Abstract
Background:
Cordyceps militaris fraction (CMF) has been shown to possess in vitro antitumor activity against human chronic myeloid leukemia K562 cells in our previous research.
Materials and Methods:
The in vitro inhibitory activities of CMF on the growth of KB cells were evaluated by viability assay. The apoptotic and cell cycle influences of CMF were detected by 4′,6-diamidino-2-phenylindole staining and flow cytometry assay. The expression of different apoptosis-associated proteins and cell cycle regulatory proteins was examined by Western blot assay. The nuclear localization of c-Jun was observed by fluorescence staining.
Objective:
The objective of this study was to investigate the antiproliferative effect of CMF as well as the mechanism underlying the apoptosis and cell cycle arrest it induces in KB cells.
Results:
CMF suppressed KB cells’ proliferation in a dose- and time-dependent manner. Flow cytometric analysis indicated that CMF induced G2/M cell cycle arrest and apoptosis. Western blot analysis revealed that CMF induced caspase-3, caspase-9, and PARP cleavages, and increased the Bax/Bcl-2 ratio. CMF also led to increased expression of p21, decreased expression of cyclin B1, mitotic phosphatase cdc25c, and mitotic kinase cdc2, as well as unchanged expression of p53. In addition, CMF stimulated c-Jun N-terminal kinases (JNK) protein phosphorylations, resulting in upregulated expression of c-Jun and nuclear localization of c-Jun. Pretreatment with JNK inhibitor SP600125 suppressed CMF-induced apoptosis and G2/M arrest.
Conclusions:
CMF is capable of modulating c-Jun caspase and Bcl-2 family proteins through JNK-dependent apoptosis, which results in G2/M phase arrest in KB cells. CMF could be developed as a promising candidate for the new antitumor agents.
SUMMARY
CMF exhibited strong anticancer activity against oral squamous carcinoma KB cells
CMF inhibited KB cells’ proliferation via induction of apoptosis and G2/M cell cycle arrest
CMF activated JNK signaling pathway and promoted the nuclear localization of c-Jun
CMF regulated the apoptosis- and cell cycle-related proteins in a manner dependent on JNK/c-Jun pathway.
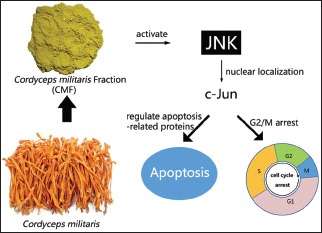
Abbreviations used: CMF: Cordyceps militaris fraction; OSCC: Oral squamous cell carcinoma; JNK: c-Jun N-terminal kinase.
Keywords: Apoptosis, cell cycle arrest, Cordyceps militaris fraction, c-Jun N-terminal kinases/c-Jun, KB cells
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the sixth most common tumor in the world.[1] There are several strategies for OSCC treatments involving chemotherapy, surgery, radiation, or a combination of these methods. However, the adequate understanding of cell biology of oral oncogenesis has not been explored, and the development of drug resistance to cancer chemotherapy has been the most critical problem.[2] Therefore, finding the new type of agents to treat OSCC and elucidating their potential mechanisms have great scientific and practical values.[3]
To find potential anticancer agents from natural products and their derivatives is one of the most convenient and valuable methods.[4] In traditional Asian medicine, Cordyceps militaris has attracted a great attention. Some constituents obtained from C. militaris, such as nucleosides and polysaccharides, were reported to have antitumor,[5,6] immunomodulatory,[7,8] and anti-inflammatory[9,10] activities. In our original research, C. militaris fraction (CMF) has been demonstrated to possess antiproliferative property in human chronic myeloid leukemia K562 cells.[11]
In the last two decades, many studies have proposed that diverse phytochemicals and various botanical formulations have potential anticancer effects via inducing apoptosis.[12] Thus, activating the process of cell death has been proved to be a valuable method in cancer therapy.[13] The intrinsic or mitochondrial apoptotic pathway is controlled by the proteins of Bcl-2 family which regulate the permeability of mitochondrial membrane.[14] The released cytochrome c could recruit Apaf-1 and activate caspase-9 and caspase-3, resulting in apoptosis. Additionally, induction of cell cycle arrest is another way to control tumor. The G2/M cell cycle procedure is positively regulated by the members of cyclin-dependent kinase (CDK) family.[15] In particular, the phosphorylation of Tyr15 of cdc2 suppresses the activity of cdc2/cyclin B1 kinase complex, while the dephosphorylation of Tyr15 of cdc2 by cdc25 phosphatases decides cell entry into mitosis.[16] The G2 phase is also can be regulated by the CDK inhibitor (CKI), which can induce cell cycle arrest in G2 phase, thereby inhibiting cell proliferation.[17] Cell cycle checkpoint kinase 2 (CHK2), a serine/threonine protein kinase, contributes to phosphorylate a number of proteins involved in cell cycle arrest, apoptosis, and DNA repair.[18]
Additionally, the mitogen-activated protein kinase (MAPK) family has been identified to play pivotal roles in a variety of cell functions, including cell cycle and apoptosis, and different MAPK members have different functions.[15] Studies have shown that c-Jun N-terminal kinases (JNK) is sensitive to stress signals, which mediate cellular steps in the apoptosis of some cell types.[19,20] As a target of JNK pathway, the specifically phosphorylated c-Jun plays a central role in diverse functions of AP-1 complex.[21]
In the present study, we investigated the antiproliferative effect of CMF and to explore its mechanism in oral squamous carcinoma KB cells. Furthermore, we first demonstrated that the inhibition of proliferation of KB cells by CMF was involved with the induction of apoptosis and G2/M phase arrest via JNK activation.
MATERIALS AND METHODS
Fraction preparation and reagents
Cultured C. militaris was purchased from Honghao Biological Company of Jiangmen (Guangdong, China). CMF was isolated, identified, and purified as per our previous report.[11] CMF stock solution was prepared into 1000 μg/ml concentration in 1640 complete medium and stored at 4°C. The antibodies for p-c-Jun, c-Jun, p-ERK, ERK, p-p38, p38, p-JNK, JNK, GAPDH, PARP, p-p53, cyclin B1, cdc2, cdc25c, caspase-3, and caspase-9 were obtained from Cell Signaling Technology, Inc. (Boston, MA, USA). Antibodies for Bax, Bcl-2, and cytochrome c were purchased from Abcam Ltd. (Cambridge, UK). SB203580, SP600125, and 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich, Inc. (Saint Louis, MO, USA). Roswell Park Memorial Institute (RPMI)-1640 medium and fetal bovine serum (FBS) were purchased from Gibco/Life Technologies Inc. (Carlsbad, CA, USA).
Cell culture and treatment
The human oral squamous carcinoma cancer (KB) cells were purchased from the American Type Culture Collection (Manassas, VA, USA), cells were cultured in RPMI-1640 supplemented with 10% FBS, and incubated at 37°C with 5% CO2 and 95% humidity.
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay for cell viability
The KB cells were seeded in 96-well plates 3.5 × 103 cells per well and then treated with 0.625, 1.25, 2.5, 5, 10, and 20 μg/ml of CMF for 24, 48, or 72 h. After the treatment, 20 μl of MTT working solution was added to each well for another 4 h. The culture supernatant was removed from the well and 200 μl of dimethyl sulfoxide was added to resuspend the dark formazan crystals, and the absorbance was measured at 570 nm.
Quantification of apoptotic cells by flow cytometry
Apoptotic/necrotic cells were quantitatively detected with Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (Nanjing KeyGen Biotech Co. Ltd., Nanjing, China).[3] Briefly, KB cells were treated with CMF at concentrations of 2.5, 5, 10, and 20 μg/ml for 48 h. Then, cells were suspended in binding buffer and stained with Annexin V-FITC and PI in the dark at room temperature. Cell fluorescence was detected by a flow cytometer after 10 min. When SP600125 was used, KB cells were incubated with the desired concentration of the JNK inhibitor for 1 h before addition of CMF.
4’,6-diamidino-2-phenylindole staining assay
Cells were plated at a density of 5 × 104 cells/ml and exposed to different concentrations of CMF for 48 h. Treated cells for the indicated time period were collected, washed three times, and fixed with 4% paraformaldehyde for 10 min at room temperature. Fixed cells were washed and stained with 4′,6-diamidino-2-phenylindole (DAPI) solution for 15 min in the dark. The morphological changes were then observed via a fluorescence microscope (Carl Zeiss, Germany). Ten fields were randomly selected to observe and images were taken.
Cell cycle analysis
Cells were treated with CMF at concentrations of 2.5, 5, 10, and 20 μg/ml for 24 h and the whole cells were harvested, pelleted at 2000 rpm, and fixed with 70% alcohol on ice. Fixed cells were resuspended and washed again with PBS and incubated with RNase A (100 mg/ml) for 30 min, and then PI Flow Cytometry Kit was added for another 30 min at room temperature.[22] The cell cycle distribution was then detected using a flow cytometer. The percentage of cells on each phase was determined using MultiCycle AV for Windows Version 295 (Beckman Coulter, Brea, CA, USA).
Western blot analysis
A total of 2.0 × 105 cells were cultured in 6-well plates. After treatment with indicated concentrations of CMF for 8 or 24 h, the adherent and nonadherent KB cells were harvested by 200 μl of RIPA lysis buffer supplemented with protease inhibitors (1 mM Na3VO4 and 1 mM phenylmethanesulfonyl fluoride) for 30 min in ice to incubate. The protein content was measured by bicinchoninic acid (BCA) protein assay kit (Beyotime, Nanjing, China). For Western blot analysis, the equal amount of protein was loaded onto SDS-PAGE Running Buffer and transferred to polyvinylidene difluoride membrane (Millipore Bedford, MA, USA) by electroblotting, and the membranes were treated with the desired primary antibodies overnight, incubated with the diluted enzyme-linked secondary antibody for 2 h, and detected with an ECL detection kit.[23] GAPDH was used as an internal control.
Fluorescence staining for confocal imaging
KB cells were treated with different concentrations of CMF for 24 h, and then the immunofluorescence staining was performed as described previously.[24,25] Cells were fixed using 2% paraformaldehyde at 4°C for 10 min, followed by permeabilized 0.1% Triton X-100 in TBS and blocking with 1% bovine serum albumin for 2 h. The cells were incubated with the primary antibody against p-c-Jun (1:800) overnight at 4°C and with Alexa Fluor® 488 secondary antibody (Invitrogen, Grand Island, NY, USA) for 2 h. Samples were mounted and analyzed by the use of Confocal Microscope Detection System, Leica TCS SP5 (LeicaBiosystems Nussloch GmbH, Heidelberg, Germany).
Statistical analysis
Data were expressed as means ± standard deviation of more than three separate assays. Statistical Package for the Social Sciences version 20.0 software (SPSS, Chicago, IL, USA) and GraphPad Prism Version 6.0 (GraphPad Software Inc., San Diego, CA, USA) were used for statistical analysis. Differences between treatment groups were calculated using Student's t-test, with the following symbols of significance levels: *P < 0.05 and **P < 0.01.
RESULTS
Cordyceps militaris fraction inhibits proliferation of KB cells
The viability of KB cells was determined by MTT assay. IC50 values of KB cell line were around 18.1 μg/ml for 24 h treatment, 4.32 μg/ml for 48 h treatment, and 3.94 μg/ml for 72 h treatment [Figure 1].
Figure 1.
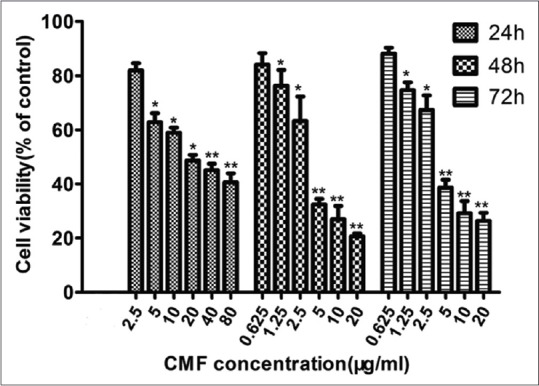
Cordyceps militaris fraction inhibits proliferation of KB cells. Cells were seeded at a density of 3.5 × 103 cells/well in 96-well plates overnight and then treated with the different concentrations of Cordyceps militaris fraction for 24, 48, or 72 h. Cell viability was assessed by 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. Each value represents the mean ± standard deviation of three independent experiments. *P < 0.05, **P < 0.01, compared to the control
Cordyceps militaris fraction induces apoptosis through the intrinsic apoptotic pathway
To examine whether CMF inhibits KB cell survival through the induction of apoptosis, the morphological changes of KB cells were preliminarily investigated. As indicated by arrows in Figure 2a, apoptotic shrinkage and chromatin condensation occurred in the cells treated with CMF, whereas these features of apoptotic cells were not observed in control cells. Apoptotic cell death was further detected by flow cytometry. As shown in Figure 2b, CMF induced the proportions of late apoptosis cells, which is consistent with the results by DAPI staining.
Figure 2.
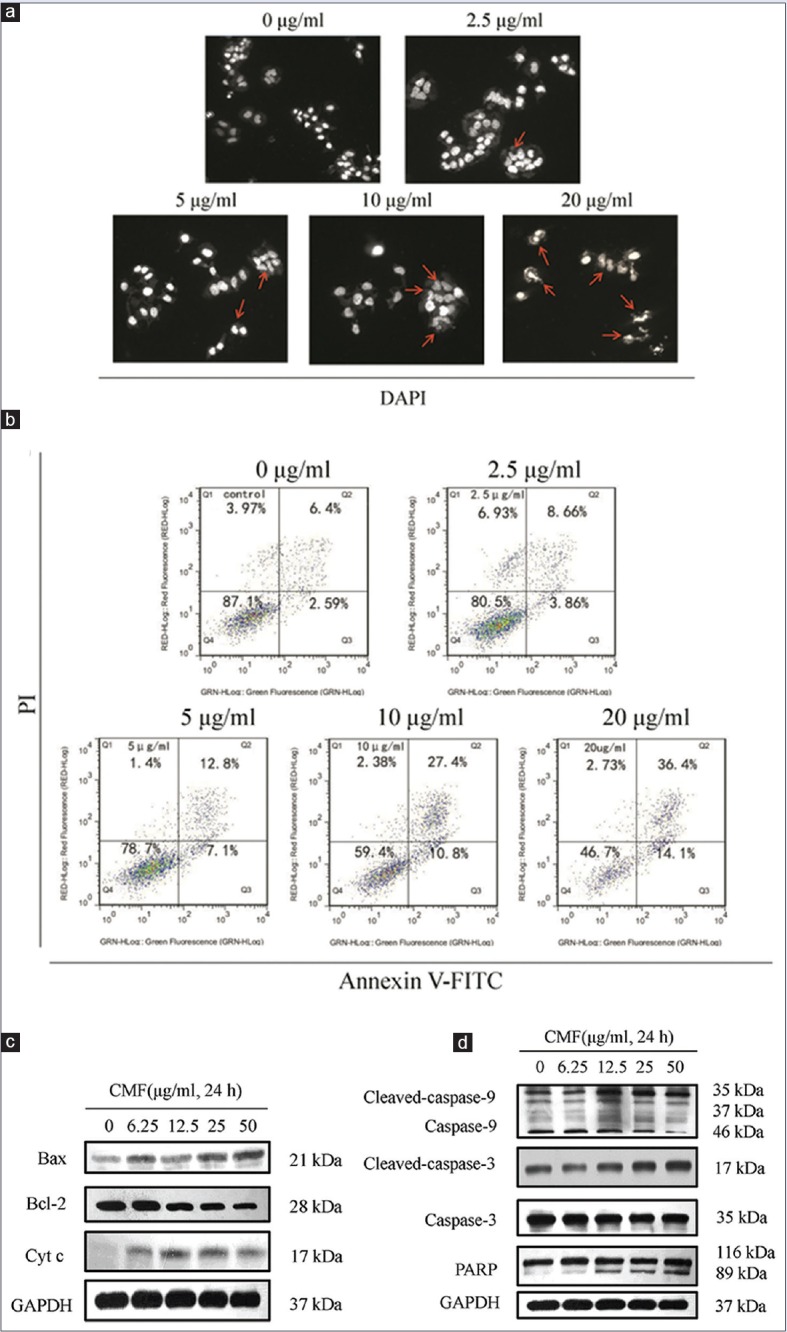
Cordyceps militaris fraction induces apoptosis and activates the intrinsic apoptotic pathway in KB cells. (a) Apoptotic morphological changes in KB cells. Apoptotic nuclei manifested condensed or fragmented DNA brightly stained by 4′,6-diamidino-2-phenylindole (48 h) (magnification, ×200). Arrows pointed to morphological changes in the cells. (b) Determination of apoptosis of KB cells by flow cytometry. After treatment of cells with Cordyceps militaris fraction, the percentage of apoptotic cells was detected by flow cytometry using Annexin V/propidium iodide staining. (c and d) Effect of Cordyceps militaris fraction on the expression of apoptosis-related proteins in KB cells. Cells were treated with the indicated concentrations of Cordyceps militaris fraction for 24 h, and cell lysates were analyzed by Western blot analysis
Substantial evidence supports the idea that Bcl-2 family members regulate the release of mitochondrial proteins.[26] Cytochrome c is a key component for the activation of the caspase cascade.[27] To further unravel the mechanism of apoptotic signaling in CMF-treated KB cells, the intrinsic signaling pathway of apoptosis was investigated. As shown in Figure 2c, the level of cytochrome c was increased and the pro-apoptosis protein Bax as the last gateway of cytochrome c release was subsequently increased. In contrast, CMF suppressed Bcl-2 expression. Furthermore, CMF significantly increased the expression of cleaved caspase-9, caspase-3, and PARP [Figure 2d]. These results suggested that an increased ratio of Bax/Bcl-2 can induce cytochrome c release, thereby increasing apoptosis.
Cordyceps militaris fraction induces G2/M arrest in KB cells and inhibits the expression of key mitotic proteins
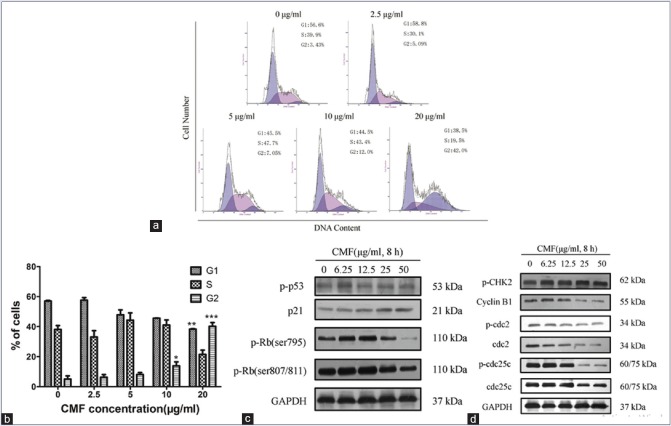
To test if CMF induce cell cycle arrest, we determined the cell cycle distribution. The results demonstrated that an increase in G2 phase from 5.09% to 42.0% and a decrease in G1 phase from 58.8% to 38.5% in comparison with the control, after the cells were treated with CMF (20 μg/ml) for 24 h [Figure 3a and b].
Figure 3.
Cordyceps militaris fraction induces G2/M phase arrest and regulates the expression of cell cycle-related mitotic proteins in KB cells. (a) Effect of Cordyceps militaris fraction on cell cycle distribution of KB cells. After treatment of cells with Cordyceps militaris fraction, the cell cycle proportions were detected by flow cytometry using propidium iodide staining. (b) Histograms of cell cycle distribution in nontreated and treated KB cells. Each value represents the mean ± standard deviation of three independent experiments. *P < 0.05 and **P < 0.01, compared to the control group. (c and d) Effect of Cordyceps militaris fraction on the expression of cell cycle-related mitotic proteins in KB cells. Cells were treated with the indicated concentrations of Cordyceps militaris fraction for 8 h, and cell lysates were analyzed by Western blot analysis
Next, the effects of CMF on the expression of cell cycle-related proteins were determined. As shown in Figure 3c and d, CMF concentration dependently increases the expression of p21 and CHK2, while the expression of phosphorylated retinoblastoma protein (p-Rb) was downregulated. Report has shown that p53 was involved in response to DNA damage by upregulated transcription of p21 gene.[28] However, CMF had no effect on the expression of p53. It suggested that CMF upregulated the expression of p21 through the p53-independent pathway.
The important role of CDKs and cyclins in regulate cell cycle progression was observed.[15] The cyclin B1/cdc2 complex was also proposed to control the G2/M phase, which is regulated by cdc25c. And then, the effects of CMF modulating the expression of cell cycle regulatory molecules were determined. The result showed that the levels of cyclin B1, cdc2, and cdc25c were significantly decreased by CMF [Figure 3d]. The data were consistent with those of cell cycle analysis by flow cytometry. These results suggested that CMF was proposed to inhibit the proliferation of KB cells through the cell cycle arrest at G2/M phase.
The activation of JNK and c-Jun in KB cells was proposed to be induced by CMF
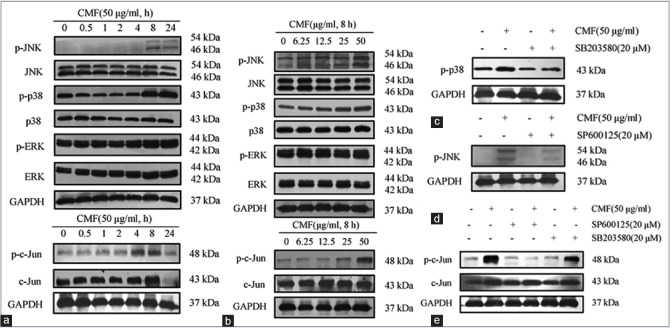
The effect of CMF on activation of MAPKs and c-Jun was examined to identify the molecular target of CMF in KB cells. Western blot experiment demonstrated that phosphorylation of JNK, p38, and c-Jun was significantly increased by CMF, whereas phosphorylation level of ERK was not changed [Figure 4a and b].
Figure 4.
Cordyceps militaris fraction induces activation of c-Jun N-terminal kinase and c-Jun in KB cells. (a and b) Effect of Cordyceps militaris fraction on the expression of phosphor mitogen-activated protein kinases and p-c-Jun in KB cells. Cells were treated with 50 μg/ml of Cordyceps militaris fraction for indicated hours (a) or with indicated concentrations of Cordyceps militaris fraction for 8 h (b), and cell lysates were analyzed by Western blot analysis. (c) Effect of p-38 inhibitor SB203580 on Cordyceps militaris fraction-regulated expression of p-p38 in KB cells. Cells were treated with 20 μM SB203580 for 1 h prior to exposure to 50 μg/ml of Cordyceps militaris fraction for 6 h, and cell lysates were analyzed by Western blot analysis. (d) Effect of c-Jun N-terminal kinase inhibitor SP600125 on Cordyceps militaris fraction-regulated expression of p-c-Jun N-terminal kinase in KB cells. Cells were treated with 20 μM SP600125 for 1 h prior to exposure to 50 μg/ml of Cordyceps militaris fraction for 6 h, and cell lysates were analyzed by Western blot analysis. (e) Effects of c-Jun N-terminal kinase inhibitor SP600125 and p-38 inhibitor SB203580 on Cordyceps militaris fraction-regulated expression of p-c-Jun in KB cells. Cells were treated with 20 μM SP600125 or 20 μM SB203580 for 1 h prior to exposure to 50 μg/ml of Cordyceps militaris fraction for 6 h, and cell lysates were analyzed by Western blot analysis
We then determined whether these phosphorylation events can be suppressed by specific kinase inhibitors, KB cells were incubated with CMF in the presence of SP600125 (JNK inhibitor) or SB203580 (p38 inhibitor). The pretreatment with SP600125 markedly decreased CMF-induced p-JNK and p-c-Jun induction, while treated with SB203580 did not change the expression of p-p38 and p-c-Jun [Figure 4c–e].
The experiment results indicated that JNK pathway plays an important role in CMF-induced apoptosis. It is also proposed that JNK, not p38 and ERK, was induced by CMF for the expression of c-Jun.
Cordyceps militaris fraction increases the nuclear localization of c-Jun
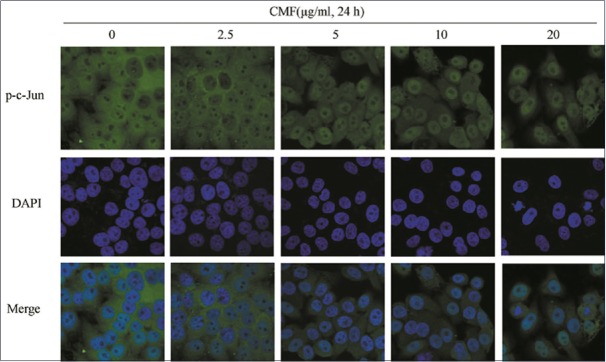
The immunofluorescence microscopic analysis was performed to further corroborate the notion that KB cells are defective in c-Jun upregulation in response to DNA damage and cell cycle arrest. As shown in Figure 5, p-c-Jun mostly localized in nuclei in a dose-dependent manner after KB cells was treated with CMF. It demonstrated that JNK/c-Jun pathway could be responding to CMF, which then results in a series of related reactions.
Figure 5.
Cordyceps militaris fraction increases the nuclear localization of c-Jun. Exposure to Cordyceps militaris fraction causes nuclear accumulation of phospho-c-Jun in KB cells. Cells were treated with Cordyceps militaris fraction for 24 h, and then fixed and immunostained with phospho-c-Jun antibody. Nuclei were stained with 4′,6-diamidino-2-phenylindole. Localization was visualized using laser scanning confocal microscope. Images were taken under × 40 objective
Cordyceps militaris fraction induces apoptosis and cell cycle arrest in a manner dependent on JNK/c-Jun signaling pathway
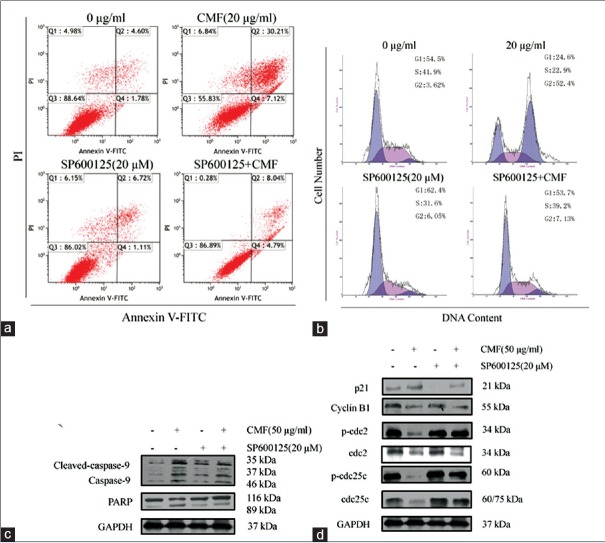
To unravel the possible roles of JNK pathway in CMF-induced apoptosis and cell cycle arrest, KB cells were incubated with CMF and JNK inhibitor. As shown in Figure 6a and b, SP600125 significantly inhibited CMF-induced apoptosis and G2/M phase cell cycle arrest. In addition, SP600125 not only inhibited the CMF-induced cleavage of PARP, but also reversed the upregulation of p21 expression and downregulation of mitotic cyclin/kinase/phosphatase-related protein levels [Figure 6c and d]. Taken together, the results show that JNK signaling pathway is directly related to the apoptosis and cell cycle arrest caused by CMF in KB cells.
Figure 6.
Cordyceps militaris fraction induces apoptosis and cell cycle arrest in a manner dependent on c-Jun N-terminal kinase/c-Jun signaling pathway. (a) Effect of c-Jun N-terminal kinase inhibitor SP600125 on Cordyceps militaris fraction-induced apoptosis in KB cells. Cells were treated with 20 μg/ml of Cordyceps militaris fraction alone or in combination with 20 μM SP600125 for 48 h, and then Annexin V-FITC/PI double-staining analysis was performed. (b) Effect of c-Jun N-terminal kinase inhibitor SP600125 on Cordyceps militaris fraction-induced G2/M arrest in KB cells. Cells were treated with 20 μg/ml of Cordyceps militaris fraction alone or in combination with 20 μM SP600125 for 24 h, and then PI staining analysis was performed. (c) Effect of c-Jun N-terminal kinase inhibitor SP600125 on Cordyceps militaris fraction-regulated expression of caspase-9 and PARP in KB cells. Cells were cotreated with 20 μg/ml of Cordyceps militaris fraction and 20 μM SP600125, and cell lysates were analyzed by Western blot analysis. (d) Effect of c-Jun N-terminal kinase inhibitor SP600125 on Cordyceps militaris fraction-regulated expression of mitotic cyclin/kinase/phosphatase-related proteins in KB cells. Cells were cotreated with 20 μg/ml of Cordyceps militaris fraction and 20 μM SP600125, and cell lysates were analyzed by Western blot analysis
DISCUSSION
Natural products have drawn more and more attention due to their growth inhibition of various types of cancers through complex signaling pathways.[29] The results proposed that CMF could efficiently inhibit the proliferation of KB cells by targeting JNK-mediated signaling pathways. It also changed a broad spectrum of signaling effectors, including c-Jun, Bcl-2, Bax, and p21, thus regulate cell cycle and cell survival.
We are aware that a successful cancer therapy depends on the complex network of cell signaling pathways, and activation of the MAPK cascade precedes apoptosis and cell cycle arrest.[30] Some investigations have shown that JNK might decrease cell proliferation by activating its downstream effector c-Jun.[31] At the present experiment, the results showed that the levels of phosphorylated JNK, p38, and c-Jun were significantly increased by CMF. In another experiment, CMF-induced c-Jun expression was abrogated by the pharmacological JNK inhibitor SP600125. However, no obvious effect on c-Jun expression was observed while using p38 inhibitor SB203580 in CMF-treated KB cells. Therefore, we analyzed that p38 MAP kinase signaling was not mediated by growth inhibition in KB cells. Additionally, CMF stimulated the activation of JNK, leading to increased nuclear localization of c-Jun, supporting a role of JNK, not p38 and ERK, in controlling the expression of c-Jun in KB cells.
Besides c-Jun nuclear localization, JNK-induced apoptosis can also be regulated through JNK cytoplasmic substrates, such as proapoptotic proteins Bax, Puma, and Bim activity,[25] or can be regulated via Bcl-2 family proteins Bcl-2, Bcl-x L, and Mcl-1 activity.[32] In this work, the pro-apoptosis effects of CMF were also shown in KB cells. We observed that CMF significantly increased the ratio of Bcl-2/Bax and induced the release of cytochrome c, thus resulting in a significant proteolytic cleavage of caspase-9 and PARP. In addition, JNK inhibitor significantly abrogated the cleavage of caspase-9 and PARP caused by CMF and consequently reduced the level of apoptosis [Figure 6a and c]. Altogether, these data suggested that activation of the intrinsic apoptotic pathway might be a mechanism underlying CMF-induced antiproliferative effect in KB cells.
Many types of stimuli have been shown to inhibit cell cycle progression from one phase to another. During G2 phase, the main regulator is the cdc2/cyclin B1 complex. The decreased formation of cdc2/cyclin B1 complex inhibits cell cycle progression from G2 phase to M phase, and this complex can be kept inactive via phosphorylation at Thr14 and Tyr15 of cdc2,[33] or inhibited by p21. Additionally, CHK2 as a key operator in eliciting DNA repair, cell cycle arrest, or apoptosis in response to DNA damage[15,18] and both p-Rb and p53 are key cell cycle regulatory proteins in mediating certain functions of p21. In the present study, CMF resulted in G2/M phase arrest in a dose-dependent manner. CMF decreased the expression of cyclin B1, cdc2, cdc25c, and p-Rb, whereas it induced an increase in the protein levels of p21 and CHK2. However, CMF had no effect on the expression of p53. It is well known that the JNK pathway has antiproliferative effects and regulates the cell cycle.[15,34] The treatment with CMF plus JNK inhibitor led to an increase in cdc2/cyclin B1 and a decrease in the expression of p21, and consequently reduced the levels of cell cycle arrest compared with CMF treatment alone [Figure 6d]. These results suggested that CMF enhanced p21 expression, which induced G2/M phase arrest by a p53-independent pathway.
Interestingly, previous reports had shown that c-Jun transcription factor could control a wide range of molecular targets, such as Bim,[30] Bcl-2,[35] caspase-3,[36] and CDK inhibitors, which could regulate the cellular processes, including proliferation, differentiation, migration, survival, or death. Thus, we propose that CMF activated JNK signaling pathway, promoted the nuclear localization of c-Jun, and then regulated the key apoptosis-related proteins and cell cycle-related proteins, eventually inhibiting KB cell proliferation.
CONCLUSIONS
The present paper reported the potent anticancer activities of CMF fraction. The mechanism of action might result from CMF-induced apoptosis and G2/M cell cycle arrest through JNK pathway. This preliminary elucidating of JNK and c-Jun also provide new insights for further development of this agent. Therefore, CMF may possess great potential as a candidate for therapy of OSCC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ruggieri V, Agriesti F, Scrima R, Laurenzana I, Perrone D, Tataranni T, et al. Dichloroacetate, a selective mitochondria-targeting drug for oral squamous cell carcinoma: A metabolic perspective of treatment. Oncotarget. 2015;6:1217–30. doi: 10.18632/oncotarget.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Sun C, He S, Guo X, Xu H, Zeng X, et al. Apoptotic effects of diosgeninlactoside on oral squamous carcinoma cells in vitro and in vivo. Biol Pharm Bull. 2014;37:1450–9. doi: 10.1248/bpb.b14-00122. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Yang RL. A phthalide derivative isolated from endophytic fungi Pestalotiopsis photiniae induces G1 cell cycle arrest and apoptosis in human HeLa cells. Braz J Med Biol Res. 2013;46:643–9. doi: 10.1590/1414-431X20132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshikawa N, Nakamura K, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Antitumour activity of cordycepin in mice. Clin Exp Pharmacol Physiol. 2004;31(Suppl 2):S51–3. doi: 10.1111/j.1440-1681.2004.04108.x. [DOI] [PubMed] [Google Scholar]
- 6.Jeong MH, Lee CM, Lee SW, Seo SY, Seo MJ, Kang BW, et al. Cordycepin-enriched Cordyceps militaris induces immunomodulation and tumor growth delay in mouse-derived breast cancer. Oncol Rep. 2013;30:1996–2002. doi: 10.3892/or.2013.2660. [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, Hong EK. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int Immunopharmacol. 2011;11:1226–33. doi: 10.1016/j.intimp.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Jing Y, Cui X, Chen Z, Huang L, Song L, Liu T, et al. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohydr Polym. 2014;102:288–96. doi: 10.1016/j.carbpol.2013.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006;545:192–9. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JL, Xu Y, Shen J. Cordycepin inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production via activating amp-activated protein kinase (AMPK) signaling. Int J Mol Sci. 2014;15:12119–34. doi: 10.3390/ijms150712119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian T, Song L, Zheng Q, Hu X, Yu R. Induction of apoptosis by Cordyceps militaris fraction in human chronic myeloid leukemia K562 cells involved with mitochondrial dysfunction. Pharmacogn Mag. 2014;10:325–31. doi: 10.4103/0973-1296.137374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar M, Kaur V, Kumar S, Kaur S. Phytoconstituents as apoptosis inducing agents: Strategy to combat cancer. Cytotechnology. 2016;68:531–63. doi: 10.1007/s10616-015-9897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HH, Park C, Jeong JW, Kim MJ, Seo MJ, Kang BW, et al. Apoptosis induction of human prostate carcinoma cells by cordycepin through reactive oxygen species-mediated mitochondrial death pathway. Int J Oncol. 2013;42:1036–44. doi: 10.3892/ijo.2013.1762. [DOI] [PubMed] [Google Scholar]
- 14.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004 30;305:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Kim SK, Choi WS, Kim WJ, Moon SK. Cordycepin causes p21WAF1-mediated G2/M cell-cycle arrest by regulating c-Jun N-terminal kinase activation in human bladder cancer cells. Arch Biochem Biophys. 2009;490:103–9. doi: 10.1016/j.abb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson C, Katich S, Hagting A, Hoffmann I, Pines J. Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis. J Cell Biol. 1999;146:573–84. doi: 10.1083/jcb.146.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, Wang Y, Li J, Jin H, Song S, Huang C. The Chinese herb isolate yuanhuacine (YHL-14) induces G2/M arrest in human cancer cells by up-regulating p21 protein expression through an p53 protein-independent cascade. J Biol Chem. 2014;289:6394–403. doi: 10.1074/jbc.M113.513960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yutori H, Semba S, Komori T, Yokozaki H. Restoration of fragile histidine triad expression restores Chk2 activity in response to ionizing radiation in oral squamous cell carcinoma cells. Cancer Sci. 2008;99:524–30. doi: 10.1111/j.1349-7006.2007.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J Biol Chem. 2002;277:29792–802. doi: 10.1074/jbc.M201299200. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, Wang JY, Pan BS, Mu YF, Lai MS, So EC, et al. Cordycepin enhances cisplatin apoptotic effect through caspase/MAPK pathways in human head and neck tumor cells. Onco Targets Ther. 2013;6:983–98. doi: 10.2147/OTT.S45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 22.Hu X, Zhang Z, Liu T, Song L, Zhu J, Guo Z, et al. Polypeptide fraction from Arca subcrenata induces apoptosis and G2/M phase arrest in HeLa cells via ROS-mediated MAPKs pathways. Evid Based Complement Alternat Med. 2015;2015:930249. doi: 10.1155/2015/930249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HH, Jeong JW, Lee JH, Kim GY, Cheong J, Jeong YK, et al. Cordycepin increases sensitivity of Hep3B human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by inactivating the JNK signaling pathway. Oncol Rep. 2013;30:1257–64. doi: 10.3892/or.2013.2589. [DOI] [PubMed] [Google Scholar]
- 24.Ko BS, Lu YJ, Yao WL, Liu TA, Tzean SS, Shen TL, et al. Cordycepin regulates GSK-3ß/ß-catenin signaling in human leukemia cells. PLoS One. 2013;8:e76320. doi: 10.1371/journal.pone.0076320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng JR, Bai LY, Chiu CF, Wang YC, Tsai MH. The dietary phytochemical 3,3'-diindolylmethane induces G2/M arrest and apoptosis in oral squamous cell carcinoma by modulating Akt-NF-κB, MAPK, and p53 signaling. Chem Biol Interact. 2012;195:224–30. doi: 10.1016/j.cbi.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Esmaeili MA, Farimani MM, Kiaei M. Anticancer effect of calycopterin via PI3K/Akt and MAPK signaling pathways, ROS-mediated pathway and mitochondrial dysfunction in hepatoblastoma cancer (HepG2) cells. Mol Cell Biochem. 2014;397:17–31. doi: 10.1007/s11010-014-2166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dotto GP. p21(WAF1/Cip1): More than a break to the cell cycle? Biochim Biophys Acta. 2000;1471:M43–56. doi: 10.1016/s0304-419x(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 29.Shebaby WN, Bodman-Smith KB, Mansour A, Mroueh M, Taleb RI, El-Sibai M, et al. Daucus carota pentane-based fractions suppress proliferation and induce apoptosis in human colon adenocarcinoma HT-29 cells by inhibiting the MAPK and PI3K pathways. J Med Food. 2015;18:745–52. doi: 10.1089/jmf.2014.3225. [DOI] [PubMed] [Google Scholar]
- 30.Tomicic MT, Meise R, Aasland D, Berte N, Kitzinger R, Krämer OH, et al. Apoptosis induced by temozolomide and nimustine in glioblastoma cells is supported by JNK/c-Jun-mediated induction of the BH3-only protein BIM. Oncotarget. 2015;6:33755–68. doi: 10.18632/oncotarget.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui H, Shen J, Lu D, Zhang T, Zhang W, Sun D, et al. 4-Aryl-1,3,2-oxathiazolylium-5-olate: A novel GST inhibitor to release JNK and activate c-Jun for cancer therapy. Cancer Chemother Pharmacol. 2008;62:509–15. doi: 10.1007/s00280-007-0632-3. [DOI] [PubMed] [Google Scholar]
- 32.Tan M, Li Z, Ma S, Luo J, Xu S, Lu A, et al. Heroin activates Bim via c-Jun N-terminal kinase/c-Jun pathway to mediate neuronal apoptosis. Neuroscience. 2013;233:1–8. doi: 10.1016/j.neuroscience.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100:71–8. doi: 10.1016/s0092-8674(00)81684-0. [DOI] [PubMed] [Google Scholar]
- 34.Lee SJ, Moon GS, Jung KH, Kim WJ, Moon SK. c-Jun N-terminal kinase 1 is required for cordycepin-mediated induction of G2/M cell-cycle arrest via p21WAF1 expression in human colon cancer cells. Food Chem Toxicol. 2010;48:277–83. doi: 10.1016/j.fct.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Bourguignon LY. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol Cancer. 2014;13:52. doi: 10.1186/1476-4598-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song B, Xie B, Wang C, Li M. Caspase-3 is a target gene of c-Jun: ATF2 heterodimers during apoptosis induced by activity deprivation in cerebellar granule neurons. Neurosci Lett. 2011;505:76–81. doi: 10.1016/j.neulet.2011.09.060. [DOI] [PubMed] [Google Scholar]