Abstract
Previous studies have shown that oxidative stress and corporal fibrosis in penile tissues of rats were key pathological factors of erectile dysfunction induced by diabetic mellitus (DMED). Lipoxin A4 (LXA4) was reported to inhibit oxidative stress and fibrosis diseases, while whether it could exert a protective role on erectile function was not clear. Type I diabetic mellitus (DM) was induced in thirty male 10-week-old Sprague-Dawley rats using streptozotocin. Ten weeks later, twenty-two rats with DMED confirmed by an apomorphine test were divided into two groups: the DMED group (n = 11) and the DMED + LXA4 group (n = 11; LXA4 injection daily for 4 weeks). In addition, another ten age-matched rats formed the Control group. We found that erectile function was significantly impaired in the DMED group compared with the Control group, but was improved in the DMED + LXA4 group. Similarly, the over-activated oxidative stress and impaired endothelial function in the DMED group were both improved in the DMED + LXA4 group. Moreover, the DMED group showed serious corporal fibrosis, which was also inhibited by the treatment of LXA4 in the DMED + LXA4 group. Taken together, LXA4 could exert an inhibition role on oxidative stress and fibrosis to improve DMED effectively.
Keywords: diabetic mellitus, erectile dysfunction, fibrosis, oxidative stress
INTRODUCTION
Erectile dysfunction (ED), defined as the inability to achieve and maintain a penile erection adequate for satisfactory sexual intercourse, is a chronic condition that exerts a negative effect on male self-esteem and nearly all life domains including interpersonal and family relationships.1 It is a distressing common complication of diabetic mellitus (DM), and about 35%–90% of patients with DM suffer it, which starts about 10–15 years earlier than in the population without DM.2 In the Massachusetts Male Aging study, ED was three times more common in men with DM compared with nondiabetic men, and the annual, age-adjusted incidence of ED in men with DM was twice than that in nondiabetic men.3,4 Moreover, ED induced by DM (DMED) can be caused by various pathological factors such as endothelial dysfunction, atherosclerosis, and fibrosis.5,6 Together, DMED is difficult to treat, and brings men a poor response to the first-line drugs, phosphodiesterase type 5 inhibitors (PDE5Is), because of the complex pathological factors involved.7 Thus, a new treatment method for complex DMED is urgently needed.
It is widely accepted that nitric oxide (NO) induces the activation of soluble guanylyl cyclase and the accumulation of cyclic guanosine monophosphate (cGMP), resulting in smooth muscle relaxation and penile erection.8,9 Further, hyperglycemic conditions in diabetes cause oxidative stress inhibiting the activity of endothelial nitric oxide synthase (eNOS) and the formation of NO, which impairs the endothelial function of corpus cavernosum (CC) in the penis.7 Similarly, previous studies have revealed that reactive oxygen species (ROS) play an important role in the pathogenesis of DMED and provide a rationale for the use of antioxidant therapy in the treatment of DMED.10,11,12 Recently, it was found that Lipoxin A4 (LXA4), a metabolite of arachidonic acid found in human leukocytes, exhibits a strong anti-oxidative role via blocking ROS generation in endothelial cells.13 Moreover, LXA4, as a possible endogenous anti-diabetic molecule, could attenuate renal fibrosis and lung fibrosis through its inhibition role on the key factors of fibrosis including transforming growth factor beta (TGF-β) and connective tissue growth factor (CTGF).14,15 However, whether LXA4 might exert any effect on DMED is not clear.
Here, we generated a rat model of DMED and determined the effect of LXA4 treatment. We then explored the underlying mechanism of how LXA4 improves DMED, which might be beneficial to the treatment of DMED in humans.
MATERIALS AND METHODS
Animals
A total of forty male Sprague-Dawley (SD) rats were used for the experiment, and the rat model of type I DM was a classic one used by many other researchers for many years.10,11,12 Thirty male SD rats, 10 weeks of age and weighing 300–350 g, were injected with streptozotocin (STZ; 60 mg kg−1; Sigma-Aldrich; St. Louis, MO, USA) intraperitoneally, and another ten male age-matched SD rats, used as the Control group, were injected with vehicle (citrate phosphate buffer 0.1 mol l−1; pH 4.2) as in our previous study.16 Blood glucose levels of all rats were tested through a blood glucose meter (ACCU-CHEK Performa; Roche Diagnostics, Shanghai, China) 3 and 7 days after injection. Only rats with a fasting glucose level >16.7 mmol l−1 on both days were considered as having DM.
Ten weeks later, 28 rats with DM had survived. We evaluated the erectile function of all rats through an apomorphine (APO) test and only rats with the negative results of APO tests were confirmed to have DMED and used for further experiments (n = 22). These were injected intraperitoneally with LXA4 (10 μg kg−1 day−1; Cayman Chemical, Ann Arbor, MI, USA; DMED + LXA4 group; n = 11) or vehicle (0.9% normal saline; DMED group; n = 11) daily for another 4 weeks. This dose of LXA4 we use was as reported in previous studies.17,18,19 All procedures were approved by the Animal Care and Use Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China).
In vivo evaluation of erectile function
Erectile function of all rats was tested immediately after 4 weeks' treatment of LXA4 through electric stimulation as reported previously.11 First, they were anesthetized using pentobarbital (40 mg kg−1). Then, we exposed the left carotid artery of rats and cannulated it with a PE-50 tube. The tube was also connected to a data acquisition system (AD Instruments Powerlab/4SP, Bella Vista, NSW, Australia) to record mean arterial blood pressure (MAP) continuously. Next, the cavernous nerves at the posterolateral aspect of the prostate on both sides were exposed, followed by the electrical stimulation with a bipolar electrode at 15 Hz frequency, with a 1.2 ms width for 1 min. A 25-gauge needle was inserted into the right crus of the penis to monitor intracavernous pressure (ICP) continuously, which was measured at different voltages (2.5 V and 5.0 V), with a 3-min interval before the next stimulation. The ratio of maximal ICP and area under the curve (AUC) to MAP was used to evaluate erectile function.
Each rat's CC was cut into four pieces after discarding the place inserted by the needle. One of the three complete pieces was harvested and maintained in 4% paraffin for subsequent histologic studies, and the remaining three pieces were stored at −80°C for Western blotting, real-time reverse transcription quantitative polymerase chain reaction (RT-PCR), and other further assay, respectively.
Western blotting
As in our previous study,11 penile tissues were homogenized in RIPA buffer (Beyotime Biotechnology, Haimen, China) containing a protease inhibitor cocktail and phosphatase inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Concentrations of soluble proteins were measured using a bicinchoninic acid assay (Beyotime Biotechnology). Equal amounts of protein samples (30 μg per lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes, followed by blocking in Tris-buffered saline-Tween (TBST) with 5% bovine serum albumin (BSA) for 1 h. Then, membranes were incubated with primary antibodies against: eNOS (1:1000; ab5589, Abcam, Cambridge, MA, USA), p-eNOS (Ser1177; 1:1000; 9571; Cell Signaling Technology, Danvers, MA, USA), neuronal nitric oxide synthase (nNOS; 1:1000; ab76067; Abcam), p22phox (1:500; sc-271968, Santa Cruz, Dallas, Texas, USA), p47phox (1:1000; D154042, Sangon Biotech, Shanghai, China), gp91phox (1:500, 19013-1-AP, Proteintech); p40phox (1:500; D154073, Sangon Biotech), p67phox (1:1000; 15551-1-AP, Proteintech), α-smooth muscle actin (α-SMA; 1:1000; ab5694; Abcam), Collagen I (1:500; PB0981, Boster, Wuhan, China), Collagen IV (1:500; 19797-1-AP, Proteintech), TGF-β1 (1:1000; ab92486, Abcam), Smad2/3 (1:1000; 8685, Cell Signaling Technology), p-Smad2/3 (1:1000; 8828, Cell Signaling Technology), CTGF (1:1000; 23936-1-AP, Proteintech), and β-actin (1:1000, 20536-1-AP, Proteintech). Then, membranes were washed in TBST three times (10 min each), followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:5000) for 1 h at room temperature. After another 30 min of washing, membranes were analyzed using an enhanced chemiluminescence detection system (Pierce, Thermo Fisher Scientific, Rockford, IL, USA).
Examination of antioxidant activity
Penile tissues were stored at −80°C for assaying antioxidant activity. Test kits for measuring malondialdehyde (MDA, S0131, Beyotime Biotechnology) and superoxide dismutase (SOD, S0101, Beyotime Biotechnology) were obtained from Beyotime Biotechnology and used according to standard protocol. Penile MDA level and SOD activities were normalized to the wet weight of penile tissues.
Analysis of NO levels
For the analysis of NO levels, penile tissues were homogenized in cold phosphate-buffered saline (PBS). The supernatant was collected, followed by measurement of the total protein content using a BCA protein assay (P0012S, Beyotime Biotechnology). NO is extremely unstable and is quickly metabolized to nitrate and nitrite, and the nitrate is reduced to nitrite by nitrite reductase according to the nitrate-nitrite assay kit (S0024, Beyotime Biotechnology), so we can calculate the total NO level by the classic Griess Reagent and by detecting the absorbance of each sample at A540 nm. It is an indirect measurement of NO levels, which were standardized to the protein concentration.
Detection of cGMP
The cGMP concentration was detected using an enzyme-linked immunosorbent assay kit (F15182, R and D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The cGMP concentration was standardized by dividing it by the protein concentration of each sample.
Immunohistochemistry
As we previously described,11 the location and expression of target proteins such as eNOS and α-SMA were investigated by immunohistochemical staining within 5-μm tissue sections of the corpus cavernous. Sections were incubated at 37°C for 1 h with primary antibodies against eNOS (1:100; Abcam) and α-SMA (1:100; Abcam). Next, they were incubated with a biotinylated secondary antibody applied according to the standard protocol. Semi-quantitative analysis was also conducted to test the staining intensity using Image-Pro plus software (Media Cybernetics Inc., Silver Spring, MD, USA).
Masson's trichrome staining
Penile tissue sections (5-μm thick) were deparaffinized with xylene and rehydrated in a grade ethanol series. Masson's trichrome staining was performed according to a standard protocol. Semi-quantitative analysis was performed to evaluate intensity using Image-Pro plus software.
Immunofluorescence
Rat penile sections were dewaxed in dimethylbenzene and washed thrice with PBS. The slides were blocked and incubated with nNOS (1:100, Abcam). Slides were then incubated with the appropriate secondary antibodies. Digital images were obtained using an Olympus DP71 fluorescence microscope.
Real-time RT-PCR
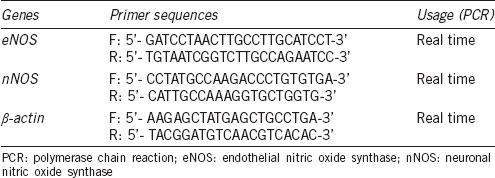
Total RNA was extracted using multisource RNA miniprep kits (08114KD1, Corning Inc., New York, NY, USA) and converted to cDNA using PrimeScript™ RT reagent kit (RR037A, TaKaRa, Dalian, China). Real-time qPCR was performed using SYBR Premix Ex Taq (TaKaRa) on an MX3000P quantitative PCR system (Agilent, Santa Clara, CA, USA). The level of β-actin mRNA expression was measured as an endogenous reference and used for normalization. Relative quantification of the expression levels of each transcript for each group was calculated using the 2−ΔΔCt method. The primer sequences of eNOS, nNOS, and β-actin are listed in Table 1.
Table 1.
Primers used in conventional polymerase chain reaction and real-time reverse transcriptase-polymerase chain reaction
Statistical analysis
Results were analyzed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) and were expressed as mean ± standard error of the mean. Data were analyzed using one-way analysis of variance followed by the Tukey–Kramer test for post hoc comparisons. P < 0.05 was considered to have a statistically significant difference among groups.
RESULTS
Metabolic meters and assessment of erectile function
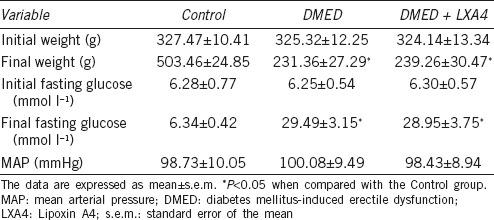
No differences were found on the initial body weights and serum glucose concentrations among all the three groups (P > 0.05). However, the DMED and the DMED + LXA4 groups showed lower final body weights and higher final serum glucose concentrations compared with that of the Control group. There were no differences on the final body weights and serum glucose concentrations between the DMED and the DMED + LXA4 groups (P > 0.05; Table 2).
Table 2.
Metabolic parameters
Erectile function was assessed by measuring maximum ICP, MAP, and AUC. There was a great decrease of the erectile response in the DMED group compared with the other two groups. Treatment of LXA4 in the DMED + LXA4 group partly, but obviously, improved erectile dysfunction (all P < 0.05; Figure 1). MAP did not differ significantly among all the three groups (P > 0.05).
Figure 1.
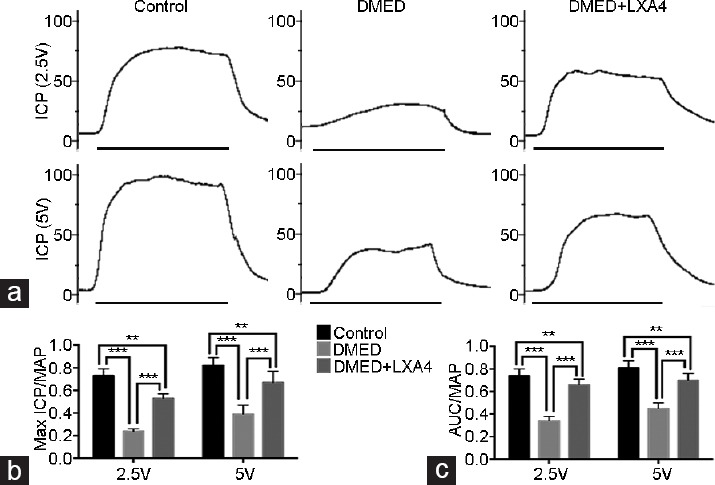
Treatment of LXA4 improves erectile dysfunction elicited by electrical stimulation of the cavernous nerve. (a) Representative ICP tracings were measured through stimulation of 2.5 and 5 V for 1 min, respectively. Ratios of max ICP and AUC to MAP of all the three groups were presented through bar graphs: (b) for max ICP/MAP and (c) for AUC/MAP. Data are expressed as mean ± s.e.m. (ncontrol = 10, nDMED = 11, and nDMED + LXA4 = 11). **P < 0.01 and ***P < 0.001 when comparing two groups. DMED: diabetes mellitus-induced erectile dysfunction; LXA4: Lipoxin A4; ICP: intracavernous pressure; AUC: area under the curve; MAP: mean arterial pressure. Scale bars = 1 min; s.e.m.: standard error of the mean.
Treatment of LXA4 inhibits oxidative stress in CC of DMED rats
We detected the oxidative stress levels in all the three groups. Western blot was performed to detect the expression level of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunits (a crucial enzyme that catalyzes the production of ROS). The expression levels of NADPH oxidase subunits gp91phox, p47phox, and p67phox were higher in the DMED group compared with the other two groups, while LXA4 treatment in the DMED + LXA4 group partly, but obviously, reduced these subunits' expression levels (all P < 0.05). There was no significant difference among all the three groups for the other units (p22phox and p40phox) (P > 0.05; Figure 2a and 2b). In consistent with the Western blot results, the DMED group also showed a lower SOD activity and a higher MDA level compared with the other two groups, while the change on the SOD activity and MDA level was partly, but significantly diminished in the DMED+LXA4 group (all P < 0.05; Figure 2c and 2d).
Figure 2.
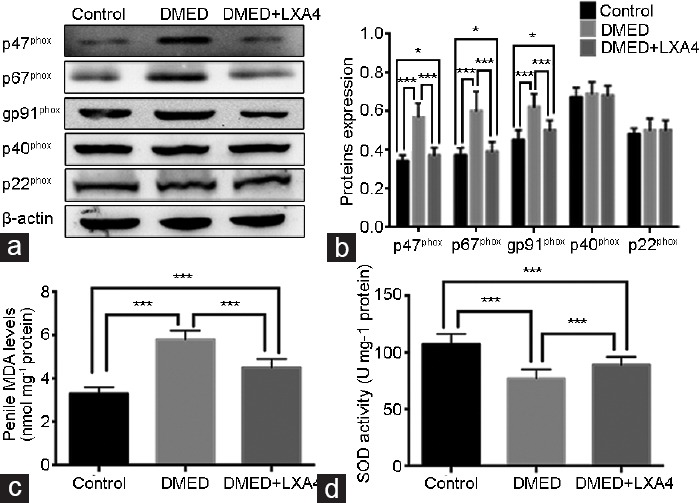
Treatment of LXA4 inhibits oxidative stress in corpus cavernous of DMED rats. (a) Representative Western blot results for the p22phox, p47phox, gp91phox, p40phox, and p67phox in rats of all the three groups. (b) Expressions of p22phox, p47phox, gp91phox, p40phox, and p67phox with β-actin as the loading control in all the three groups were presented through bar graphs. (c) MDA levels determined by the ELISA method in all the three groups. (d) SOD activities determined by the ELISA method in all the three groups. Data are expressed as mean ± s.e.m. (ncontrol = 10, nDMED = 11, and nDMED + LXA4 = 11). *P < 0.05 and ***P < 0.001 when comparing two groups. DMED: diabetes mellitus-induced erectile dysfunction; LXA4: Lipoxin A4; ELISA: enzyme-linked immunosorbent assay; SOD: superoxide dismutase; MDA: malondialdehyde; s.e.m.: standard error of the mean.
Treatment of LXA4 activates the NO/cGMP signaling pathway in CC of DMED rats
Real-time RT-PCR showed that the eNOS and nNOS expression levels in the DMED group were lower than that of the other two groups, while they were partly increased in the DMED + LXA4 group (P < 0.05; Figure 3a and 4d). Western blot analysis was also conducted to determine target proteins' expression levels. Experimental results showed that the expression levels of eNOS and nNOS were both lower in the DMED group compared with the other two groups, while they were partly elevated in the DMED + LXA4 group. Moreover, the phosphorylation level of eNOS in the DMED group was sharply attenuated at the site of serine 1177 (S1177) compared with that of the Control, while the change was greatly diminished in the DMED + LXA4 group (all P < 0.05; Figure 3b–3d and 4b, 4c). In consistent with the Western blot results, immunohistochemistry result of eNOS also showed a lower eNOS expression in the DMED group, which was partly elevated in the DMED + LXA4 group (P < 0.05; Figure 3e), and immunofluorescence result of nNOS also showed a lower nNOS expression in the DMED group, which was also partly elevated in the DMED + LXA4 group (P < 0.05; Figure 4a). Finally, the NO and cGMP concentrations were also dramatically reduced in the DMED group compared with that of the other two groups, while they were partly elevated in the DMED + LXA4 group (P < 0.05; Figure 4e and 4f).
Figure 3.
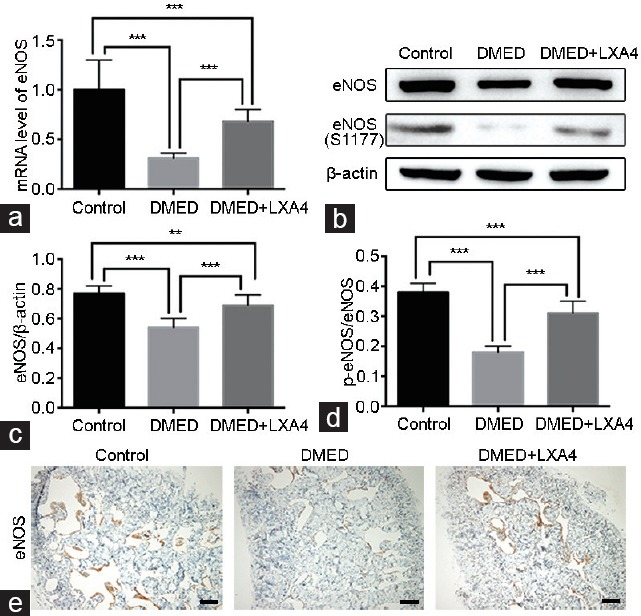
Treatment of LXA4 increases eNOS activation in corpus cavernous of DMED rats. (a) eNOS expression level with β-actin as the loading control in penile tissues of all groups by real-time RT-PCR using the 2-ΔΔCt method. (b) Representative Western blot results for eNOS and p-eNOS (S1177) of rats in the Control, DMED, and DMED + LXA4 groups after 4 weeks' treatment. Expressions of eNOS (c) with β-actin as the loading control and its phosphorylation level at the S177 site (d) in all the three groups were presented through bar graphs. (e) Immunohistochemical staining with antibody to eNOS (×100). Scale bars = 100 μm. Data are expressed as mean ± s.e.m. (ncontrol = 10, nDMED = 11, and nDMED + LXA4 = 11). **P < 0.01 and ***P < 0.001 when comparing two groups. DMED: diabetes mellitus-induced erectile dysfunction; LXA4: Lipoxin A4; RT-PCR: reverse transcriptase-polymerase chain reaction; eNOS: endothelial nitric oxide synthase; s.e.m.: standard error of the mean.
Figure 4.
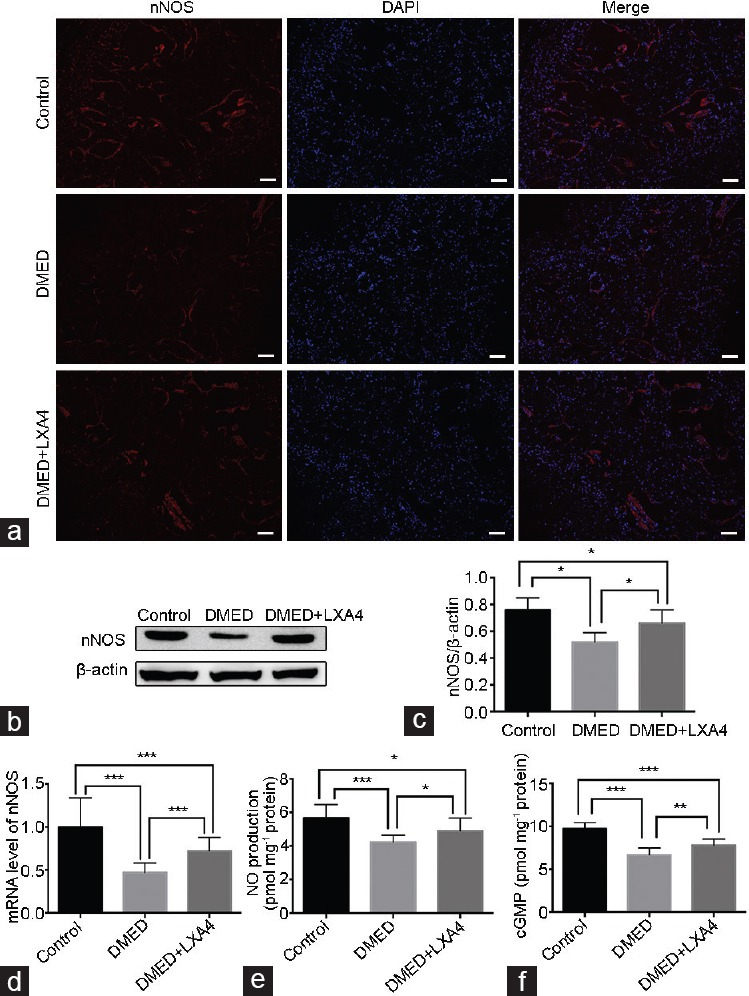
Treatment of LXA4 increases nNOS expression, thus activates the NO/cGMP signaling pathway. (a) Immunofluorescence results of nNOS in all the three groups after 4 weeks' treatment (×100). Scale bars = 100 μm. (b) Representative Western blot results for nNOS of rats in the Control, DMED, and DMED + LXA4 groups after 4 weeks' treatment. (c) Expressions of nNOS with β-actin as the loading control in all the three groups were presented through bar graphs. (d) nNOS expression level with β-actin as the loading control in penile tissues of all groups by real-time RT-PCR using the 2-ΔΔCt method. (e) The NO concentrations in all the three groups determined by the ELISA method. (f) The cGMP concentrations in all the three groups determined by the ELISA method. Data are expressed as mean ± s.e.m. (ncontrol = 10, nDMED = 11, and nDMED + LXA4 = 11). *P < 0.05, **P < 0.01, and ***P < 0.001 when comparing two groups. DMED: diabetes mellitus-induced erectile dysfunction; LXA4: Lipoxin A4; nNOS: neuronal nitric oxide synthase; NO: nitric oxide; cGMP: cyclic guanosine monophosphate; RT-PCR: reverse transcriptase-polymerase chain reaction; s.e.m.: standard error of the mean.
Treatment of LXA4 attenuates corporal fibrosis in CC of DMED rats
As shown in Figure 5a (×100) and 5b (×400), CC of rats in the DMED group showed a lower smooth muscle/collagen ratio compared with those of the Control group, while treatment of LXA4 greatly increased this ratio in the DMED + LXA4 group compared with the DMED group (P < 0.05). Similarly, immunohistochemistry results showed a lower expression of α-SMA in the DMED group and a partially increased expression level in the DMED + LXA4 group (P < 0.05; Figure 5c). Western blot analysis showed lower α-SMA expression levels and higher expression levels of Collagen I and Collagen IV in the DMED group compared with the other two groups, while treatment of LXA4 partly reduced these histological changes of target proteins in the DMED group (P < 0.05; Figure 5d–5g).
Figure 5.
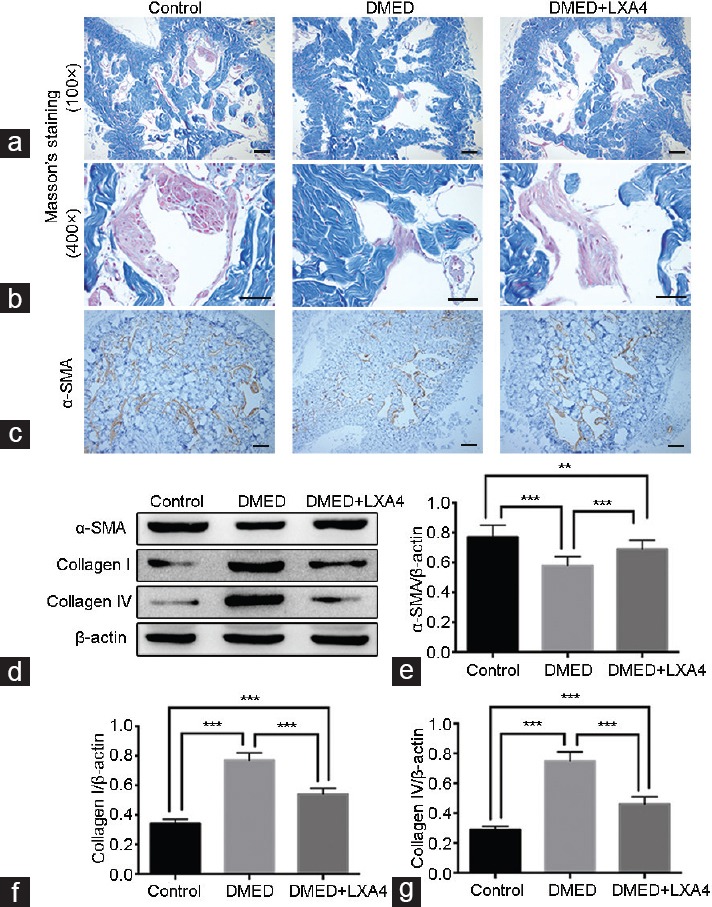
Treatment of LXA4 inhibits corporal fibrosis in corpus cavernous of DMED rats. Masson's trichrome staining of magnification ×100 (a) and magnification ×400 (b) was performed to assess the corporal fibrosis level in rat cavernous of all the three groups after 4 weeks' treatment (the area of smooth muscle is represented by red stain and the area of collagen by blue stain). Scale bars = 100 μm. (c) Immunohistochemistry results of α-SMA (×100) in all the three groups. Scale bars = 100 μm. (d) Representative Western blot results for a-SMA, Collagen I, and Collagen IV in all the three groups. Expressions of (e) α-SMA, (f) Collagen I, and (g) Collagen IV with β-actin as the loading control in all the three groups were presented through bar graphs. Data are expressed as mean ± s.e.m. (ncontrol = 10, nDMED = 11, and nDMED + LXA4 = 11). **P < 0.01 and ***P < 0.001 when comparing two groups. DMED: diabetes mellitus-induced erectile dysfunction; LXA4: Lipoxin A4; α-SMA: α-smooth muscle actin; s.e.m.: standard error of the mean.
Treatment of LXA4 inhibits the TGF-β/Smad/CTGF signaling pathway in CC of DMED rats
Western blot results demonstrated that the TGF-β/Smad/CTGF signaling pathway activity was elevated in the DMED group compared with that of the other two groups, while it was partly reduced in the DMED + LXA4 group (P < 0.05; Figure 6a–6c and 6e). In addition, there were no significant differences on the expression levels of p-Smad3/Smad3 (P > 0.05; Figure 6d).
Figure 6.
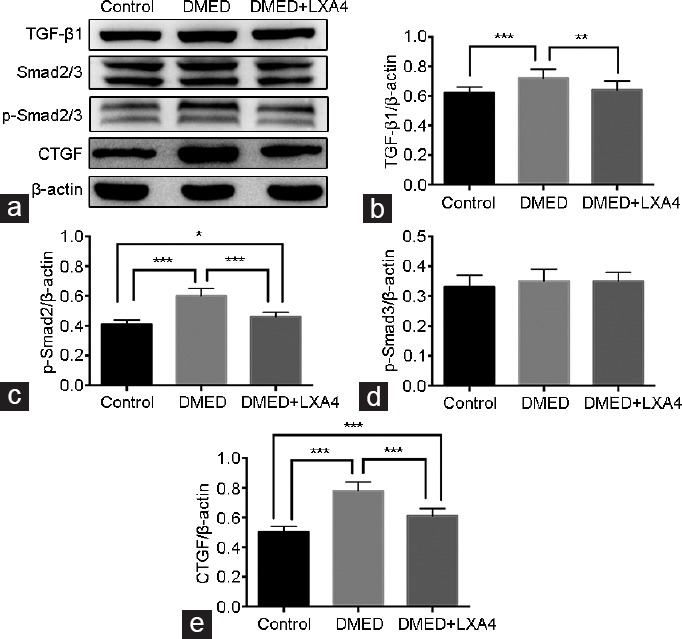
Treatment of LXA4 inhibits the TGF-β1/Smad/CTGF signaling pathway activity. (a) Representative Western blot results for TGF-β1, Smad2/3, p-Smad2/3, and CTGF in all the three groups after 4 weeks' treatment. Expressions of (b) TGF-β1, (c) p-Smad2, (d) p-Smad3, and (e) CTGF with β-actin as the loading control in all the three groups were presented through bar graphs. Data are expressed as mean ± s.e.m. (ncontrol = 10, nDMED = 11, and nDMED + LXA4 = 11). *P < 0.05, **P < 0.01, and ***P < 0.001 when comparing two groups. DMED: diabetes mellitus-induced erectile dysfunction; LXA4: Lipoxin A4; TGF-β1: transforming growth factor-beta 1; CTGF: connective tissue growth factor; s.e.m.: standard error of the mean.
DISCUSSION
In the present study, we initially demonstrated the restorative role of LXA4 for rats with DMED. The results suggested that overactivated oxidative stress, impaired endothelial function, and severe corporal fibrosis in rats' penile tissues are all involved in the pathogenesis of DMED. However, treatment of LXA4 could partly reduce oxidative stress, activate the NO/cGMP signaling pathway, and inhibit corporal fibrosis and its related signaling pathway activity, thus improving DMED in rats.
Lipoxins are generated from arachidonic acid via sequential actions of lipoxygenases (LOs) during cell-cell interactions and appear to act at both temporal and spatially distinct sites from eicosanoids produced during the onset of the inflammatory response. Previous studies have reported the protective role of LXA4 including anti-oxidant, anti-inflammatory, and anti-fibrotic activities in rat models.15,17,20 Moreover, Angulo et al.12 had reported that a novel antioxidant, AC3065, could reverse DMED in rats. Similarly, our previous study also verified the role of an antioxidant, apocynin, in the treatment of DMED in rats. Together, previous studies had suggested the potential role of antioxidants in the treatment of DMED. In the present study, we generated a rat model of type I DM through injection of STZ on rats. Then, we performed an APO test and only rats with the negative result were confirmed to have DMED. These data suggested that high serum glucose concentration induced by DM might impair the normal erectile response in rats. We treated these DMED rats with LXA4 for another 4 weeks and determined the effect of LXA4 on DMED. Erectile function of rats in the DMED + LXA4 group was greatly improved compared with that of the DMED group. Although there were also differences on erectile function between the DMED + LXA4 and the Control group, these data confirmed that treatment of LXA4 might partly, but obviously, improve DMED. We only determined the effect of LXA4 treatment for 4 weeks in the present study, and the long-term effect (8 and 12 weeks) would be observed in our following study to further verify the role of LXA4 on DMED. Moreover, LXA4 is a trihydroxy fatty acid containing a conjugated tetraene with a low bioavailability. We lacked related pharmacokinetic data to prove that it has reached the target tissues, so we observed the effect of LXA4 on the erectile function to verify that LXA4 could reach the target tissues and improved DMED, which was a limitation in the present study.
Oxidative stress is an important component of DM and is involved in the pathogenesis of many diseases.6,21 The major sources of ROS generated in the vessel wall are the vascular NADPH oxidases. Those multienzymatic complexes are similar in structure to the neutrophil NADPH oxidase and the most common isoforms are p22phox, p40phox, p47phox, p67phox, and gp91phox. In addition, a low-molecular-weight G protein (Rac) participates in assembly of the active complex. A previous study reported that excessive ROS in the CC might attenuate NO bioavailability, thereby decreasing cGMP levels and inducing apoptosis of CC smooth muscle cells (CCSMCs), finally leading to ED.7 Similarly, Angulo et al.12 also reported that oxidative stress has a relevant role in the pathophysiology of DMED and AC3056 could reverse DMED in rats and further improve NO-mediated response in penile tissue from diabetic men. In the present study, the higher MDA levels and the lower SOD activity in the CC of rats in the DMED group compared with the other two groups showed that overactivated oxidative stress was involved in the pathogenesis of DMED, while treatment of LXA4 could attenuate oxidative stress presented by the lower MDA levels and the higher SOD activities in the DMED + LXA4 group compared with the DMED group. Moreover, Western blot results showed that the higher serum glucose concentration in the DMED group could induce the higher expressions of p47phox, p67phox, and gp91phox, which was the same as the MDA and SOD results, further proving that overactivated oxidative stress was an important factor in DMED.
Endothelial dysfunction, mainly manifested as a decrease in NO release and low activity in the NO/cGMP signaling pathway, is a key component of DMED.7 In the NO/cGMP signaling pathway, NO is generated by three different isoforms of enzyme NOS: eNOS, nNOS, and inducible NOS (iNOS). Among these, eNOS and nNOS are strongly associated with ED.22,23,24 Moreover, Bivalacqua et al.25 reported that hyperglycemic conditions in cases of DM caused oxidative stress, inhibiting the activity of eNOS and the formation of NO, which impaired the endothelial function of CC.25 Angulo et al.26 also suggested that an exacerbated reduction of NO/cGMP signaling pathway could be responsible for ED men and would explain their reduced response to treatment. In addition, the eNOS activity was mediated by its phosphorylation site (S1177). Our previous study also revealed that the low eNOS activity in the CC of aged rats was characterized by a decrease in the eNOS phosphorylation level at the S1177 site.27 Treatment of LXA4 in the present study increased eNOS expression in the CC, which might suggest its potential protective role in endothelial function. Otherwise, LXA4 supplementation also increased the phosphorylation level of eNOS at the S1177 site, leading to the activation of eNOS and the increase of NO and cGMP concentrations in the CC of rats.
Some conventional antioxidants, such as Vitamin E and tempol, can improve DMED through modulating the scavenging activities of ROS in many animal models.28,29 However, clinical trials on the subject of antioxidant vitamins and cardiovascular disease have concluded that there is insufficient evidence to justify their use for reducing cardiovascular disease risks, suggesting that a more comprehensive approach rather than merely scavenging ROS might be more beneficial.30 Therefore, we selected LXA4 as the treatment method mainly because of its variety of effects. Martini et al.31 suggested that the LXA4 was a potent agonist for neuromodulation, neurological protection, and repair of the diseased nervous system. Therefore, it was worthwhile to study whether treatment of LXA4 could exert a neuroprotective role against DMED in rats. We found decreased expression of nNOS in the DMED group compared with the other two groups, suggesting that hyperglycemic conditions in DM might induce cavernous nerve injury. Treatment of LXA4 could increase the nNOS expression according to the Western blot and immunofluorescence results, which might be also responsible for the increase of NO and cGMP concentrations. Our study verified the potential neuroprotective role of LXA4 on the cavernous nerve.
Another important effect of LXA4 is the inhibitory role for tissue fibrosis. This is associated with many complications of DM, including cardiomyopathy and nephropathy. There is a well-recognized theory that corporal fibrosis contributes to DMED, and that this might be a potent reason for the poor response of DMED to conventional antioxidants and PDE5 inhibitors.7 Previous studies have suggested an inhibitory role of LXA4 for renal and lung fibrosis.15,32 However, there have been no studies on the effects of LXA4 on corporal fibrosis induced by DM. Here, a dramatic decrease of α-SMA expression and higher Collagen I and Collagen IV expression levels in the CC of DMED rats verified that corporal fibrosis is a common pathological feature of DMED. Treatment of LXA4 effectively reduced these histopathological changes in the CC, which demonstrated the restrained role of LXA4 for corporal fibrosis induced by DM.
TGF-β1, as an important pro-fibrotic cytokine, induces vascular fibrosis through Smad and non-Smad signaling pathways.33 It has also been recognized as the main factor involved in the development of corporal fibrosis.34 Brennan et al.15 suggested that LXA4 could mediate the suppression of TGF-β1-induced human renal fibrosis with a possible role in modulating fibrosis-related diseases. Similarly, Roach et al.32 showed that LXA4 inhibited many TGF-β1-dependent pro-fibrotic responses, which might result from the inhibition of nuclear translocation by Smad2/3. Wu et al.14 reported that LXA4 inhibited CTGF-induced fibrosis in a rat model. Therefore, LXA4 might inhibit fibrosis diseases through suppression of the key pro-fibrotic factors such as TGF-β1 and CTGF. Our previous study revealed that the Smad signaling pathway (TGF-β1/Smad2/3/CTGF) was involved in the DMED-related corporal fibrosis.35,36 Zhou et al.37 also suggested that upregulation of the TGF-β1/Smad/CTGF signaling pathway might play an important role in diabetes-induced fibrous muscular structural changes and in deteriorating erectile function. Thus, treatment of LXA4 might inhibit corporal fibrosis through suppression of the TGF-β1/Smad2/3/CTGF signaling pathway activity. We found that the higher activity of TGF-β1/Smad2/3/CTGF signaling pathway presented by the higher expression levels of TGF-β1, p-Smad2, and CTGF was induced by the higher serum glucose concentrations in the DMED group compared with those in the Control group, suggesting that this signaling pathway is involved in the development of DMED-related corporal fibrosis. Treatment of LXA4 could suppress the activities of target proteins, thereby inhibiting the signaling pathway activity and the pathological development of corporal fibrosis.
Although we showed here that treatment of LXA4 could partially improve DMED through inhibition of oxidative stress and corporal fibrosis, and potential protection of endothelial function and cavernous nerve function, some limitations remain. One is the absence of the cellular-based experiments and further in vitro experiments are needed to validate our conclusions. Another limitation is our study was restricted to a single drug dose. Whether a lower dose of LXA4 could reach a similar effect or a higher dose could be better was not studied. Further study would be conducted to explain the role of LXA4 on DMED.
CONCLUSION
Treatment of LXA4 might improve DMED in rats through its anti-oxidant capacity, neuroprotective function and anti-fibrosis effect. This study provides evidence that LXA4 supplementation might be an alternative therapy for DMED, thanks to its multiple effects.
AUTHOR CONTRIBUTIONS
KC participated in the design of the trial, conducted the data acquisition, interpreted and analyzed the data, and drafted and revised the manuscript. ZT, CCL, and TW designed the study and contributed to the study materials. KR and SGW pointed out deficiencies and ameliorated the manuscript. JHL and ZC guided the experiment directions and drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Natural Science Foundation of China (NSFC #81270690 & NSFC #81372759).
REFERENCES
- 1.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–65. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 2.Shiri R, Koskimaki J, Hakkinen J, Tammela TL, Huhtala H, et al. Effects of age, comorbidity and lifestyle factors on erectile function: Tampere Ageing Male Urological Study (TAMUS) Eur Urol. 2004;45:628–33. doi: 10.1016/j.eururo.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 4.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, et al. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts Male Aging Study. J Urol. 2000;163:460–3. [PubMed] [Google Scholar]
- 5.Andersen I, Heitmann BL, Wagner G. Obesity and sexual dysfunction in younger Danish men. J Sex Med. 2008;5:2053–60. doi: 10.1111/j.1743-6109.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- 6.Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6:1232–47. doi: 10.1111/j.1743-6109.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 7.Francis SH, Corbin JD. PDE5 inhibitors: targeting erectile dysfunction in diabetes. Curr Opin Pharmacol. 2011;11:683–8. doi: 10.1016/j.coph.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–13. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 9.Burnett AL, Musicki B. The nitric oxide signaling pathway in the penis. Curr Pharm Des. 2005;11:3987–94. doi: 10.2174/138161205774913381. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Zhuan L, Wang T, Rao K, Yang J, et al. Apocynin improves erectile function in diabetic rats through regulation of NADPH oxidase expression. J Sex Med. 2012;9:3041–50. doi: 10.1111/j.1743-6109.2012.02960.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Wang T, Yang J, Rao K, Zhan Y, et al. S-allyl cysteine restores erectile function through inhibition of reactive oxygen species generation in diabetic rats. Andrology. 2013;1:487–94. doi: 10.1111/j.2047-2927.2012.00060.x. [DOI] [PubMed] [Google Scholar]
- 12.Angulo J, Peiro C, Cuevas P, Gabancho S, Fernandez A, et al. The novel antioxidant, AC3056 (2,6-di-t-butyl-4-((dimethyl-4-methoxyphenylsilyl)methyloxy)phenol), reverses erectile dysfunction in diabetic rats and improves NO-mediated responses in penile tissue from diabetic men. J Sex Med. 2009;6:373–87. doi: 10.1111/j.1743-6109.2008.01088.x. [DOI] [PubMed] [Google Scholar]
- 13.Nascimento-Silva V, Augusta Arruda M, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost. 2006;97:88–98. [PubMed] [Google Scholar]
- 14.Wu SH, Wu XH, Lu C, Dong L, Zhou GP, et al. Lipoxin A4 inhibits connective tissue growth factor-induced production of chemokines in rat mesangial cells. Kidney Int. 2006;69:248–56. doi: 10.1038/sj.ki.5000025. [DOI] [PubMed] [Google Scholar]
- 15.Brennan EP, Nolan KA, Borgeson E, Gough OS, McEvoy CM, et al. Lipoxins attenuate renal fibrosis by inducing let-7c and suppressing TGFbetaR1. J Am Soc Nephrol. 2013;24:627–37. doi: 10.1681/ASN.2012060550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan Y, Li M, Wang T, Yang J, Rao K, et al. Taurine supplementation improves erectile function in rats with streptozotocin-induced Type 1 diabetes via amelioration of penile fibrosis and endothelial dysfunction. J Sex Med. 2016;13:778–85. doi: 10.1016/j.jsxm.2016.02.164. [DOI] [PubMed] [Google Scholar]
- 17.Zhou XL, Yang QS, Ni SZ, Tu XP, Zhao Y, et al. Protective effects of lipoxin A4 in testis injury following testicular torsion and detorsion in rats. Mediators Inflamm. 2014;2014:898056. doi: 10.1155/2014/898056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Guan H, Cai C, Li F, Xiao J. Lipoxin A4 suppresses osteoclastogenesis in RAW264.7 cells and prevents ovariectomy-induced bone loss. Exp Cell Res. 2017;352:293–303. doi: 10.1016/j.yexcr.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Zong H, Li X, Lin H, Hou C, Ma F. Lipoxin A4 pretreatment mitigates skeletal muscle ischemia-reperfusion injury in rats. Am J Transl Res. 2017;9:1139–50. [PMC free article] [PubMed] [Google Scholar]
- 20.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–62. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Jones RW, Rees RW, Minhas S, Ralph D, Persad RA, et al. Oxygen free radicals and the penis. Expert Opin Pharmacother. 2002;3:889–97. doi: 10.1517/14656566.3.7.889. [DOI] [PubMed] [Google Scholar]
- 22.Aversa A, Bruzziches R, Francomano D, Natali M, Gareri P, et al. Endothelial dysfunction and erectile dysfunction in the aging man. Int J Urol. 2010;17:38–47. doi: 10.1111/j.1442-2042.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- 23.Chiou WF, Liu HK, Juan CW. Abnormal protein expression in the corpus cavernosum impairs erectile function in type 2 diabetes. BJU Int. 2010;105:674–80. doi: 10.1111/j.1464-410X.2009.08852.x. [DOI] [PubMed] [Google Scholar]
- 24.Cui K, Luan Y, Tang Z, Rao K, Wang T, et al. Involvement of DDAH/ADMA/NOS/cGMP and COX-2/PTGIS/cAMP pathways in human tissue kallikrein 1 protecting erectile function in aged rats. PloS One. 2017;12:e0170427. doi: 10.1371/journal.pone.0170427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003;24:S17–37. doi: 10.1002/j.1939-4640.2003.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 26.Angulo J, Gonzalez-Corrochano R, Cuevas P, Fernandez A, La Fuente JM, et al. Diabetes exacerbates the functional deficiency of NO/cGMP pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J Sex Med. 2010;7:758–68. doi: 10.1111/j.1743-6109.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- 27.Luan Y, Ruan Y, Wang T, Zhuan L, Wen Z, et al. Preserved erectile function in the aged transgenic rat harboring human tissue kallikrein 1. J Sex Med. 2016;13:1311–22. doi: 10.1016/j.jsxm.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Gocmen C, Secilmis A, Kumcu EK, Ertug PU, Onder S, et al. Effects of Vitamin E and sodium selenate on neurogenic and endothelial relaxation of corpus cavernosum in the diabetic mouse. Eur J Pharmacol. 2000;398:93–8. doi: 10.1016/s0014-2999(00)00242-9. [DOI] [PubMed] [Google Scholar]
- 29.De Young L, Yu D, Bateman RM, Brock GB. Oxidative stress and antioxidant therapy: their impact in diabetes-associated erectile dysfunction. J Androl. 2004;25:830–6. doi: 10.1002/j.1939-4640.2004.tb02862.x. [DOI] [PubMed] [Google Scholar]
- 30.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martini AC, Forner S, Bento AF, Rae GA. Neuroprotective effects of lipoxin A4 in central nervous system pathologies. Biomed Res Int. 2014;2014:316204. doi: 10.1155/2014/316204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roach KM, Feghali-Bostwick CA, Amrani Y, Bradding P. Lipoxin A4 attenuates constitutive and TGF-beta1-dependent profibrotic activity in human lung myofibroblasts. J Immunol. 2015;195:2852–60. doi: 10.4049/jimmunol.1500936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samarakoon R, Higgins PJ. Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thromb Haemost. 2008;100:976–83. [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Cadavid NF. Mechanisms of penile fibrosis. J Sex Med. 2009;6(Suppl 3):353–62. doi: 10.1111/j.1743-6109.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Chen Z, Wang T, Yang J, Li R, et al. Treatment of diabetes mellitus-induced erectile dysfunction using endothelial progenitor cells genetically modified with human telomerase reverse transcriptase. Oncotarget. 2016;7:39302–15. doi: 10.18632/oncotarget.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui K, Ruan Y, Wang T, Rao K, Chen Z, et al. FTY720 Supplementation partially improves erectile dysfunction in rats with streptozotocin-induced type 1 diabetes through inhibition of endothelial dysfunction and corporal fibrosis. J Sex Med. 2017;14:323–35. doi: 10.1016/j.jsxm.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Zhou F, Li GY, Gao ZZ, Liu J, Liu T, et al. The TGF-beta1/Smad/CTGF pathway and corpus cavernosum fibrous-muscular alterations in rats with streptozotocin-induced diabetes. J Androl. 2012;33:651–9. doi: 10.2164/jandrol.111.014456. [DOI] [PubMed] [Google Scholar]


