Abstract
In men, obesity and metabolic complications are associated with lower serum testosterone (T) and dihydrotestosterone (DHT) and an increased risk of, and mortality from, multiple chronic diseases in addition to cardiovascular disease (CVD). The causal interrelationships between these factors remain a matter of debate. In men with untreated congenital and lifelong forms of hypogonadotropic hypogonadism, there appears to be no increased risk. Men with Klinefelter's syndrome have an increased risk of various types of cancers, as well as CVD, which persist despite T therapy. In the absence of pathology of the hypothalamic–pituitary–gonadal axis, the effect of modest reductions in serum T in aging men is unclear. The prevalence of low serum T concentrations is high in men with cancer, renal disease, and respiratory disease and is likely to be an indicator of severity of systemic disease, not hypogonadism. Some population-based studies have found low serum T to be associated with a higher risk of deaths attributed to cancer, renal disease, and respiratory disease, while others have not. Although a meta-analysis of longitudinal studies has shown an association between low serum T and all-cause mortality, marked heterogeneity between studies limited a firm conclusion. Therefore, while a decrease in T particularly occurring later in life may be associated with an increase in all-cause and specific types of mortality in men, the differential effects, if any, of T and other sex steroids as compared to health and lifestyle factors are unknown at the current time.
Keywords: hypogonadism, mortality, testosterone
INTRODUCTION
This review aims to provide a perspective on the evolving evidence base relating to endogenous circulating testosterone (T) and its association with all-cause mortality and cause-specific mortality by examining data relating to long-standing treated and untreated forms of congenital hypogonadism, hypogonadism acquired late in life, and from longitudinal population-based cohort studies.
HYPOGONADISM INDUCED BY ANDROGEN DEPRIVATION THERAPY (ADT) IN MEN WITH PROSTATE CANCER (PCa)
Men with PCa who are treated with surgical or medical castration (resulting in extremely low or undetectable serum concentrations of T and its major metabolites, estradiol, and dihydrotestosterone) have a higher prevalence of all-cause mortality1 as well as cardiovascular disease (CVD)-specific mortality.1,2,3,4,5 Men with a history of any form of cardiac or cerebrovascular disease or diabetes are at greatest risk of all-cause mortality independent of the level of risk from the PCa, even with short course of ADT.2,3,4,5 Furthermore, the combination of a low pretreatment serum T level and multiple preexisting comorbidities is associated with decreased overall survival following ADT for PCa.4,6,7,8 This suggests an interaction between T and other risk factors to increase all-cause mortality in these men.
CONGENITAL HYPOGONADOTROPIC HYPOGONADISM (HH)
Men with congenital forms of HH untreated to their late teens and early twenties have increased adiposity, endothelial dysfunction, inflammation, insulin resistance,9 and carotid intima-media thickness (CIMT)10 compared to age-matched healthy controls. After 6 months of T-replacement therapy, cardiometabolic risk markers appear to worsen rather than improve,9 but after 9 months of treatment, improvements compared to baseline are seen.11 There is limited mortality data on lifelong untreated HH, but there appears to be no increased risk of CVD.12 After T treatment has been instituted, a short-term (2-week) withdrawal of T leads to an increase in fasting insulin.13
KLINEFELTER'S SYNDROME
Klinefelter's syndrome is the most common sex chromosome disorder and cause of hypogonadism affecting 1 in 600 men.14 Men with Klinefelter's syndrome have a 40%–50% increased risk of early mortality, with a loss of between 2 and 5 years in lifespan compared to age- and sex-matched population controls.15,16 All-cause mortality risk appears to be similar across all ages.15 While mortality is increased for most causes (relative risk 1.63), specific causes of death include infectious causes, cancer, diabetes mellitus, vascular insufficiency of the intestine and cardiovascular (CV), neurological, urological, and pulmonary diseases.15,17 Significantly raised risk is seen for lung cancer, breast cancer, mediastinal tumors, and non-Hodgkin's lymphoma.16,17,18,19
Men with Klinefelter's syndrome have an increased risk of CVD including nonischemic heart disease and cerebrovascular disease, but whether risk can be attributed to hypogonadism or the syndrome itself remains unclear and there is a lack of evidence that T therapy abrogates CVD risk.17,20,21 Hypogonadism may be an important factor in determining metabolic syndrome, and men with Klinefelter's syndrome have a higher risk of type 2 diabetes mellitus and obesity.
At least in part, the increased risk may be conferred by the chromosomal abnormality.20,21,22 Androgen sensitivity is influenced by gene polymorphisms encoding the X-linked androgen receptor and by the number of CAG repeats in the receptor. Klinefelter's syndrome genotypes associated with polymorphisms of the androgen receptor and increased numbers of CAG repeats result in decreased androgen sensitivity.22 Studies of mice with intact testis showed that the variation in the number of X chromosomes contributed to sex differences in CVD, a finding that might explain the increased CVD risk in men with Klinefelter's syndrome.23
ENDOGENOUS T AND ITS MAJOR SEX STEROID METABOLITES
Although T is the primary sex steroid in men, tissue-specific effects of T may be mediated or modulated by dihydrotestosterone (DHT) or estradiol (E2) depending on the presence of 5α-reductase or aromatase. Serum E2 concentrations in adult men do not accurately reflect tissue activity as T is aromatized to E2 within many tissues and E2 is partly metabolized in situ. Differential associations of each of the endogenous sex steroids on markers of CV risk and CV outcomes have been observed, and further the effects might depend on the specific group of men studied. For example, in community-dwelling men, higher serum T is associated with favorable CIMT and lower prevalence of carotid plaque, while the opposite is shown with higher serum E2. In men with coronary artery disease, higher DHT or E2 levels are associated with less carotid plaque.24 Furthermore, in elderly men, higher baseline serum T and DHT were associated with reduced risk of stroke, and higher DHT was associated with lower mortality from ischemic heart disease, but serum E2 was not associated with either.25,26
ENDOGENOUS T AND ALL-CAUSE MORTALITY
A meta-analysis and systematic review of 12 observational studies, of which 11 examined all-cause mortality, found low endogenous T levels to be associated with an increased risk of all-cause mortality. However, there was a significant heterogeneity between studies, and the authors concluded that it is likely that low endogenous T levels are a marker of poor general health status.27
More recent prospective longitudinal population studies in community-dwelling men have reported associations between low serum T and all-cause mortality; however, some have not (Table 1). A longitudinal study by Pye et al.28 of 2599 men aged 40–79 years found mortality in men with late-onset hypogonadism (LOH), defined as a clinical and biochemical state of hypogonadism, had a higher mortality rate of 30.9% compared to that in the entire cohort of 5.7%. Nearly two-thirds of deaths in men with LOH were due to CVD and one-third from cancer, which was different than that observed from the total cohort with 38.1% of deaths caused by CVD and 40.8% from cancer. The association between LOH and all-cause mortality showed that over time, survival continued to decline. After adjustment for confounders, men with severe LOH had a 5-fold increased risk of all-cause mortality and an 8-fold increased risk of CVD mortality. Men with a serum T of <8.0 nmol l−1, irrespective of sexual symptoms, had a 2-fold increased risk of all-cause mortality, compared to eugonadal men. This risk increased 3-fold in men with a serum T of <8.0 nmol l−1 who also reported sexual symptoms. Therefore again, the likely explanation is that LOH represents a prognostic marker of poor health in aging men.
Table 1.
Endogenous testosterone and all-cause mortality outcomes in men
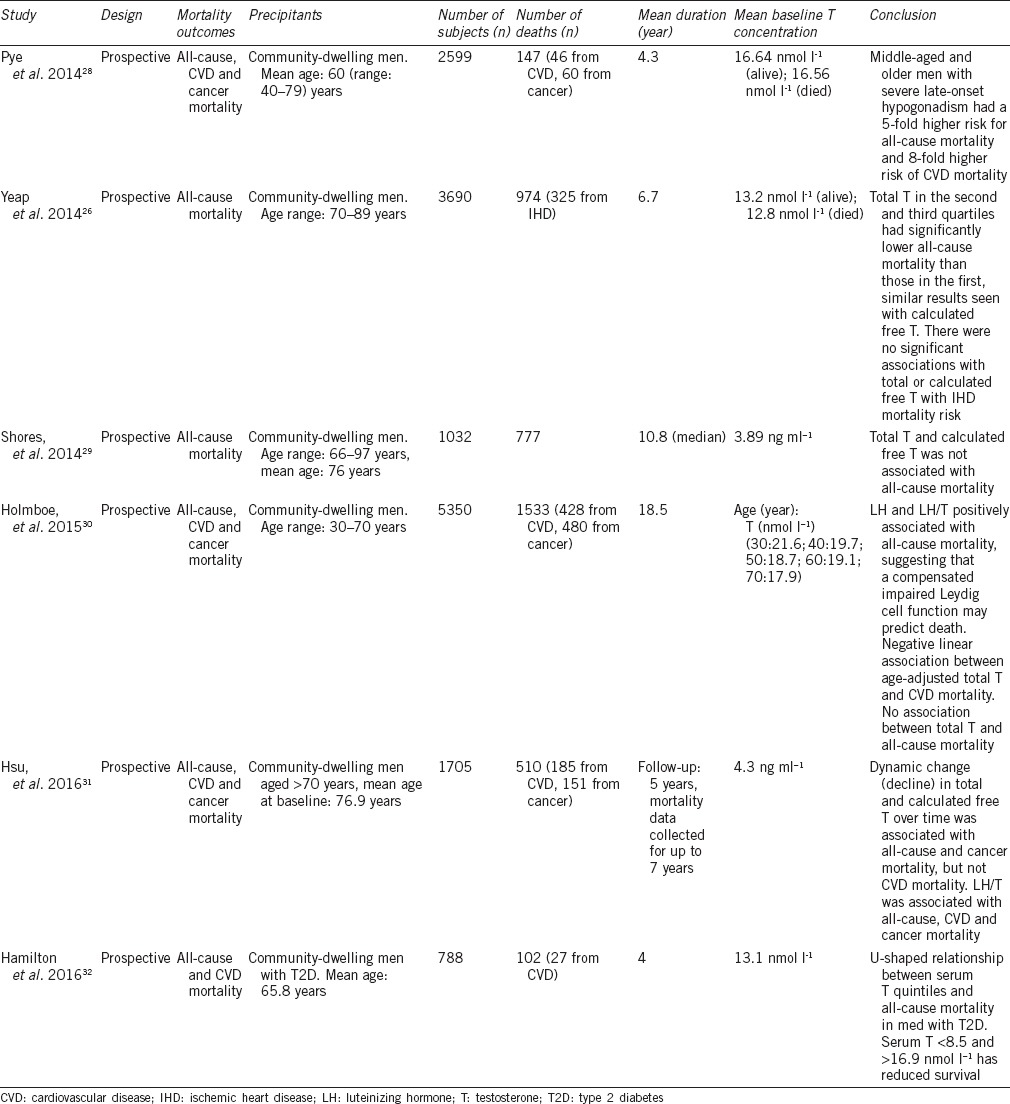
A prospective study by Holmboe et al.30 of 5350 men at different ages between 30 and 70 years were followed for a mean period of 18.5 years after baseline sex hormone levels. A positive association was observed between luteinizing hormone (LH) and the ratio of LH to T with all-cause and cancer mortality. While total and free T decreased with age, neither was associated with all-cause or cancer mortality, but low T was a predictor of CVD mortality. It was surmised that primary Leydig cell dysfunction, even when compensated by increased LH, is a risk factor for mortality in men. In contrast, secondary decreased Leydig cell function may be a marker of increased CVD mortality risk. It was concluded that the measurement of LH in addition to T in men with androgen insufficiency may inform poorer health and comorbidity risk that may otherwise be overlooked.
Dynamic changes in serum T in older men were found to be associated with all-cause mortality. Serum T was measured in men aged ≥70 years at baseline, with repeated levels taken at 2 and 5 years. Men with the lowest quartile of baseline serum T, E2, DHT, and calculated free testosterone (cFT) had the highest cumulative all-cause mortality risk. A progressive decline in serum T over time was associated with all-cause mortality (RR: 1.18, 95% CI: 1.05–1.32, P = 0.004) and cancer-specific mortality (RR: 1.32, 95% CI: 1.06–1.64, P = 0.01), which were significant even after adjusting for age, BMI, and smoking comorbidities. Serum T decline over time was also significant for CVD mortality, however after adjusting for confounders, this was no longer significant. A key finding was the association of progressively declining serum T and death from cancer within the 7-year period in which mortality data were collected.31
Not only low but also high serum T has been found to be associated with an increase in mortality in both community-dwelling men with and without type 2 diabetes.26,32 In older men aged 70–89 years, cumulative mortality was highest for men with total T within the lowest quartile of values, with the second highest rate seen in men with total T within the highest quartile.26 This U-shaped association with total T- and all-cause mortality has also been demonstrated in men with type 2 diabetes with all-cause mortality greatest in those with a serum T in the lowest and highest quintile (serum T <8.6 and >16.9 nmol l−1, respectively), and lowest risk in those within the middle quintile (serum T: 11.1–13.7 nmol l−1).32 Men with low serum T are more likely to be obese and have metabolic syndrome. A longitudinal study of 581 men with type 2 diabetes mellitus by Muraleedharan et al.33 was the first to demonstrate that low baseline T of <10.4 nmol l−1 is associated with an increased mortality risk. Men were followed for a mean period of 5.8 years, while some were receiving T therapy, mortality rates were increased within the group with T <10.4 nmol l−1 of 17.2%, compared to 9% in men with T >10.4 nmol l−1 (P = 0.003). Within the low T group, after separating those who received T therapy (mean duration: 41.6 months) from men who did not, there was a significant increase in mortality of 20.11% in the untreated group compared to 9.38% in the treated group (P = 0.002) and 9.12% in men with T >10.4 nmol l−1. After excluding deaths within the first 6 months, the majority of deaths were from CVD.33 A prospective cohort study of 1239 Chinese men with type 2 diabetes mellitus and median disease duration of 9 years were followed for 4.8 years. While men within the low T group with a mean serum T of 6.89 nmol l−1 had a higher prevalence of CVD and metabolic syndrome, there was not an association between T and all-cause mortality.34
Thus, epidemiological studies have found that men with low endogenous T have an increased risk of all-cause mortality compared to those with higher T levels, of which death from CVD remains the predominant cause. Men with low serum T are more likely to be obese and have metabolic syndrome, and men with type 2 diabetes mellitus and low endogenous T have an increase in all-cause mortality. It is not known if T deficiency is due to the disease and reflects the state of ill health or contributes to the underlying pathogenesis of these conditions.
ENDOGENOUS T CONCENTRATIONS AND DEATHS FROM NON-CV CAUSES
Cancer
The prevalence of T deficiency among men with cancer is high and is due to either the underlying disease or the treatment.35 Men with metastatic disease have a high prevalence of androgen deficiency, and up to 90% of those receiving opioids for cancer pain are T deficient.36,37 Men with advanced malignancies have lower T, and this was associated with higher LH levels, suggesting a testicular insufficiency within terminal stages of the disease.37 Opioids inhibit the pituitary–gonadal axis;38,39 certain chemotherapy agents, including the alkylating and platinum-based therapies, impair testicular function; and high levels of inflammatory cytokines which are observed in advanced malignancy inhibit the pituitary–gonadal axis.37 Androgen deficiency impacts on appetite and negatively affects metabolism, further aggravating cancer-related cachexia.
While some longitudinal studies have shown a relationship between low baseline serum T and cancer mortality,40,41 others have not.30,42,43 Men with progressively declining serum T had a greater mortality due to cancer.31 The EPIC-Norfolk study found an association with serum T and cancer mortality, however this was no longer significant after adjusting for covariates. Of the 304 cancer-related deaths, 55 were due to lung, 50 prostate, 37 colorectal, 15 esophageal and 11 stomach cancers.41 Men with Klinefelter's syndrome are also at an increased risk of cancer, but with a different at-risk cancer profile compared with breast, lung, and mediastinal cancer, and non-Hodgkin's lymphoma being more common.16,17,18,19,44
Therefore, serum T is frequently low in men with cancer. The reasons for this are multifactorial and include the effects of the underlying malignancy and/or associated treatment. The extent to which androgen deficiency contributes to cancer morbidity and mortality and whether treatment with testosterone is beneficial remains to be determined.
Renal disease
Chronic kidney disease (CKD) leads to dysregulation of the hypothalamic–pituitary–gonadal (HPG) axis and subsequent androgen deficiency in men.45 The estimated prevalence of androgen deficiency in men with CKD is approximately 48%–60%.46 The degree of T deficiency is proportional to the severity of the underlying CKD however, whether this is a causal effect or an epiphenomenon remains in contention. It is not well established if androgens have a direct effect on the kidney, but it is postulated that low T might indirectly contribute to the pathogenesis of CKD through vascular and metabolic pathways. Longitudinal population studies showed CKD and low serum T to be additive risk factors for mortality.47 A prospective cohort study of 143 males with CKD not receiving dialysis, receiving dialysis and post renal transplant, were followed up for a median of 5.8 years. Fifty-two (36%) patients died, of which 17 were with CKD, 28 were receiving dialysis, and 7 were post a renal transplant. Those who died during follow-up had a lower median T (9.9 nmol l−1) compared to those who survived or received a kidney transplant (11.7 nmol l−1, P = 0.002). Low serum T was an independent predictor of mortality (P = 0.02), and a decrease in serum T of 1 nmol l−1 was associated with a 9.8% increase in mortality. T was inversely associated with renal function, men on dialysis had a lower T compared to men with CKD and renal transplant recipients.48
Respiratory disease
Low serum T has been reported in pulmonary disease, and some longitudinal studies have shown an increased mortality due to respiratory disease.42,43 A population-based study of 1686 men followed up for a mean of 15.3 years found that 9.6% of men died from respiratory diseases. Low calculated free serum T, but not total T, was associated with increased respiratory disease mortality. Men with the lowest free T quintile were five times more likely to die from respiratory disease compared to men with the highest quintile.43 Another prospective population study of 794 men, with a median age of 73.6 years followed up to 20 years (mean follow-up period of 11.8 years), found that those with a low baseline serum total and free T were associated with an elevated 20-year risk of death due to respiratory disease.42
CONCLUSIONS
It remains unclear whether low serum T concentrations in men are an independent risk factor for CVD or a marker of the presence of CVD.49 Genetic, lifestyle, behavioral and concomitant disease-related factors are likely more important in determining CVD risk than direct effects of sex steroids. What is clear, however, is that when low T is the consequence of obesity and lifestyle factors, substantial weight loss and exercise improve or normalize T concentrations7,50,51,52,53 improving CV risk. Older, overweight men with borderline low serum T concentrations can be expected to derive more CV benefit from a healthy diet and exercise regimen and statin and aspirin therapy, as appropriate, than they would from T therapy. There appears to be an association between low serum T and all-cause and CV mortality in older men; however, heterogeneity between studies limited a firm conclusion. Men with cancer, CKD, and respiratory disease have a higher prevalence of hypogonadism. Longitudinal population-based studies of community-dwelling men have found low serum T to be associated with a higher risk of death from cancer and renal and respiratory disease, while other studies have not found this association. Taken together, these data suggest that low serum T later in life may be associated with an increase in all-cause and specific types of mortality in men, this body of evidence is evolving and further research is required.
AUTHOR CONTRIBUTIONS
The planning for the manuscript was done by EJM and GW. The literature search and preparation of the manuscript was undertaken by EJM with review and editing by GW.
COMPETING INTEREST
Gary Wittert has received funding from: Weight Watchers, Bayer Schering, Eli Lilly, ResMed Foundation, and Lawley Pharmaceuticals; Payments for lectures from Novo Nordisk, Bayer, MSD, Merck, Astra Zeneca, Roche, AbbVie, Besins, and Amgen; Honoraria as member of an International Advisory Board for Elsevier, Editor-in-Chief of Obesity Research and Clinical Practice, and Independent Chair of the Weight Management Council of Australia.
ACKNOWLEDGMENT
Gary Wittert has received: grant funding or in-kind support from the National Health and Medical Research Council (NH&MRC project grant 627227).
REFERENCES
- 1.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, et al. Fifteen-year survival outcomes following primary androgen-deprivation therapy for localized prostate cancer. JAMA Intern Med. 2014;174:1460–7. doi: 10.1001/jamainternmed.2014.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, D'Amico AV, Berger P, Clark PE, Eckel RH, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–40. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes JH, Chen MH, Moran BJ, Braccioforte MH, Dosoretz DE, et al. Androgen-suppression therapy for prostate cancer and the risk of death in men with a history of myocardial infarction or stroke. BJU Int. 2010;106:979–85. doi: 10.1111/j.1464-410X.2010.09273.x. [DOI] [PubMed] [Google Scholar]
- 5.Parekh A, Chen MH, D'Amico AV, Dosoretz DE, Ross R, et al. Identification of comorbidities that place men at highest risk of death from androgen deprivation therapy before brachytherapy for prostate cancer. Brachytherapy. 2013;12:415–21. doi: 10.1016/j.brachy.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Shi Z, Araujo AB, Martin S, O'Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013;98:3289–97. doi: 10.1210/jc.2012-3842. [DOI] [PubMed] [Google Scholar]
- 7.Camacho EM, Huhtaniemi IT, O'Neill TW, Finn JD, Pye SR, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168:445–55. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- 8.Taira AV, Merrick GS, Galbreath RW, Butler WM, Wallner KE. Factors impacting all-cause mortality in prostate cancer brachytherapy patients with or without androgen deprivation therapy. Brachytherapy. 2010;9:42–9. doi: 10.1016/j.brachy.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Sonmez A, Haymana C, Aydogdu A, Tapan S, Basaran Y, et al. Endothelial dysfunction, insulin resistance and inflammation in congenital hypogonadism, and the effect of testosterone replacement. Endocr J. 2015;62:605–13. doi: 10.1507/endocrj.EJ15-0125. [DOI] [PubMed] [Google Scholar]
- 10.Tuna MM, Dogan BA, Karakilic E, Arduc A, Isik S, et al. Evaluation of adipocytokine levels and vascular functions in young aged to middle aged men with idiopathic hypogonadotrophic hypogonadism. Neuro Endocrinol Lett. 2014;35:640–4. [PubMed] [Google Scholar]
- 11.Mao JF, Wu XY, Li NS, Lu SY, Jing ZM, et al. Testosterone replacement in hypogonadotropic hypogonadal young male improves insulin sensitivity. Zhonghua Yi Xue Za Zhi. 2008;88:2550–2. Article in Chinese. [PubMed] [Google Scholar]
- 12.Nieschlag E, Nieschlag S, Behre HM. Lifespan and testosterone. Nature. 1993;366:215. doi: 10.1038/366215a0. [DOI] [PubMed] [Google Scholar]
- 13.Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, et al. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–9. doi: 10.1210/jc.2007-0454. [DOI] [PubMed] [Google Scholar]
- 14.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88:622–6. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- 15.Bojesen A, Juul S, Birkebaek N, Gravholt CH. Increased mortality in Klinefelter syndrome. J Clin Endocrinol Metab. 2004;89:3830–4. doi: 10.1210/jc.2004-0777. [DOI] [PubMed] [Google Scholar]
- 16.Price WH, Clayton JF, Collyer S, de Mey R. Mortality ratios and life expectancy in X chromatin positive males. J Epidemiol Community Health. 1985;39:33–8. doi: 10.1136/jech.39.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swerdlow AJ, Hermon C, Jacobs PA, Alberman E, Beral V, et al. Mortality and cancer incidence in persons with numerical sex chromosome abnormalities: a cohort study. Ann Hum Genet. 2001;65:177–88. doi: 10.1017/S0003480001008569. [DOI] [PubMed] [Google Scholar]
- 18.Swerdlow AJ, Schoemaker MJ, Higgins CD, Wright AF, Jacobs PA. Cancer incidence and mortality in men with Klinefelter syndrome: a cohort study. J Natl Cancer Inst. 2005;97:1204–10. doi: 10.1093/jnci/dji240. [DOI] [PubMed] [Google Scholar]
- 19.Hasle H, Mellemgaard A, Nielsen J, Hansen J. Cancer incidence in men with Klinefelter syndrome. Br J Cancer. 1995;71:416–20. doi: 10.1038/bjc.1995.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salzano A, Arcopinto M, Marra AM, Bobbio E, Esposito D, et al. Klinefelter syndrome, cardiovascular system, and thromboembolic disease: review of literature and clinical perspectives. Eur J Endocrinol. 2016;175:R27–40. doi: 10.1530/EJE-15-1025. [DOI] [PubMed] [Google Scholar]
- 21.Pasquali D, Arcopinto M, Renzullo A, Rotondi M, Accardo G, et al. Cardiovascular abnormalities in Klinefelter syndrome. Int J Cardiol. 2013;168:754–9. doi: 10.1016/j.ijcard.2012.09.215. [DOI] [PubMed] [Google Scholar]
- 22.Bojesen A, Hertz JM, Gravholt CH. Genotype and phenotype in Klinefelter syndrome – Impact of androgen receptor polymorphism and skewed X inactivation. Int J Androl. 2011;34:e642–8. doi: 10.1111/j.1365-2605.2011.01223.x. [DOI] [PubMed] [Google Scholar]
- 23.Arnold AP, Reue K, Eghbali M, Vilain E, Chen X, et al. The importance of having two X chromosomes. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150113. doi: 10.1098/rstb.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan YX, Knuiman MW, Hung J, Divitini ML, Handelsman DJ, et al. Testosterone, dihydrotestosterone and estradiol are differentially associated with carotid intima-media thickness and the presence of carotid plaque in men with and without coronary artery disease. Endocr J. 2015;62:777–86. doi: 10.1507/endocrj.EJ15-0196. [DOI] [PubMed] [Google Scholar]
- 25.Yeap BB, Alfonso H, Chubb SA, Hankey GJ, Handelsman DJ, et al. In older men, higher plasma testosterone or dihydrotestosterone is an independent predictor for reduced incidence of stroke but not myocardial infarction. J Clin Endocrinol Metab. 2014;99:4565–73. doi: 10.1210/jc.2014-2664. [DOI] [PubMed] [Google Scholar]
- 26.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99:E9–18. doi: 10.1210/jc.2013-3272. [DOI] [PubMed] [Google Scholar]
- 27.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, et al. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–19. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pye SR, Huhtaniemi IT, Finn JD, Lee DM, O'Neill TW, et al. Late-onset hypogonadism and mortality in aging men. J Clin Endocrinol Metab. 2014;99:1357–66. doi: 10.1210/jc.2013-2052. [DOI] [PubMed] [Google Scholar]
- 29.Shores MM, Biggs ML, Arnold AM, Smith NL, Longstreth WT, Jr, et al. Testosterone, dihydrotestosterone, and incident cardiovascular disease and mortality in the cardiovascular health study. J Clin Endocrinol Metab. 2014;99:2061–8. doi: 10.1210/jc.2013-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmboe SA, Vradi E, Jensen TK, Linneberg A, Husemoen LL, et al. The association of reproductive hormone levels and all-cause, cancer, and cardiovascular disease mortality in men. J Clin Endocrinol Metab. 2015;100:4472–80. doi: 10.1210/jc.2015-2460. [DOI] [PubMed] [Google Scholar]
- 31.Hsu B, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, et al. Temporal changes in androgens and estrogens are associated with all-cause and cause-specific mortality in older men. J Clin Endocrinol Metab. 2016;101:2201–10. doi: 10.1210/jc.2016-1025. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton EJ, Davis WA, Makepeace A, Lim EM, Yeap BB, et al. Prevalence and prognosis of a low serum testosterone in men with type 2 diabetes: the Fremantle Diabetes Study Phase II. Clin Endocrinol (Oxf) 2016;85:444–52. doi: 10.1111/cen.13087. [DOI] [PubMed] [Google Scholar]
- 33.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–33. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 34.Cheung KK, Lau ES, So WY, Ma RC, Ozaki R, et al. Low testosterone and clinical outcomes in Chinese men with type 2 diabetes mellitus – Hong Kong Diabetes Registry. Diabetes Res Clin Pract. 2017;123:97–105. doi: 10.1016/j.diabres.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Fleishman SB, Khan H, Homel P, Suhail MF, Strebel-Amrhein R, et al. Testosterone levels and quality of life in diverse male patients with cancers unrelated to androgens. J Clin Oncol. 2010;28:5054–60. doi: 10.1200/JCO.2010.30.3818. [DOI] [PubMed] [Google Scholar]
- 36.Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, Kaur G, Bruera E. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer. 2004;100:851–8. doi: 10.1002/cncr.20028. [DOI] [PubMed] [Google Scholar]
- 37.Garcia JM, Li H, Mann D, Epner D, Hayes TG, et al. Hypogonadism in male patients with cancer. Cancer. 2006;106:2583–91. doi: 10.1002/cncr.21889. [DOI] [PubMed] [Google Scholar]
- 38.Pimpinelli F, Parenti M, Guzzi F, Piva F, Hokfelt T, et al. Presence of delta opioid receptors on a subset of hypothalamic gonadotropin releasing hormone (GnRH) neurons. Brain Res. 2006;1070:15–23. doi: 10.1016/j.brainres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Mendelson JH, Ellingboe J, Judson BA, Goldstein A. Plasma testosterone and luteinizing hormone levels during levo-alpha-acetylmethadol maintenance and withdrawal. Clin Pharmacol Ther. 1984;35:545–7. doi: 10.1038/clpt.1984.75. [DOI] [PubMed] [Google Scholar]
- 40.Haring R, Volzke H, Steveling A, Krebs A, Felix SB, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J. 2010;31:1494–501. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- 41.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 42.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, et al. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med. 2007;167:1252–60. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- 44.Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006;91:1254–60. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- 45.Handelsman DJ, Dong Q. Hypothalamo-pituitary gonadal axis in chronic renal failure. Endocrinol Metab Clin North Am. 1993;22:145–61. [PubMed] [Google Scholar]
- 46.Carrero JJ, Stenvinkel P. The vulnerable man: impact of testosterone deficiency on the uraemic phenotype. Nephrol Dial Transplant. 2012;27:4030–41. doi: 10.1093/ndt/gfs383. [DOI] [PubMed] [Google Scholar]
- 47.Haring R, Nauck M, Völzke H, Endlich K, Lendeckel U, et al. Low serum testosterone is associated with increased mortality in men with stage 3 or greater nephropathy. Am J Nephrol. 2011;33:209–17. doi: 10.1159/000324562. [DOI] [PubMed] [Google Scholar]
- 48.Grossmann M, Hoermann R, Ng Tang Fui M, Zajac JD, Ierino FL, et al. Sex steroids levels in chronic kidney disease and kidney transplant recipients: associations with disease severity and prediction of mortality. Clin Endocrinol. 2015;82:767–75. doi: 10.1111/cen.12656. [DOI] [PubMed] [Google Scholar]
- 49.Ruige JB, Mahmoud AM, De Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97:870–5. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- 50.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–53. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 51.Stanik S, Dornfeld LP, Maxwell MH, Viosca SP, Korenman SG. The effect of weight loss on reproductive hormones in obese men. J Clin Endocrinol Metab. 1981;53:828–32. doi: 10.1210/jcem-53-4-828. [DOI] [PubMed] [Google Scholar]
- 52.Khoo J, Piantadosi C, Worthley S, Wittert GA. Effects of a low-energy diet on sexual function and lower urinary tract symptoms in obese men. Int J Obes (Lond) 2010;34:1396–403. doi: 10.1038/ijo.2010.76. [DOI] [PubMed] [Google Scholar]
- 53.Pritchard J, Despres JP, Gagnon J, Tchernof A, Nadeau A, et al. Plasma adrenal, gonadal, and conjugated steroids before and after long-term overfeeding in identical twins. J Clin Endocrinol Metab. 1998;83:3277–84. doi: 10.1210/jcem.83.9.5136. [DOI] [PubMed] [Google Scholar]


