Abstract
Introduction:
Melanin is synthesized by melanocytes, which are located in the basal layer of the skin. After synthesis, melanin is further deposited on the surface of the skin to form black spots or chloasma. Tyrosinase is a rate-limiting enzyme that plays an important role in melanogenesis. Currently, there are many drugs that inhibit tyrosinase expression to further reduce melanogenesis. Nevertheless, some of these could reverse the pharmacological effect of other drugs, when used simultaneously.
Materials and Methods:
B16 mouse melanoma cells were treated with the tyrosinase inhibitors licochalcone A and β-arbutin, alone or in combination with capsaicin, an alkaloid found in peppers. Cytotoxicity, melanin content, and tyrosinase activity and expression were determined.
Results:
Licochalcone A/β-arbutin inhibited tyrosinase expression and further hindered melanin synthesis when applied individually to B16 mouse melanoma cells. However, licochalcone A/β-arbutin combined with 50 μmol/L capsaicin enhanced the expression of tyrosinase in these cells and further increased melanin content.
Conclusion:
Our data implied that capsaicin could reverse the inhibitory effect of licochalcone A/β-arbutin on tyrosinase expression in B16 mouse melanoma cells.
SUMMARY
B16 mouse melanoma cells were treated with the tyrosinase inhibitors licochalcone A and β-arbutin, alone or in combination with capsaicin, an alkaloid found in peppers. Cytotoxicity, melanin content, and tyrosinase activity and expression were determined. Licochalcone A/β-arbutin inhibited tyrosinase expression and further hindered melanin synthesis when applied individually to B16 mouse melanoma cells. However, licochalcone A/β-arbutin combined with 50 μmol/L capsaicin enhanced the expression of tyrosinase in these cells and further increased melanin content. Our research implied that capsaicin could reverse the inhibitory effect of licochalcone A/β-arbutin on tyrosinase expression in B16 mouse melanoma cells.
Abbreviations used: B16: B16 mouse melanoma cells; L-DOPA: 3, 4-L-dihydroxyphenylalanine; TYR: Tyrosinase; USP: United States Pharmacopeia; FBS: Fetal bovine serum; EDTA: Ethylenediaminetetraacetic acid; DMSO: Dimethyl sulfoxide; RPMI: Roswell Park Memorial Institute; MTT3: 4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, NaOH: Sodium hydroxide; PBS: Phosphate-buffered saline; RIPA: Radio-immunoprecipitation assay; PMSF: Phenylmethanesulfonyl fluoride or phenylmethylsulfonyl fluoride; SDS: Sodium dodecyl sulfate, sodium salt; PVDF: Polyvinylidene fluoride; ECL: Enhanced chemiluminescence.
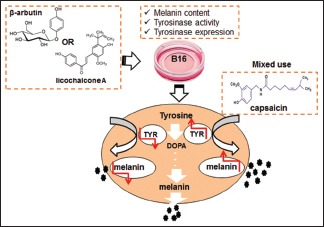
Keywords: Capsaicin, licochalcone A, melanin, melanocyte, tyrosinase, β-arbutin
INTRODUCTION
Melanin is a protein derivative that can be divided into two categories, specifically, pitchy pigment, which is called eumelanin, and reddish-brown pigment, which is called pheomelanin.[1,2] Both the biological pigments are synthesized by melanocytes, and the formation of melanin is dependent on tyrosine, tyrosinase, and the concentration of oxygen. Tyrosinase, a rate-limiting enzyme that plays an important role in melanogenesis,[3] catalyzes the hydroxylation of tyrosine to 3, 4-L-dihydroxyphenylalanine (L-DOPA) and the further oxidation of L-DOPA to dopaquinone.[4]
Excess production of melanin can lead to freckles, melasma, and even malignant melanoma. To address these problems, many tyrosinase inhibitors have been applied to medications and cosmetics, such as arbutin, ellagic acid, licochalcone A, and kojic acid, among others. Licochalcone A is a flavonoid compound isolated from licorice[5] that has significant pharmacological applications such as anti-inflammatory,[6,7,8] anti-bacterial,[9,10] anticancer,[11,12,13,14] anti-angiogenic,[15] and weight loss effects,[16] among others. Additionally, Furusawa et al.[17] found that licochalcone A inhibits tyrosinase activity, and thus it has potential for use in skin-whitening cosmetics. Arbutin is one whitening ingredient that is commonly used in cosmetics.[18,19,20] According to different chemical structures, this compound can be categorized as α-arbutin and β-arbutin.[18] Currently, β-arbutin is widely used in cosmetics. Previous studies[20,21,22] have found that arbutin could inhibit the expression of tyrosinase and further reduce melanin content and the deposition of melanin in the skin. Moreover, it also greatly affects the intestinal microflora[23] and possesses anti-inflammatory properties.[24]
Capsaicin is a vanillin amine alkaloid found in chili pepper[25,26] of the Solanaceae family and mainly exists in the placenta and flesh of the pepper fruit at a concentration of 0.2%–1.0%. Capsaicin has been shown to have various biological functions. For example, it can induce nociceptive neuron desensitization and possesses anti-inflammatory and analgesic properties.[27] It also inhibits platelet aggregation[28] and it plays an important role in lowering blood pressure and preventing atherosclerosis.[29] Peppin et al.[30] found that a transdermal patch containing 8% capsaicin could be used for the treatment of peripheral neuralgia and its effect was obvious and without the side effects of opioid analgesics. A capsaicin transdermal patch is a prescription drug used for relieving neuralgia, and it has been included in the United States Pharmacopeia. Recently, studies have also found that capsaicin has many pharmacological effects such as anticancer,[31] carcinogenic,[32,33] anti-obesity,[34,35] antibacterial,[36] antioxidant,[33] and liver protective,[37] among others.
At first, since capsaicin is the main compound responsible for the spicy, pungent taste of chili peppers,[38] we hypothesized that it could be a potential permeation enhancer. To verify this, we performed preliminary experiments. We found that β-arbutin inhibited the expression of tyrosinase and further reduced melanin contents. However, after treating with capsaicin and β-arbutin simultaneously, the melanin content of B16 mouse melanoma cells increased. The results of this preliminary experiment were not consistent with that of the previous hypothesis. Therefore, we aspired to ascertain the reason as to why melanin content decreased after treatment with β-arbutin alone, but increased with a combination of β-arbutin and capsaicin.
The present study aimed to investigate whether capsaicin could reverse the inhibitory effects of licochalcone A/β-arbutin on tyrosinase expression in B16 mouse melanoma cells.
MATERIALS AND METHODS
Materials
B16 mouse melanoma cells were purchased from the Laboratory Animal Center of Sun Yat-sen University (China). Roswell Park Memorial Institute (RPMI)-1640 medium, fetal bovine serum (FBS), penicillin-streptomycin, and trypsin-EDTA were purchased from Thermo Fisher Scientific (Gibco, USA). In addition, 60-mm tissue culture dishes and 96-well plates were purchased from Corning (USA). Capsaicin, licochalcone A, and β-arbutin were purchased from Chroma Biotechnology (China). Dimethyl sulfoxide (DMSO), TritonX-100, sodium dodecyl sulfate, sodium salt (SDS)-polyacrylamide gels, polyvinylidene fluoride (PVDF) membranes, mouse anti-β-actin antibody (A1978), and rabbit anti-goat antibody conjugated with horseradish peroxidase were purchased from Sigma-Aldrich (USA). Phosphate-buffered saline (PBS), NaOH, L-DOPA, and normal saline were purchased from Koyos Biotechnology (China). (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide [MTT]) and the BCA protein quantification kit were purchased from Beyotime Biotechnology (China). Cell lysis solution (1 mL radio-immunoprecipitation assay [RIPA] with 10 μL phenylmethanesulfonyl fluoride or phenylmethylsulfonyl fluoride [PMSF]) was purchased from Fdbio Science (China). The mouse anti-tyrosinase antibody (ab180753) was purchased from Abcam (England). An anti-mouse antibody, conjugated with horseradish peroxidase, was purchased from Cell Signaling Technology (USA). Multiskan™ was purchased from Thermo Fisher Scientific (USA).
Cell culture
B16 mouse melanoma cells were cultured in RPMI-1640 medium containing 10% FBS and 1% penicillin-streptomycin in a 37°C thermostat incubator with 5% CO2. The cells were seeded in 60-mm tissue culture dishes to be used for subsequent experiments.
Experimental groups
The experimental groups were divided into control group (culture medium only), drug control group (culture medium containing the highest concentration of the agent), negative control group (culture medium with cells and vehicle control), and experimental group (culture medium, cells, and different concentrations of the agent, specifically, 800, 400, 200, 100, and 50 μmol/L). Capsaicin and licochalcone A were dissolved in DMSO, and β-arbutin was dissolved in normal saline.
Cytotoxicity assays
The cytotoxicity against B16 mouse melanoma cells was determined by MTT-assays.[39] First, cells were digested with a 0.25% trypsin-EDTA solution and then seeded in 96-well plates at a density of 5 × 104 cells per 100 μL of cell suspension. Subsequently, the cells were incubated in a 37°C thermostat incubator with 5% CO2. For all experimental groups, 100 μL of capsaicin, licochalcone A, or β-arbutin, at different defined concentrations, were added after 24 h, and cells were cultured for an additional 48 h. At this time, 30 μL of MTT solution at a concentration of 5 mg/mL was added to each well, and samples were incubated for 4 h. After this, 100 μL of DMSO was added to each well. The rate of cell proliferation was calculated based on measurements at 490 nm[39] using a Multiskan™.
Measurement of relative melanin contents
The relative content of melanin in B16 mouse melanoma cells was measured by performing an NaOH assay.[40] For the first experiment, after 24 h, 100 μL capsaicin, licochalcone A, or β-arbutin at different concentrations were individually added to the cells; in another experiment, 50 μL of 50 μmol/L capsaicin and 100 μL of capsaicin, licochalcone A, or β-arbutin of different concentrations in combination were added to the cells, which were cultured for an additional 48 h. After this, the cells were washed with PBS. Subsequently, 100 μL of 1 mol/L NaOH solution was added to each well, and samples were incubated for 1 h at 37°C. Finally, the samples were measured at 460 nm using a Multiskan™, and relative contents of melanin were calculated.
Measurement of the tyrosinase activity
Tyrosine activity in B16 mouse melanoma cells was determined by performing an L-DOPA oxidation assay.[41] After 24 h, 100 μL capsaicin, licochalcone A, or β-arbutin at different concentrations were added to the first experiment; 50 μL of 50 μmol/L capsaicin and 100 μL of different concentrations of licochalcone A or β-arbutin were added for a different experiment and cells were cultured for 48 h. After treatment, cells were washed with PBS and 100 μL of 1% Triton™ X-100 was added to each well; samples were agitated for 5 min and transferred to freeze at -20°C for 1 h. After the cells were thawed at room temperature for complete lysis, 100 μL of 0.1% L-DOPA was added to each well and cells were cultured for 2 h. Finally, samples were measured at 460 nm using a Multiskan™, and tyrosinase activity was calculated.
Western blotting
Cells were divided into six groups, which were different from those of previous experiments; cells were seeded at a density of 5 × 105 cells/well of a 60-mm dish. After 24 h, 3 mL of drug and 1 mL of 10% RPMI-1640 medium were added to each culture dish, except for the blank control group, and cells were cultured for 48 h. After treatment, cells were washed with PBS and 400 μL of cell lysis solution (1 mL RIPA and 10 μL PMSF) was added to each 60-mm dish to lyse the cells; samples were then centrifuged at 12,000 rpm for 5 min. Protein lysates were quantified using a BCA protein quantification kit. Subsequently, 30 μL of each sample was separated by 8.0% SDS-polyacrylamide gel electrophoresis and transferred onto a PVDF membrane. The membrane was soaked in blocking reagent for 1 h, and the membrane was incubated overnight with an anti-tyrosinase antibody (1:1000) at 4°C, and washed with PBS with Tween-20 (PBST) four times. The next day, the membrane was incubated with anti-goat antibody conjugated with horseradish peroxidase (1:30,000) for 1 h at 37°C, and washed with PBST five times. Finally, the membrane was developed using enhanced chemiluminescence.
Statistical analysis
Student's t-test was applied to compare differences between groups, with cutoff P < 0.05, using SPSS statistical software 22.0 (IBM). Data are shown as mean ± standard deviation.
RESULTS
Cytotoxic effects of drugs on B16 mouse melanoma cells
To study the cytotoxic effects of capsaicin, licochalcone A, and β-arbutin, cells were treated with different drugs at different concentrations, and cell proliferation rates are shown in Table 1. The results showed that the cell proliferation rates were above 85% after treatment with 0–800 μmol/L licochalcone A/β-arbutin, and as such, neither licochalcone A nor β-arbutin was cytotoxic for the cells. When cells were treated with 100–800 μmol/L capsaicin, cell proliferation rates were below 70%, but upon treatment with 50 μmol/L capsaicin, the cell proliferation rate was 86.9%. This indicated that this compound lacked cytotoxicity only at 50 μmol/L. To verify this, cells were simultaneously treated with 50 μmol/L capsaicin and different concentrations of β-arbutin. The results showed that 50 μmol/L capsaicin is not cytotoxic for B16 mouse melanoma cells.
Table 1.
Cell proliferation rate of B16 mouse melanoma cells with different treatments (mean±standard deviation)

Effects of drugs on melanogenesis in B16 mouse melanoma cells
Cells from the first experiment were treated with licochalcone A or β-arbutin at concentrations of 800, 400, 200, 100, and 50 μmol/L. Cells from the second experiment were treated with 50 μmol/L capsaicin and licochalcone A/β-arbutin simultaneously. The melanin content of B16 mouse melanoma cells is shown in Table 2 and Figure 1. The results indicated that with increasing concentrations of licochalcone A/β-arbutin, the melanin content decreased. However, in contrast, when cells were treated with 50 μmol/L capsaicin and licochalcone A/β-arbutin at different concentrations, the melanin content increased.
Table 2.
Melanin content of B16 mouse melanoma cells with different treatments (mean±standard deviation)
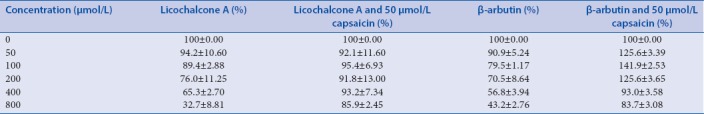
Figure 1.
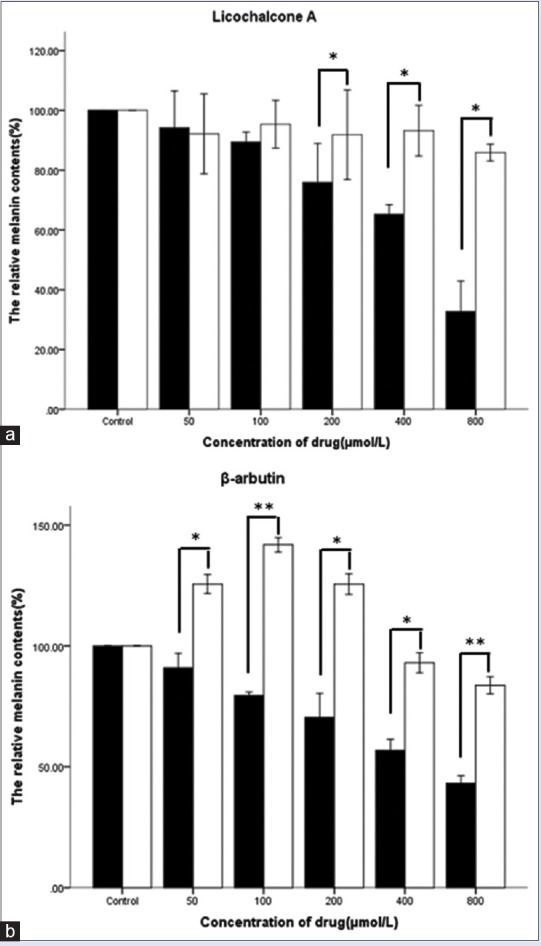
Effects of licochalcone A (a) or β-arbutin (b) on melanogenesis in B16 mouse melanoma cells simultaneously treated with or without 50 μmol/L capsaicin. Melanin production was assessed by performing a NaOH assay, and cells were treated for 48 h. All data are shown as mean ± standard deviation (n = 3). *P < 0.05, ** P < 0.001, compared to the control group
Effects of drugs on tyrosinase activity in B16 mouse melanoma cells
Cells were treated as stated previously. Tyrosinase activity in B16 mouse melanoma cells, after treatment with drugs at different concentrations, is shown in Table 3 and Figure 2. Variations in tyrosinase activity were consistent with melanin content. Thus, regarding melanogenesis and tyrosinase activity, results of the aforementioned experiments indicated that capsaicin and licochalcone A/β-arbutin probably have antagonistic effects.
Table 3.
Tyrosinase activity in B16 mouse melanoma cells with different treatments (mean±standard deviation)

Figure 2.
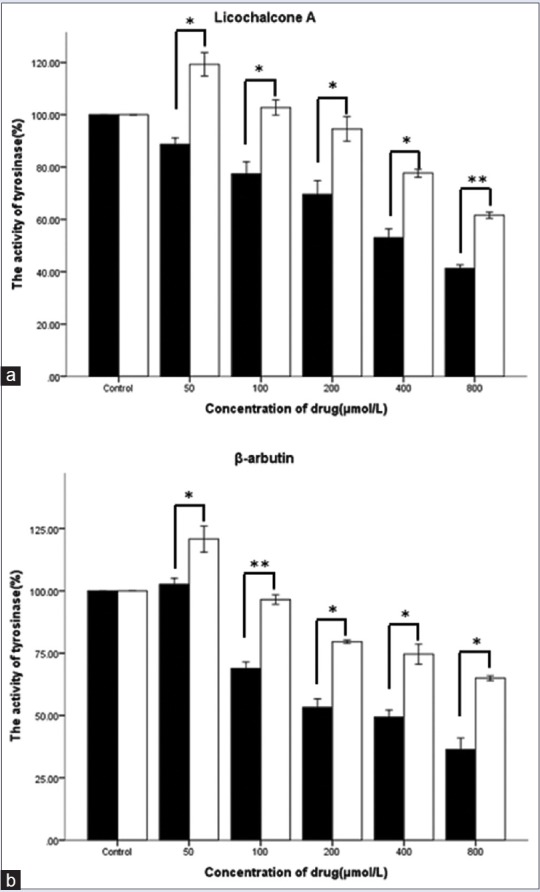
Effects of licochalcone A (a) or β-arbutin (b) on tyrosinase activity in B16 mouse melanoma cells simultaneously treated with or without 50 μmol/L capsaicin. Tyrosinase activity was assessed by performing an L-DOPA-oxidation-assay 48 h after treatment. All data are shown as mean ± SD (n = 3); *P < 0.05, **P < 0.001, compared to the control group
Effects of drugs on tyrosinase expression in B16 mouse melanoma cells
As is known, the expression of tyrosinase in B16 mouse melanoma cells can be determined by Western blot analysis. The results [Figure 3] implied that tyrosinase protein expression was significantly inhibited after treatment with β-arbutin, licochalcone A, or capsaicin, individually. Moreover, the expression of tyrosinase protein was augmented by treatment with capsaicin and licochalcone A/β-arbutin simultaneously.
Figure 3.
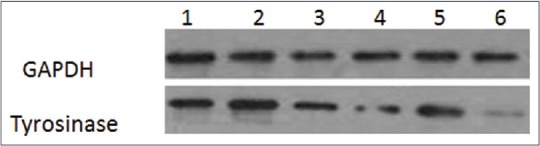
Western blot showing tyrosinase expression in B16 mouse melanoma cells after treatment with licochalcone A or β-arbutin simultaneously with or without 50 μmol/L capsaicin. (1): The blank control group, (2): licochalcone A and capsaicin, (3): licochalcone A, (4): capsaicin, (5): β-arbutin and capsaicin, (6): β-arbutin
DISCUSSION
Currently, more and more consumers, especially women around the world, desire to brighten or whiten their skin. Consequently, with respect to dermal pigmentation, many active ingredients such as β-arbutin have been studied and applied to cosmetics. If the effects of cosmetics were inferior, many people would question their efficacy; however, undoubtedly, diet also probably has an important role. Interestingly, in an experiment in which B16 mouse melanoma cells were treated with β-Arbutin, the tyrosinase activity was obviously inhibited; however, the opposite result was observed when capsaicin and β-arbutin were used simultaneously.
In this study, we discovered that when licochalcone A, 50 μmol/L capsaicin, and β-arbutin were used individually, the expression of tyrosinase was inhibited, and melanogenesis was reduced. However, capsaicin and licochalcone A/β-arbutin together enhanced the expression of tyrosinase when used simultaneously, which indicates that capsaicin can reverse the function of licochalcone A/β-arbutin with respect to inhibiting the expression of tyrosinase. Since it is an interesting phenomenon, which nobody reported it, our work is currently ongoing to identify the mechanism of this reversal effect.
SUMMARY
The results of our study serve as a reminder to pay attention to dietary factors, such as food that contain capsaicin, when using whitening cosmetics, as this compound potentially inhibits the whitening effect. It also provides theoretical evidence for drug incompatibility between whitening cosmetics and capsaicin that could be further explored in the future.
Financial support and sponsorship
We sincerely thank the National Natural Science Foundation of China (8157140020), College Chinese Cosmetics Engineering Centre Construction of Guangdong Province (GCZX-A1007), Natural Science Foundation of Guangdong Province (2014A030310342), and Science and Technology Planning Project of Guangdong Province (2016A010105007 and 2014A010107013) for financial support.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We sincerely thank the National Natural Science Foundation of China (81573611), College Chinese Cosmetics Engineering Centre Construction of Guangdong Province (GCZX-A1007), Natural Science Foundation of Guangdong Province (2014A030310342), and Science and Technology Planning Project of Guangdong Province (2016A010105007 and 2014A010107013) for financial support.
REFERENCES
- 1.Ito S, Wakamatsu K. Chemistry of mixed melanogenesis – Pivotal roles of dopaquinone. Photochem Photobiol. 2008;84:582–92. doi: 10.1111/j.1751-1097.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 2.Ramsden CA, Riley PA. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg Med Chem. 2014;22:2388–95. doi: 10.1016/j.bmc.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 3.Sturm RA, Teasdale RD, Box NF. Human pigmentation genes: Identification, structure and consequences of polymorphic variation. Gene. 2001;277:49–62. doi: 10.1016/s0378-1119(01)00694-1. [DOI] [PubMed] [Google Scholar]
- 4.Tripathi RK, Hearing VJ, Urabe K, Aroca P, Spritz RA. Mutational mapping of the catalytic activities of human tyrosinase. J Biol Chem. 1992;267:23707–12. [PubMed] [Google Scholar]
- 5.Liu H, Wang J, Zhou W, Wang Y, Yang L. Systems approaches and polypharmacology for drug discovery from herbal medicines: An example using licorice. J Ethnopharmacol. 2013;146:773–93. doi: 10.1016/j.jep.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Funakoshi-Tago M, Nakamura K, Tsuruya R, Hatanaka M, Mashino T, Sonoda Y, et al. The fixed structure of licochalcone A by alpha, beta-unsaturated ketone is necessary for anti-inflammatory activity through the inhibition of NF-kappaB activation. Int Immunopharmacol. 2010;10:562–71. doi: 10.1016/j.intimp.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Furusawa J, Funakoshi-Tago M, Tago K, Mashino T, Inoue H, Sonoda Y, et al. Licochalcone A significantly suppresses LPS signaling pathway through the inhibition of NF-kappaB p65 phosphorylation at serine 276. Cell Signal. 2009;21:778–85. doi: 10.1016/j.cellsig.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Furusawa J, Funakoshi-Tago M, Mashino T, Tago K, Inoue H, Sonoda Y, et al. Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS signaling pathway. Int Immunopharmacol. 2009;9:499–507. doi: 10.1016/j.intimp.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Mishra LC, Bhattacharya A, Bhasin VK. Phytochemical licochalcone A enhances antimalarial activity of artemisinin in vitro. Acta Trop. 2009;109:194–8. doi: 10.1016/j.actatropica.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Friis-Møller A, Chen M, Fuursted K, Christensen SB, Kharazmi A. In vitro antimycobacterial and antilegionella activity of licochalcone A from Chinese licorice roots. Planta Med. 2002;68:416–9. doi: 10.1055/s-2002-32087. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y, Hsieh TC, Guo J, Kunicki J, Lee MY, Darzynkiewicz Z, et al. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem Biophys Res Commun. 2004;322:263–70. doi: 10.1016/j.bbrc.2004.07.094. [DOI] [PubMed] [Google Scholar]
- 12.Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni SJ, et al. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011;302:69–75. doi: 10.1016/j.canlet.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Park MR, Kim SG, Cho IA, Oh D, Kang KR, Lee SY, et al. Licochalcone-A induces intrinsic and extrinsic apoptosis via ERK1/2 and p38 phosphorylation-mediated TRAIL expression in head and neck squamous carcinoma FaDu cells. Food Chem Toxicol. 2015;77:34–43. doi: 10.1016/j.fct.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi AY, Choi JH, Hwang KY, Jeong YJ, Choe W, Yoon KS, et al. Licochalcone A induces apoptosis through endoplasmic reticulum stress via a phospholipase cγ1-, ca(2+)-, and reactive oxygen species-dependent pathway in HepG2 human hepatocellular carcinoma cells. Apoptosis. 2014;19:682–97. doi: 10.1007/s10495-013-0955-y. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, Shin EK, Kim DH, Lee HH, Park JH, Kim JK, et al. Antiangiogenic effect of licochalcone A. Biochem Pharmacol. 2010;80:1152–9. doi: 10.1016/j.bcp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Won S, Kim S, Kim Y, Lee P, Ryu J, Kim J, et al. Licochalcone A: A lipase inhibitor from the roots of Glycyrrhiza uralensis. Food Res Int. 2007;40:1046–50. [Google Scholar]
- 17.Fu B, Li H, Wang X, Lee FS, Cui S. Isolation and identification of flavonoids in licorice and a study of their inhibitory effects on tyrosinase. J Agric Food Chem. 2005;53:7408–14. doi: 10.1021/jf051258h. [DOI] [PubMed] [Google Scholar]
- 18.Migas P, Krauze-Baranowska M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem Lett. 2015;13:35–40. [Google Scholar]
- 19.Boissy RE, Visscher M, DeLong MA. DeoxyArbutin: A novel reversible tyrosinase inhibitor with effective in vivo skin lightening potency. Exp Dermatol. 2005;14:601–8. doi: 10.1111/j.0906-6705.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 20.Chawla S, Kvalnes K, deLong MA, Wickett R, Manga P, Boissy RE, et al. DeoxyArbutin and its derivatives inhibit tyrosinase activity and melanin synthesis without inducing reactive oxygen species or apoptosis. J Drugs Dermatol. 2012;11:e28–34. [PubMed] [Google Scholar]
- 21.Jin YH, Lee SJ, Chung MH, Park JH, Park YI, Cho TH, et al. Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Arch Pharm Res. 1999;22:232–6. doi: 10.1007/BF02976355. [DOI] [PubMed] [Google Scholar]
- 22.Maeda K, Fukuda M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J Pharmacol Exp Ther. 1996;276:765–9. [PubMed] [Google Scholar]
- 23.Khanal T, Kim HG, Hwang YP, Kong MJ, Kang MJ, Yeo HK, et al. Role of metabolism by the human intestinal microflora in arbutin-induced cytotoxicity in HepG2 cell cultures. Biochem Biophys Res Commun. 2011;413:318–24. doi: 10.1016/j.bbrc.2011.08.094. [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, Kim KW. Anti-inflammatory effects of arbutin in lipopolysaccharide-stimulated BV2 microglial cells. Inflamm Res. 2012;61:817–25. doi: 10.1007/s00011-012-0474-2. [DOI] [PubMed] [Google Scholar]
- 25.Govindarajan VS, Sathyanarayana MN. Capsicum – Production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Crit Rev Food Sci Nutr. 1991;29:435–74. doi: 10.1080/10408399109527536. [DOI] [PubMed] [Google Scholar]
- 26.Reyes-Escogido Mde L, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E. Chemical and pharmacological aspects of capsaicin. Molecules. 2011;16:1253–70. doi: 10.3390/molecules16021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain. 1991;47:285–94. doi: 10.1016/0304-3959(91)90217-L. [DOI] [PubMed] [Google Scholar]
- 28.Wang JP, Hsu MF, Teng CM. Antiplatelet effect of capsaicin. Thromb Res. 1984;36:497–507. doi: 10.1016/0049-3848(84)90189-0. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 30.Peppin JF, Majors K, Webster LR, Simpson DM, Tobias JK, Vanhove GF, et al. Tolerability of NGX-4010, a capsaicin 8% patch for peripheral neuropathic pain. J Pain Res. 2011;4:385–92. doi: 10.2147/JPR.S22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh AK, Basu S. Tumor macrophages as a target for capsaicin mediated immunotherapy. Cancer Lett. 2012;324:91–7. doi: 10.1016/j.canlet.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Zhu P, Tao Y, Shen C, Wang S, Zhao L, et al. Cancer-promoting effect of capsaicin on DMBA/TPA-induced skin tumorigenesis by modulating inflammation, Erk and p38 in mice. Food Chem Toxicol. 2015;81:1–8. doi: 10.1016/j.fct.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Kwon HJ, Kim GE, Cho MH, Yoon SY, Davies AJ, et al. Attenuation of natural killer cell functions by capsaicin through a direct and TRPV1-independent mechanism. Carcinogenesis. 2014;35:1652–60. doi: 10.1093/carcin/bgu091. [DOI] [PubMed] [Google Scholar]
- 34.Mun JM, Ok HM, Kwon O. Corn gluten hydrolysate and capsaicin have complimentary actions on body weight reduction and lipid-related genes in diet-induced obese rats. Nutr Res. 2014;34:458–65. doi: 10.1016/j.nutres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Reinbach HC, Smeets A, Martinussen T, Møller P, Westerterp-Plantenga MS. Effects of capsaicin, green tea and CH-19 sweet pepper on appetite and energy intake in humans in negative and positive energy balance. Clin Nutr. 2009;28:260–5. doi: 10.1016/j.clnu.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Molina-Torres J, García-Chávez A, Ramírez-Chávez E. Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: Affinin and capsaicin. J Ethnopharmacol. 1999;64:241–8. doi: 10.1016/s0378-8741(98)00134-2. [DOI] [PubMed] [Google Scholar]
- 37.Giri TK, Mukherjee P, Barman TK, Maity S. Nano-encapsulation of capsaicin on lipid vesicle and evaluation of their hepatocellular protective effect. Int J Biol Macromol. 2016;88:236–43. doi: 10.1016/j.ijbiomac.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 38.Gannon NP, Lambalot EL, Vaughan RA. The effects of capsaicin and capsaicinoid analogs on metabolic molecular targets in highly energetic tissues and cell types. Biofactors. 2016;42:229–46. doi: 10.1002/biof.1273. [DOI] [PubMed] [Google Scholar]
- 39.Sadri A, Changizi V, Eivazadeh N. Evaluation of glioblastoma (U87) treatment with ZnO nanoparticle and X-ray in spheroid culture model using MTT assay. Radiat Phys Chem. 2015;115:17–21. [Google Scholar]
- 40.Shirasugi I, Kamada M, Matsui T, Sakakibara Y, Liu MC, Suiko M, et al. Sulforaphane inhibited melanin synthesis by regulating tyrosinase gene expression in B16 mouse melanoma cells. Biosci Biotechnol Biochem. 2010;74:579–82. doi: 10.1271/bbb.90778. [DOI] [PubMed] [Google Scholar]
- 41.Maack A, Pegard A. Populus nigra (Salicaceae) absolute rich in phenolic acids, phenylpropanoïds and flavonoids as a new potent tyrosinase inhibitor. Fitoterapia. 2016;111:95–101. doi: 10.1016/j.fitote.2016.04.001. [DOI] [PubMed] [Google Scholar]


