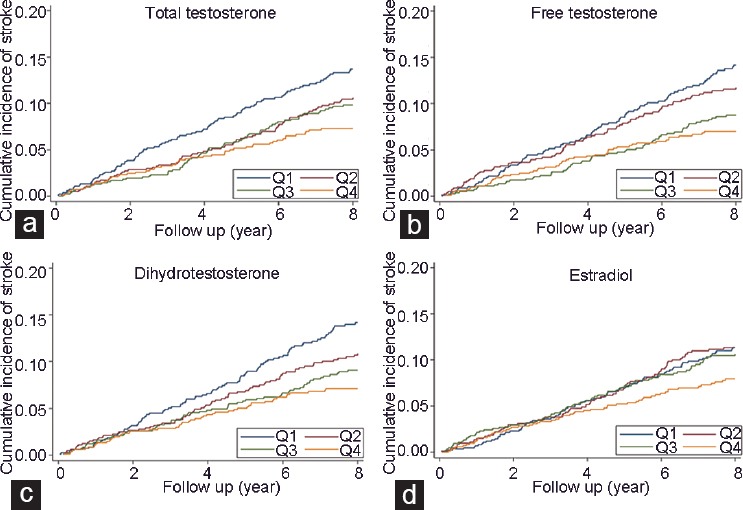
Nelson–Aalen plots showing the cumulative incidence of nonfatal or fatal stroke according to (a) quartiles of T, (b) calculated free T, (c) DHT, and (d) E2 in 3690 community dwelling men aged 70–89 years. T, DHT, and E2 were measured using liquid chromatography-mass spectrometry. Figure reproduced with the permission of the publisher.21 DHT: dihydrotestosterone; E2: estradiol; T: testosterone.
Confirmatory studies
Two subsequent studies examining endogenous T and incident stroke have been reported. Holmegard et al.28 analyzed 4602 men aged 57 years from the Copenhagen study, with 20 years of follow-up during which there were 560 stroke events. In that study, T was measured by immunoassay and men with T in the lowest 10% of values had a higher incidence of ischemic stroke.28 E2 also measured using immunoassay was not associated with ischemic stroke. This confirms the findings from HIMS both with respect to the association of lower T with incident stroke21,26 and the negative or neutral association of E2 with this outcome.21 A further analysis by Srinath et al.29 from the CHS did not show any association of T measured using mass spectrometry with the incidence of stroke, but there were only 79 outcome events in that analysis.
Summary: sex steroids and stroke
Overall, these epidemiological studies identify lower circulating T as an independent risk predictor for the incidence of stroke in men.21,26,28 Lower DHT is also an independent predictor of incident stroke.21,27 The initial study suggesting that higher E2 may be associated with incident stroke25 has not been confirmed with two subsequent larger analyses showing no association.21,28 While the utility of lower T and to an extent lower DHT as a biomarker for stroke risk has been established, additional studies are necessary to ascertain whether lower T or DHT might contribute causally to stroke risk.
CARDIOVASCULAR DEATHS
Cohort studies using immunoassays for T, DHT, and E2
Prospective cohort studies in predominantly middle-aged and older men, which have examined associations between sex hormones and deaths related to CVD, are summarized in chronological order in Table 2 (studies which reported overall or all-cause mortality without analyzing CVD-related deaths have not been included). Five of the studies summarized in Table 1 also reported CVD-related deaths as a separate outcome.17,18,19,22,27 Of studies using immunoassays for sex steroids, neither Smith et al.18 nor Vikan et al.19 found any association of T with ischemic heart disease mortality, nor was E2 associated.19 By contrast, Khaw et al.30 in a prospective case–control analysis found that higher baseline T was associated with lower risk of dying from CVD (Table 2). Contrastingly, Araujo et al.31 reported an association of higher calculated free T with mortality from ischemic heart disease. In that study, there was a borderline association of lower DHT with increased mortality from IHD.31 Of note, two prospective cohort studies, by Laughlin et al.32 and Haring et al.,33 found that lower T was associated with increased all-cause and CVD-related mortality. Of two studies that reported associations with calculated free T, Menke et al.34 reported associations of lower calculated free T with higher all-cause and CVD-related mortality, and an association of lower E2 with higher CVD mortality. Hyde et al.35 found that lower calculated free T predicted all-cause and CVD-related mortality. Therefore, overall, these analyses based on immunoassay of T tend to support an association of lower T, or lower calculated free T, with higher CVD mortality, while results for E2 are limited and inconsistent.
Table 2.
Cohort studies examining associations between sex hormones and cardiovascular disease-related mortality in middle-aged and older men
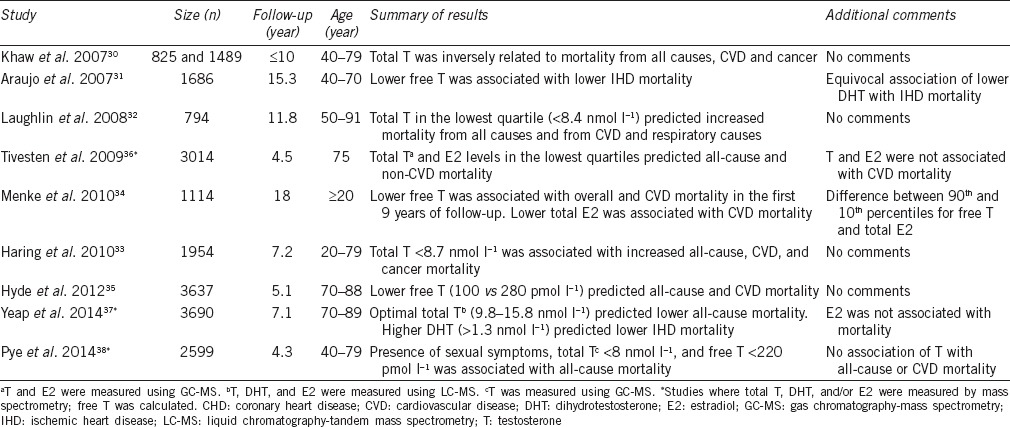
Cohort studies using mass spectrometry for T, DHT, and E2
Prospective cohort studies analyzing the associations of sex steroids assayed by mass spectrometry with the outcome of deaths from CVD are summarized in Table 1 and 2.
Tivesten et al.36 analyzed 3014 men aged 75 years followed up for 4.5 years during which time 383 men died, of which 144 from CVD. In that study, lower T and lower E2 were associated with all-cause mortality but not with deaths from CVD.36 The analysis from HIMS involved 3690 men aged 70–89 years followed up for 7.1 years during which time there were 974 deaths, of which 325 were due to ischemic heart disease.37 In that study with larger numbers of outcome events, having T in the middle of the range (25th–75th percentiles: 9.8–15.8 nmol l−1) predicted lower mortality, as did DHT in the 3rd quartile (1.3–1.8 nmol l−1).37 Interestingly, having plasma DHT in the 3rd and 4th quartiles (>1.3 nmol l−1) predicted lower risk of death due to ischemic heart disease.37 This association of DHT with CVD-related mortality was confirmed by the analysis by Shores et al.27 from the CHS, in which men with lower DHT had increased CVD-related mortality while T was not associated.
By contrast, Pye et al.38 analyzed data from 2599 men aged 40–79 years followed up for 4.3 years, during which time there were 147 deaths, of which 56 were due to CVD. In that analysis, men with sexual symptoms (decreased frequency of morning erections and sexual thoughts, and erectile dysfunction) and lower T had higher all-cause mortality, but no association was seen for T in the absence of symptoms with either all-cause or CVD mortality.38 This finding raises the prospect that the combination of symptoms with a low T concentration carries prognostic weight beyond that of low circulating T on its own. Srinath et al.22 in their analysis from ARIC did not find any association between T and CVD-related mortality. Of note, these analyses had limited numbers of outcome events with 56 and 29 deaths due to CVD, respectively.22,38 The analysis by Chan et al.17 showed neutral associations of T, DHT, and E2 with CVD mortality with 141 deaths from CVD.
Summary: sex steroids and cardiovascular mortality
While not all studies were uniformly positive, several studies have reported the associations of lower T or calculated free T with an increased risk of CVD-related deaths.32,33,35 A large prospective cohort study using mass spectrometry for assay of sex steroids with large numbers of outcome events identified lower DHT as a risk predictor for deaths due to ischemic heart disease,37 a finding supported by an independent analysis from a different study.27 This association may be more robust in larger studies of older men, compared to studies including younger or middle-aged men. The possible interpretations for these findings are that lower T or DHT might predispose to more severe CVD events leading to death, or that DHT might be a resilience factor with men having lower DHT being more vulnerable to dying when an ischemic heart disease event occurs.
DIFFERENTIAL ASSOCIATIONS OF T, DHT, AND E2 WITH CVD-RELATED OUTCOMES
Androgens not estrogen associated with CVD-related outcomes
These studies illuminate a clear distinction between androgens and estrogen in relation to risk of CVD in men. While lower T and DHT have both been shown to predict CVD events14,15,16 and incidence of stroke,21,26,27,28 there are no consistent associations of E2 with these outcomes. Thus, for further studies of sex hormones and risk of CVD, attention should be focused on androgens rather than on estrogens.
Distinct associations of T versus DHT with specific CVD-related outcomes
In studies where both T and DHT have been measured using mass spectrometry, similar as well as differential associations are seen. For example, both lower T and lower DHT were associated with the incidence of stroke in the HIMS analysis.21 This would be consistent with the correlation between circulating T and DHT in men,5 and their binding to the single androgen receptor.39 However, in the analysis from CHS, a U-shaped association was noted with both lower and higher DHT concentrations associated with CVD events and with stroke.16,27 Furthermore, in HIMS, higher DHT rather than T was associated with lower mortality from ischemic heart disease.37 The differential association of DHT compared to T with a common outcome has also been reported in other settings. For example, community dwelling men with higher T were less likely to have carotid plaque, but in men with angiographically proven coronary artery disease, higher DHT was associated with lower risk of carotid plaque.40 Intriguingly, higher DHT rather than T has been associated with longer leukocyte telomere length, a marker for slower biological aging, in community dwelling men.41 DHT can be regarded as a paracrine factor as it is synthesized within androgen-sensitive organs such as the prostate, leading to higher intraprostatic concentrations compared to circulating concentrations.42 Nevertheless, DHT is a more potent ligand for the androgen receptor compared to T,39 and being present in the circulation may possess a classical endocrine role.43 Further research is needed to determine whether exposure to DHT might lead to differential effects within the vasculature compared to T.
CONCLUSIONS
In large prospective cohort studies of predominantly middle-aged and older men, lower circulating T predicts higher incidence of cardiovascular events. Both lower circulating T and lower DHT are associated with higher incidence of stroke. Lower circulating T has been shown to predict higher CVD-related mortality, as has lower circulating DHT. Thus, epidemiological studies support the utility of lower circulating androgens as a risk predictor for CVD. However, causality cannot be assumed and needs to be proven. The difficulty is that none of the randomized controlled trials of T carried out to date have been sufficiently powered to have cardiovascular events as a prespecified end point. In the BHS, observed 5-year risks were 11% for CVD events, 3% for stroke events, and 3% for myocardial infarction events in men aged 50–69 years. Consequently, a study of about 6000 men per group treated for 5 years would be needed to detect differences in the rates of myocardial infarction or stroke (Knuiman M, personal communication). Such large randomized controlled trials have yet to be attempted for T therapy. Smaller trials have reported on the occurrence of cardiovascular adverse events in T versus placebo recipients. One randomized controlled trial of T in older men with limited mobility was stopped due to an excess of cardiovascular adverse events,44 but no such signal was seen in another trial in older intermediate-frail or frail men nor in the larger US T Trials.45,46 Meta-analyses of randomized controlled trials of T in general do not show an excess of cardiovascular adverse events, except possibly in men aged ≥65 years in the first year.47 Therefore, additional carefully designed mechanistic studies performed in vivo will be important and informative. Ultimately, a large randomized controlled trial would be needed to determine whether T, directly or via its conversion to DHT, would reduce the risk of cardiovascular events such as stroke in middle-aged or older men.
COMPETING INTERESTS
The author has received research support from Bayer and Lawley Pharmaceuticals, and speaker and advisory committee honoraria from Besins and Lilly.
ACKNOWLEDGMENT
Prof. Bu Yeap is a recipient of National Health and Medical Research Council of Australia Project Grants 1030123, 1045710, 1060557, 1121548 and 1128083.
REFERENCES
- 1.Handelsman DJ, Yeap BB, Flicker L, Martin S, Wittert GA, et al. Age-specific population centiles for androgen status in men. Eur J Endocrinol. 2015;173:809–17. doi: 10.1530/EJE-15-0380. [DOI] [PubMed] [Google Scholar]
- 2.Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the USA and Europe. J Clin Endocrinol Metab. 2017;102:1161–73. doi: 10.1210/jc.2016-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Z, Araujo AB, Martin S, O'Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013;98:3289–97. doi: 10.1210/jc.2012-3842. [DOI] [PubMed] [Google Scholar]
- 4.Camacho EM, Huhtaniemi IT, O'Neill TW, Finn JD, Pye SR, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168:445–55. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- 5.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, et al. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab. 2012;97:4030–9. doi: 10.1210/jc.2012-2265. [DOI] [PubMed] [Google Scholar]
- 6.Sikaris K, McLachlan RI, Kazlauskas R, de Kretser D, Holden CA, et al. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab. 2005;90:5928–36. doi: 10.1210/jc.2005-0962. [DOI] [PubMed] [Google Scholar]
- 7.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. 3. Cardiovascular diseases Circulation. 2011;123:e35–59. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeap BB, Araujo AB, Wittert GA. Do low testosterone levels contribute to ill-health during male ageing? Crit Rev Clin Lab Sci. 2012;49:168–82. doi: 10.3109/10408363.2012.725461. [DOI] [PubMed] [Google Scholar]
- 9.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 10.Ly LP, Sartorius G, Hull L, Leung A, Swerdloff RS, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol. 2010;73:382–8. doi: 10.1111/j.1365-2265.2010.03804.x. [DOI] [PubMed] [Google Scholar]
- 11.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499–503. [PubMed] [Google Scholar]
- 12.Arnlov J, Pencina MJ, Amin S, Nam BH, Benjamin EJ, et al. Endogenous sex hormones and cardiovascular disease incidence in men. Ann Intern Med. 2006;145:176–84. doi: 10.7326/0003-4819-145-3-200608010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Haring R, Teng Z, Xanthakis V, Coviello A, Sullivan L, et al. Associations of sex steroids, gonadotropins, and their trajectories with clinical cardiovascular disease and all-cause mortality in elderly men from the Framingham Heart Study. Clin Endocrinol. 2013;78:629–34. doi: 10.1111/cen.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soisson V, Brailly-Tabard S, Helmer C, Rouaud O, Ancelin ML, et al. A J-shaped association between plasma testosterone and risk of ischemic arterial event in elderly men: the French 3C Cohort Study. Maturitas. 2013;75:282–8. doi: 10.1016/j.maturitas.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Ohlsson C, Barrett-Connor E, Bhasin S, Orwoll E, Labrie F, et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. J Am Coll Cardiol. 2011;58:1674–81. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Shores MM, Biggs ML, Arnold AM, Smith NL, Longstreth WT, et al. Testosterone, dihydrotestosterone, and incident cardiovascular disease and mortality in the cardiovascular health study. J Clin Endocrinol Metab. 2014;99:2061–8. doi: 10.1210/jc.2013-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan YX, Knuiman MW, Hung J, Divitini ML, Beilby JP, et al. Neutral associations of testosterone, dihydrotestosterone and estradiol with fatal and non-fatal cardiovascular events, and mortality in men aged 17-97 years. Clin Endocrinol. 2016;85:575–82. doi: 10.1111/cen.13089. [DOI] [PubMed] [Google Scholar]
- 18.Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, et al. Cortisol, testosterone, and coronary heart disease. Prospective evidence from the Caerphilly Study. Circulation. 2005;112:332–40. doi: 10.1161/CIRCULATIONAHA.104.489088. [DOI] [PubMed] [Google Scholar]
- 19.Vikan T, Schirmer H, Njolstad I, Svartberg J. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromsø study. Eur J Endocrinol. 2009;161:435–42. doi: 10.1530/EJE-09-0284. [DOI] [PubMed] [Google Scholar]
- 20.Hyde Z, Norman PE, Flicker L, Hankey GJ, McCaul KA, et al. Elevated luteinizing hormone predicts ischaemic heart disease events in older men.The Health in Men Study. Eur J Endocrinol. 2011;164:569–77. doi: 10.1530/EJE-10-1063. [DOI] [PubMed] [Google Scholar]
- 21.Yeap BB, Alfonso H, Chubb SA, Hankey GJ, Handelsman DJ, et al. In older men, higher plasma testosterone or dihydrotestosterone is an independent predictor for reduced incidence of stroke but not myocardial infarction. J Clin Endocrinol Metab. 2014;99:4565–73. doi: 10.1210/jc.2014-2664. [DOI] [PubMed] [Google Scholar]
- 22.Srinath R, Golden SH, Carson KA, Dobs A. Endogenous testosterone and its relationship to preclinical and clinical measures of cardiovascular disease in the Atherosclerosis Risk in Communities Study. J Clin Endocrinol Metab. 2015;100:1602–8. doi: 10.1210/jc.2014-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JS, Nah HW, Park SM, Kim SK, Cho KH, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012;43:3313–8. doi: 10.1161/STROKEAHA.112.658500. [DOI] [PubMed] [Google Scholar]
- 24.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–38. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 25.Abbott RD, Launer LJ, Rodriguez BL, Ross GW, Wilson PW, et al. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68:563–8. doi: 10.1212/01.wnl.0000254473.88647.ca. [DOI] [PubMed] [Google Scholar]
- 26.Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, et al. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94:2353–9. doi: 10.1210/jc.2008-2416. [DOI] [PubMed] [Google Scholar]
- 27.Shores MM, Arnold AM, Biggs ML, Longstreth WT, Smith NL, et al. Testosterone and dihydrotestosterone and incident ischaemic stroke in men in the Cardiovascular Health Study. Clin Endocrinol. 2014;81:746–53. doi: 10.1111/cen.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmegard HN, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A, Benn M. Sex hormones and ischemic stroke: a prospective cohort study and meta-analyses. J Clin Endocrinol Metab. 2016;101:69–78. doi: 10.1210/jc.2015-2687. [DOI] [PubMed] [Google Scholar]
- 29.Srinath R, Gottesman RF, Golden SH, Carson KA, Dobs A. Association between endogenous testosterone and cerebrovascular disease in the ARIC Study (Atherosclerosis Risk in Communities) Stroke. 2016;47:2682–8. doi: 10.1161/STROKEAHA.116.014088. [DOI] [PubMed] [Google Scholar]
- 30.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men.European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116:2694–701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 31.Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, et al. Sex steroids and cause-specific mortality in men. Arch Intern Med. 2007;167:1252–60. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- 32.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haring R, Volzke H, Steveling A, Krebs A, Felix SB, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J. 2010;31:1494–501. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- 34.Menke A, Guallar E, Rohrmann S, Nelson WG, Rifai N, et al. Sex steroid concentrations and risk of death in US men. Am J Epidemiol. 2010;171:583–92. doi: 10.1093/aje/kwp415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyde Z, Norman PE, Flicker L, Hankey GJ, Almeida OP, et al. Low free testosterone predicts mortality from cardiovascular disease but not other causes: the Health in Men Study. J Clin Endocrinol Metab. 2012;97:179–89. doi: 10.1210/jc.2011-1617. [DOI] [PubMed] [Google Scholar]
- 36.Tivesten A, Vandenput L, Labrie F, Karlsson MK, Ljunggren O, et al. Low serum testosterone and estradiol predict mortality in elderly men. J Clin Endocrinol Metab. 2009;94:2482–8. doi: 10.1210/jc.2008-2650. [DOI] [PubMed] [Google Scholar]
- 37.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality, and higher dihydrotestosterone with reduced ischaemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99:E9–18. doi: 10.1210/jc.2013-3272. [DOI] [PubMed] [Google Scholar]
- 38.Pye SR, Huhtaniemi IT, Finn JD, Lee DM, O'Neill TW, et al. Late-onset hypogonadism and mortality in ageing men. J Clin Endocrinol Metab. 2014;99:1357–66. doi: 10.1210/jc.2013-2052. [DOI] [PubMed] [Google Scholar]
- 39.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem Rev. 2005;105:3352–70. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan YX, Knuiman MW, Hung J, Divitini ML, Handelsman DJ, et al. Testosterone, dihydrotestosterone and estradiol are differentially associated with carotid intima-media thickness and carotid plaque in men with and without coronary artery disease. Endocrine J. 2015;62:777–86. doi: 10.1507/endocrj.EJ15-0196. [DOI] [PubMed] [Google Scholar]
- 41.Yeap BB, Knuiman MW, Divitini ML, Hui J, Arscott GM, et al. Epidemiological and Mendelian randomisation studies of dihydrotestosterone and estradiol, and leucocyte telomere length in men. J Clin Endocrinol Metab. 2016;101:1299–306. doi: 10.1210/jc.2015-4139. [DOI] [PubMed] [Google Scholar]
- 42.Swerdloff RS, Dudley RE, Page ST, Wang C, Salameh WA. Dihydrotestosterone: biochemistry, physiology, and clinical implications of elevated blood levels. Endocr Rev. 2017;38:220–54. doi: 10.1210/er.2016-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anawalt BD. Is dihydrotestosterone a classic hormone? Endocr Rev. 2017;38:170–2. doi: 10.1210/er.2017-00091. [DOI] [PubMed] [Google Scholar]
- 44.Basaria S, Coviello AD, Travison TG, Stoere TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srinivas-Shankar U, Roberts SA, Connolly MJ, O'Connell MD, Adams JE, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 46.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–24. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol. 2016;85:436–43. doi: 10.1111/cen.13084. [DOI] [PubMed] [Google Scholar]


