Abstract
We report the largest single-center experience with robotic-assisted microscopic varicocelectomy (RAMV) in male infertility. From August 2012 to February 2015, men with infertility of at least a year and varicoceles underwent RAMV by a single surgeon. Varicocele was diagnosed on physical examination and confirmed by ultrasound by a single ultrasonographer. Preoperative hormone panel, semen analyses, and testicular Doppler ultrasound were obtained from all men and repeated at 3 months. One hundred and forty consecutive men (258 varicocelectomies) were included. Mean age and duration of infertility was 36.4 and 2.8 years, respectively. Median total and free testosterone increased by 145 ng dl−1 and 4.3 pcg ml−1 (44.3%), respectively (P < 0.0001). Median sperm concentration increased by 37.3% (P < 0.03). Median sperm motility and morphology did not significantly change. Median left and right testicular volume increased by 22.3% (P < 0.0001) and 12.6% (P < 0.0006), respectively. Hydroceles occurred 0.8% of procedures. We had no testicular artery injuries. Persistence of varicocele by Doppler ultrasound was 9.6%. Only 37.3% of patients required pain medications postoperatively. We concluded that RAMV is a safe and effective alternative for varicocele repair with outcomes comparable to historical traditional microsurgical approach.
Keywords: male infertility, reproductive technology, robot-assisted microsurgery, varicocele, varicocelectomy
INTRODUCTION
A varicocele is a pathologic dilation of the testicular veins within the spermatic cord. Varicoceles can be found in 15% of the adult male population and in over 35% of men presenting for infertility evaluation.1,2 Varicoceles can also contribute to low testosterone (T), orchialgia, and testicular atrophy.3
Many explanations for the pathophysiology have been described to account for testicular dysfunction due to varicoceles. Reflux of adrenal metabolites and increased testicular hypoxia have been demonstrated.4,5 Contralateral testicular dysfunction has also been shown with histological studies and unilateral varicoceles.6 Varicoceles are known to disrupt the temperature countercurrent regulation of the testicles, disrupting key enzymes involved in spermatogenesis.7 DNA damage in spermatids from reactive oxygen species (ROS) is also more prominent with varicoceles.8,9,10,11
Numerous studies have shown the benefits from varicocele ligation. Improvements in semen analyses with male factor infertility, improvements in orchialgia, increasing T levels, and growth in testicular size have all been reported.12,13,14,15,16,17,18,19,20,21 Recent meta-analyses have confirmed that subinguinal traditional microsurgical varicocele repair has the highest success rate and lowest complication rate as compared to macroscopic inguinal or laparoscopic abdominal approaches.22 This approach has become the gold standard for varicocelectomy.
Recently, the da Vinci® robot (DR; Intuitive Surgical, Inc., Sunnyvale, CA, USA) has been used to successfully perform microsurgical vasectomy reversals and varicocelectomy.23,24 Advantages of robotics include elimination of tremor, retraction with third arm, and stable immersive 3D microscopic vision, all contributing to precision of surgery. One of the earliest experiences by Shu et al.23 demonstrated successful robotic-assisted microscopic varicocelectomy (RAMV) without complications in eight patients. Operative times were similar to those of traditional microscopic varicocelectomy (TMV). In another study, RAMV improved sperm counts in 76% of oligospermic patients and converted 18% of azoospermic patients to oligospermic with low failure and low complication rates.24
In this study, we aim to describe our outcomes for RAMV in men with infertility. To date, this is the largest series reported for RAMV.
PATIENTS AND METHODS
We retrospectively reviewed charts of 140 patients who underwent RAMV from March 2012 to July 2014 presenting for symptomatic hypogonadism, testicular atrophy, infertility, or orchialgia. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the Albany Medical College. This retrospective study was not deemed to require patient consents.
All patients were referred to our men's health clinic for infertility, hypogonadism, and/or symptomatic varicocele. All men had an initial history and physical by a urologist (Andrew McCullough). A morning hormone panel including T, free testosterone (Tfree), estradiol (E2), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and sex hormone binding globulin (SHBG) was obtained. The laboratory analyses were not done at a single laboratory but were done at reference laboratories approved by the patients’ insurance plans. Karyotype and microdeletion studies were performed on men suspected of Klinefelter and sperm counts of <5 × 106 ml−1. Men included in the study had normal karyotype and negative microdeletion studies. Men with an examination suspicious for a varicocele underwent a testicular duplex Doppler ultrasound. They were assessed for the presence of a varicocele, varicocele size, testicular parenchyma, and size. All ultrasounds were performed by a single ultrasonographer (Leon Elebyjian) with the same equipment (Acuson 128 with 7.5 MHz electronic linear probe, Acuson 128 XP10, Siemens Healthcare GmbH, Erlangen, Germany). The ultrasound was performed in a warm room (>24°C). Patients were examined in the supine and standing position with and without valsalva. The spermatic cord and the testicles were examined bilaterally, recording the maximum diameter of the venous vessels and the presence and speed of retrograde venous flow during Valsalva's maneuver. Gray scale and Doppler evaluation of the testes with measurement of testicular dimensions for volume calculation was performed. Volume was calculated as per Lambert (length × width × height × 0.7).25 Patients with clinical varicoceles but without duplex Doppler corroboration of retrograde blood flow were not offered varicocele repair. Only men with varicoceles documented by ultrasound with hypogonadism or an abnormal sperm concentration, motility, or morphology were offered surgery. All surgeries were performed by the same surgeon (Andrew McCullough).
Patients who presented with infertility underwent pre- and post-operative semen analyses that were obtained at one of two independent in vitro fertilization (IVF) center Andrology laboratories. Postoperatively, an ultrasound was repeated at 3 months by the same ultrasonographer. Persistent retrograde flow was documented and considered a failure regardless of the physical examination. All men had a hormone panel repeated 3 months postoperatively. Complications and use of pain medications were recorded.
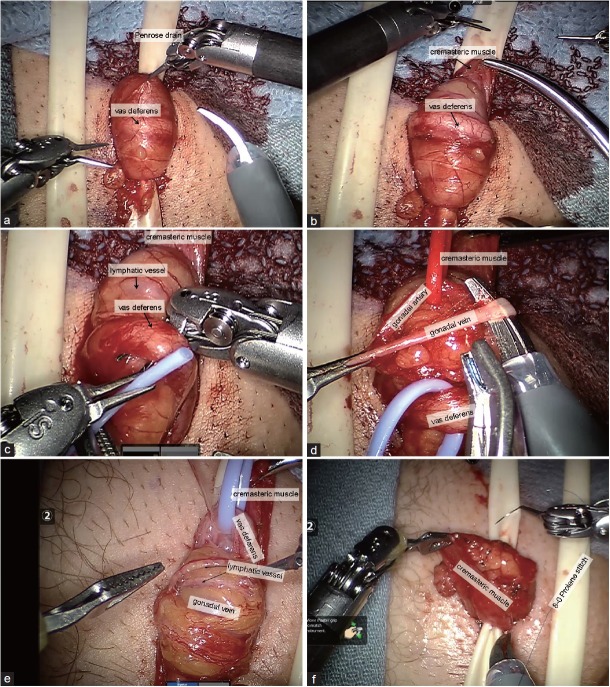
RAMV was performed in the following fashion. A 2-cm horizontal incision was made 3 cm from the base of the penis and 3 cm lateral to the midline (Figure 1). The spermatic cord was isolated through the incision using 3.5 × loupe magnification. It was elevated from the incision and suspended extracorporally using a Penrose drain (Figure 2a). For bilateral varicoceles, both cords were isolated before the robot was docked. The robot was then docked perpendicular to the patient on his left side. The first arm was loaded with black diamond forceps and aligned with the right anterior superior iliac spine toward the incision. The third arm was loaded with black diamond forceps and aligned with the ipsilateral knee to the incision. The second arm was loaded with monopolar scissors and was ninety degrees from the first arm in between arms one and three (Figure 1). A bedside assistant was responsible for irrigation, clipping the veins and presenting sutures and vessel loops to the surgeon. Next, after opening and fastening the cremasteric fascia to the Penrose drain (Figure 2b), the vas deferens was isolated with vessel loop and retracted off the field (Figure 2c). The testicular artery (ies) was/were then located with direct visualization without Doppler ultrasound and isolated with another vessel loop (Figure 2d). The lack of field motion and clear magnification allowed identification of the pulsating arteries. Papaverine was used to enhance pulsations. The veins were then dissected, ligated with titanium clips, and divided (Figure 2d). Lymphatics were preserved (Figure 2e). At completion of the varicocelectomy, only the testicular artery, lymphatics, and vas deferens remained. Vessel loops were removed, cremasteric fascia was reapproximated with 8-0 nylon sutures (Figure 2f), and the cord was freed of the Penrose drain and allowed to retract into the incision. For bilateral procedures, the robot arms were shifted to the contralateral incision without redraping and an identical procedure was performed on the contralateral side. After completion, the robot was undocked, the perifuniclar space was infiltrated with 10 cc of a 50/50 mixture of 1% lidocaine and 0.25% bupivacaine and the skin was closed with subcuticular stitches. Statistics were performed with JMP software (SAS Institute Inc., Cary, NC, USA) by Student's t-test.
Figure 1.
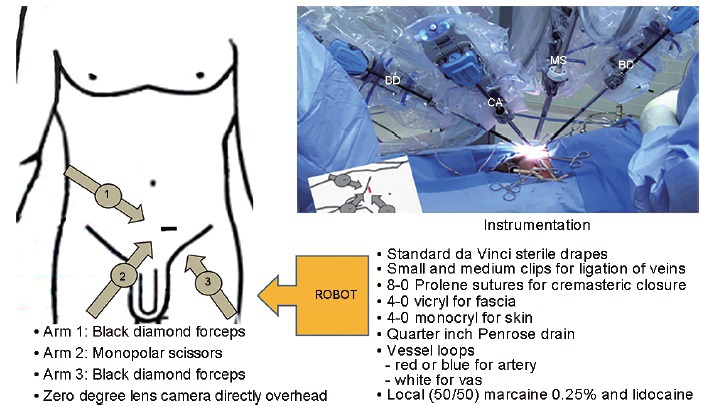
Schematic of robot docking position, actual docked robot and instrumentation required. BD: black diamond forceps; CA: zero-degree lens camera; MS: monopolar scissors
Figure 2.
Robotic-assisted microsurgical varicocelectomy surgery. (a) Cord is exposed and suspended extracorporally with Penrose. (b) Cremastric muscle fastened to Penrose exposing the vas deferens. (c) Vas being isolated with vessel loop. (d) Vein being isolated with artery and vas identified and individually retracted. (e) Identification and preservation of lymphatic. (f) Closure of cremasteric with 8-0 sutures.
RESULTS
Of 214 patients who underwent RAMV during that time period, 140 (65.4%) presented with infertility of at least 1 year and varicoceles. We are presenting only our data on the 140 men who presented with infertility. Baseline comorbidities are shown in Table 1. Mean age ± standard deviation (s.d.) and duration of infertility ± s.d. was 34.5 ± 5.8 and 2.8 ± 2.9 years, respectively. Oligozoospermia (<15 × 106 ml−1) was not required if teratozoospermia, asthenozoospermia or hypogonadism was present (T <350 ng dl−1). Seventy-three (52.1%) patients were oligozoospermic with a mean sperm count (SCmean) of 4.1 × 106 ml−1. A total of 118 (84.3%) and 22 (15.7%) patients underwent bilateral and unilateral repair, respectively, for a total of 258 varicocelectomies. Fifty-seven men (40.7%), 21 (15.0%), and 62 (44.3%) were eugonadal (T >350 ng dl−1), borderline hypogonadal (300 ng dl−1 ≤ T ≤350 ng dl−1), and hypogonadal (T <300 ng dl−1), respectively. Infertility was primary in 76 (54.3%) patients. Median T and Tfree increased by 44.3% (P < 0.001) (Table 2). Testicular volume increased significantly bilaterally by at least 12.5% (Table 2). Whereas median sperm concentration increased by 37.3% (P < 0.03), motility, Kruger, and World Health Organization (WHO) morphology were virtually unchanged (Table 2).
Table 1.
Baseline demographics and comorbidities in 140 infertile men with varicoceles
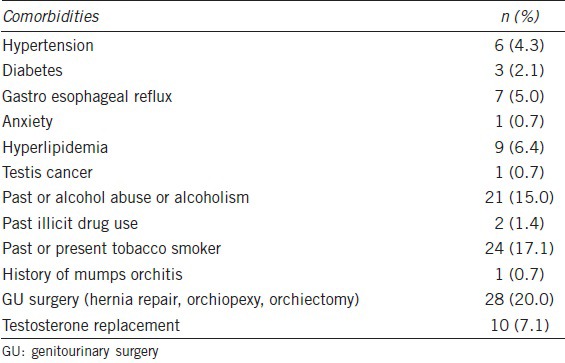
Table 2.
Comparison of pre- and post-operative hormone profile, semen analysis, and ultrasound parameters at 3 months in 140 infertile men with varicoceles
Operative time was recorded in our first 69 cases and compared to TMV. Operative time per side was 49 ± 13 min and 57 ± 16 min for TMV and RMV, respectively, reflecting our learning curve and reaching its nadir at 15 cases. For a bilateral case, robotic dock time averaged 39 ± 9 min. Throughout our series, we had 9/258 complications (3.5%) including 7 (2.7%) hematomas and 2 (0.8%) hydroceles. There were no injuries to the vas deferens or testicular artery on any of the testicular units (Table 3). All of these complications were managed conservatively. Persistent venous flow on postoperative ultrasound was seen in 9.7% (25) of testicular though dramatically reduced (Table 2). Intraoperatively with direct visualization, one testicular artery was identified on 80.7% of testicular units, two arteries on 15.2%, and three arteries on 4.1%. Postoperatively, only 37.3% patients used pain medications >24 h.
Table 3.
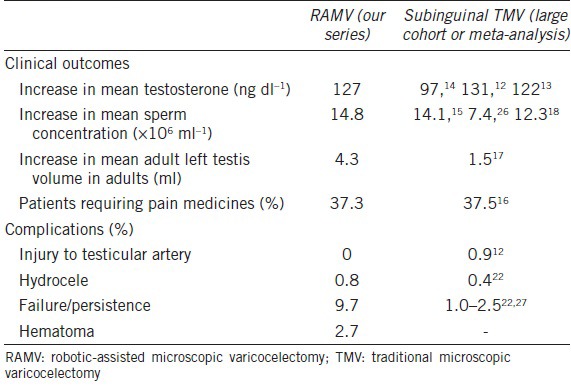
Comparison of robotic-assisted microsurgical varicocelectomy with subinguinal traditional microscopic varicocelectomy
DISCUSSION
We describe our series of RAMV on 140 infertile men, a subset of our larger series. As can be seen from the baseline characteristics, this represents a highly select group of men. With the robotic approach, complication rates were low without any injury to testicular arteries. Minimal persistent flow demonstrated that duplex Doppler was seen in 9.6% of testicular units. Serum T significantly improved.
The incidence of bilaterality of varicoceles in this series is higher than other varicocele surgical series and is most likely a reflection of the author's referral pattern and the use of reflux on ultrasound as a criterion for surgical candidacy. Our high percentage of bilaterality has been previously reported. In a study by Gat et al.,28 81% of 286 infertile men were confirmed to have bilateral disease by venography. Whereas physical examination detected right-sided varicoceles in 7.3% of men, Doppler ultrasound detected right-sided varicoceles in 66%.28 Testis duplex Doppler ultrasonography has 97% sensitivity and 94% specificity when compared to venography, the more invasive “gold standard” for the diagnosis of varicoceles.29 The European Association of Urology (EAU) guidelines support the use of testis Doppler ultrasound to determine the presence of varicocele and reflux.30 It is the authors’ opinion that the lack of objectivity in the diagnosis of varicoceles pre- and post-operatively may be contributory to the inconsistent results reported about benefits of varicocele surgery in the literature. The reliance on a classification system described in 197131 may be anachronistic in the era of sophisticated and more objective ultrasound imagery.
Most of the men with Doppler documented in our study, did, in fact, have clinical preoperative varicoceles, though the comparison of the clinical versus ultrasound diagnosis was not the objective of the paper.
Our recurrence rate is higher than previously reported in series. In our series, we found a 9.6% “failure rate.” This is higher than previously reported for microsurgical subinguinal repair 1.4%,32 3%,33 2%,34 0.82%,35 and 1.05%.22 As the presence of documentable venous reflux was a preoperative criterion, we defined recurrence as any measurable postoperative retrograde flow which may account for our higher recurrence rate. As shown in Table 2, our residual venous flow is extremely low. Other microsurgical subinguinal series use clinically detectable varicoceles as the definition of success. In addition to the lower sensitivity of clinical examination in the detection of varicoceles, the clinical examination of varicoceles as a postoperative end point is subjective and operator dependent and has never been intra- or inter-observer critically validated.36
Our observed T changes are similar to previously published studies involving TMV (Table 3). Chan et al.12 reviewed their series of 2012 men who underwent subinguinal TMV. They observed an increased mean T from 362 ng dl−1 to 493 ng dl−1. Hsiao et al.13 noted a similar increase in T from 309 to 431 ng dl−1 in men with preoperative T <400 ng dl−1. Both of these studies included patients with subinguinal TMV only. Patients were evaluated for symptomatic varicoceles or for infertility in these studies. A meta-analysis by Li et al.14 showed increase in T by 97.5 ng dl−1 in 814 patients in nine studies. Most, but not all (6 out of 9), of the studies exclusively included subinguinal TMV and have increases in T similar to our findings (Table 3).
Two prospective randomized controlled trials showed increased mean sperm concentration after subinguinal TMV by 14.1 × 106 and 7.4 × 106 ml−1.15,26 A large meta-analysis of 22 studies with various techniques by Baazeem et al.18 showed a significant increase in concentration by 12.3 × 106 ml−1. Similarly, another meta-analysis observed an increase in concentration by 13.7 × 106 ml−1; however, this was not statistically significant. This meta-analysis incorporated 3 randomized controlled trails and 4 nonrandomized trials, using various varicocelectomy techniques.19 These prospective studies demonstrate findings similar to our mean sperm concentration increase of 14.7 × 106 ml−1 (16.9 × 106 ± 23.4 × 106 vs 31.6 × 106 ± 66.5 × 106 ml−1).
We observed a significant increase in testicular size bilaterally based on testicular ultrasonography performed by a single ultrasonographer, pre- and post-operatively. A previously reported study examined the change in testicular volume in 44 men after varicocelectomy and demonstrated an increase of 1.5 ml.17 While 25 out of 44 of their patients underwent bilateral varicocelectomy, 21 underwent subinguinal TMV, 17 underwent laparoscopic varicocelectomy, and 6 underwent sclerotherapy. The study included a population of various techniques and a higher proportion of unilateral repairs; however, it is the only study evaluating testicular size in adults with a portion of patients who underwent subinguinal TMV. Zucchi et al.20 demonstrated only a 0.6 ml increase in testicular size in 43 patients who underwent either sclerotherapy or open inguinal approach. The various surgical techniques, patient selection, and the methodology of testicular size measurement might explain inconsistent reported increases in testicular size.
Hydroceles were observed as 0.8%, which is comparable to other subinguinal TMV series showing 0.44%,22 0.4%,12 1.6%,37 0.3%,32 and 0.2%35 rates of postoperative hydroceles. Injury to the testicular artery is rare with no occurrences in our population. In a large series of over 2100 patients, injury occurred in only 0.9% of cases.12 This slightly higher incidence is likely due to a larger study size compared to our series. In contrast to a smaller series of only 42 patients, one testicular artery injury occurred with immediate repair.38 Only 37.3% of patients needed pain medication for more than 24 h postoperatively. This result is similar to another study evaluating postoperative pain requirements. Pan et al.16 only had 37.5% of patients requiring pain medications in their series of subinguinal TMV.
The high capital cost of the DR is used as an argument against its use in the repair of varicoceles. Without a doubt the purchase costs for DR are more than an operating microscope (OM). From a practical viewpoint, unlike the OM, the DR is shared by all services. Frequently service-specific OMs differ in quality and features and are reluctantly shared. The urologic microsurgeon and operating room staff frequently have to familiarize themselves with a different microscope on the day of the procedure, decreasing OR efficiency. Admittedly the use of the robot precludes performing the procedure in outpatient surgery units, where the availability of the robot is unlikely. Although robot access for the urologic microsurgeon might be difficult, the relatively short length of the case makes it administratively attractive. The procedure-specific expenses for the RAMV are the 2 multiple use black diamond and monopolar scissors amounting to below US dollar $1000 per procedure. The drapes are comparable to those used for the standard microsurgical or laparoscopic approach. This study is not intended to condone the purchase and use of the DR where one is not available or it is a demonstration of the feasibility of the technique, if one is available. A cost analysis was not performed in this study, but the surgeon encountered no administrative resistance in scheduling cases. In terms of surgical training, a typical urology resident is unlikely to use microsurgical skills achieved during residency after training. Few urology residents enter into the area of male infertility. On the other hand, robotic skills learned in residency will continued to be used in performing robotic prostatectomies, nephrectomies, or urogynecologic procedures. As resident staff is already familiar with the DR equipment, their learning curve for the procedure is likely less than standard microsurgery. It seems instinctive that the micro-robotic skills developed handling 8-0 and 9-0 sutures and isolating 1 mm gonadal arteries will only improve macro-robotic skills. Improvement in robotic skills was not evaluated in this study. Additional training may be required for a traditional microsurgeon to master the technique with the DR, a problem faced by traditional open prostatectomists decades ago. Familiarity with microsurgical techniques and currently available training software were found to be of tremendous benefit to the surgeon (Andrew McCullough) in mastering the technique and minimizing the learning curve. Ergonometrically there is no question that operating from a console is superior to the awkward position during a typical OM varicocelectomy. Surgeon fatigue for attending and resident is minimized.
Weaknesses of this study include the retrospective nature of the study and comparison to historical controls rather than a simultaneous comparison. As the hormone values were done at different reference laboratories, the reference ranges may be different from one patient to the next. Patients had follow-up hormonal studies done at the same laboratory that was used for the preoperative laboratories. Though pre- and post-operative semen analyses were always done at the same IVF Andrology laboratory, no attempt was made to standardize the analyses between the two centers. Laboratories were done as the specifications and standards of the American Society for Reproductive Medicine.
Strengths were the rigorous clinical follow-up and use of also used Doppler ultrasound pre- and post-operatively to evaluate outcomes.
CONCLUSION
This study demonstrates that RAMV is comparable to subinguinal TMV. RAMV can be used as an alternative to TMV where a robot is available without compromise of patient care. More prospective studies are needed to evaluate cost differentials and the benefits to resident training.
AUTHOR CONTRIBUTIONS
AM came up with study concept, worked as principal surgeon, collected data, performed statistical analysis, and prepared manuscript. LE performed testicular ultrasounds. JE worked as surgical assistant, collected data, and reviewed manuscript. CM collected data, performed statistical analysis, and reviewed manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We would like to thank Barry Kogan and Sijo Parekattil for their support, encouragement, and guidance.
REFERENCES
- 1.Clarke BG. Incidence of varicocele in normal men and among men of different ages. JAMA. 1966;198:1121–2. [PubMed] [Google Scholar]
- 2.Greenberg SH, Lipshultz LI, Wein AJ. Experience with 425 subfertile male patients. J Urol. 1978;119:507–10. doi: 10.1016/s0022-5347(17)57531-x. [DOI] [PubMed] [Google Scholar]
- 3.WHO The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. World Health Organization. Fertil Steril. 1992;57:1289–93. [PubMed] [Google Scholar]
- 4.Comhaire F, Vermeulen A. Varicocele sterility: cortisol and catecholamines. Fertil Steril. 1974;25:88–95. doi: 10.1016/s0015-0282(16)40159-7. [DOI] [PubMed] [Google Scholar]
- 5.Reyes JG, Farias JG, Henriquez-Olavarrieta S, Madrid E, Parraga M, et al. The hypoxic testicle: physiology and pathophysiology. Oxid Med Cell Longev. 2012;2012:929285. doi: 10.1155/2012/929285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadziselimovic F, Leibundgut B, Da Rugna D, Buser MW. The value of testicular biopsy in patients with varicocele. J Urol. 1986;135:707–10. doi: 10.1016/s0022-5347(17)45826-5. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein M, Eid JF. Elevation of intratesticular and scrotal skin surface temperature in men with varicocele. J Urol. 1989;142:743–5. doi: 10.1016/s0022-5347(17)38874-2. [DOI] [PubMed] [Google Scholar]
- 8.Aitken RJ, Clarkson JS, Hargreave TB, Irvine DS, Wu FC. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J Androl. 1989;10:214–20. doi: 10.1002/j.1939-4640.1989.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987;8:338–48. doi: 10.1002/j.1939-4640.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 10.Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod. 1998;13:896–900. doi: 10.1093/humrep/13.4.896. [DOI] [PubMed] [Google Scholar]
- 11.Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 1998;13:1429–36. doi: 10.1093/humrep/13.6.1429. [DOI] [PubMed] [Google Scholar]
- 12.Chan PT, Wright EJ, Goldstein M. Incidence and postoperative outcomes of accidental ligation of the testicular artery during microsurgical varicocelectomy. J Urol. 2005;173:482–4. doi: 10.1097/01.ju.0000148942.61914.2e. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao W, Rosoff JS, Pale JR, Greenwood EA, Goldstein M. Older age is associated with similar improvements in semen parameters and testosterone after subinguinal microsurgical varicocelectomy. J Urol. 2011;185:620–5. doi: 10.1016/j.juro.2010.09.114. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Yue H, Yamaguchi K, Okada K, Matsushita K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol. 2012;19:149–54. doi: 10.1111/j.1442-2042.2011.02890.x. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Meguid TA, Al-Sayyad A, Tayib A, Farsi HM. Does varicocele repair improve male infertility.An evidence-based perspective from a randomized, controlled trial? Eur Urol. 2011;59:455–61. doi: 10.1016/j.eururo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Pan F, Pan L, Zhang A, Liu Y, Zhang F, Dai Y. Comparison of two approaches in microsurgical varicocelectomy in Chinese infertile males. Urol Int. 2013;90:443–8. doi: 10.1159/000345606. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto H, Saito K, Ogawa Y, Yoshida H. Effects of varicocele repair in adults on ultrasonographically determined testicular volume and on semen profile. Urology. 2008;71:485–9. doi: 10.1016/j.urology.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 18.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Zhou T, Zhang W, Chen Q, Li L, Cao H, et al. Effect of varicocelectomy on testis volume and semen parameters in adolescents: a meta-analysis. Asian J Androl. 2015;7:1012–6. doi: 10.4103/1008-682X.148075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zucchi A, Mearini L, Mearini E, Fioretti F, Bini V, et al. Varicocele and fertility: relationship between testicular volume and seminal parameters before and after treatment. J Androl. 2006;27:548–51. doi: 10.2164/jandrol.05200. [DOI] [PubMed] [Google Scholar]
- 21.Shridharani A, Lockwood G, Sandlow J. Varicocelectomy in the treatment of testicular pain: a review. Curr Opin Urol. 2012;22:499–506. doi: 10.1097/MOU.0b013e328358f69f. [DOI] [PubMed] [Google Scholar]
- 22.Cayan S, Shavakhabov S, Kadioglu A. Treatment of palpable varicocele in infertile men: a meta-analysis to define the best technique. J Androl. 2009;30:33–40. doi: 10.2164/jandrol.108.005967. [DOI] [PubMed] [Google Scholar]
- 23.Shu T, Taghechian S, Wang R. Initial experience with robot-assisted varicocelectomy. Asian J Androl. 2008;10:146–8. doi: 10.1111/j.1745-7262.2008.00354.x. [DOI] [PubMed] [Google Scholar]
- 24.Parekattil SJ, Gudeloglu A. Robotic assisted andrological surgery. Asian J Androl. 2013;15:67–74. doi: 10.1038/aja.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambert B. The frequency of mumps and of mumps orchitis and the consequences for sexuality and fertility. Acta Genet Stat Med. 1951;2:1–166. [PubMed] [Google Scholar]
- 26.Abdel-Maguid AF, Othman I. Microsurgical and nonmagnified subinguinal varicocelectomy for infertile men: a comparative study. Fertil Steril. 2010;94:2600–3. doi: 10.1016/j.fertnstert.2010.03.063. [DOI] [PubMed] [Google Scholar]
- 27.Rotker K, Sigman M. Recurrent varicocele. Asian J Androl. 2016;18:229–33. doi: 10.4103/1008-682X.171578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gat Y, Bachar GN, Everaert K, Levinger U, Gornish M. Induction of spermatogenesis in azoospermic men after internal spermatic vein embolization for the treatment of varicocele. Hum Reprod. 2005;20:1013–7. doi: 10.1093/humrep/deh706. [DOI] [PubMed] [Google Scholar]
- 29.Belay RE, Huang GO, Shen JK, Ko EY. Diagnosis of clinical and subclinical varicocele: how has it evolved? Asian J Androl. 2016;18:182–5. doi: 10.4103/1008-682X.169991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, et al. European association of urology guidelines on male infertility: the 2012 update. Eur Urol. 2012;62:324–32. doi: 10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 31.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–9. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 32.Jungwirth A, Gogus C, Hauser G, Gomahr A, Schmeller N, et al. Clinical outcome of microsurgical subinguinal varicocelectomy in infertile men. Andrologia. 2001;33:71–4. doi: 10.1046/j.1439-0272.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- 33.Orhan I, Onur R, Semercioz A, Firdolas F, Ardicoglu A, et al. Comparison of two different microsurgical methods in the treatment of varicocele. Arch Androl. 2005;51:213–20. doi: 10.1080/01485010590919648. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R, Gupta NP. Subinguinal microsurgical varicocelectomy: evaluation of the results. Urol Int. 2003;71:368–72. doi: 10.1159/000074087. [DOI] [PubMed] [Google Scholar]
- 35.Marmar JL, Kim Y. Subinguinal microsurgical varicocelectomy: a technical critique and statistical analysis of semen and pregnancy data. J Urol. 1994;152:1127–32. doi: 10.1016/s0022-5347(17)32521-1. [DOI] [PubMed] [Google Scholar]
- 36.Lund L, Roebuck DJ, Lee KH, Sorensen HT, Yeung CK. Clinical assessment after varicocelectomy. Scand J Urol Nephrol. 2000;34:119–22. doi: 10.1080/003655900750016733. [DOI] [PubMed] [Google Scholar]
- 37.Ghanem H, Anis T, El-Nashar A, Shamloul R. Subinguinal microvaricocelectomy versus retroperitoneal varicocelectomy: comparative study of complications and surgical outcome. Urology. 2004;64:1005–9. doi: 10.1016/j.urology.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 38.Leung L, Ho KL, Tam PC, Yiu MK. Subinguinal microsurgical varicocelectomy for male factor subfertility: ten-year experience. Hong Kong Med J. 2013;19:334–40. doi: 10.12809/hkmj133884. [DOI] [PubMed] [Google Scholar]