Abstract
Abiraterone acetate is approved for the treatment of castration-resistant prostate cancer (CRPC); however, its effects vary. An accurate prediction model to identify patient groups that will benefit from abiraterone treatment is therefore urgently required. The Chi model exhibits a good profile for risk classification, although its utility for the chemotherapy-naive group is unclear. This study aimed to externally validate the Chi model and develop a new nomogram to predict overall survival (OS). We retrospectively analyzed a cohort of 110 patients. Patients were distributed among good-, intermediate-, and poor-risk groups, according to the Chi model. The good-, intermediate-, and poor-risk groups had a sample size of 59 (53.6%), 34 (30.9%), and 17 (15.5%) in our dataset, and a median OS of 48.4, 29.1, and 10.5 months, respectively. The C-index of external validation of Chi model was 0.726. Univariate and multivariate analyses identified low hemoglobin concentrations (<110 g l−1), liver metastasis, and a short time interval from androgen deprivation therapy to abiraterone initiation (<36 months) as predictors of OS. Accordingly, a new nomogram was developed with a C-index equal to 0.757 (95% CI, 0.678–0.836). In conclusion, the Chi model predicted the prognosis of abiraterone-treated, chemotherapy-naive patients with mCRPC, and we developed a new nomogram to predict the overall survival of this group of patients with less parameters.
Keywords: abiraterone acetate, castration-resistant prostate cancer, external validation, nomogram, survival
INTRODUCTION
Prostate cancer (PCa) is a significant burden on public health and a major cause of morbidity and mortality among men worldwide. It is the most frequently diagnosed cancer and the second leading cause of mortality in men in the United States.1 In China, the incidence of prostate cancer has rapidly increased. Although PCa is sensitive to androgen deprivation therapy (ADT) upon initial diagnosis, most patients with PCa progress to a castration-resistant stage after 2–3 years, which is characterized by a continuous rise in prostate-specific antigen (PSA) concentrations, insensitivity to first-line ADT, or the appearance of new lesions.
Until recently, only docetaxel significantly prolonged overall survival (OS) of patients with castration-resistant prostate cancer (CRPC) patients. New and more effective agents are now available, such as Sipuleucel-T immunotherapy, radium-223, enzalutamide, and abiraterone acetate.2,3,4,5,6,7 For example, abiraterone, an inhibitor of CYP17, a critical enzyme in testosterone synthesis, significantly prolongs OS in combination with prednisone compared with prednisone alone. Moreover, abiraterone can be used to treat patients after chemotherapy and those who are chemotherapy naive. Although abiraterone combined with prednisone is considered the standard of care for patients with metastatic CRPC (mCRPC),8 its efficacy varies.6,7 Here, we focused on the identification of a patient group that can maximally benefit from abiraterone.
The prognostic model proposed by Chi et al.9 (referred to as the Chi model) comprises six factors that can be employed for routine patient management. The Chi model can predict the OS of patients with mCRPC after treatment using abiraterone and external validation of the Chi model supports the clinical implementation for assessing patients with mCRPC after abiraterone therapy, although the model indicates poorer performance when applied to chemotherapy-naive patients who receive abiraterone.10 Here, we externally validated the predictive efficacy of the Chi model in a study of chemotherapy-naive, abiraterone-treated patients with mCRPC and further developed a new prognostic nomogram comprising three clinical factors that can be determined during routine patient management to predict OS.
PATIENTS AND METHODS
Patients and treatment
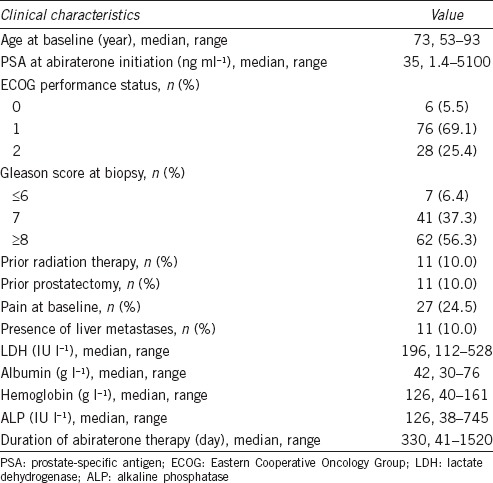
We conducted a retrospective study of a consecutive cohort of 110 patients with prostate cancer treated using abiraterone combined with prednisone from June 2009 to November 2016 at Fudan University Shanghai Cancer Center. Patients (aged ≥18 years) were included if they were diagnosed with histologically or cytologically confirmed prostate adenocarcinoma. The patients had PSA progression according to the criteria of the Prostate Cancer Clinical Trials Working Group (PCWG2),11 or radiographic progression in bone or soft tissue, and ongoing ADT with serum testosterone <1.7 nmol l−1. Patients were examined before abiraterone therapy, which included a physical examination, determination of Eastern Cooperative Oncology Group Performance Status (ECOG PS), routine clinical laboratory tests, measurement of serum PSA and testosterone levels, a whole-body bone scan, and if required, chest, abdominal, and pelvic computed tomography (CT), or magnetic resonance imaging (MRI). During follow-up, patients' PSA and testosterone levels were measured. Further, monthly tests of liver and kidney function were performed, and abdominal ultrasound, chest X-ray every 3 months, and CT or MRI of the pelvic cavity and bone scan were performed, the latter as required.
Patients who met the eligibility criteria received 1000 mg of oral abiraterone acetate once daily plus 5 mg prednisone twice each day. Abiraterone was administered continuously unless radiographic progression was detected or intolerable toxicities occurred. Radiographic disease progression was defined as increases in the number of evaluable lesions observed using CT or MRI, as defined by the modified Response Evaluation Criteria in Solid Tumors12 or progression detected using a bone scan as adopted by the PCWG2. OS was defined as the time from admission for treatment to death from any cause, or to the last follow-up. This study was approved by the Ethics and Scientific Committee of our institution, and written informed consent to participate was obtained from all patients.
Chi prognostic model
The patients were categorized into good-, intermediate-, and poor-risk groups according to the Chi model, which includes the risk factors as follows: lactate dehydrogenase (LDH), ECOG PS, presence of liver metastases, albumin, alkaline phosphatase (ALP), and the time interval from ADT to initiation of abiraterone therapy. LDH concentrations greater than the upper limit of normal (ULN) (250 IU l−1), ECOG PS of 2, liver metastases, albumin ≤4 g dl−1, ALP > ULN (160 IU l−1), and the time interval from ADT to initiation of abiraterone therapy (≤36 months) are considered to adversely affect OS. Therefore, patients with 0 or 1, 2 or 3, or ≥4 risk factors were classified into good-, intermediate-, and poor-risk groups, respectively.
Statistical analyses
OS was estimated using the Kaplan–Meier method. Univariate and multivariate Cox regression models were used to identify significant associations between clinically relevant baseline factors and OS. The six risk factors described in the Chi model were collected and grouped in the same way. Continuous data were divided into two groups according to the model. The performance of the model in our validation cohort was assessed through estimates of discrimination and calibration. A concordance index (C-index) was estimated as a measure of the discriminative ability of the model.13 A C-index equal to 0.50 represents a random prediction (no better than chance), and a C-index equal to 1.0 represents perfect discriminative ability. Calibration refers to how closely the probability of 2-year survival predicted by the model agrees with the observed value and was assessed via a calibration plot of the model's predicted 2-year survival probability versus a patient's observed probability of survival calculated using the Kaplan–Meier method.14 If the model is perfectly accurate, e.g., predicted and observed 2-year survival probabilities agree over the entire range of probabilities, the slope of the curve is 45°. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 21.0 (SPSS Inc., Chicago, IL, USA) and R statistics package, version 3.2.2 (http://www.r-project.org/).
RESULTS
The present study included 110 patients whose baseline characteristics are listed in Table 1. The median age was 73 (range: 53–93) years, and median OS was 30.8 (range: 2.5–87.4) months. The median duration of abiraterone therapy was 330 (range: 41–1520) days. We found that 77 (70.0%) patients exhibited a PSA response and that the most common adverse events were osteodynia (43/110, 39.1%), hypodynamia (40/110, 36.4%), and hypokalemia (32/110, 29.1%).
Table 1.
Clinical characteristics of 110 patients
External validation of the Chi model
OS associated with each Chi-model risk factor is shown in Figure 1. There were significant associations between OS and the six Chi-model risk factors of ECOG PS, ALP, liver metastases, albumin, and time interval from ADT to initiation of abiraterone therapy, but not LDH (P = 0.055) (Table 2). The good-, intermediate-, and poor-risk groups included 59 (53.6%), 34 (30.9%), and 17 (15.5%) patients, respectively, each with a median OS of 48.4, 29.1, and 10.5 months, respectively (Figure 2). During follow-up, 56 (50.9%) patients died, and 14, 25, and 17 were members of the good-, intermediate-, and poor-risk groups, respectively. The nomogram C-index in our dataset was 0.726 (95% CI, 0.642–0.809), which was higher than the C-index of the Chi model (0.70 ± 0.014), indicating that the Chi model possessed good discriminative ability.
Figure 1.
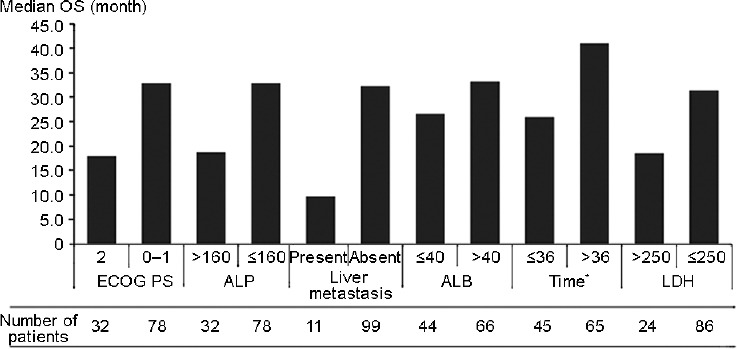
The OS and number of patients of each risk factor according to Chi model. OS: overall survival; ECOG: Eastern Cooperative Oncology Group; ALP: alkaline phosphatase (IU l-1); ALB: albumin (g l-1); LDH: lactate dehydrogenase (IU l-1). *Time interval from androgen deprivation therapy to abiraterone initiation (month).
Table 2.
Univariate and multivariate analyses for overall survival associated with major prognostic factors
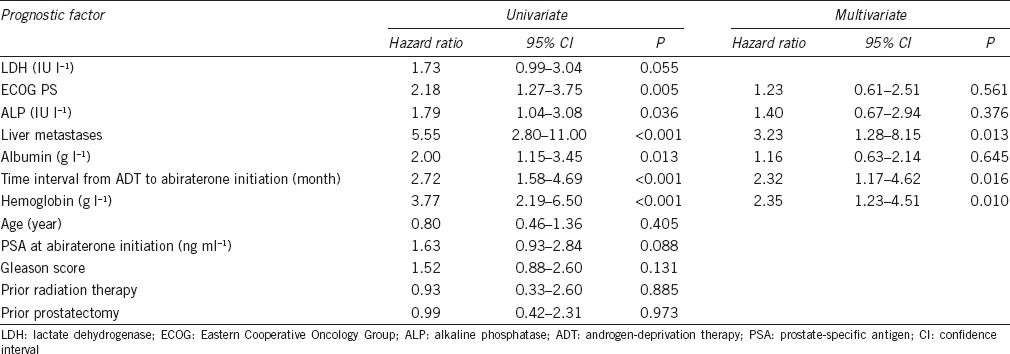
Figure 2.
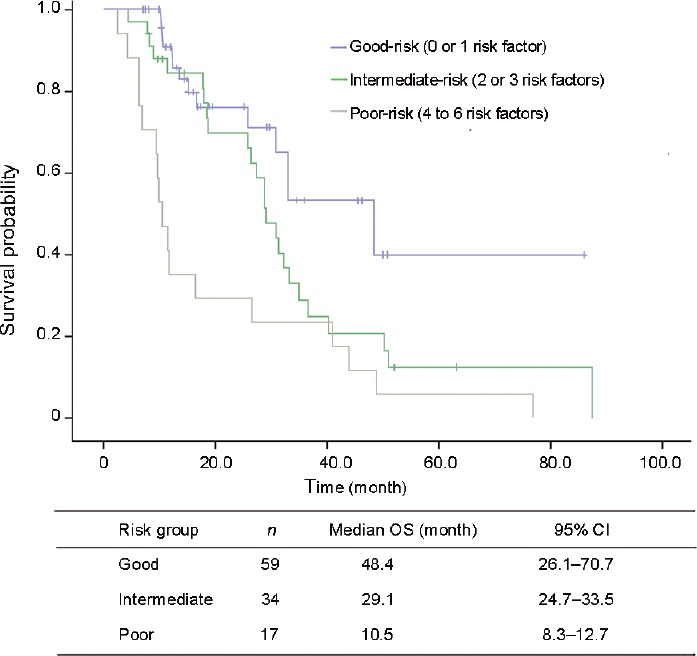
Overall survival curves according to Chi prognostic model.
Development of a new nomogram
When we further estimated the significance of each risk factor using univariate Cox regression analysis, we found that the six baseline clinical and laboratory factors were significantly associated with OS (P ≤ 0.05) (Table 2). Further, the multivariate Cox regression model identified liver metastases, hemoglobin concentration, and time interval from ADT to initiation of abiraterone therapy as independent predictors of OS, which were included in the new nomogram to predict the OS of abiraterone-treated, chemotherapy-naive patients with mCRPC (Figure 3). The C-index of the new nomogram was 0.757 (95% CI, 0.678–0.836). Supplementary Figure 1 (111.1KB, tif) presents how the predictions from the model at 24 months compared with the actual survival probability for the 110 patients in our analysis (calibration).
Figure 3.
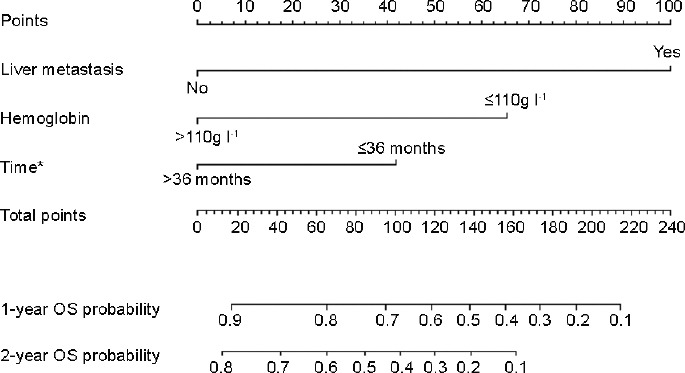
Overall survival nomogram. *Time interval from androgen deprivation therapy to abiraterone initiation. OS: overall survival. It needs two steps to get the 1- and 2-year overall survival probability from the nomogram above: (I) draw a line straight up to the “points” axis from the value (e.g., for hemoglobin the values are “<110g/L” and “≤110g/L”) of each risk factor (hemoglobin, liver metastasis and time from ADT to abiraterone initiation). (II) Add the point scores of each risk factor and locate the total score on the axis of “total points”. Then draw a vertical line from the total score down to the 1- or 2-year overall survival probability axes to obtain the probability.
Calibration plots for predicted (by the new nomogram) and observed 2-year overall survival probability. OS: overall survival.
DISCUSSION
ADT is one of the main strategies for patients newly diagnosed with advanced prostate cancer;15 however, the effective treatment time is short, and disease progresses to CRPC, accompanied by increasing serum PSA concentrations, local recurrence, or distant metastasis.16,17 Docetaxel chemotherapy can lengthen survival, although serious adverse reactions reduce its benefit.2 Abiraterone is an effective treatment for patients with CRPC, which is well tolerated, and causes only mild adverse reactions. Specifically, abiraterone increases the median OS of patients who receive chemotherapy and for those who are chemotherapy naive by 4.6 and 4.4 months, respectively.18,19
However, certain problems associated with abiraterone treatment must be solved. For example, the median effective treatment is 9–10 months, and most patients then experience an increase in serum PSA concentrations and disease recurrence.19 In the COU-301 trial, the median time to PSA progression was 8.5 months (95% CI 8.3–9.7).18 While in the COU-AA-302 trial, this time was 11.1 months.19 These data indicate the variable effect of abiraterone.
Prognostic models are available for evaluating therapeutic strategies. For example, a nomogram Smaletz et al.20 developed from a study of 409 patients with mCRPC from 1989 to 2000, included the factors as follows: Karnofsky performance status, hemoglobin, ALP, albumin, LDH, age, and PSA. Multivariate analysis identified the first five factors as significantly associated with survival (P < 0.05) and achieved a C-index equal to 0.67 when applied to the external validation data set. Another model developed by Halabi et al.21 included 1101 patients with mCRPC from 1991 to 2001 considered LDH, PSA, ALP, Gleason score, ECOG PS, hemoglobin, and visceral disease. The area under the receiver operating characteristic curve is 0.68. Further, patients were grouped into quartiles according to the median of the predicted survival, which is helpful for stratifying patients with mCRPC patients in future randomized Phase III trials. These two nomograms mentioned above were very classic to predict survival of CRPC patients, and we also applied the two models into our current study, and C-index are: 0.763 (95% CI: 0.689–0.838), and 0.765 (95% CI: 0.688–0.842), respectively. These results indicated that although docetaxel, abiraterone and other new drugs directed to CRPC emerged constantly since the two models were proposed in 2002, because of the multicenter and large scale characteristics, they are still useful in today's therapeutic strategies.
However, there are few prognostic models for evaluating abiraterone therapy for patients with mCRPC. Ravi et al.10 conducted an external validation of the Chi model for predicting patients' prognoses after undergoing chemotherapy. However, the data do not conclusively prove whether the Chi model is applicable for patients with chemotherapy naive: in Ravi et al.'s study, they also assessed the prognostic value of the model for chemotherapy-naive patients; however, because of the small number of patients (n = 64), the intermediate and high-risk groups were combined, decreasing the detection efficiency of the model.
The results of the present external validation indicate that certain risk factors included in the Chi model played an important role in predicting prognosis. Specifically, we identified statistically significant differences in OS among the good-, intermediate-, and poor-risk groups, which were similar to those of the Chi model. The C-index for OS was 0.726, indicating that the Chi model achieved good discriminatory ability. Thus, although the Chi model was originally used to predict the efficacy of abiraterone for patients after chemotherapy, our data demonstrate that in the chemotherapy-naive group, the model could be applied to predict and judge the effect.
In the present study, liver metastases and time interval from ADT to initiation of abiraterone therapy were identified as independent prognostic factors of OS. An equally plausible explanation is that liver metastasis and the short time interval from ADT to initiation of abiraterone therapy indicate a highly malignant phenotype, or neuroendocrine differentiation.22,23,24 Therefore, hormone therapy for these patients may not serve as an ideal treatment strategy.
We note that in the univariate and multivariate analyses, two of the six predictors of Chi model were confirmed as statistically significant. A potential explanation for this difference is that the present study included a small sample size from a single center. These results show that an informed approach to the determination of the optimal prognosis model remains a critical clinical issue.
Then, we show here that lower hemoglobin values indicated worse prognosis, and the risk of poor OS for patients with hemoglobin <110 g l−1 was twice that compared with patients with hemoglobin >110 g l−1. So, the factor of hemoglobin was also included in the final nomogram.
Therefore, we used these three predictors to successfully construct an individualized predictive nomogram model (C-index = 0.757) that allows stratification of progression risks and provides a numerical estimate to help clinicians and patients manage disease. The value of the C-index of our nomogram is slightly higher compared with that of the Chi model, whereas because of the limitations imposed by the derivation of our nomogram from a single-center dataset, smaller sample size, measurement errors, and fewer parameters, we cannot conclude therefore that our prediction model is more accurate compared with the Chi model.25 Then, we note that the value of the C-index of our nomogram, using our dataset, is slightly higher compared with that of the external validation of the Chi model. Moreover, fewer parameters are required. Therefore, our nomogram is more conducive to clinical application.
This manuscript has strictly followed the guidelines of the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) Statement which is aimed to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis.26
There are some limitations to our study. This was a retrospective, Chinese single-center study, on one hand, the treatment in China for PCa, especially for mCRPC is deviated from guideline, such as Sipuleucel-T, enzalutamide and cabazitaxel are not accessible in China, so the treatment options for Chinese patients with mCRPC is limited; on the other hand, the number of patients is smaller compared with that used to develop the Chi model and the two nomograms described above.9,20,21 Further, few patients underwent local treatment, which may be explained as follows: first, most patients had metastatic disease, and the concept of oligometastasis was relatively new, particularly during the preceding years. Therefore, patients with metastatic disease did not undergo radical prostatectomy. Second, radiation therapy in China is not used as frequently compared with other countries, and is not preferred by patients. Therefore, patients typically undergo relatively conservative hormone therapy.
CONCLUSIONS
In summary, we conducted an external validation to identify the prognostic value of the Chi model for chemotherapy-naive patients and developed a novel nomogram model to assess the benefits and risks of abiraterone treatment for mCRPC patients, especially for Chinese mCRPC patients. Furthermore, the parameters included in our novel prognostic model can be easily got in clinical practice, and the model is helpful to predict individual survival probabilities and stratify mCRPC patients in future randomized Phase III trials.
AUTHOR CONTRIBUTIONS
YJY and GWL designed the study, collected, analyzed, and interpreted the clinical data. YJY wrote and revised the manuscript. GXL, JLW, and HYX helped perform the external validation and build the new nomogram. GWL and YZ helped with patient follow-up. BD and DWY designed the study, supervised the project, and revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 4.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 5.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ®). Prostate Cancer. Ver. 1.2017. National Comprehensive Cancer Network Website. 2017. [Last accessed on 2016 Dec 16]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf .
- 9.Chi KN, Kheoh T, Ryan CJ, Molina A, Bellmunt J, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol. 2016;27:454–60. doi: 10.1093/annonc/mdv594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravi P, Mateo J, Lorente D, Zafeiriou Z, Altavilla A, et al. External validation of a prognostic model predicting overall survival in metastatic castrate-resistant prostate cancer patients treated with abiraterone. Eur Urol. 2014;66:8–11. doi: 10.1016/j.eururo.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 14.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–38. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loblaw DA, Mendelson DS, Talcott JA, Virgo KS, Somerfield MR, et al. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J Clin Oncol. 2004;22:2927–41. doi: 10.1200/JCO.2004.04.579. [DOI] [PubMed] [Google Scholar]
- 16.Salonen AJ, Viitanen J, Lundstedt S, Ala-Opas M, Taari K, et al. Finnish multicenter study comparing intermittent to continuous androgen deprivation for advanced prostate cancer: interim analysis of prognostic markers affecting initial response to androgen deprivation. J Urol. 2008;180:915–20. doi: 10.1016/j.juro.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Chi KN, Bjartell A, Dearnaley D, Saad F, Schroder FH, et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment targets. Eur Urol. 2009;56:594–605. doi: 10.1016/j.eururo.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 19.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 20.Smaletz O, Scher HI, Small EJ, Verbel DA, McMillan A, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–82. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Li B, Zhang P, Yao Y, Chang J. Clinical characteristics and prognostic factors of prostate cancer with liver metastases. Tumour Biol. 2014;35:595–601. doi: 10.1007/s13277-013-1083-6. [DOI] [PubMed] [Google Scholar]
- 23.Sagnak L, Topaloglu H, Ozok U, Ersoy H. Prognostic significance of neuroendocrine differentiation in prostate adenocarcinoma. Clin Genitourin Cancer. 2011;9:73–80. doi: 10.1016/j.clgc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Cornelio A, Gonzalez-Perez J, Tabares-Garcia FJ, Ramos-Salgado F, Alvarado-Cabrero I, et al. Androgen-deprivation therapy in the management of neuroendocrine prostate cancer. Cir Cir. 2009;77:293–9. 273–8. Article in English, Spanish. [PubMed] [Google Scholar]
- 25.Kattan MW. Factors affecting the accuracy of prediction models limit the comparison of rival prediction models when applied to separate data sets. Eur Urol. 2011;59:566–7. doi: 10.1016/j.eururo.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 26.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. Eur Urol. 2015;67:1142–51. doi: 10.1016/j.eururo.2014.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calibration plots for predicted (by the new nomogram) and observed 2-year overall survival probability. OS: overall survival.


