Abstract
The numbers of testosterone prescriptions written have increased several-fold worldwide, but the incidence of pathological hypogonadism due to hypothalamic, pituitary, and testicular disease has remained unchanged. Most of these prescriptions are being dispensed to middle-aged and older men who have experienced age-related decline in serum testosterone levels; a subset of the population in which benefits of testosterone replacement is at best, modest. Recently, some randomized controlled trials have reported increased cardiovascular events in men (mainly older men and those with prevalent cardiovascular disease) with testosterone use, and a few recent meta-analyses have confirmed these findings. In this review, we discuss trials of testosterone therapy that have reported higher cardiovascular events, relevant trials that have not reported increased cardiovascular events and large trials that have focused on cardiovascular risk (mainly atherosclerosis progression) as their main outcome. We also review findings from meta-analyses that have evaluated cardiovascular events in various testosterone trials. Finally, we discuss some potential mechanisms by which testosterone use might result in an increased cardiovascular risk. As none of the trials conducted to date were adequately powered to evaluate cardiovascular events, no firm conclusions can be drawn regarding the cardiovascular safety of testosterone therapy at this time. In the interim, we hope that this review will help practitioners make informed decisions regarding the care of their patients.
Keywords: atherosclerosis, cardiovascular, frailty, testosterone, thromboembolism
INTRODUCTION
The number of testosterone prescriptions written in the United States in the first decade of this century has progressively increased, with most of the prescriptions dispensed to middle-aged and older men.1 In 2011, approximately 3% of men over the age of 40 years received at least one prescription,2 and recent data suggest that this increase in dispensing is a global trend.3 Considering that (i) there has not been a population-level increase in the incidence of pathological ("classical") androgen deficiency (as a result of congenital or acquired hypothalamic, pituitary, or testicular disease), (ii) no new formal indications for testosterone therapy have emerged, and (iii) guidelines for male androgen deficiency have not changed,4,5,6,7 this increase in prescription rate is mainly driven by clinicians who prescribe testosterone to treat age-related low serum testosterone levels in middle-aged or older men (many who present with nonspecific symptoms) and is a result of a sophisticated marketing campaign by the industry.8 Interestingly (and unfortunately), one in six men who are prescribed testosterone therapy do not have a baseline serum testosterone measured, suggesting that practice patterns do not conform to published guidelines.9
There is general consensus that testosterone replacement is beneficial in young androgen-deficient men with an organic etiology for their androgen deficiency; these benefits include increased muscle mass and strength,10,11,12,13 increased bone mineral density,12,14,15 improvement in sexual function,12,16,17 and energy.12 Additionally, physiological testosterone replacement in young men is associated with a low frequency of side effects that include acne, oily skin, transient breast tenderness or gynecomastia, and erythrocytosis; these side effects are dose-dependent and are more common with short-acting intramuscular injections.7 In contrast, in older men with age-related low testosterone, benefits of testosterone replacement are at best, modest. Although some recent large trials of testosterone therapy in older men have demonstrated modest improvements in sexual function,18,19 mood,19 bone density (although anti-fracture efficacy is unknown),20,21 and anemia (per se not an indication for testosterone therapy);22 no improvement was seen in cognitive function23,24 while effects on physical function have been inconsistent.18,19,25,26 Although benefits of testosterone therapy in older men are modest, its potential effect on prostate safety remains unknown. Furthermore, some recent trials have reported an increased incidence of cardiovascular adverse events in men on testosterone therapy, mainly involving older men and those with prevalent cardiovascular disease.27,28,29,30,31 Although none of these trials were adequately powered to assess cardiovascular events, these studies have raised questions regarding the cardiovascular safety of testosterone therapy in this patient population.
In this review, we discuss (i) clinical trials of testosterone therapy that have reported cardiovascular events, (ii) relevant trials that did not show an increased risk, and (iii) large trials that have evaluated atherosclerosis progression as the primary outcome. We summarize meta-analyses of testosterone trials that have evaluated cardiovascular events. Finally, we discuss some potential mechanisms that might be involved in cardiovascular events reported in some studies.
SMALL MECHANISTIC STUDIES SHOWING POTENTIAL CARDIOVASCULAR BENEFIT OF TESTOSTERONE THERAPY
In a few small mechanistic studies, testosterone therapy has shown benefits in some select cardiovascular parameters. In a placebo-controlled study of 50 men with ST-segment depression on exercise testing at baseline, treatment with intramuscular testosterone cypionate at 200 mg per week for 8 weeks showed a 51% reduction in the sum of ST segment depression at the end of treatment.32 In another 12-week trial of 5 mg transdermal testosterone patch in 22 men with stable angina, the time to 1 mm ST-segment depression on the treadmill exercise test improved with testosterone.33 Beneficial effects of testosterone on myocardial ischemia were also reported in a small 12-month study of 15 men; 7 of whom received intramuscular testosterone undecanoate.34 The results of these trials seem to suggest that testosterone has beneficial effects on the coronary vasculature; this was verified in another small study of 13 men with angiographically proven coronary artery disease showing coronary vasodilation in response to intracoronary administration of testosterone.35
Limited data, mainly from small trials, also suggest beneficial effect of testosterone on aerobic function. A trial of 76 men with congestive heart failure given transdermal testosterone patch for 12 months improved their performance on the incremental shuttle walk test.36 However, only a quarter of these men had low testosterone levels at baseline and only 42 men completed the 12-month intervention. Another 12-week trial of long-acting intramuscular testosterone preparation in older men with chronic heart failure improved aerobic capacity, baroreflex sensitivity, and 6-min walking distance.37 Similarly, in a subset analysis of 64 mobility-limited older participants in the Testosterone in Older Men with Mobility Limitation (TOM) Trial, daily application of testosterone gel for 6 months attenuated the age-related decline in aerobic function.38
Together, these mechanistic studies suggest that testosterone therapy might be beneficial to the cardiovascular system; however, the findings from these studies should be interpreted with caution as - (i) these trials enrolled a small number of men, (ii) all enrolled subjects did not have low serum testosterone levels, and (iii) some studies used routes of testosterone administration that cannot be replicated in clinical practice (e.g., intra-coronary infusion).
CARDIOVASCULAR EVENTS IN CLINICAL TRIALS OF TESTOSTERONE REPLACEMENT
In this section, we discuss trials that have reported higher incidence of cardiovascular events in men treated with testosterone, relevant trials of testosterone therapy that have not reported increased cardiovascular events and relatively large clinical trials that assessed atherosclerosis progression as their primary outcome.
The copenhagen study group: testosterone in alcoholic cirrhosis trial
One of the first trials that reported a higher mortality with testosterone treatment evaluated the effects of 600 mg of micronized testosterone compared with placebo on survival in 221 men with alcoholic cirrhosis.39 Participants were followed for a median of 28 months. The trial was stopped prematurely as the mortality data revealed an increased death rate in the testosterone group (17% higher than placebo); the majority of the deaths were apparently related to hepatic complications. This trial was neither designed to evaluate cardiovascular risk nor did it demonstrate increased cardiovascular event rate (only one confirmed myocardial infarction); however, it deserves mention as it is frequently cited and included in meta-analyses reviewing cardiovascular safety of testosterone therapy.
Clinical trial in men 65 years and older
In a 36-month trial of transdermal testosterone replacement in men aged 65 years and older,40,41 a higher number of cardiovascular events were seen in the testosterone arm (9 vs 5 in placebo group), although this difference was not statistically significant.42 Interestingly, 3 of the events in men receiving testosterone were arrhythmias. A secondary analysis of this trial showed that testosterone therapy did not significantly influence any of the lipoprotein parameters.42 Although the total number of events in this trial was small, this trial is also frequently included in meta-analyses.
Clinical trial in men with anemia of chronic renal disease
This frequently-cited trial evaluated whether treatment with transdermal testosterone gel reduces the requirement for recombinant human erythropoietin in forty men on hemodialysis who had low serum testosterone and anemia of renal failure.43 A slightly higher number of cardiovascular events occurred in the testosterone group compared with placebo (7 vs 3); details regarding these events were not reported. This trial was also not designed to evaluate cardiovascular events.
TOM trial
The TOM trial was published in 2010 and reported an increased incidence of cardiovascular-related events in older men receiving testosterone therapy.29 The trial enrolled men 65 years of age or older with limitations in mobility (based on subjective and objective criteria) and serum total testosterone <350 ng dl−1 or free testosterone <50 pg ml−1 who either received 100 mg of transdermal testosterone gel or placebo gel for 6 months. The original goal of the trial was to randomize 252 men; however, the trial was stopped prematurely (after 209 randomizations) by the trial's Data and Safety Monitoring Board because of higher incidence of cardiovascular-related events in the testosterone arm (23 vs 5 in the placebo arm). The cardiovascular-related events seen were diverse and included both atherosclerotic and nonatherosclerotic events; major adverse cardiovascular events were only seen in the testosterone group.29 Interestingly, the difference in the frequency of cardiovascular events between the two groups was apparent within weeks of randomization, suggesting an acute mechanism behind these events (Figure 1). In the testosterone arm, men who experienced cardiovascular-related events had higher on-treatment circulating serum total testosterone concentrations compared with men who did not;29 however, secondary analyses showed that only changes in serum free testosterone levels were associated with events.44
Figure 1.
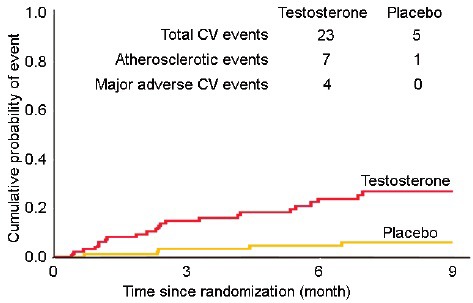
Cardiovascular events seen in the TOM Trial. The Kaplan–Meier plot shows divergence of the curves weeks after randomization (adapted from Basaria et al.29). TOM: Testosterone in Older Men with Mobility Limitation.
A few aspects of the TOM Trial should be highlighted. First, the participants in the TOM Trial had a high prevalence of cardiovascular risk factors at baseline; not an unexpected observation as the burden of comorbidities is high in mobility-limited men. Indeed, nearly 25% of the participants had diabetes mellitus, half were obese, >80% had hypertension and approximately 50% of the participants had preexisting heart disease. Second, the starting dose of testosterone gel in the TOM Trial was higher than the dose at which treatment is initiated in clinical practice. However, the on-treatment circulating mean serum testosterone levels in the TOM Trial were comparable to the levels seen in older men who participated in other trials;29 however, the majority of other studies enrolled “healthy” older men. Finally, the diversity of cardiovascular-related events seen in the TOM Trial does not suggest a single mechanism; though the rapidity with which the events occurred suggests an acute mechanism. Indeed, this early increase in cardiovascular risk was also reported in a large population study that showed a 2-fold increase in the risk of cardiovascular events within the first 90 days following the initiation of testosterone therapy.45 In contrast, early cardiovascular events have not been reported in other epidemiologic studies or in the Testosterone Trials (TTrials).19
Clinical trial in intermediate-frail and frail men
In an elegant clinical trial from Manchester, United Kingdom, 274 intermediate-frail and frail men (based on the Fried criteria) aged 65 years and older with serum testosterone concentration less than 345 ng dl−1 were randomized to topical testosterone gel or placebo for 6 months; the starting daily dose was 50 mg.18 This trial did not find a higher incidence of cardiovascular events in the testosterone group (one case each of myocardial infarction and ruptured aneurysm [death] in the placebo group while one case each of constrictive pericarditis [death], angina, pulmonary embolism, and abdominal aneurysm surgery in the testosterone arm). This trial is frequently cited along with the TOM Trial, mainly to contrast the cardiovascular findings between the two trials; however, unlike the TOM Trial (in which all participants were mobility-limited), only 15% of men in this trial met the criteria of frailty while the remaining men were intermediate-frail.18 Consideration of this characteristic of this trial's population is important as frailty is associated with a greater burden of subclinical cardiovascular disease compared to subjects who are prefrail.46 In addition, in contrast to the TOM Trial, the average baseline serum testosterone concentrations in men participating in this trial were in the low-normal range; therefore, the findings of this trial cannot be extrapolated to men with unequivocally low serum testosterone levels.
The Testosterone's Effect on Atherosclerosis Progression in Aging Men (TEAAM) Trial
The Testosterone's Effects on Atherosclerosis Progression in Aging Men (TEAAM) trial was designed to evaluate the effect of testosterone therapy in 308 men, 60 years and older, with low or low-normal total testosterone (100–400 ng dl−1) or free testosterone <50 pg ml−1 on progression of subclinical atherosclerosis in the common carotid artery (assessed by sonographic measurement of common carotid intima-media thickness) and the coronary arteries (assessed by measurement of coronary artery calcium score with computerized tomography scan).47 Men received either 7.5 g of 1% transdermal testosterone gel or placebo gel daily for 3 years. Overall, there were small number of cardiovascular adverse events in the trial, and the major adverse cardiac events did not differ between the two groups: three men in the testosterone versus two in the placebo arm had myocardial infarction, five men in the testosterone versus two in the placebo arm underwent coronary revascularization, three men in the testosterone versus zero in the placebo arm experienced a stroke, and one man in the testosterone versus zero in the placebo group died of a cardiovascular-related event.47 No significant difference in the rates of change in either common carotid artery intima-media thickness or coronary artery calcium scores was observed between the groups (Figure 2).47 These findings suggest that testosterone treatment is not associated with progression of carotid intima-media thickness or progression of calcified coronary plaques; noncalcified coronary plaques were not measured in this trial. Of note, the mean baseline serum testosterone concentrations in men participating in the TEAAM trial was in the low-normal range; therefore, these findings cannot be extrapolated to men with low serum testosterone levels.
Figure 2.
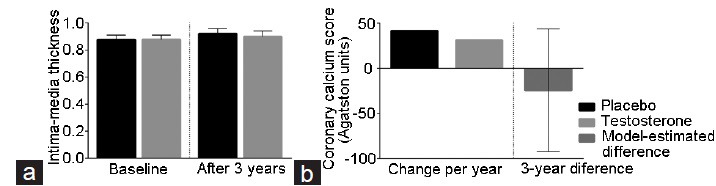
Mean change from baseline in the common carotid intima-media thickness (a) and in the calcified coronary atherosclerotic plaques (b) in the TEAAM trial (adapted from Basaria et al.47). Error bars represent 95% confidence interval. The model-estimated between-group difference represents the change from baseline to the end-of-treatment in coronary artery calcium scores in participants who completed the intervention. The model controls stratification by age group and study center, using mixed-effects regression model in which subjects were nested within study center (random effect). Age-group, time-in-treatment, and treatment-by-time interaction were included as fixed effects, with the latter quantifying the estimated treatment effect. The missing data were accommodated using multiple imputations in which missing outcomes values were estimated using predictive mean matching. TEAAM: Testosterone's Effect on Atherosclerosis Progression in Aging Men.
Considering that some studies have shown that cardiovascular adverse events occur early after initiation of testosterone treatment,29,45 the TEAAM trial investigators also evaluated the effect of testosterone treatment on electrocardiographic parameters, mainly ventricular repolarization, as assessed by the corrected QT interval (QTc).48 As prolongation of the QT interval is a risk factor for ventricular arrhythmias and sudden death,49,50,51 the TEAAM trial provided an opportunity to assess the long-term effect of testosterone treatment on cardiac conduction system. In addition to the TEAAM trial, the same investigators also evaluated the effect of testosterone therapy on QTc in the Testosterone and Pain (TAP) trial,52 a 14-week trial assessing the efficacy of testosterone replacement on pain perception in men with opioid-induced androgen deficiency. The investigators found that testosterone treatment did not prolong QTc duration in either trial. Furthermore, testosterone therapy was associated with attenuation of the expected age-related increase in QTc duration in the TEAAM trial. These findings suggest that testosterone administration is not associated with arrhythmogenic changes,48 making it an unlikely mechanism behind acute cardiovascular events.
The TTrials
The TTrials were a group of seven coordinated multicenter, double-blind, placebo-controlled trials evaluating the efficacy of testosterone replacement in 790 men, 65 years or older, with an average of two total testosterone values <275 ng dl−1.19 Men were randomized to daily application of topical testosterone gel or placebo gel for 1 year. At the end of the intervention phase of the trial, there was no difference in the number of cardiovascular events between the two groups; seven men in each group experienced a major adverse cardiovascular event (myocardial infarction, stroke or death related to cardiovascular disease).19
One of the seven coordinated trials of the TTrials was the Cardiovascular Trial, which evaluated the effect of testosterone treatment on the progression of both calcified and noncalcified coronary artery plaque volume using computerized tomography angiography scan in a subset of 138 participants.53 After 12 months of intervention, testosterone treatment was associated with a greater increase in the volume of noncalcified plaque compared with placebo (Figure 3); there was no significant difference in the change in calcified plaque between the groups. Although definitive evidence regarding cardiovascular safety of testosterone therapy will only emerge from large, adequately powered trials that are designed to assess cardiovascular event rate, based on these findings of greater progression in noncalcified plaque, one might speculate that the rupture (and thrombosis) of the soft plaque might explain some of the cardiovascular events that occur early after initiation of testosterone therapy.
Figure 3.
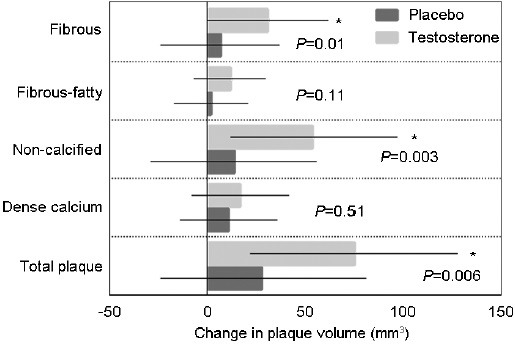
Change from baseline in the volume of various components of the coronary atherosclerotic plaques in the Cardiovascular Trial of the Testosterone Trials (adapted from Budoff et al.53). Bars are adjusted least square means and error bars represent 95% confidence interval.
In summary, the number of cardiovascular events reported in TTrials are low; in fact, it is lower than the expected rate for the age and comorbidities of the participants.54,55 None of the trials discussed above was powered to evaluate cardiovascular events and most enrolled a small number of participants, making any small differences between groups inconclusive. Furthermore, the recording and reporting of adverse events in previous testosterone trials have not followed a standardized format, underscoring the need for standardization of adverse event reporting in future trials.56
META-ANALYSES OF RANDOMIZED TTRIALS REPORTING CARDIOVASCULAR EVENTS
Several meta-analyses have attempted to elucidate the association between testosterone administration and cardiovascular events; however, it is difficult to draw firm conclusions as these analyses have pooled trials that were of low-to-medium quality, enrolled men with variable characteristics, used different testosterone doses and formulations, duration of treatment was not uniform, and none of these trials were powered to evaluate cardiovascular events.57 As a result, some meta-analyses of placebo-controlled trials have not found an association between testosterone therapy and cardiovascular events,54,55,58,59 whereas others have found such an association (Figure 4).27,31
Figure 4.
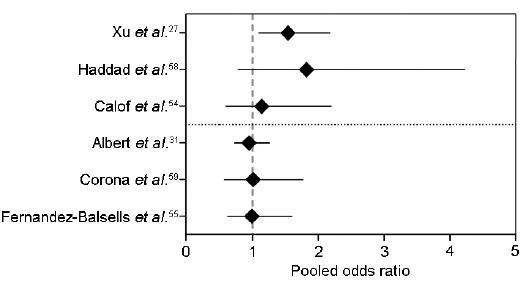
Forest-plot of meta-analyses discussed in this review showing the pooled odds ratio for cardiovascular events
Meta-analyses showing neutral effects of testosterone therapy
In 2005, a meta-analysis of 19 randomized controlled trials of men 45 years or older with low or low-normal testosterone who were treated with testosterone (n = 651) or placebo (n = 433) for at least 90 days was published.54 The pooled analyses showed 18 cardiovascular events in the testosterone arm (including four myocardial infarctions and three strokes), whereas 16 events were observed in the placebo group (including three myocardial infarctions and four strokes); the difference in event rate with testosterone treatment was not significantly different (pooled odds ratio [OR] for all cardiovascular events 1.14, 95% confidence interval [CI]: 0.59 – 2.20). Although these findings seem to be reassuring, this meta-analysis included relatively young men. Another study reviewed thirty clinical trials comprising a total pool of 1642 men;58 however, the authors concluded that most of these trials were not methodologically sound and short-listed only six clinical trials (that reported allocation concealment) for their meta-analysis. In these six trials, 14 cardiovascular events occurred in 161 men who received testosterone while seven events occurred among the 147 men who were assigned to placebo, resulting in a pooled OR of 1.82 (95%CI: 0.78–4.23), which was statistically nonsignificant.58 The OR for fatal and nonfatal myocardial infarction was 2.24 (95%CI: 0.5–10.02). The authors concluded that their data weakly supports the notion that use of testosterone in men is not associated with clinically important cardiovascular events. Another systematic review and meta-analysis of 51 randomized and nonrandomized trials that included 2679 men showed no significant effect of testosterone therapy on mortality or any cardiovascular outcome (including arrhythmias, coronary bypass surgery, and myocardial infarction).55 However, these studies were considered low to medium quality, and of the 51 studies included, only nine reported cardiovascular outcomes. A recent meta-analysis of 75 randomized controlled trials also did not find an association between testosterone treatment and cardiovascular events.59
Meta-analyses showing harmful effects of testosterone therapy
In a meta-analysis by Xu et al.27 27 trials were selected, yielding a sample of 2994 men who experienced 180 cardiovascular-related events. In this study, testosterone therapy was associated with an increased risk of a cardiovascular-related event (OR: 1.54; 95%CI: 1.09–2.18). Interestingly, this risk was higher in trials that were not funded by the pharmaceutical industry (OR: 2.06; 95%CI: 1.34–3.17 vs OR: 0.89; 95%CI: 0.50-1.60 for industry-funded studies).27 Possible explanations for this discrepancy include: (i) difference in age of the participants as industry-funded trials generally enrolled younger men, and (ii) trials conducted in an academic setting usually enroll subjects with a higher degree of morbidity, as the main objective of such trials is to improve health outcomes (such as mobility-limitation, sexual dysfunction, and depression); to the contrary, the primary objective of many industry-funded trials is to evaluate the efficacy of their product in increasing serum testosterone levels, which can be achieved by enrolling hypogonadal men who are generally healthy.
Another recent systematic review and meta-analysis evaluated the risk of testosterone therapy on cardiovascular events in 5328 men who were pooled from 45 clinical trials.31 Overall, testosterone treatment was not associated with an increased cardiovascular event risk ratio (RR 1.10; 95%CI: 0.86–1.41; P = 0.45). However, in men age 65 years or older, there was a significant increase in cardiovascular event rate (RR: 2.90; 95%CI: 1.35–6.21; P = 0.006) which was evident mainly in the first 12 months of therapy. Oral (RR: 2.28; 95%CI: 2.28–8.59; P = 0.22) and transdermal testosterone therapy were associated with higher cardiovascular event rate (RR: 2.80; 95%CI: 1.38–5.68; P = 0.004) compared with intramuscular testosterone administration (RR: 0.96; 95%CI: 0.46–1.98; P = 0.91).
In summary, these meta-analyses provide limited information, as they are limited by the fact that most studies included were low-to-medium quality and none was powered to evaluate cardiovascular events. Therefore, firm conclusions regarding the cardiovascular safety of testosterone therapy cannot be drawn from these data.
POTENTIAL MECHANISMS INVOLVED IN CARDIOVASCULAR EVENTS
As cardiovascular events seen in some testosterone trials have been diverse, it is difficult to implicate a single mechanism. Preclinical data from mechanistic animal studies show both beneficial and detrimental effects of testosterone administration. For instance, animal studies have shown that androgens promote sodium reabsorption in the kidneys by up-regulating the renin-angiotensin system, which leads to expansion in extracellular volume which in turn, could lead to hypertension.60,61 To the contrary, testosterone stimulates nitric oxide (NO) production by the endothelial cells in vitro;62,63 indeed, NO-mediated vasodilation of coronary and other systemic arteries in response to testosterone administration has been shown in several animal models.64,65,66,67 Similarly, animal studies show that testosterone administration stimulates proliferation and migration of vascular smooth muscle cells in culture through both genomic and nongenomic mechanisms,68 but in androgen-deficient animal models of atherosclerosis, testosterone administration protects against plaque formation.69,70,71,72
Similar to animal studies, data from human studies are also conflicting (as discussed earlier). However, based on the types of adverse events seen in clinical trials, we discuss some potential mechanisms that might be involved in triggering these events.
Hypercoagulability and thrombosis
Some studies have suggested that testosterone administration induces platelet aggregability by increasing thromboxane A2 receptor density on human platelets,73 which is reversed by castration.74 A recent population-based case-control study reported that testosterone treatment was associated with increased risk of venous thromboembolism within the first 6 months of treatment regardless of the underlying risk factors;75 though some other population studies have not shown this association. In addition to the independent effect of testosterone on platelet aggregation, estradiol (an active metabolite of testosterone) also has direct effects on platelet aggregability and thrombosis. In the TOM Trial, men who were randomized to testosterone and experienced cardiovascular events had higher on-treatment circulating mean serum total testosterone, free testosterone, estradiol, and estrone levels compared with men in the testosterone arm who did not have an event (Figure 5).44 Though in a different context than testosterone replacement, the Coronary Drug Project and studies of men with prostate cancer, in which participants were treated with exogenous estrogens, also observed a higher incidence of myocardial infarction, stroke, and pulmonary embolism.76,77,78 Hence, it is conceivable that early events seen in some testosterone trials might be related to thrombosis.
Figure 5.
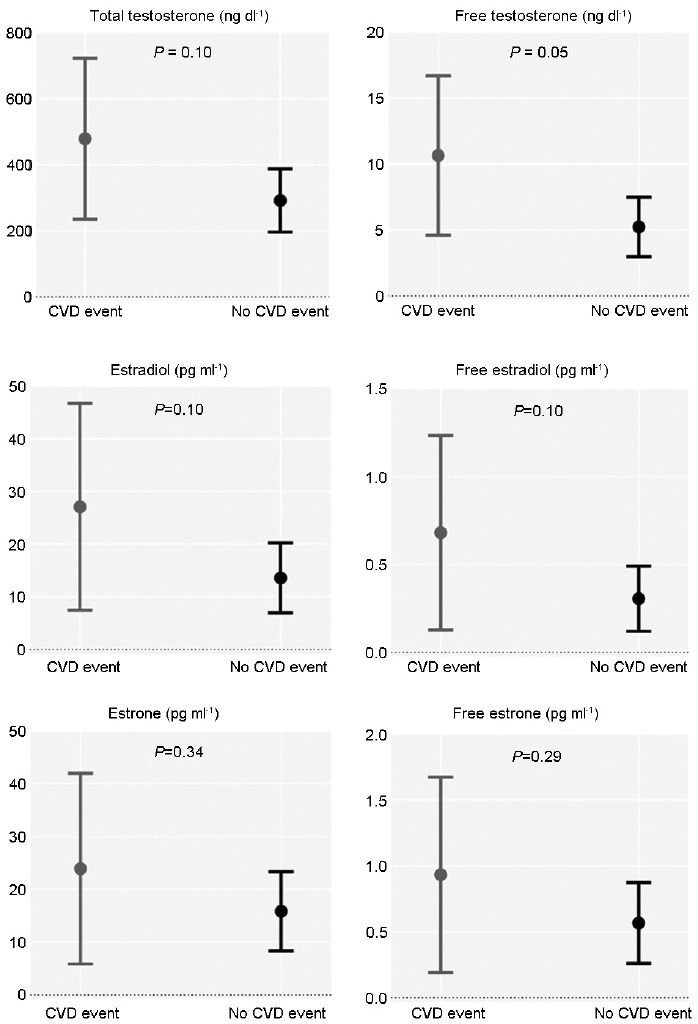
Changes in on-treatment circulating serum sex steroids from baseline to 6 months in the testosterone group of the TOM Trial according to cardiovascular event occurrence (adapted from Basaria et al.44). TOM: Testosterone in Older Men with Mobility Limitation; CVD: cardiovascular disease.
Plaque destabilization and rupture
Another potential mechanism behind acute cardiovascular events might be plaque destabilization. Erythrocytosis resulting from testosterone administration increases blood viscosity, which is a key parameter responsible for shear stress action on the endothelial wall,79 and a trigger for plaque rupture.80,81,82 Because noncalcified plaques in the coronary artery are considered unstable (and are most vulnerable to rupture), and as recent evidence from the TTrial showed the greater progression of noncalcified coronary plaques in the testosterone group,53 it is conceivable that some early events associated with testosterone treatment might be due to plaque erosion and thrombosis.
Fluid retention
Another mechanism by which testosterone might contribute to cardiovascular events is by salt and water retention. It has been known for some time that testosterone increases renal sodium reabsorption, which results in an expansion of extracellular volume83 and an increase in blood pressure.84 In healthy men, pressure natriuresis leads to excretion of this excess water, however, men with underlying cardiac or renal abnormalities may not be able to efficiently excrete excess water, which could result in fluid overload. Indeed, exacerbation of congestive heart failure has been reported in some trials of testosterone therapy in older men with underlying comorbidities.29,44
CONCLUSIONS
To date, not a single published trial of testosterone treatment was adequately powered to assess cardiovascular events. Only a large, well-designed, adequately-powered, and randomized placebo-controlled trial will definitively answer the question regarding cardiovascular safety of testosterone therapy. Until such a trial is conducted, and the results become available, the clinicians should have an open discussion with their patients regarding the available evidence of cardiovascular safety of testosterone therapy. This discussion will not only allow the clinicians to counsel men regarding their likelihood to benefit from testosterone therapy and to discuss their risk/benefit ratio, but will also help the patients in making informed decisions. In the interim, the investigators should conduct mechanistic studies to understand the effects of testosterone on coagulation parameters, platelet function, plaque stability and water metabolism. Based on the current evidence, no firm conclusions can be drawn regarding the cardiovascular safety of testosterone therapy.
AUTHOR CONTRIBUTIONS
TGJ and SB conceptualized this work together and also contributed equally to the drafting of the manuscript. Both authors read and approved the final manuscript.
COMPETING INTERESTS
Dr. Basaria has previously served as a consultant to AbbVie Pharmaceuticals. Dr. Gagliano-Jucá has no competing interests.
ACKNOWLEDGMENTS
Dr. Gagliano-Jucá and Dr. Basaria would like to thank the staff of the Section on Men's Health and also the subjects who participated in their clinical trials.
REFERENCES
- 1.Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, et al. Testosterone and “age-related hypogonadism”- FDA concerns. N Engl J Med. 2015;373:689–91. doi: 10.1056/NEJMp1506632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465–6. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199:548–51. doi: 10.5694/mja13.10111. [DOI] [PubMed] [Google Scholar]
- 4.Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, and EAU recommendations. Int J Androl. 2005;28:125–7. doi: 10.1111/j.1365-2605.2005.00553.x. [DOI] [PubMed] [Google Scholar]
- 5.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30:1–9. doi: 10.2164/jandrol.108.006486. [DOI] [PubMed] [Google Scholar]
- 7.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 8.Handelsman DJ. Testosterone and male aging: faltering hope for rejuvenation. JAMA. 2017;317:699–701. doi: 10.1001/jama.2017.0129. [DOI] [PubMed] [Google Scholar]
- 9.Jasuja GK, Bhasin S, Reisman JI, Berlowitz DR, Rose AJ. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53:746–52. doi: 10.1097/MLR.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men - A clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–75. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- 11.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, et al. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82:407–13. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 12.Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–7. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 13.Storer TW, Basaria S, Traustadottir T, Harman SM, Pencina K, et al. Effects of testosterone supplementation for 3-years on muscle performance and physical function in older men. J Clin Endocrinol Metab. 2017;102:583–93. doi: 10.1210/jc.2016-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, et al. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–65. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 15.Aversa A, Bruzziches R, Francomano D, Greco EA, Fornari R, et al. Effects of long-acting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 36 months controlled study. Aging Male. 2012;15:96–102. doi: 10.3109/13685538.2011.631230. [DOI] [PubMed] [Google Scholar]
- 16.Bancroft J, Wu FC. Changes in erectile responsiveness during androgen replacement therapy. Arch Sex Behav. 1983;12:59–66. doi: 10.1007/BF01542116. [DOI] [PubMed] [Google Scholar]
- 17.Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli G, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med. 2014;11:1577–92. doi: 10.1111/jsm.12536. [DOI] [PubMed] [Google Scholar]
- 18.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 19.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–24. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–72. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- 21.Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177:471–9. doi: 10.1001/jamainternmed.2016.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy CN, Snyder PJ, Stephens-Shields AJ, Artz AS, Bhasin S, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med. 2017;177:480–90. doi: 10.1001/jamainternmed.2016.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resnick SM, Matsumoto AM, Stephens-Shields AJ, Ellenberg SS, Gill TM, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA. 2017;317:717–27. doi: 10.1001/jama.2016.21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang G, Wharton W, Bhasin S, Harman SM, Pencina KM, et al. Effects of long-term testosterone administration on cognition in older men with low or low-to-normal testosterone concentrations: a prespecified secondary analysis of data from the randomised, double-blind, placebo-controlled TEAAM trial. Lancet Diabetes Endocrinol. 2016;4:657–65. doi: 10.1016/S2213-8587(16)30102-4. [DOI] [PubMed] [Google Scholar]
- 25.Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–10. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 26.Travison TG, Basaria S, Storer TW, Jette AM, Miciek R, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66:1090–9. doi: 10.1093/gerona/glr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borst SE, Shuster JJ, Zou B, Ye F, Jia H, et al. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC Med. 2014;12:211. doi: 10.1186/s12916-014-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onasanya O, Iyer G, Lucas E, Lin D, Singh S, et al. Association between exogenous testosterone and cardiovascular events: an overview of systematic reviews. Lancet Diabetes Endocrinol. 2016;4:943–56. doi: 10.1016/S2213-8587(16)30215-7. [DOI] [PubMed] [Google Scholar]
- 31.Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf) 2016;85:436–43. doi: 10.1111/cen.13084. [DOI] [PubMed] [Google Scholar]
- 32.Jaffe MD. Effect of testosterone cypionate on postexercise ST segment depression. Br Heart J. 1977;39:1217–22. doi: 10.1136/hrt.39.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–11. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 34.Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, et al. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161:443–9. doi: 10.1530/EJE-09-0092. [DOI] [PubMed] [Google Scholar]
- 35.Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–6. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- 36.Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, et al. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006;27:57–64. doi: 10.1093/eurheartj/ehi443. [DOI] [PubMed] [Google Scholar]
- 37.Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919–27. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- 38.Storer TW, Bhasin S, Travison TG, Pencina K, Miciek R, et al. Testosterone attenuates age-related fall in aerobic function in mobility limited older men with low testosterone. J Clin Endocrinol Metab. 2016;101:2562–9. doi: 10.1210/jc.2015-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Testosterone treatment of men with alcoholic cirrhosis: a double-blind study. The Copenhagen Study Group for Liver Diseases. Hepatology. 1986;6:807–13. [PubMed] [Google Scholar]
- 40.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966–72. doi: 10.1210/jcem.84.6.5741. [DOI] [PubMed] [Google Scholar]
- 41.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–53. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 42.Snyder PJ, Peachey H, Berlin JA, Rader D, Usher D, et al. Effect of transdermal testosterone treatment on serum lipid and apolipoprotein levels in men more than 65 years of age. Am J Med. 2001;111:255–60. doi: 10.1016/s0002-9343(01)00813-0. [DOI] [PubMed] [Google Scholar]
- 43.Brockenbrough AT, Dittrich MO, Page ST, Smith T, Stivelman JC, et al. Transdermal androgen therapy to augment EPO in the treatment of anemia of chronic renal disease. Am J Kidney Dis. 2006;47:251–62. doi: 10.1053/j.ajkd.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, et al. Risk factors associated with cardiovascular events during testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2013;68:153–60. doi: 10.1093/gerona/gls138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 47.Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314:570–81. doi: 10.1001/jama.2015.8881. [DOI] [PubMed] [Google Scholar]
- 48.Gagliano-Juca T, Icli TB, Pencina KM, Li Z, Tapper J, et al. Effects of testosterone replacement on electrocardiographic parameters in men: findings from two randomized trials. J Clin Endocrinol Metab. 2017;102:1478–85. doi: 10.1210/jc.2016-3669. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Post WS, Blasco-Colmenares E, Dalal D, Tomaselli GF, et al. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22:660–70. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noseworthy PA, Peloso GM, Hwang SJ, Larson MG, Levy D, et al. QT interval and long-term mortality risk in the Framingham Heart Study. Ann Noninvasive Electrocardiol. 2012;17:340–8. doi: 10.1111/j.1542-474X.2012.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen JB, Graff C, Rasmussen PV, Pietersen A, Lind B, et al. Risk prediction of cardiovascular death based on the QTc interval: evaluating age and gender differences in a large primary care population. Eur Heart J. 2014;35:1335–44. doi: 10.1093/eurheartj/ehu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basaria S, Travison TG, Alford D, Knapp PE, Teeter K, et al. Effects of testosterone replacement in men with opioid-induced androgen deficiency: a randomized controlled trial. Pain. 2015;156:280–8. doi: 10.1097/01.j.pain.0000460308.86819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER, 3 rd, Wenger NK, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317:708–16. doi: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–7. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–75. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 56.Basaria S. Need for standardising adverse event reporting in testosterone trials. Evid Based Med. 2014;19:32–3. doi: 10.1136/eb-2013-101402. [DOI] [PubMed] [Google Scholar]
- 57.Cunningham GR, Toma SM. Clinical review: why is androgen replacement in males controversial? J Clin Endocrinol Metab. 2011;96:38–52. doi: 10.1210/jc.2010-0266. [DOI] [PubMed] [Google Scholar]
- 58.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Bolona ER, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 59.Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13:1327–51. doi: 10.1517/14740338.2014.950653. [DOI] [PubMed] [Google Scholar]
- 60.Quan A, Chakravarty S, Chen JK, Chen JC, Loleh S, et al. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol. 2004;287:F452–9. doi: 10.1152/ajprenal.00188.2003. [DOI] [PubMed] [Google Scholar]
- 61.Quigley R. Androgens stimulate proximal tubule transport. Gend Med. 2008;5(Suppl A):S114–20. doi: 10.1016/j.genm.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Campelo AE, Cutini PH, Massheimer VL. Cellular actions of testosterone in vascular cells: mechanism independent of aromatization to estradiol. Steroids. 2012;77:1033–40. doi: 10.1016/j.steroids.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 63.Yu J, Akishita M, Eto M, Ogawa S, Son BK, et al. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology. 2010;151:1822–8. doi: 10.1210/en.2009-1048. [DOI] [PubMed] [Google Scholar]
- 64.Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, et al. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94:2614–9. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- 65.Molinari C, Battaglia A, Grossini E, Mary DA, Vassanelli C, et al. The effect of testosterone on regional blood flow in prepubertal anaesthetized pigs. J Physiol. 2002;543:365–72. doi: 10.1113/jphysiol.2002.022756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perusquia M, Greenway CD, Perkins LM, Stallone JN. Systemic hypotensive effects of testosterone are androgen structure-specific and neuronal nitric oxide synthase-dependent. Am J Physiol Regul Integr Comp Physiol. 2015;309:R189–95. doi: 10.1152/ajpregu.00110.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation. 1995;91:1154–60. doi: 10.1161/01.cir.91.4.1154. [DOI] [PubMed] [Google Scholar]
- 68.Chignalia AZ, Schuldt EZ, Camargo LL, Montezano AC, Callera GE, et al. Testosterone induces vascular smooth muscle cell migration by NADPH oxidase and c-Src-dependent pathways. Hypertension. 2012;59:1263–71. doi: 10.1161/HYPERTENSIONAHA.111.180620. [DOI] [PubMed] [Google Scholar]
- 69.Bruck B, Brehme U, Gugel N, Hanke S, Finking G, et al. Gender-specific differences in the effects of testosterone and estrogen on the development of atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1997;17:2192–9. doi: 10.1161/01.atv.17.10.2192. [DOI] [PubMed] [Google Scholar]
- 70.Nettleship JE, Jones TH, Channer KS, Jones RD. Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse: an effect that is independent of the classic androgen receptor. Circulation. 2007;116:2427–34. doi: 10.1161/CIRCULATIONAHA.107.708768. [DOI] [PubMed] [Google Scholar]
- 71.Bourghardt J, Wilhelmson AS, Alexanderson C, De Gendt K, Verhoeven G, et al. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology. 2010;151:5428–37. doi: 10.1210/en.2010-0663. [DOI] [PubMed] [Google Scholar]
- 72.Nathan L, Shi W, Dinh H, Mukherjee TK, Wang X, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci U S A. 2001;98:3589–93. doi: 10.1073/pnas.051003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91:2742–7. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- 74.Ajayi AA, Halushka PV. Castration reduces platelet thromboxane A2 receptor density and aggregability. QJM. 2005;98:349–56. doi: 10.1093/qjmed/hci054. [DOI] [PubMed] [Google Scholar]
- 75.Martinez C, Suissa S, Rietbrock S, Katholing A, Freedman B, et al. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ. 2016;355:i5968. doi: 10.1136/bmj.i5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.The Coronary Drug Project. Initial findings leading to modifications of its research protocol. JAMA. 1970;214:1303–13. [PubMed] [Google Scholar]
- 77.Hedlund PO, Johansson R, Damber JE, Hagerman I, Henriksson P, et al. Significance of pretreatment cardiovascular morbidity as a risk factor during treatment with parenteral oestrogen or combined androgen deprivation of 915 patients with metastasized prostate cancer: evaluation of cardiovascular events in a randomized trial. Scand J Urol Nephrol. 2011;45:346–53. doi: 10.3109/00365599.2011.585820. [DOI] [PubMed] [Google Scholar]
- 78.Mikkola AK, Ruutu ML, Aro JL, Rannikko SA, Salo JO. Parenteral polyoestradiol phosphate vs. orchidectomy in the treatment of advanced prostatic cancer. Efficacy and cardiovascular complications: a -year follow-up report of a national, prospective prostatic cancer study. Finnprostate Group. Br J Urol. 1998;82:63–8. doi: 10.1046/j.1464-410x.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 79.Jeong SK, Rosenson RS. Shear rate specific blood viscosity and shear stress of carotid artery duplex ultrasonography in patients with lacunar infarction. BMC Neurol. 2013;13:36. doi: 10.1186/1471-2377-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groen HC, Gijsen FJ, van der Lugt A, Ferguson MS, Hatsukami TS, et al. Plaque rupture in the carotid artery is localized at the high shear stress region: a case report. Stroke. 2007;38:2379–81. doi: 10.1161/STROKEAHA.107.484766. [DOI] [PubMed] [Google Scholar]
- 81.Tuenter A, Selwaness M, Arias Lorza A, Schuurbiers JC, Speelman L, et al. High shear stress relates to intraplaque haemorrhage in asymptomatic carotid plaques. Atherosclerosis. 2016;251:348–54. doi: 10.1016/j.atherosclerosis.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 82.Gijsen F, van der Giessen A, van der Steen A, Wentzel J. Shear stress and advanced atherosclerosis in human coronary arteries. J Biomech. 2013;46:240–7. doi: 10.1016/j.jbiomech.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Johannsson G, Gibney J, Wolthers T, Leung KC, Ho KK. Independent and combined effects of testosterone and growth hormone on extracellular water in hypopituitary men. J Clin Endocrinol Metab. 2005;90:3989–94. doi: 10.1210/jc.2005-0553. [DOI] [PubMed] [Google Scholar]
- 84.Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94:1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]


