Abstract
The aim of hormonal male contraception is to prevent unintended pregnancies by suppressing spermatogenesis. Hormonal male contraception is based on the principle that exogenous administration of androgens and other hormones such as progestins suppress circulating gonadotropin concentrations, decreasing testicular Leydig cell and Sertoli cell activity and spermatogenesis. In order to achieve more complete suppression of circulating gonadotropins and spermatogenesis, a progestin has been added testosterone to the most recent efficacy trials of hormonal male contraceptives. This review focusses on the potential effects of male hormonal contraceptives on cardiovascular risk factors, lipids and body composition, mainly in the target group of younger to middle-aged men. Present data suggest that hormonal male contraception can be reasonably regarded as safe in terms of cardiovascular risk. However, as all trials have been relatively short (< 3 years), a final statement regarding the cardiovascular safety of hormonal male contraception, especially in long-term use, cannot be made. Older men with at high risk of cardiovascular event might not be good candidates for hormonal male contraception. The potential adverse effects of hormonal contraceptives on cardiovascular risk appear to depend greatly on the choice of the progestin in regimens for hormonal male contraceptives. In the development of prospective hormonal male contraception, data on longer-term cardiovascular safety will be essential.
Keywords: cardiovascular risk, male contraception, progestins, testosterone
INTRODUCTION
Methods of hormonal contraception for women have been available for many years. Why there is a need to develop a hormonal form of contraception for men as there are non-hormonal applications such as condoms and vasectomy? There is a demand from men and women that a reversible and reliable method for men should be established for several reasons. Men have expressed a desire to gain control over their reproductive capacities and take a more active role in family planning. About 50%–70% of men in Europe, North and South America, Australia, and Asia would be willing to use a hormonal method for contraception.1 Their female partners have indicated support for male-based contraceptives so that advantages but also burdens and putative risks of such medications are shared within the couple.2
Women support the development of male-based contraceptives as an important component of women's health. During 2010–2014, an estimated 56 million induced abortions occurred each year worldwide. Complications from unsafe abortions are common in developing regions. Estimates for 2012 indicate that 6.9 million women in these regions were treated for complications from unsafe abortions, corresponding to a rate of 6.9 women treated per 1000 women aged 15–44 years. About 40% of women who experience complications never receive required follow-up treatment. Almost all abortion-related deaths occur in developing countries, with the highest number occurring in Africa. Recent studies estimate that 8%–18% of maternal deaths worldwide are due to unsafe abortion, and the number of abortion-related deaths in 2014 ranged from 22 500 to 44 000.3,4,5
However, hormonal male contraception represents an unusual class of drugs. It would be a medication that is given to one person to prevent a health event (pregnancy) in another person. Stricter ethical barriers than in the development of a drug to treat a disease might be applied in this case. Such a pharmaceutical regimen must withstand special scrutiny and pass high safety prerequisites. In light of discussions about the cardiovascular safety of hormonal treatments for hypogonadism in men and also reports of adverse effects of anabolic steroid abuse, the question of how a young man desiring to use hormonal male contraception should be counseled arises.
ENDOCRINE MECHANISM AND EFFICACY OF HORMONAL MALE CONTRACEPTION
The aim of hormonal male contraception is the reversible suppression of spermatogenesis to a level that is consistent with infertility. The principle is based on the suppression of circulating gonadotropin concentrations, thus suppressing testicular Leydig cell and Sertoli cell activity, sex steroid hormone production, and spermatogenesis. Since endogenous testosterone production is suppressed by male hormonal contraceptive regimens, these regimens must include androgens at a dosage that is sufficient for replacement therapy (Figure 1). Adequate suppression of spermatogenesis (to ≤1 × 106 ml−1) had been achieved in more than 95% of men within a few months of treatment in the six efficacy studies published to date.6,7 The goal of hormonal male contraception can be best achieved by testosterone (or a chemical derivative of testosterone) alone or by testosterone in combination with a progestin. There have been efficacy trials using testosterone alone, but they used rather high doses of testosterone and not all men suppressed their sperm counts. In order to achieve a further suppressive impact on the secretion of gonadotropins, a progestin has been added to the most recently contraceptive efficacy trials.
Figure 1.
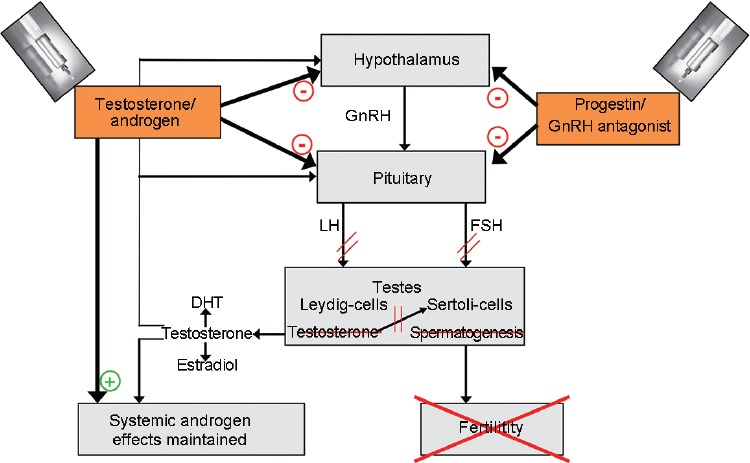
Schematic principle of the endocrine mechanism of hormonal male contraception. Testosterone or the androgen used suppresses GnRH release as well as the production of gonadotropins. This effect is augmented by additional use of a progestin or a long-acting GnRH antagonist. Testicular functions mediated by Leydig cells and Sertoli cells are attenuated and spermatogenesis is inhibited. Systemic androgen activity is maintained due to the administered androgen. DHT: double hydrogen testosterone; FSH: follicle-stimulating hormone; GnRH: gonadotropin-releasing hormone; LH: luteinizing hormone.
So far, clinical trials on potential hormonal male contraceptives have used testosterone combined with a variety of progestins including medroxyprogesterone, norethisterone, desogestrel, etonogestrel, cyproterone acetate, levonorgestrel, or nestorone. The success of this principle in terms of lowering sperm counts in semen to azoospermia or to severe oligozoospermia has been demonstrated in multiple studies.8,9,10,11,12,13
Some trials demonstrated the contraceptive efficacy of hormonal male contraception when couples used no other method of contraception; it is highly efficacious and results in failure rates of approximately 5%, provided sperm concentrations are maintained ≤1 × 106 ml−1. The failure rates compare favorably to the effectiveness of female oral contraceptives.11 Upon cessation of hormonal male contraception, sperm quality fully recovers in a predictable manner resulting in pregnancies and live birth.11,14
The most recent multicenter efficacy trial in hormonal male contraception was sponsored by the World Health Organization and Contraception Research and Development, and it tested the combination of intramuscular injections of testosterone undecanoate plus norethisterone enanthate at 8-week intervals. Of the 320 participating couples, 95.9 of 100 continuing users suppressed to a sperm concentration ≤1 × 106 ml−1 within 24 weeks. During the efficacy phase of up to 56 weeks, 4 pregnancies occurred with the rate of 1.57 per 100 continuing users. The cumulative reversibility of suppression of spermatogenesis after 52 weeks of recovery was 94.8 per 100 continuing users.7 This study published in 2016 reaffirmed previous studies.11
ANDROGEN EFFECTS IN HORMONAL MALE CONTRACEPTION COMPARED TO TESTOSTERONE REPLACEMENT THERAPY OF HYPOGONADAL MEN
A potential user of any novel hormonal male contraception would have to be advised of the safety profile of the androgenic and the progestinic components.
The most prominent question is whether the androgenic effects of hormonal male contraception more closely resemble those of testosterone replacement therapy of hypogonadal men or those seen in abusers of anabolic-androgenic steroids. The dose of testosterone used in hormonal male contraception is often slightly higher than that used in testosterone replacement therapy. Nevertheless, serum levels of testosterone remain generally within the normal range with the most recently developed hormonal male contraceptives. Trough serum testosterone concentrations are lower than pretreatment concentrations with some male hormonal contraceptive regimens that have been tested.7,11,15,16,17,18,19 This effect is most likely due to the suppression of endogenous androgen production and nonphysiological androgen regimens in the regimens.
The important androgen-related aspects during the application of hormonal male contraception regarding cardiovascular health are possible changes of hematocrit, lipids, and blood pressure as peak serum and trough testosterone concentrations may vary considerably from the baseline, eugonadal concentrations of men. A general picture reported by most trials is a slight but statistically significant increase in hematocrit (mean change by 1%–2%) and a slight, statistically significant decrease in serum high-density lipoprotein (HDL) cholesterol (mean change by 0.1–0.2 mmol l−1). Changes in other lipid, blood pressure, or glucose metabolism have been reported, but the results are variable and might be due to chance.7,9,15,18,19
ARE THE EFFECTS OF HORMONAL MALE CONTRACEPTIVES COMPARABLE TO ANABOLIC ANDROGEN (AAS) SUBSTANCE ABUSE?
Abuse of AAS is widespread. A study focusing on the effects of such abuse used a cross-sectional cohort design and recruited 140 experienced male weight lifters in the age group of 34–54 years. The cohort comprised 86 men reporting ≥2 years each of cumulative lifetime AAS use and 54 controls. Transthoracic echocardiography and coronary computed tomography angiography revealed pathologic findings in the abuser group compared to controls including left ventricular systolic and diastolic function as well as increased prevalence of advanced coronary artery plaque volume.20 This is consistent with previous reviews of case reports on AAS abuse.21
Studies of AAS use are problematic because of wide interindividual choice of AAS, cyclic use (“cycling”), and use of multiple AAS (“stacking”). In addition, men who abuse androgens often use diuretics, growth hormone, thyroid hormone, and insulin to enhance performance. Steroid abuse cannot be compared to hormonal male contraception in terms of cardiovascular risk.22
PROGESTINS IN HORMONAL MALE CONTRACEPTION
Another aspect of hormonal male contraception that has to be discussed is the progestin component of the regimen. These substances have inherent properties and are necessary to suppress gonadotropin secretion to a level that allows spermatogenesis to stop in a reversible manner. Progestins bind to the progesterone receptor as well as to the androgen receptor.12,13 The putative cardiovascular risk depends on the choice of the progestin: its binding capacity to the progesterone receptor and its antiandrogenic (e.g., cyproterone acetate), neutral androgenic (e.g., nestorone, which is also known as segesterone), or androgenic properties (e.g., levonorgestrel).12,13 A recent comparative trial of progestins for hormonal male contraception demonstrated that these substances can induce hypogonadism with a decrease in hemoglobin concentrations, reduced insulin sensitivity, and increased inflammation markers. Such effects were counteracted by the application of transdermal testosterone gel normalized to serum testosterone concentrations.23
It has been shown in male contraceptive clinical trials comparing testosterone alone versus testosterone plus norethisterone24 that individuals in the progestin-containing study arms exhibited increased plasma levels of pro-coagulant substances and also higher plasma concentrations of inflammatory markers than those in the testosterone only group.25,26 Inflammation is most likely promoting cardiovascular disease, and recent approaches involve anti-inflammatory treatment to reduce the incidence of cardiovascular events.27,28,29 The progestin norethisterone enanthate administered intramuscularly in combination with testosterone undecanoate was associated with an increase in pro-inflammatory interleukin-6, whereas testosterone alone resulted in decreased interleukin-6.26
A large multicenter, double-blind, placebo-controlled trial involving injectable testosterone undecanoate and implants of etonogestrel reported a slight decrease of serum HDL cholesterol (by 0.1 mmol l−1) versus placebo but no other significant changes in serum lipids.30
These results are comparable to those of the most recent large trial in hormonal male contraception also using both injectable testosterone undecanoate and norethisterone enanthate.7
In general, trials of hormonal male contraception have shown consistent findings in regard to suppression of HDL cholesterol and gaining weight. However, the pro-atherogenic LDL-cholesterol may be suppressed by androgens, too.12 There appears to be a dose-dependent effect of testosterone on serum HDL and weight; the second WHO efficacy trial that used high-dosage intramuscular testosterone alone was associated with an average decline of serum HDL of 13% and an average weight gain of 4 kg.31 Lower doses of testosterone alone cause markedly less HDL suppression (and weight gain) but are not as effective at suppressing sperm production, thus requiring an additional progestin for suppression of gonadotropins.12
When combining lower, physiological doses of testosterone with a progestin, this regimen improves sperm suppression, but HDL and weight are also adversely affected.32,33 HDL cholesterol suppression and weight gain are associated with the dosage of the progestin.12 Overall, the data suggest that the progestin component in hormonal contraceptive regimens contributes a significant proportion of the adverse effect of hormonal male contraceptives on metabolic risk factors for atherosclerotic heart disease.
The trials on hormonal male contraception have been too short to detect cardiovascular events or deep vein thrombosis, adverse events that occur with androgenic-anabolic steroid abuse,21 and it remains to be demonstrated whether the changes in HDL cholesterol, lipids, and hematocrit are clinically significant. In the trials completed to date, no safety signal has been seen. One has to remark, however, that the trials have been for only 1–2 years and the young healthy controls represent a low-risk population.
Hormonal male contraception is similar to testosterone replacement in many aspects, but hormonal male contraception includes a progestin that has important metabolic effects. In future regimens of hormonal male contraception, the choice of progestin will be paramount.23 Hormonal male contraception also is used in healthy, eugonadal men, and the effects of testosterone and sex steroids might differ from those seen in hypogonadal men.
ALTERNATIVES TO PROGESTINS IN HORMONAL MALE CONTRACEPTIVES
An alternative to the use of progestins in hormonal male contraception is long-acting gonadotropin-releasing hormone (GnRH) antagonists such as acyline that suppress the release of gonadotropins without exerting progestin-like effects. In a recent trial, dose-response impacts of various doses of transdermal testosterone gel in men having been pretreated by a GnRH antagonist resulted in corresponding serum sex steroid concentrations and were related to shifts in body composition and adipocytokines.34 This trial demonstrated that body composition changed dose dependently: lean mass increased and fat mass decreased with increasing serum concentrations of testosterone. As with other studies, weight gain was related to the testosterone dosage. There was no significant change in blood pressure in this study or previous hormonal contraceptive studies.12,34
CONCLUSIONS
Since there have not been adequately powered, long-term randomized clinical trials of hormonal contraceptive regimens, it cannot be concluded that these regimens are safe. However, the present data support the view that hormonal male contraception can be reasonably regarded as safe in terms of cardiovascular risk. However, as the trials were short of duration (not longer than a maximum of 2 years) and the participants were healthy younger men, a final statement regarding the cardiovascular safety of hormonal male contraception, especially in long-term use, cannot be made. Older men with a high risk of cardiovascular event might not be good candidates for hormonal male contraception as serum HDL cholesterol might decrease and erythrocytosis might be more likely.35,36 The choice of progestin in regimens for hormonal male contraception has to be made carefully, as these substances might increase blood coagulation and inflammation.
In the development of prospective hormonal male contraception, data on long-term cardiovascular safety will be essential.
COMPETING INTERESTS
The author declares no competing interests.
REFERENCES
- 1.Heinemann K, Saad F, Wiesemes M, White S, Heinemann L. Attitudes toward male fertility control: results of a multinational survey on four continents. Hum Reprod. 2005;20:549–56. doi: 10.1093/humrep/deh574. [DOI] [PubMed] [Google Scholar]
- 2.Glasier AF, Anakwe R, Everington D, Martin CW, van der Spuy Z, et al. Would women trust their partners to use a male pill? Hum Reprod. 2000;15:646–9. doi: 10.1093/humrep/15.3.646. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Darroch JE, Ashford LS. Adding It Up: The Costs and Benefits of Investing in Sexual and Reproductive Health. New York: Guttmacher Institute; 2014. [Google Scholar]
- 4.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 6.Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, et al. Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: an integrated analysis. J Clin Endocrinol Metab. 2008;93:1774–83. doi: 10.1210/jc.2007-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behre HM, Zitzmann M, Anderson RA, Handelsman DJ, Lestari SW, et al. Efficacy and safety of an injectable combination hormonal contraceptive for men. J Clin Endocrinol Metab. 2016;101:4779–88. doi: 10.1210/jc.2016-2141. [DOI] [PubMed] [Google Scholar]
- 8.Nieschlag E, Vorona E, Wenk M, Hemker AK, Kamischke A, et al. Hormonal male contraception in men with normal and subnormal semen parameters. Int J Androl. 2011;34:556–67. doi: 10.1111/j.1365-2605.2011.01142.x. [DOI] [PubMed] [Google Scholar]
- 9.Amory JK, Page ST, Bremner WJ. Drug insight: recent advances in male hormonal contraception. Nat Clin Pract Endocrinol Metab. 2006;2:32–41. doi: 10.1038/ncpendmet0069. [DOI] [PubMed] [Google Scholar]
- 10.Aaltonen P, Amory JK, Anderson RA, Behre HM, Bialy G, et al. 10th summit meeting consensus: recommendations for regulatory approval for hormonal male contraception. J Androl. 2007;28:362–3. doi: 10.2164/jandrol.106.002311. [DOI] [PubMed] [Google Scholar]
- 11.Piotrowska K, Wang C, Swerdloff RS, Liu PY. Male hormonal contraception: hope and promise. Lancet Diabetes Endocrinol. 2017;5:214–23. doi: 10.1016/S2213-8587(16)00034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page ST, Amory JK, Bremner WJ. Advances in male contraception. Endocr Rev. 2008;29:465–93. doi: 10.1210/er.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieschlag E, Zitzmann M, Kamischke A. Use of progestins in male contraception. Steroids. 2003;68:965–72. doi: 10.1016/s0039-128x(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 14.Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C, et al. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet. 2006;367:1412–20. doi: 10.1016/S0140-6736(06)68614-5. [DOI] [PubMed] [Google Scholar]
- 15.Nieschlag E. The struggle for male hormonal contraception. Best Pract Res Clin Endocrinol Metab. 2011;25:369–75. doi: 10.1016/j.beem.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Qoubaitary A, Meriggiola C, Ng CM, Lumbreras L, Cerpolini S, et al. Pharmacokinetics of testosterone undecanoate injected alone or in combination with norethisterone enanthate in healthy men. J Androl. 2006;27:853–67. doi: 10.2164/jandrol.106.000281. [DOI] [PubMed] [Google Scholar]
- 17.Roth MY, Ilani N, Wang C, Page ST, Bremner WJ, et al. Characteristics associated with suppression of spermatogenesis in a male hormonal contraceptive trial using testosterone and Nestorone(®) gels. Andrology. 2013;1:899–905. doi: 10.1111/j.2047-2927.2013.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilani N, Roth MY, Amory JK, Swerdloff RS, Dart C, et al. A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J Clin Endocrinol Metab. 2012;97:3476–86. doi: 10.1210/jc.2012-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu Y, Liang X, Wu W, Liu M, Song S, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1910–5. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- 20.Baggish AL, Weiner RB, Kanayama G, Hudson JI, Lu MT, et al. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation. 2017;135:1991–2002. doi: 10.1161/CIRCULATIONAHA.116.026945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieschlag E, Vorona E. Doping with anabolic androgenic steroids (AAS): Adverse effects on non-reproductive organs and functions. Rev Endocr Metab Disord. 2015;16:199–211. doi: 10.1007/s11154-015-9320-5. [DOI] [PubMed] [Google Scholar]
- 22.Nieschlag E, Vorona E. MECHANISMS IN ENDOCRINOLOGY: Medical consequences of doping with anabolic androgenic steroids: effects on reproductive functions. Eur J Endocrinol. 2015;173:R47–58. doi: 10.1530/EJE-15-0080. [DOI] [PubMed] [Google Scholar]
- 23.Zitzmann M, Rohayem J, Raidt J, Kliesch S, Kumar N, et al. Impact of various progestins with or without transdermal testosterone on gonadotropin levels for non-invasive hormonal male contraception: a randomized clinical trial. Andrology. 2017;5:516–26. doi: 10.1111/andr.12328. [DOI] [PubMed] [Google Scholar]
- 24.Kamischke A, Plöger D, Venherm S, von Eckardstein S, von Eckardstein A, et al. Intramuscular testosterone undecanoate with or without oral levonorgestrel: a randomized placebo-controlled feasibility study for male contraception. Clin Endocrinol (Oxf) 2000;53:43–52. doi: 10.1046/j.1365-2265.2000.01024.x. [DOI] [PubMed] [Google Scholar]
- 25.Zitzmann M, Junker R, Kamischke A, Nieschlag E. Contraceptive steroids influence the hemostatic activation state in healthy men. J Androl. 2002;23:503–11. [PubMed] [Google Scholar]
- 26.Zitzmann M, Erren M, Kamischke A, Simoni M, Nieschlag E. Endogenous progesterone and the exogenous progestin norethisterone enanthate are associated with a proinflammatory profile in healthy men. J Clin Endocrinol Metab. 2005;90:6603–8. doi: 10.1210/jc.2005-0847. [DOI] [PubMed] [Google Scholar]
- 27.Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrington RA. Targeting inflammation in coronary artery disease. N Engl J Med. 2017;377:1197–8. doi: 10.1056/NEJMe1709904. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 30.Mommers E, Kersemaekers WM, Elliesen J, Kepers M, Apter D, et al. Male hormonal contraception: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2572–80. doi: 10.1210/jc.2008-0265. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligospermia in normal men. Fertil Steril. 1996;65:821–9. [PubMed] [Google Scholar]
- 32.Gonzalo IT, Swerdloff RS, Nelson AL, Clevenger B, Garcia R, et al. Levonorgestrel implants (Norplant II) for male contraception clinical trials: combination with transdermal and injectable testosterone. J Clin Endocrinol Metab. 2002;87:3562–72. doi: 10.1210/jcem.87.8.8710. [DOI] [PubMed] [Google Scholar]
- 33.Bebb RA, Anawalt BD, Christensen RB, Paulsen CA, Bremner WJ, et al. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J Clin Endocrinol Metab. 1996;81:757–62. doi: 10.1210/jcem.81.2.8636300. [DOI] [PubMed] [Google Scholar]
- 34.Thirumalai A, Rubinow KB, Cooper LA, Amory JK, Marck BT, et al. Dose-response effects of sex hormone concentrations on body composition and adipokines in medically castrated healthy men administered graded doses of testosterone gel. Clin Endocrinol (Oxf) 2017;87:59–67. doi: 10.1111/cen.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachman E, Feng R, Travison T, Li M, Olbina G, et al. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95:4743–7. doi: 10.1210/jc.2010-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]


