Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising agent for anticancer therapy. The identification of small molecules that can establish the sensitivity of prostate cancer (PCa) cells to TRAIL-induced apoptosis is crucial for the targeted treatment of PCa. PC3, DU145, JAC-1, TsuPr1, and LNCaP cells were treated with Andrographolide (Andro) and TRAIL, and the apoptosis was measured using the Annexin V/PI double staining method. Real time-polymerase chain reaction (PCR) and Western blot analysis were performed to measure the expression levels of target molecules. RNA interference technique was used to down-regulate the expression of the target protein. We established a nude mouse xenograft model of PCa, which was used to measure the caspase-3 activity in the tumor cells using flow cytometry. In this research study, our results demonstrated that Andro preferentially increased the sensitivity of PCa cells to TRAIL-induced apoptosis at subtoxic concentrations, and the regulation mechanism was related to the up-regulation of DR4. In addition, it also increased the p53 expression and led to the generation of reactive oxygen species (ROS) in the cells. Further research revealed that the DR4 inhibition, p53 expression, and ROS generation can significantly reduce the apoptosis induced by the combination of TRAIL and Andro in PCa cells. In conclusion, Andro increases the sensitivity of PCa cells to TRAIL-induced apoptosis through the generation of ROS and up-regulation of p53 and then promotes PCa cell apoptosis associated with the activation of DR4.
Keywords: andrographolide, DR4, p53, reactive oxygen species, tumor necrosis factor-related apoptosis-inducing ligand
INTRODUCTION
Prostate cancer (PCa) is the most common cancer in men with a high incidence. However, past research studies have not been successful in establishing a standard treatment for PCa. Traditional therapy using anti-male hormone is not only insufficient to treat PCa but also may promote the deterioration of PCa into hormonal refractory prostate cancer (HRPC).1 Many studies have indicated that tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) can induce the apoptosis of cancer cells.2 A clinical phase I trial has been performed and reported that the adenoviral vectors encoding the TRAIL transgene caused local inflammation and apoptosis in PCa patients.3 Therefore, TRAIL can be the new therapeutic biologic agent for PCa.
TRAIL, as a related apoptosis inducing ligand, was associated with tumor necrosis factor family, which can induce apoptosis through the activation of the caspases pathway4 and interaction with the related cell surface receptors DR4 and DR5 (also called TRAIL-R1 and TRAIL-R2, respectively).5 TRAIL was able to bind the tumor cell death receptors DR4 and DR5 that promoted the formation of the death-inducing signaling complex and the activation of caspase-3 and caspase-8, inducing tumor cell death.6 Theoretically, TRAIL can be used as an apoptosis-inducing factor in many human cancers as well as an ideal target for cancer therapy. However, many types of cancer cells were found to be insensitive or resistant to TRAIL, according to recent studies,7 including PCa cells.8 The mechanism by which PCa cells resist TRAIL-induced apoptosis is complex and is currently under study. The main underlying mechanisms were found to be receptor gene mutation, hypermethylation, downstream inhibitor interference, and the competitive binding of the decoy receptor and the DR4 and DR5 receptors for binding to ligands. All these may lead to the loss of function of the normal signaling pathway with respect to the proliferation of PCa cells, making the cells relatively nonsensitive to TRAIL-induced apoptosis. Therefore, it is vital to enhance the sensitivity of PCa cells to TRAIL-induced apoptosis and promote TRAIL-mediated apoptosis of the PCa cells. Studies have consistently identified several compounds and agents that can improve the sensitivity of cells to TRAIL-induced apoptosis, and these have been defined as anticancer agents.
Andrographolide (Andro) is a diterpene lactone compound from Andrographis paniculata which has been widely used for its anti-inflammatory and antibacterial action, cardiovascular protection and immune regulation in clinical settings. Recent studies have shown that Andro is a powerful anti-cancer agent, which works by promoting the apoptosis of cancer cells.9 Andro has been found to induce programmed cell death in breast cancer cells.10 In vitro, Andro enhanced the radiosensitivity of esophageal cancer cells, causing severe apoptosis.11 Similarly, Andro had also been found to reverse the resistance phenotype of cancer cells to anticancer drugs to increase the effectiveness of drug treatment.12 Previous studies have reported that Andro has a role on inhibition of PCa through cell cycle regulation. It is noteworthy that when combined with anticancer drugs or biological agents, Andro can play a greater role in restricting tumor initiation and promotion. In addition, Andro usually increases the related sensitivity to apoptosis in cancer cells. However, the role and mechanisms of Andro in the TRAIL-induced apoptosis of PCa cells are unclear. Therefore, this study attempted to investigate whether Andro can increase the sensitivity of PCa cells to TRAIL-induced apoptosis. We found that Andro regulated the expressions of apoptosis-related proteins, making PCa cells sensitive to TRAIL-induced apoptosis.
MATERIALS AND METHODS
Cell culture and treatment
PCa cells PC3, DU145, JCA-1, TsuPr1, LNCaP and human renal tubular epithelial cells 293T and human prostate stromal cells PS30 were purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). All PCa cell lines were maintained in Roswell Park Memorial Institute-1640 medium (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), 100 U ml−1 penicillin and 100 U ml−1 streptomycin (Hyclone, Logan, UT, USA). The culture condition of 293T cells was Dulbecco's Modified Eagle Medium (DMEM) (Gibco, Carlsbad, CA, USA), containing 10% FBS and 1% penicillin-streptomycin (Hyclone, Logan, UT, USA). All cells were cultured at 37°C in 5% CO2. Andro (Sigma, Milwaukee, WI, USA) was dissolved with dimethyl sulfoxide (DMSO; Sigma, Milwaukee, WI, USA), the stock solution at 100 μmol l−1, and the human TRAIL (Sigma, Milwaukee, WI, USA) solution with a concentration of 100 mg ml−1 was prepared by PBS (Hyclone, Logan, UT, USA) containing 0.1% bovine serum albumin (Hyclone, Logan, UT, USA). Caspases inhibitors were formulated as solutions with suitable working concentration according to the manufacturer's instructions.
Apoptosis assay
The treated cells were collected and washed with PBS; thereafter, they were centrifuged to obtain the cells. The cells were resuspended in 100 μl labeling solution in the Annexin-V-FLUOS Kit (Roche, Basel, Switzerland). After an incubation period of 10 min in the dark, the supernatant was removed by centrifugation. The cell precipitate was added to a fluorescent solution and incubated at 4°C for 20 min. Fluorescein isothiocyanate (FITC)-fluorescence and PI were detected at 515 nmol l−1 and 560 nmol l−1 wavelengths, respectively, using flow cytometry.
Western blot
The cells were collected and lysed with RIPA Lysis Buffer (BioTNT, Shanghai, China). Total protein was extracted, and the BCA Protein Assay Kit (Beyotime, Shanghai, China) was used to detect the total protein concentration. Polyacrylamide gel was prepared based on the size and molecular weight of the target proteins. Total protein was diluted to the appropriate concentration using sample buffer, and the protein samples were injected into the gel. After separating the target proteins using electrophoresis, the proteins were transferred onto the polyvinylidene difluoride (PVDF) membrane (Invitrogen, Carlsbad, CA, USA) from the gel. The PVDF membrane was immersed overnight in 5% skimmed milk (Hyclone, Logan, UT, USA) at 4°C. The proteins were incubated with the primary and secondary antibodies and subjected to chromogenic reactions using the Pierce™ ECL Plus Western Blotting Substrate (Thermo Scientific, Waltham, MA, USA) to detect the expressions of the target proteins.
Flow cytometry
The cells were digested with trypsin and harvested; they were washed two times with PBS, and the supernatant was removed by centrifugation at 800 rpm min−1 for 5 min. The cell suspension in 0.1 ml PBS was added to 1 μl first antibodies, and incubated for 1 h at room temperature. After the first antibodies were washed away by PBS, the cells and secondary antibodies with fluorescent were incubated for another 1 h. The secondary antibodies were removed and washed two times; thereafter, cells were re-suspended in 0.2 ml PBS. The cell suspension was subjected to flow cytometry, and the results were analyzed.
Transfection siRNA
The cells were cultured in 6-well plates for 24 h. Lipofectamine 2000 and siRNA were added to the cell culture system with the appropriate working concentration and mixed sufficiently; thereafter, the mixed system was statically cultured for 24 h at 37°C. The culture medium was discarded; the fresh medium was then added and allowed to culture for 48 h. The interference efficiency was detected using the expression of the target protein. In the present study, all lipofectamine 2000, si-RNA and its control were designed and synthesized by Invitrogen (Carlsbad, CA, USA). Sequences of siRNA were: DR4-siRNA-GCGCGGAGCATTGATCCACG; p53-siRNA-GCAUGAACCGGAGGCCCAU-30.
Tumor growth in vivo
All animal experiments were conducted as per the standard guidelines for the care and use of laboratory animals of Xi’an Jiaotong University, and the study was approved by the Research Ethics Committee at the first affiliated hospital of the Xi’an Jiaotong University. BALB/c mice (5–6-week-old) were purchased from the animal center of Xi’an Jiaotong University. PC3 cells were inoculated into nude mice to produce xenograft models. Mice were challenged with 100 μg TRAIL, 10 mg kg−1 Andro or both once every 3 days for 24 consecutive days. Tumor volume and animal weight were measured once every 3 days. After treatment, tumors were removed, and the caspase-3 activity in the tumor cells was measured with flow cytometry using the FITC-conjugated caspase-3 substrate.
Statistical analysis
All experiments were repeated at least three times. The data were from one representative experiment, and we have performed this experiment for at least three times and got similar results. GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA) was used to perform statistical description and data analysis. The groups were compared using the Student's t-test. The value of P ≤ 0.05 was considered statistically significant. Data are represented as mean ± standard deviation.
RESULTS
Effects of Andro and TRAIL on the apoptosis of PCa cells
To investigate the effect of Andro on the apoptosis of PCa cells, we examined five PCa cell lines (PC3, DU145, JCA-1, TsuPr1, and LNCaP) with 293T cells as the control group. When PCa cell lines and 293T cells were treated with different concentrations (0 μmol l−1, 10 μmol l−1, 20 μmol l−1 and 30 μmol l−1) of Andro alone, there was no significant effect on cell apoptosis. However, the apoptosis of PCa cells was significantly increased after 24 h of co-treatment with Andro and TRAIL (20 ng ml−1), and the apoptosis was positively correlated with the Andro concentration. The apoptosis with 30 μmol l−1 Andro was significantly higher than that without Andro treatment (Figure 1a). PC3, DU145, JCA-1, TsuPr1 and LNCaP cells were treated with 20 μmol l−1 Andro, 20 ng ml−1 TRAIL, or both for 0, 8, 12, 16, 24, and 30 h; untreated cells comprised the blank control group. From 8 h after the treatment, the interaction of Andro and TRAIL significantly increased cell apoptosis (Figure 1b–1f).The percentage of apoptotic cells increased gradually with the time, and all PCa cell lines showed a similar trend of apoptosis. Furthermore, compared to the effect on PCa cells, treatment of only Andro or Andro combined with TRAIL had no significant effects on the apoptosis of normal cells, including normal prostate epithelial cell line RWPE-1, human prostate stromal cells PS30, human hepatocytes HL7702 and 293T cells (Figure 1g).
Figure 1.
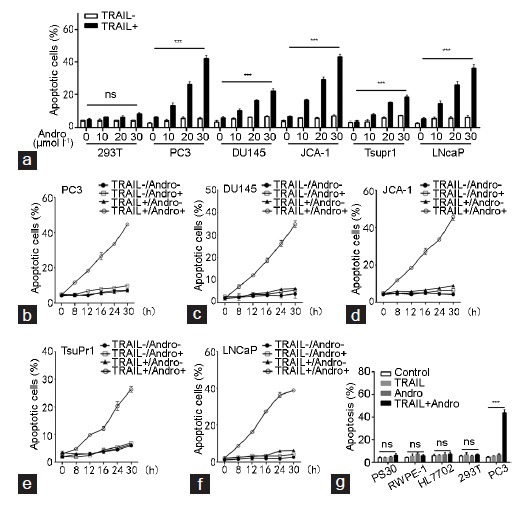
Effects of Andro and TRAIL on the apoptosis in PCa cells and normal cells. (a) The apoptosis in PCa cell lines (PC3, DU145, JCA-1, TsuPr1 and LNCaP) and 293T under different concentrations (0 µmol l−1, 10 µmol l−1, 20 µmol l−1, 30 µmol l−1) of Andro treatment with or without TRAIL (20 ng ml−1) for 24 h. TRAIL vs TRAIL (0), *P < 0.05. The apoptosis of (b) PC3, (c) DU145, (d) JCA-1, (e) TsuPr1 and (f) LNCaP treated with 20 µmol l−1 Andro, 20 ng ml−1 TRAIL, or both for 0, 8, 12, 16, 24 and 30 h, and untreated cells that comprised as the blank control group. (g) Effects of Andro and TRAIL on the apoptosis of PCa cells and normal cells. PC3, normal prostate epithelial cell line RWPE-1, human prostate stromal cells PS30, human hepatocytes HL7702 and 293T cells were treated with 30 µmol l−1 Andro, 20 ng ml−1 TRAIL or both for 24 h, and untreated cells comprised the blank control group. TRAIL + Andro vs control or TRAIL or Andro, *P < 0.05. Andro: andrographolide; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; ns: not significant.
The role of caspases and DR4 in Andro mediated apoptosis-enhancement
PC3 cells were treated with 20 μmol l−1 Andro, 20 ng ml−1 TRAIL, or both for 12 h; the expression of apoptosis-related proteins was detected using western blot analysis. The combined Andro and TRAIL treatment considerably increased the expressions of cleaved caspase-8, cleaved caspase-3 and cleaved PARP compared to the other treatments, whereas FLIP expression was decreased (Figure 2a). PC3 cells were pretreated with 5 μmol l−1 caspases inhibitors before being treated with TRAIL (20 ng ml−1) and Andro (20 μmol l−1). Caspases inhibitors effectively reduced the apoptosis induced by Andro and TRAIL (Figure 2b). Similarly, the interaction of Andro and TRAIL increased the protein expression levels of DR4 and DR5 and raised the expression of DR4 on the cell surface (Figure 2c and 2d). SiRNA-DR4 transfection into PC3 cells can effectively reduce the expression of DR4 (Figure 2e). PC3 cells were transfected with siRNA-DR4 before the combined Andro and TRAIL treatment, and cell apoptosis was significantly decreased compared with that in the control group (Figure 2f).
Figure 2.
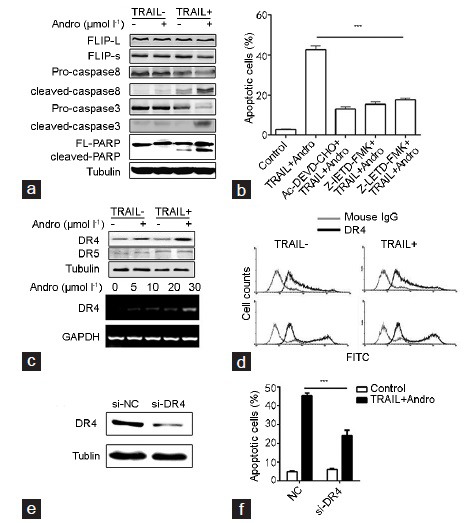
The role of caspases and DR4 in Andro-mediated apoptosis enhancement. (a) The expressions of apoptosis-related proteins in the PC3 cells treated with 20 μmol l−1 Andro, 20 ng ml−1 TRAIL, or both for 12 h. (b) The apoptosis of PC3 cells pretreated with 5 μmol l−1 caspases inhibitors for 30 min, and then treated with TRAIL (20 ng ml−1) and Andro (20 μmol l−1) for 24 h. TRAIL+Andro vs Ac-DEVD-CHO+TRAIL+Andro or Z-IETD-FMK+TRAIL+Andro or Z-LETD-FMK+TRAIL+Andro, *P < 0.05. After the PC3 cells were treated with Andro (20 μmol l−1) and TRAIL (20 ng ml−1) or both for 12 h, (c) the DR5 expression at the mRNA level and the DR4 expression in both mRNA-protein, (d) the expression of DR4 on the cell surface were detected. (e) The expression of DR4 in PC3 cells transfected with control siRNA or DR4 siRNA for 24 h. (f) Apoptosis of PC3 cells transfected with control siRNA or DR4 siRNA for 24 h, and then treated with control or combined TRAIL (20 ng ml−1) and Andro (20 μmol l−1) for another 24 h. si-DR4 vs NC, *P < 0.05. Andro: andrographolide; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; FITC: fluorescein isothiocyanate; NC: negative control for DR4 siRNA.
The role of p53 in Andro-mediated apoptosis enhancement
PC3 cells were treated with different concentrations of Andro and TRAIL for 12 h. Results of the western blot analysis showed that the expressions of DR4, p53, and p-p53 in the PC3 cells treated with TRAIL were significantly different than the expressions of those in the untreated control group; the combined Andro and TRAIL treatment of PC3 cells significantly increased the expressions of DR4 and p53 in the cells (Figure 3a). The expression of p53 was inhibited effectively by the transfection of siRNA-p53 (Figure 3b). In the control group, Andro increased the expression of DR4 and p53; when the expression of p53 was suppressed, the expressions of DR4 and p53 were not significantly changed under the conditions of Andro treatment compared with the control group (Figure 3c). The inhibition of p53 expression significantly reduced the cell apoptosis induced by Andro and TRAIL (Figure 3d).
Figure 3.
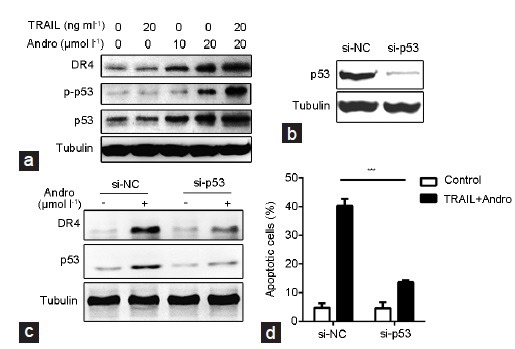
The role of p53 in Andro mediated apoptosis-enhancement. (a) The expression of p53 and DR4 in PC3 cells treated as indicated for 12 h. (b) The expression of p53 in PC3 cells transfected with control siRNA or p53 siRNA for 24 h. (c) The expression levels of p53 and DR4 in PC3 cells transfected with control siRNA or p53 siRNA for 24 h and then treated (or not treated) with Andro for 12 h. (d) The apoptosis of PC3 cells transfected with control siRNA or p53 siRNA for 24 h, and then treated with control or both TRAIL (20 ng ml−1) and Andro (20 µmol l−1) for another 24 h. si-p53 vs NC, *P < 0.05. Andro: andrographolide; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; NC: negative control for p53 siRNA.
ROS generation induced by Andro in PC3 cells
Flow cytometry was used to detect the ROS generation in PC3 cells treated with Andro and TRAIL. Andro treatment alone or in combination with TRAIL promoted ROS generation in the cells (Figure 4a). However, Andro and NAC co-treatment of the cells, Andro had no significant effect on ROS generation and ROS generation level reduced obviously (Figure 4b); meanwhile, NAC significantly reduced the high expressions of DR4 and p53 which were induced by Andro (Figure 4c), and promoted the expressions of PARP and caspase-3 (Figure 4d). Similarly, NAC significantly inhibited the cell apoptosis induced by Andro and TRAIL (Figure 4e).
Figure 4.
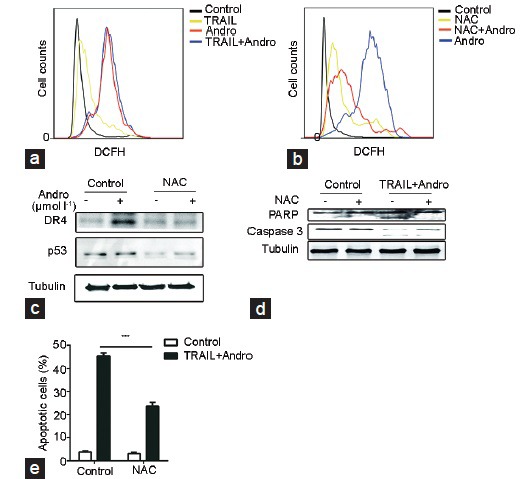
ROS generation induced by Andro in PC3 cells. (a) ROS generation in PC3 cells treated with Andro (20 μmol l−1) and TRAIL (20 ng ml−1) or both for 12 h detected using flow cytometry. PC3 cells were pretreated with or without 5 mmol l−1 NAC for 30 min and then treated with or without Andro (20 μmol l−1) for 12 h, detection of (b) ROS generation using flow cytometry, (c) and the expression of p53 and DR4 using western blot analysis. PC3 cells were pretreated with or without 5 mmol l−1 NAC for 30 min and then treated with or without both TRAIL (20 ng ml−1) and Andro (20 μmol l−1), detection of (d) the expressions of PARP and caspase-3, and (e) the apoptosis of PC3 cells. NAC vs control, *P < 0.05. Andro: andrographolide; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; ROS: reactive oxygen species; NAC: N-acetyl-cysteine.
Effects of Andro and TRAIL on tumors in nude mice xenograft models
Tumor volume and animal weight were measured every 3 days for 24 consecutive days; the following results were obtained: under the combined Andro and TRAIL treatment, the tumor size was the smallest and the growth rate was slow (Figure 5a); the four groups showed no significant differences in the weight of the mice, which remained stable (Figure 5b); the activity of caspase-3 in tumor cells was the highest; it was significantly higher than that in the other treatment groups and the control group (Figure 5c).
Figure 5.
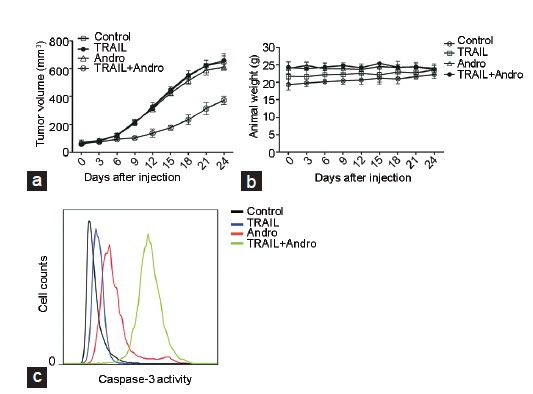
Effects of Andro and TRAIL on tumors in nude mice xenograft models. (a) Tumor volume and (b) animal weight (measured once every 3 days) in mice injected with 100 µg TRAIL, 10 mg kg−1 Andro or both once every 3 days for 24 consecutive days. (c) After treatment, flow cytometry was used to detect the activity of caspase-3 in tumor cells. Andro: andrographolide; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand.
DISCUSSION
TRAIL was first discovered and cloned successfully in 1995, and it was a new type of apoptotic molecule belonging to the tumor necrosis factor (TNF) family. TRAIL can specifically induce apoptosis in human transformed and malignant cells,13 whereas normal cells can escape its killing effect. The above characteristics indicate that the precise regulation of the function of TRAIL may provide a novel therapy treatment for cancers. However, the resistance to TRAIL in cancer cells is an important and difficult challenge that needs to be overcome for its effective clinical application. According to recent studies, many types of cancer cells, including PCa cells, are insensitive to TRAIL.14 At present, the combined use of TRAIL and other agents has proven effective for overcoming this challenge. Here, certain agents were used to improve the sensitivity of the cells to TRAIL-induced apoptosis, whereas TRAIL was responsible for the promotion of cancer cell apoptosis.15 Studies have consistently found that several compounds and agents can improve the sensitivity of cells to TRAIL-induced apoptosis; these are defined as anticancer agents. Among them, Andro, a diterpenoid lactone compound from Andrographis paniculata, is a type of anticancer agent which is currently being widely researched. Several studies have confirmed the effectiveness and specificity of Andro in the promotion of cancer cell apoptosis.16 More importantly, Andro can also increase the related sensitivity to apoptosis in cancer cells to fulfill the anti-cancer effect. Andro significantly inhibits the growth of hepatoma tumor.17 Similarly, Andro influences the expressions of apoptosis-related proteins, leading to the apoptosis of colon cancer cells.18 Previous studies have reported that Andro has a role in PCa inhibition through cell cycle regulation.19 Andro increases the radiation sensitivity of ovarian cancer cells, enhancing apoptosis and autophagy.20 In this study, we found that Andro significantly improved the sensitivity of PCa cells to TRAIL-induced apoptosis. Andro or TRAIL alone had no significant effect on the apoptosis of PCa cells or normal cells. However, the combined treatment of Andro and TRAIL significantly increased apoptosis. On the other hand, there was no significant difference in the apoptosis of normal cells. This result is consistent with the reported properties of TRAIL in terms of no apoptotic effect on normal cells.
Further study found that Andro promoted TRAIL-induced apoptosis by regulating the expression of apoptosis-related proteins. This result is consistent with those of the majority studies that have described the classic caspase-dependent apoptotic pathway as the underlying mechanism for the apoptosis induced by the adjunctive agents and TRAIL. We detected a significant increase in the expressions of cleaved caspase-8, cleaved caspase-3 and cleaved PARP which are markers of apoptosis. The lower procaspase-8 expression indicated that most of the caspase-8 were activated, which may have mediated cell apoptosis. Many studies have demonstrated that the sensitivity of the cells to TRAIL-induced apoptosis was related to the expressions of DR4 and DR5.21 We also proved that Andro increased the sensitivity of PCa cells to TRAIL-induced apoptosis depending on DR4 rather than on DR5. Andro can significantly increase DR4 expression; when DR4 expression was inhibited, cell sensitivity to TRAIL-induced apoptosis mediated by Andro decreased considerably. Therefore, it can be concluded that the regulatory mechanism of Andro-mediated apoptosis enhancement is blocked with the down-regulation of DR4.
P53, a tumor suppressor gene, plays an important role in cancer cell apoptosis via the activation of death receptors. We found that Andro simultaneously promoted DR4 and p53 expression in PCa cells. Inhibition of p53 expression, Andro showed no significant effect on the expressions of p53 and DR4, and Andro-mediated apoptosis enhancement was dramatically inhibited. Higher than normal levels of reactive oxygen species (ROS) can cause oxidative stress, which can lead to cell damage or apoptosis.22 The activation and metabolism of exogenous compounds into the cells can increase ROS generation, resulting in oxidative damage. We used a similar method to investigate the role of ROS generation in the Andro-mediated apoptosis enhancement mechanism. We found that Andro promoted ROS generation. The inhibition of ROS generation can significantly reduce p53 and DR4 expressions and decrease the Andro-induced apoptosis enhancement. We concluded that ROS up-regulated p53 expression, influencing DR4. Mice model experiments also confirmed that Andro obviously improved the sensitivity of the PCa cells to TRAIL-induced apoptosis, thus inhibiting tumor growth.
CONCLUSION
The study investigated the effect of Andro on TRAIL-induced PCa cell apoptosis and its specific regulatory mechanisms. Our results indicated that Andro can significantly improve the sensitivity of PCa cells to TRAIL-induced apoptosis. Therefore, the results provide a theoretical basis for considering Andro as an adjuvant agent for the treatment of PCa using TRAIL and a new research direction for PCa therapy.
AUTHOR CONTRIBUTIONS
XSZ put forward the concept of the study, designed the study, prepared and revised this manuscript and contributed to the statistical analysis. RJW conducted the data acquisition and contributed to the quality control of data and algorithms. DLH analyzed and interpreted the data, and edited the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All the authors declare no competing interests.
REFERENCES
- 1.Lin TP, Chang YT, Lee SY, Campbell M, Wang TC, et al. REST reduction is essential for hypoxia-induced neuroendocrine differentiation of prostate cancer cells by activating autophagy signaling. Oncotarget. 2016;7:26137–51. doi: 10.18632/oncotarget.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai FL, Tian H, Yu YH, Yin JC, Ren GP, et al. TNF-related apoptosis-inducing ligand delivered by rNDV is a novel agent for cancer gene therapy. Technol Cancer Res Treat. 2015;14:737–46. doi: 10.7785/tcrt.2012.500446. [DOI] [PubMed] [Google Scholar]
- 3.Wedrowska E, Wandtke T, Dyczek A, Wozniak J. Viral transfer of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in gene therapy. Postepy Hig Med Dosw (Online) 2015;69:1411–22. Article in Polish. [PubMed] [Google Scholar]
- 4.Das D, Persaud L, Dejoie J, Happy M, Brannigan O, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) activates caspases in human prostate cancer cells through sigma 1 receptor. Biochem Biophys Res Commun. 2016;470:319–23. doi: 10.1016/j.bbrc.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Park MH, Kim JH, Chung YH, Lee SH. Bakuchiol sensitizes cancer cells to TRAIL through ROS- and JNK-mediated upregulation of death receptors and downregulation of survival proteins. Biochem Biophys Res Commun. 2016;473:586–92. doi: 10.1016/j.bbrc.2016.03.127. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava RK. TRAIL/Apo-2L: mechanisms and clinical applications in cancer. Neoplasia. 2001;3:535–46. doi: 10.1038/sj.neo.7900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang JS, Lee HC, Oh SC, Lee DH, Kwon KH. Shogaol overcomes TRAIL resistance in colon cancer cells via inhibiting of survivin. Tumour Biol. 2015;36:8819–29. doi: 10.1007/s13277-015-3629-2. [DOI] [PubMed] [Google Scholar]
- 8.Plante MK, Arscott WT, Folsom JB, Tighe SW, Dempsey RJ, et al. Ethanol promotes cytotoxic effects of tumor necrosis factor-related apoptosis-inducing ligand through induction of reactive oxygen species in prostate cancer cells. Prostate Cancer Prostatic Dis. 2013;16:16–22. doi: 10.1038/pcan.2012.37. [DOI] [PubMed] [Google Scholar]
- 9.Mishra SK, Tripathi S, Shukla A, Oh SH, Kim HM. Andrographolide and analogues in cancer prevention. Front Biosci (Elite Ed) 2015;7:255–66. doi: 10.2741/E732. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee M, Chattopadhyay S, Choudhuri T, Bera R, Kumar S, et al. Cytotoxicity and cell cycle arrest induced by andrographolide lead to programmed cell death of MDA-MB-231 breast cancer cell line. J Biomed Sci. 2016;23:40. doi: 10.1186/s12929-016-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang ZM, Kang YH, Yang X, Wang JF, Zhang Q, et al. Andrographolide radiosensitizes human esophageal cancer cell line ECA109 to radiation in vitro. Dis Esophagus. 2016;29:54–61. doi: 10.1111/dote.12255. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Ong CN, Hur GM, Shen HM. Inhibition of the JAK-STAT3 pathway by andrographolide enhances chemosensitivity of cancer cells to doxorubicin. Biochem Pharmacol. 2010;79:1242–50. doi: 10.1016/j.bcp.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Elmallah MI, Micheau O. Marine drugs regulating apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Mar Drugs. 2015;13:6884–909. doi: 10.3390/md13116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gang X, Wang Y, Wang Y, Zhao Y, Ding L, et al. Suppression of casein kinase 2 sensitizes tumor cells to antitumor TRAIL therapy by regulating the phosphorylation and localization of p65 in prostate cancer. Oncol Rep. 2015;34:1599–604. doi: 10.3892/or.2015.4123. [DOI] [PubMed] [Google Scholar]
- 15.Crowder RN, Dicker DT, El-Deiry WS. The deubiquitinase inhibitor PR-619 sensitizes normal human fibroblasts to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated cell death. J Biol Chem. 2016;291:5960–70. doi: 10.1074/jbc.M115.713545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu SH, Lin CH, Liang FP, Chen PF, Kuo CD, et al. Andrographolide downregulates the v-Src and Bcr-Abl oncoproteins and induces Hsp90 cleavage in the ROS-dependent suppression of cancer malignancy. Biochem Pharmacol. 2014;87:229–42. doi: 10.1016/j.bcp.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Ji L, Zheng Z, Shi L, Huang Y, Lu B, et al. Andrographolide decreased VEGFD expression in hepatoma cancer cells by inducing ubiquitin/proteasome-mediated cFos protein degradation. Biochim Biophys Acta. 2015;1850:750–8. doi: 10.1016/j.bbagen.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee A, Ahmed H, Yang P, Czinn SJ, Blanchard TG. Endoplasmic reticulum stress and IRE-1 signaling cause apoptosis in colon cancer cells in response to andrographolide treatment. Oncotarget. 2016;7:41432–44. doi: 10.18632/oncotarget.9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mir H, Kapur N, Singh R, Sonpavde G, Lillard JW, Jr, et al. Andrographolide inhibits prostate cancer by targeting cell cycle regulators, CXCR3 and CXCR7 chemokine receptors. Cell Cycle. 2016;15:819–26. doi: 10.1080/15384101.2016.1148836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Qiu X. Andrographolide radiosensitizes human ovarian cancer SKOV3 xenografts due to an enhanced apoptosis and autophagy. Tumour Biol. 2015;36:8359–65. doi: 10.1007/s13277-015-3578-9. [DOI] [PubMed] [Google Scholar]
- 21.Oikonomou E, Pintzas A. The TRAIL of oncogenes to apoptosis. Biofactors. 2013;39:343–54. doi: 10.1002/biof.1112. [DOI] [PubMed] [Google Scholar]
- 22.Jian KL, Zhang C, Shang ZC, Yang L, Kong LY. Eucalrobusone C suppresses cell proliferation and induces ROS-dependent mitochondrial apoptosis via the p38 MAPK pathway in hepatocellular carcinoma cells. Phytomedicine. 2017;25:71–82. doi: 10.1016/j.phymed.2016.12.014. [DOI] [PubMed] [Google Scholar]


