Abstract
Background:
Hypertension is a great global health challenge and it mostly requires drug combination therapy with the various advantages. Metoprolol (MP) and paeoniflorin are both commonly used for the treatment of hypertension. However, whether they exert synergistic effects on antihypertension or not remains unclear, especially on vascular endothelial function.
Objective:
The purpose of the study is to investigate the advantages of the combined antihypertensive effects of paeoniflorin enriched extract from Radix Paeoniae Alba (RE) and MP in spontaneously hypertensive rats (SHR).
Materials and Methods:
SHR divided into six groups (n = 8 each group), animals in each group were administrated orally with distilled water, MP (6 and 20 mg/kg), RE (30 and 90 mg/kg), and MP (6 mg/kg) combined with RE (30 mg/kg) (MP + RE), respectively, daily for 6 weeks. Blood pressure (BP) and microcirculation were assessed. The organ bath experiment and hematoxylin and eosin staining were, respectively, performed for the functional and pathological vascular function analysis. Immunohistochemistry was applied to detect endothelial nitric oxide synthase (eNOS) expression in aorta, heart, and kidney. Further, high-performance liquid chromatography was employed to quantitatively determine paeoniflorin in RE and MP + RE sample solvent, as well as in plasma of Sprague-Dawley rats (SD) after single-dose administration of them.
Results:
The results showed that MP + RE significantly reduced BP, increased microcirculation, improved vascular function and pathological changes, and upregulated eNOS expression. MP was also found to increase the blood concentration of paeoniflorin in SD.
Conclusion:
The combination of RE and MP could be used for the treatment of hypertension and could improve microcirculation, upregulate eNOS expression, and mitigate endothelial dysfunction in SHR.
SUMMARY
Paeoniflorin enriched extract from Radix Paeoniae Alba and metoprolol exert synergistic antihypertensive effects.
Abbreviations used: RE: Paeoniflorin enriched extract from Radix Paeoniae Alba, MP: Metoprolol, MP + RE: MP combined with RE, NC: Normal control, MC: Model control, SHR: Spontaneously hypertensive rats, SD: Sprague-Dawley rats, H and E: Hematoxylin and eosin, BP: Blood pressure, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, MBP: Mean arterial blood pressure, NA: Norepinephrine, ACh: Acetylcholine, SNP: Nitroprusside, NO: Nitric oxide, eNOS: Endothelial nitric oxide synthase, RPA: Radices Paeoniae Alba, IHC: Immunohistochemistry, Cmax: Peak concentration, Tmax: The time to reach Cmax, t½: Half-life, AUC0-t: Area under the curve of 0-t time; MRT0-t: Mean residence of 0-t time; CL: Clearance rate.
Keywords: Endothelial nitric oxide synthase, hypertension, metoprolol, paeoniflorin, synergy, vascular function
INTRODUCTION
Hypertension is a common disease characterized by elevated arterial pressure. It is becoming a serious global health challenge, due to its high prevalence and risk of cardiovascular disease. In the year of 2010, an estimated 29.8% of the world's adult population had hypertension.[1] The incidence of hypertension in people older than 18 is 25.2% in China in 2012, and the figure reached as high as 35.5% in Beijing.[2] Hypertension is a dominant and independent risk factor for cardiovascular disease associated with atherosclerosis, the manifestations, of which include brain small vessel disease, heart failure, and renal failure.[3,4] At least 760 million people worldwide die per year from cardiovascular disease associated with hypertension, representing 13.5% of the total cause of death.[5] The death associated with chronic kidney disease resulting from hypertension increased >100% from 1990 to 2013.[6]
Although common hypertension treatments, such as calcium-channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers (ARB), diuretics, and β-blockers are indeed helpful in controlling blood pressure (BP), each comes with its own limitations. Only approximately 30-40% of hypertensive patients taking an antihypertensive drug benefit from BP-lowering effects and about 70% of patients must combine two types or more different drugs to see their BP reach the standard level.[7] There are various advantages of combination therapy versus monotherapy for the treatment of hypertension including low-dose drug combinations, antihypertensive mechanism complementary effects, efficacy of superposition, and ultimately reduction in the disease's adverse effects.[8,9] Drugs containing β-blockers can be associated with diuretics, calcium-channel antagonists, α-blockers, and other drugs acting on the central nervous system to treat hypertension.
Metoprolol (MP), a selective β1-receptor blocker, is among the most commonly used medications in the treatment of hypertension. By blocking the myocardial β1-receptors, it primarily functions through reduction of heart rate and cardiac output to reduce BP.[10] In 2014, the Eighth Joint National Committee (JNC eight) removed β-blockers from the first-line treatment partially due to its associated side effect in the cardiovascular event because of an increased rate of cardiovascular event associated with the drugs.[11] Endothelial dysfunction is prevalent in various cardiovascular disorders, including hypertension.[12,13] Endothelial nitric oxide synthase (eNOS) is often suppressed during the development of cardiovascular diseases, resulting in decreasing quantity of endogenous nitric oxide (NO), and thus vascular endothelial dysfunction, leading to atherosclerosis and hypertension.[14] MP might not have this beneficial effect on improving vascular endothelial cell functions.[15] Likewise, MP was proven unable to restore eNOS activity in diabetic and hypertensive rats.[16,17] This may be one of the reasons for the cardiovascular risk associated with MP.
Radix Paeoniae Alba (Baishao, RPA), such as other traditional Chinese herbal medicines, is the major constituent material of many complex preparations used in the treatment of hypertension in China. RPA, the dry root of Paeonia lactiflora pall., is mainly composed of paeoniflorin.[18] Research has shown that paeoniflorin has a wide variety of pharmacological benefits such as anti-inflammatory, antioxidative, and immune-strengthening qualities. Moreover, paeoniflorin exerts a positive effect on blood vessel wall function by releasing the relaxation factor of NO.[19] Paeoniflorin was reported to induce the expression of eNOS in the various scenario. For instance, paeoniflorin improved myocardial ischemia-reperfusion injury by activating eNOS/NO pathway[20] and directly increased the expression of eNOS in pulmonary microvascular endothelial cells in vitro.[21] Our previous studies indicated that RE, with 53% paeoniflorin, exhibit antihypertension and blood lipid regulation effects, and these effects are associated with elevated serum NO and endothelin level.[22] Hence, paeoniflorin can not only lower BP but also increase the expression of eNOS to benefit cardiovascular function.
The current study explored the potential advantages of combining paeoniflorin enriched extract from Radix Paeoniae Alba (RE) and MP, in treating hypertension, especially in regards to vascular endothelial protection. Spontaneously, hypertensive rats (SHR) were used to investigate the combined antihypertensive effects and the endothelial protection of RE plus MP including vascular function, pathological changes, and eNOS expression. We also investigated the paeoniflorin content variation of RE mixed with MP samples, and the pharmacokinetics of paeoniflorin after oral one-dose administration of RE alone or mixed with MP in Sprague-Dawley rats (SD).
MATERIALS AND METHODS
Materials and chemicals
Antibody against eNOS (Lot No.: D9A5 L) was purchased from Cell Signaling Technology (Beverly, MA); Mouse and Rabbit Specific HRP/DAB (ABC) Detection Immunohistochemistry (IHC) kit (ab64264) was purchased from Abcam (Cambridge, USA). The paeoniflorin enriched extract from Radix Paeoniae Alba (RE), containing >50% of paeoniflorin detecting by High-performance liquid chromatography(HPLC) analysis, was purchased from Zelang Medical Technology Co. (Nanjing, Jiangsu, China). MP tartrate tablets (25 mg/tablet, 1304136) were purchased from AstraZeneca Pharmaceutical Co., Ltd. Hematoxylin (Lot No.: 20140919) and Eosin (Lot No.: 20140919) were all purchased from Nanjing Jiangcheng Technology Co., Ltd.(Jiangsu, China).
Animals
Male SD rats were obtained from Animal Supply Center of Zhejiang Academy of Medical Science (SCXK2008-0033, Hangzhou, China). SHRs were purchased from Vital River Laboratory Animal Inc. (SCXK2012-0001, Beijing, China). All the animals were raised in standard environmental conditions obeying the rules for the Use and Care of Laboratory Animals promulgate by the Zhejiang province in 2009. The environmental conditions were remained at a statute temperature, and humidity, as well as 12/12 h light/dark cycle.
High-performance liquid chromatography-DAD analysis of paeoniflorin
The RE and RE combined with MP were analyzed with HPLC-DAD. Sample concentrations were 30.01 mg of RE alone or mixed with 6.03 mg MP adequately dissolved in 10 ml of water, then diluted 100-fold with distilled water and filtered with a 0.22-μm membrane filter for analysis. The Agilent HPLC1200 (Agilent Technologies Inc., Palo Alto, American) was used to determinate paeoniflorin with a C18 (4.6 mm × 250 mm, 5 μm) chromatographic column. The column oven temperature maintained was at 25°C. The mobile phase consisted of 0.1% phosphoric acid in water and acetonitrile (81:19, V/V), and detection wavelength was 230 nm. The HPLC chromatogram of sample solutions and paeoniflorin standard were shown in Figure 1.
Figure 1.
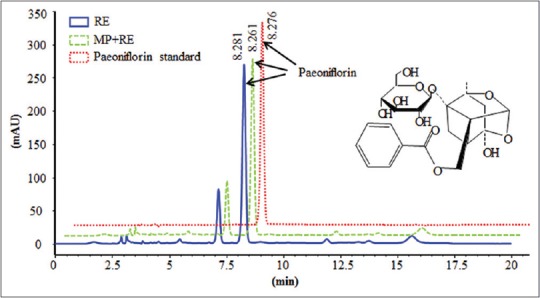
High-performance liquid chromatography chromatogram of sample solutions and paeoniflorin standard. RE: Paeoniflorin enriched extract from Radix Paeoniae Alba; metoprolol + RE: Metoprolol combined with RE. Paeoniflorin (C23H28O11, molecular weight 480)
Animal grouping
Group 1 (G1) was SD rats set as the normal control group (NC, n = 8). SHRs were randomly assigned to six groups of eight rats each (G2, G3, G4, G5, G6, and G7) based on systolic BP (SBP). G2 was model control group (MC); G3 was received the high dose of MP (MP-H, 20 mg/kg, p.o.); G4 was received the low dose of MP (MP-L, 6 mg/kg, p.o.); G5 was received the high dose of RE (RE-H, 90 mg/kg, p.o.); G6 was received the low dose of RE (RE-H, 30 mg/kg, p.o.); and G7 was given 6 mg/kg MP combined with 30 mg/kg RE (MP + RE, p.o.). Throughout the experiment, body weight was monitored (Data were not shown). Distilled water was administered in normal and MC groups.
Blood pressure measurement
BP including SBP, diastolic BP (DBP), and mean arterial BP (MBP) were measured by a noninvasive method of tail-cuff plethysmography (Softron Biotechnology Ltd., Beijing China) before the experiment and after administrated at the 2nd and 4th week. To get accurate BP, at least six consecutive determinations were recorded.
High-resolution laser Doppler perfusion imaging
Microcirculatory blood flow was assessed by laser Doppler with a moor FLPI V2.1 software (Moor Instruments Ltd, Millwey, Axminster, Devon, UK). Briefly, the distance of the scanner and tail was set as 13 cm. One 1.4 cm × 1.4 cm area was selected on the tail root. The reflective signal of laser beam was received by a detector positioned in the scanner head and converted into electrical signal to represent the blood perfusion condition.[23]
Vasodilation analysis
At the end of experiments, animals were anesthetized with pentobarbital sodium, and the descending thoracic aorta was dissect out and placed in Krebs solution (4°C). The solution (118.1 mM NaCl, 4.7 mM KCl, 1.2 mM KH2 PO4, 1.2 mM MgSO4, 1.8 mM CaCl2, 25.0 mM NaHCO3, 11.1 mM glucose; pH = 7.4) were prepared according to the report. Surrounding fat and attachments were removed from the aortic, which were cut transversely into three to five mm width rings. Isolated aortic rings were fixed on stainless steel hooks in organ baths, and soaked in 10 ml of Krebs solution aerated with 95% O2 and 5% CO2 at 37°C. During experiments, special attention should be paid to avoid damaging the endothelium. The aortic rings were equilibrated for 1 h (the solution was changed every 15 min), then contraction was induced by norepinephrine (NA, 10−6 M). The relaxant responses were then studied in the NA precontracted rings by acetylcholine (ACh, from 10−8 to 10-4 M with one logarithmic increment) and nitroprusside (SNP, from 10− 9 to 10-5 M with one logarithmic increment). When they reached at the steady state contraction, drugs were flushed with Krebs solution to allow aortic ring to return to the basic situation.
Aorta histology examination with hematoxylin and eosin staining
An additional thoracic aortic segment from each animal were fixed in 4% neutral buffered formalin and embedded in paraffin. Tissues were cutted into 4-μm serial sections and then processed for H and E staining. The thickness of the thoracic aorta was determined by Image-Pro Plus 5.1 software (Media Cybernetics Inc., Bethesda, MD, USA) (×40). The media thickness, the distance from the internal elastic lamina to the external elastic lamina, and the lumen inner diameter was determined from four points (12, 3, 6, and 9 o'clock positions). The media thickness/lumen diameter ratio was calculated based on the measured lumen inner diameter and the media thickness data.[24]
Vascular endothelial nitric oxide synthase expression with immunohistochemistry staining
IHC was performed according to the kit instructions. After deparaffinized and rehydrated, formalin-fixed paraffin-embedded tissue sections were added enough hydrogen peroxide block for 10 min, and washed two times in phosphate-buffered saline (PBS). Then, the sections went through antigen retrieval with citrate buffer liquid (PH 6.0). After 3 washes in PBS, the sections were blocked for 10 min and then incubation with primary antibody of eNOS (1:200, dilution) overnight at 4°C. After 3 washes in PBS, the sections were incubated with biotinylated goat anti-polyvalent and streptavidin-peroxidase. The signals were visualized by DAB staining and the nuclei were counterstained with hematoxylin. Positive staining presented yellow color under microscope and sections incubated without primary antibody was used as negative control.
Pharmacokinetic studies of paeoniflorin
Ten SD rats were divided evenly into two groups. All rats were fast for 12 h before drug intervention. One group was administrated with RE (30 mg/kg) and the other with 6 mg/kg MP combined with 30 mg/kg RE (MP + RE). The blood were taken and prepared the plasma after administrated for 0, 5, 15, 30, 45, 60, 90, 120, 180, 240, 360, 480, and 720 min. The Agilent HPLC1200 (Agilent Technologies Inc., Palo Alto, American) was used to determinate the content of paeoniflorin in plasma by C18 (4.6 mm × 250 mm, 5 μm) with column oven temperature at 25°C. The mobile phase was consisted of 0.1% phosphoric acid in water and acetonitrile (84:16, V/V). The concentration versus time curve was plotted. All the pharmacokinetic parameters, including the maximum plasma concentration (Cmax), the time to reach Cmax (Tmax), half-life (t½) etc., were calculated by DAS 2.0 statistical software (Mathematical Pharmacology Professional Committee of China, Shanghai, China).
Statistical analysis
All data were expressed as the mean ± standard deviation and one-way analysis of variance was employed by SPSS 15.0 software (SPSS Inc., Chicago, IL, USA). P < 0.05 was regarded as significant statistically.
RESULTS AND DISCUSSION
RE mixed with metoprolol in water didn't affect the content of paeoniflorin
Paeoniflorin in RE alone or mixed with MP adequately dissolved in water and paeoniflorin standard detected at 230 nm was well resolved at retention times of 8.281, 8.261, and 8.276 min, respectively [Figure 1]. The paeoniflorin content in RE alone or mixed with MP adequately dissolved in water were 53.15% and 52.88%. Those data suggested that RE mixed with MP in water had no effect on the content of paeoniflorin.
RE combined with metoprolol synergistically lowered blood pressure in spontaneously hypertensive rats
SHRs were nurtured form Wistar rats by Okamoto in 1963, which is internationally recognized the most comparable in characteristics with human essential hypertension. Our previous studies indicated that RE with the dose of 75 mg/kg, but not the dose of 25 mg/kg, exerts lowering BP (SBP, DBP, and MBP) effect in SHR after 4 weeks treatment.[22] Moreover, MP is commonly used in the treatment of hypertension with the dose of 10–30 mg/kg in rats, according to the dose conversion factor between human and rat.[10,17] First, to evaluate whether the low-doses of RE (30 mg/kg) and MP (6 mg/kg) exerts a synergistic effect on reducing SBP, DBP, and MBP in SHR.
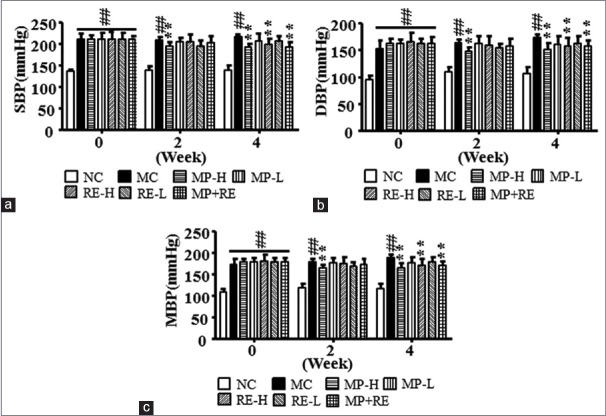
Before the experiment, SBP, DBP, and MBP among all SHR groups had no significant differences, were significantly high than those in NC group (P < 0.01) [Figure 2]. Since the 4th week of treatment, MP-H, RE-H, and MP + RE significantly decreased SBP, DBP, and MBP in SHR compared with MC group (P < 0.01). There was no significant difference after administrated with MP-L and RE-L. RE combined with MP had more antihypertension effect compared with the lower dose of RE or MP alone. It indicated that the combination of RE and MP achieve a synergistic effect on reducing SBP, DBP, and MBP.
Figure 2.
Effect of RE and metoprolol on systolic blood pressure, diastolic blood pressure, and MBP. (a) Systolic blood pressure of all groups. (b) Diastolic blood pressure of all groups. (c) Mean blood pressure of all groups. Values are expressed as the mean ± standard deviation (n = 8).#P < 0.05,##P < 0.01 versus normal control group; *P < 0.05, **P < 0.01 vs. model control group. SBP: Systolic blood pressure; DBP: Diastolic blood pressure; MBP: Mean blood pressure; RE: Paeoniflorin enriched extract from Radix Paeoniae alba; NC: Normal control; MC: Model control; MP-H: Metoprolol high dose (20 mg/kg, p.o.); MP-L: Metoprolol low dose (6 mg/kg, p.o.); RE-H: RE high dose (90 mg/kg, p.o.); RE-L: RE low dose (30 mg/kg, p.o.); MP + RE: metoprolol combined with RE (6 mg/kg + 30 mg/kg, p.o.)
RE combined with metoprolol increased microcirculatory blood flow in spontaneously hypertensive rats
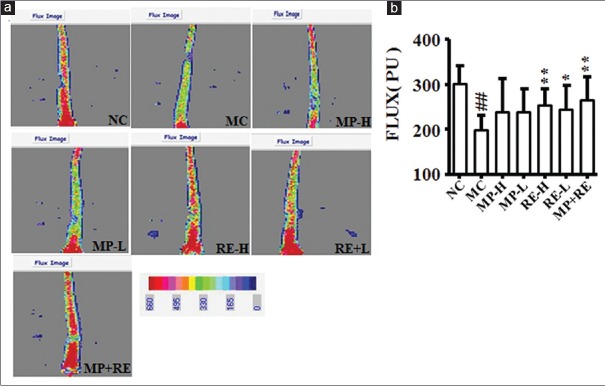
Microcirculation in hypertension may be a new target for the treatment.[25] Moreover, our previous research suggested that laser Doppler with a moor FLPI V2.1 software is a powerful tool for microvascular blood flow evaluation.[23] Taking advantage of the laser Doppler perfusion imaging (LDPI) system, we analyzed the microcirculatory blood flow in the tail of rats administrated with different drugs for 4 weeks, tail perfusion increased significantly in RE-H, RE-L, and MP + RE groups compared with MC group (P < 0.05, 0.01) in the LDPI [Figure 3a and b].
Figure 3.
Effect of RE and metoprolol on microcirculation. (a) Representative images of tails acquired by scanning Doppler perfusion imager. (b) Analysis laser of microvascular blood perfusion. Values are expressed as the mean ± standard deviation (n = 8).#P < 0.05,##P < 0.01 versus normal control group; *P < 0.05, **P < 0.01 versus MC group. RE: Paeoniflorin enriched extract from Radix Paeoniae alba; NC: Normal control; MC: Model control; MP-H: Metoprolol high dose (20 mg/kg, p.o.); MP-L: Metoprolol low dose (6 mg/kg, p.o.); RE-H: RE high dose (90 mg/kg, p.o.); RE-L: RE low dose (30 mg/kg, p.o.); MP + RE: metoprolol combined with RE (6 mg/kg + 30 mg/kg, p.o.)
It may be highly worthwhile to appreciate the role of microcirculation in the pathophysiology and treatment of hypertension. Microcirculation, by definition, involves arterial vessel physiology (rather than diameter or structure) in response to increase internal pressure.[25] Hypertensive rats induced with long-term alcohol consumption with high-sugar/fat diet and SHR all exhibited lower microcirculatory blood flow in the tail.[26,27] In the present study, microvascular blood flow significantly decreased in the tail from untreated SHR. After 4 weeks treatment, RE-H/L and MP + RE reversed the suppression of microcirculation in SHR, but not MP-H/L. It manifested that the combination of RE and MP may improve microcirculation in the treatment of hypertension.
RE combined with metoprolol improved vascular function in spontaneously hypertensive rats
Endothelial dysfunction takes part in the occurrence and progression of hypertension,[13] and improvement of endothelial responses in patients suffered hypertension has been associated with decreasing cardiovascular events and mortality. ACh-induced endothelium-dependent vasorelaxation mainly contributes to the production of NO from arginine in endothelial cells, and SNP-induced endothelium-independent vasorelaxation directly releases NO from SNP, then both acts with the vascular smooth muscle to cause vasodilatation.[28] The previous reports have indicated vascular dysfunction in SHR of different ages.[29,30] Hence, to evaluate the effect of RE combined with MP on vascular function of SHR, we determined endothelium-dependent or-independent relaxation of the rat thoracic aorta.
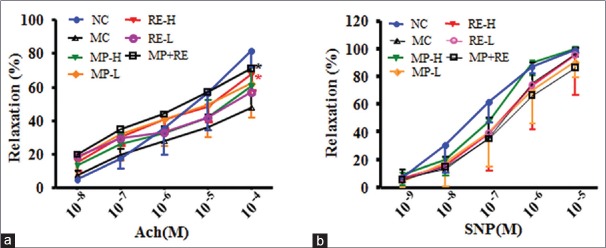
In intact thoracic aortas, endothelial relaxation to ACh (10−9–10−4 M) was significantly reduced in RE-H and MP + RE group compared with MC group (P < 0.05) [Figure 4a]. Meanwhile, endothelium-independent relaxation aroused by SNP (10−10–10−5 M), as well as contraction to 10−6 M NA, was the same tendency in experimental groups [Figure 4b] after treatment for 6 weeks. The data suggested that RE combined with metoprolol, amending vascular endothelial function in SHR, might have beneficial effects for the overall health of patients with hypertension.
Figure 4.
Effect of RE and metoprolol on improvements in vascular function. (a) Endothelium-dependent relaxation to ACh (10−8–10−4 M). (b) Endothelium-independent relaxation to SNP (10−9–10−5 M). Values are expressed as the mean ± standard deviation (n = 6–8).#P < 0.05,##P < 0.01 versus normal control group; *P < 0.05, **P < 0.01 versus modal control group. ACh: Acetylcholine; SNP: Nitroprusside; RE: Paeoniflorin enriched extract from Radix Paeoniae alba; NC: Normal control; MC: Model control; MP-H: Metoprolol high dose (20 mg/kg, p.o.); MP-L: Metoprolol low dose (6 mg/kg, p.o.); RE-H: RE high dose (90 mg/kg, p. o.); RE-L: RE low dose (30 mg/kg, p.o.); MP + RE: MP combined with RE (6 mg/kg + 30 mg/kg, p.o.)
RE combined with metoprolol improved vascular pathomorphology
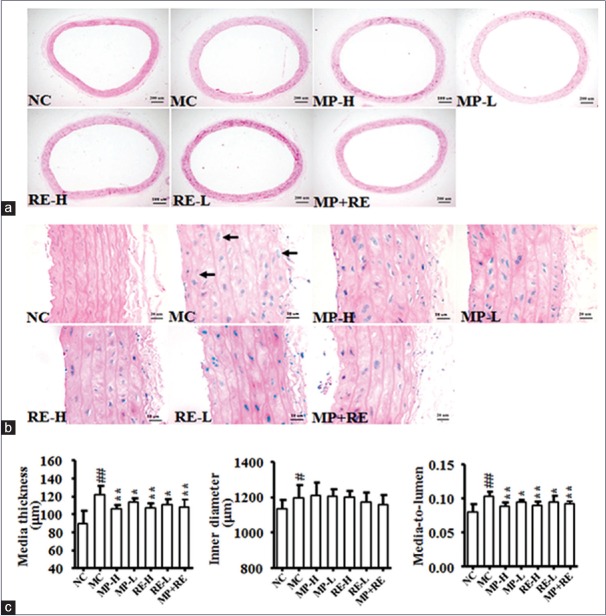
Vascular injury participates in the development and progression of hypertension.[31] The media-to-lumen ratio was used as an index estimated vascular injury.[24] To evaluate the effect of RE combined with MP on the vascular remodeling of SHR, we determined the media thickness of vascular wall and lumen inner diameter in rat aorta using the HE staining [Figure 5]. Although the aortic media thickness and media-to-lumen were marked reducing in all treat groups compared with MC group (P < 0.05, 0.01) [Figure 5c and e], the arrangement of elastic fibers and cells hypertrophy were ameliorated more remarkably in RE-H and MP + RE groups [Figure 5b]. Of note, there was no significant difference in the lumen inner diameter of all treatment and MC groups [Figure 5d]. It indicated that the combination of RE and MP may ameliorate vascular injury.
Figure 5.
Effect of RE and metoprolol on the thoracic aorta pathomorphology. (a) Representative photomicrograph of thoracic aorta sections stained with hematoxylin and eosin (H and E, ×40). (b) Representative photomicrograph of thoracic aorta sections stained with hematoxylin and eosin (H and E, ×400). (c) The media thickness. (d) The inner diameter. (e) The media to lumen ratio. Values are expressed as the mean ± standard deviation (n = 8).#P < 0.05,##P < 0.01 versus normal control group; *P < 0.05, **P < 0.01 versus model control group. RE: Paeoniflorin enriched extract from Radix Paeoniae alba; NC: Normal control; MC: Model control; MP-H: Metoprolol high dose (20 mg/kg, p.o.); MP-L: Metoprolol low dose (6 mg/kg, p.o.); RE-H: RE high dose (90 mg/kg, p.o.); RE-L: RE low dose (30 mg/kg, p.o.); MP + RE: Metoprolol combined with RE (6 mg/kg + 30 mg/kg, p.o.)
RE combined with MP increased vascular endothelial nitric oxide synthase expression
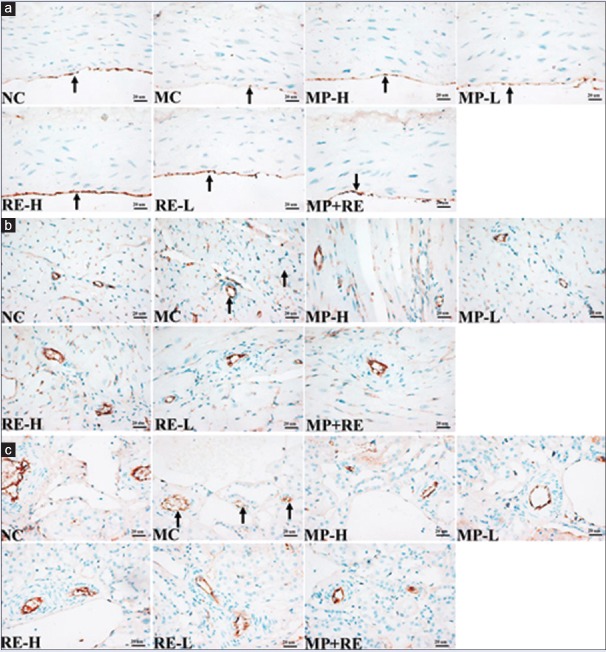
The eNOS can regulate SBP by the production of NO from L-arginine within normal endothelium.[30] Impaired endothelium-derived NO production contributes to vascular dysfunction in many cardiovascular disorders, including hypertension.[12,13] The generation of reactive oxygen species, suppression of PI3K/Akt/eNOS signal pathway,[32] upregulation of NADPH oxidase subunits,[30] inflammation, and other factors suppress of eNOS expression in SHR,[33] and the cumulative evidence supports an important role for eNOS in exerting the beneficial effect on microcirculation.[34] To further prove that RE combined with MP can increase the expression of vascular eNOS and it was contributed to this effect of paeoniflorin, we then performed the expression of eNOS in the thoracic aortic and small vessel of heart and kidney with IHC.
As a result, the expression of eNOS in the aorta endothelium decreased in MC group compared with NC group. In contrast, RE-H, RE-L, and MP + RE could significantly upregulate eNOS expression in the thoracic aortic and small vessel of heart and kidney, but not MP-H and MP-L [Figure 6]. This is consistent with the previous reports about the failure of MP in improving vascular endothelial cell functions and upregulation of eNOS expression.[15,16] It hinted that RE could compensate for the failure of MP to affect the expression of eNOS in SHR, which may be one mechanism of RE combined with MP improving vascular function, vascular injury, and microcirculation.
Figure 6.
Effect of RE and metoprolol on the endothelial nitric oxide synthase expression in thoracic aortic and small vessels of heart and kidney. (a) Representative photomicrograph of the endothelial nitric oxide synthase expression in thoracic aortic by immunohistochemistry (×400); (b) Representative photomicrograph of the endothelial nitric oxide synthase expression in small vessels of heart by immunohistochemistry (×400); (c) Representative photomicrograph of the endothelial nitric oxide synthase expression in small vessels of kidney by immunohistochemistry (×400). eNOS: Endothelial nitric oxide synthase; RE: Paeoniflorin enriched extract from Radix Paeoniae alba; NC: Normal control; MC: Model control; MP-H: Metoprolol high dose (20 mg/kg, p.o.); MP-L: Metoprolol low dose (6 mg/kg, p.o.); RE-H: RE high dose (90 mg/kg, p.o.); RE-L: RE low dose (30 mg/kg, p.o.); MP + RE: MP combined with RE (6 mg/kg + 30 mg/kg, p.o.)
Metoprolol increased the content of paeoniflorin in plasma of Sprague-Dawley rats
Pharmacokinetic interactions must be systematically investigated before the feasible application of combination therapies.[35] Drugs can interact in the metabolic process to increase or decrease the treatment efficacy. The interactions can affect the absorption, distribution, and elimination in vivo. The size of the interaction with p-gp in the small intestine, binding rate with serum protein, and the impact on drug-metabolizing enzymes all causes the concentration changes of drug cocktails in the plasma.
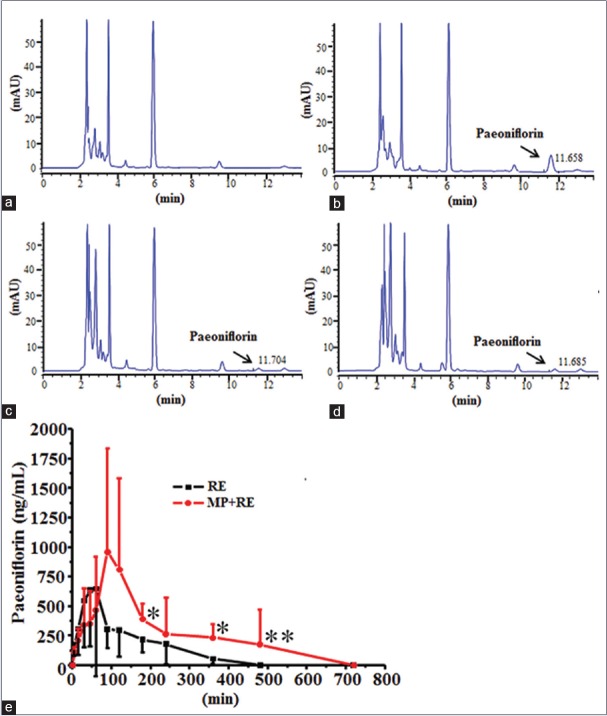
Under the conditions described above, there were representative chromatograms for blank rat plasma, blank rat plasma spiked with paeoniflorin (3 μg/ml), and the rat plasma sample obtained at 30 min after oral administration of RE (30 mg/kg) or RE (30 mg/kg) combined with MP (6 mg/kg) [Figure 7a–d]. Paeoniflorin was well resolved at retention times of 11.658, 11.704, and 11.685 min, respectively. No endogenous interference appeared at the retention times of paeoniflorin, demonstrated that the method is specific for analysis. The standard substances of paeoniflorin were prepared with different concentration following by 0.125, 0.250, 0.500, 1.000, 2.000, 3.000, and 4.000 μg/ml. The regression equation was Y = 24.257X + 0.4294, R2 = 0.9989. The standard substance of paeoniflorin was prepared with different concentration following by 0.5, 1.0, and 3.5 μg/ml, each sample was injected six times and received the recoveries of 96.39%, 102.20%, and 98.86%, respectively. After oral coadministration of RE and MP, plasma levels of paeoniflorin were significantly increased in comparison with administration of RE alone [Figure 7e]. Computation of paeoniflorin pharmacokinetic parameters with DAS 2.0 statistical software showed that Tmax, AUC0-t, and MRT0-t were dramatically altered by coadministration of RE and MP in comparison with administration of RE alone [Table 1]. These observations suggested that there was a certain concentration paeoniflorin in rat plasma after treated with RE (30 mg/kg) alone or combined with MP, and MP influenced the metabolism of paeoniflorin resulted in increase the content of paeoniflorin in rat plasma.
Figure 7.
Representative the chromatograms of paeoniflorin in rat plasma and mean plasma concentration-time profile of paeoniflorin in rat plasma. (a) blank plasma; (b) plasma spiked with paeoniflorin (3 μg/ml); (c) plasma sample obtained 30 min after oral administration with RE (30 mg/kg); (d) Plasma sample obtained 30 min after oral administration with MP + RE (6 mg/kg + 30 mg/kg); (e) Plasma concentration-time profiles of paeoniflorin after oral administration with RE (30 mg/kg) and MP + RE (6 mg/kg + 30 mg/kg), respectively. Values are expressed as the mean ± standard deviation (n = 5). *P < 0.05, **P < 0.01 versus administration with RE alone. RE: Paeoniflorin enriched extract from Radix Paeoniae alba; MP: Metoprolol
Table 1.
Pharmacokinetic parameters of paeoniflorin after oral administration with radix Paeoniae Alba alone (30 mg/kg) or combined with metoprolol (6 mg/kg) to rats
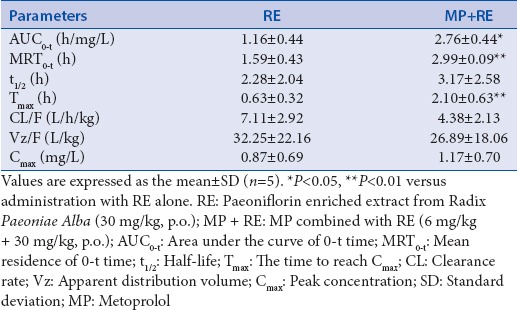
The results of this study revealed that there is a notable increase in the bioavailability of paeoniflorin administered concomitantly with MP, as evidenced clearly by significant increase in the Tmax, AUC0-t, and MRT0-t. The mechanism underlying the interaction between these two drugs is uncertain; however, various possibilities are discussed here. To date, there have been no reports showing that MP is a p-gp substrate though another β-blocker, carvedilol is known to be. Paeoniflorin is a known p-gp inhibitor.[36] It is very unlikely that MP and paeoniflorin interact at p-gp. Both paeoniflorin and MP are considered to be CYP2D6 substrate,[37,38] so they likely interact in mediating CYP metabolic enzymes to increase the bioavailability of paeoniflorin. Our results revealed that MP increased the plasma exposure of paeoniflorin in rat models. Although the mechanism of said interaction remains unclear, it may be one mechanism of the synergistic effects on antihypertension between RE and MP.
CONCLUSION
The results of this study indicated that combination of RE and MP is potentially highly feasible and effective for the therapeutic treatment of hypertension. When combined at lower doses of the two drugs, it could reduce BP, improve microcirculation, and upregulate eNOS expression thus mitigating endothelial dysfunction.
The reported work was supported by China National Natural Science Foundation (81274123 to Gui-Yuan Lv and 81374003/81673638 to Su-Hong Chen), the National Science and Technology on New Drug Creation and Development Projects (2011ZX09101-002-07 to Su-Hong Chen and 2009ZX09502-016/2014ZX09301307-013 to Gui-Yuan Lv), Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents to Su-Hong Chen and the Zhejiang Provincial Key Laboratory Project (2012E10002 to Gui-Yuan Lv).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mills KT, Bundy JD, Kelly TN, Kelly TN, Reed JE, Kearney PM, et al. Global burden and control of hypertension in 2010: Analysis of population-based studies from 89 countries. J Hypertens. 2015;33:e2. [Google Scholar]
- 2.Cai L, Zhang L, Liu A, Li S, Wang P. Prevalence, awareness, treatment, and control of hypertension among adults in Beijing. Clin Exp Hypertens. 2012;19:159–68. doi: 10.3109/10641963.2011.618206. [DOI] [PubMed] [Google Scholar]
- 3.Filomena J, Riba-Llena I, Vinyoles E, Tovar JL, Mundet X, Castañé X, et al. Short-term blood pressure variability relates to the presence of subclinical brain small vessel disease in primary hypertension. Hypertension. 2015;66:634–40. doi: 10.1161/HYPERTENSIONAHA.115.05440. [DOI] [PubMed] [Google Scholar]
- 4.Wang QZ, Gao HQ, Liang Y, Zhang J, Wang J, Qiu J, et al. Cofilin1 is involved in hypertension-induced renal damage via the regulation of NF-κB in renal tubular epithelial cells. J Transl Med. 2015;13:323. doi: 10.1186/s12967-015-0685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawes CM, Vander Hoorn S, Rodgers A. International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 6.Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu ZS, Huo Y, Wang W, Zhao LY, Zhu DL. The education guide for Chinese patients with hypertension. Chin J Hypertens. 2013;12:1123–49. [Google Scholar]
- 8.Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, et al. Single versus combined blood pressure components and risk for cardiovascular disease: The Framingham Heart Study. Circulation. 2009;119:243–50. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: Meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 10.Poirier L, Lacourcière Y. The evolving role of β-adrenergic receptor blockers in managing hypertension. Can J Cardiol. 2012;28:334–40. doi: 10.1016/j.cjca.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 12.Mahalwar R, Khanna D. Pleiotropic antioxidant potential of rosuvastatin in preventing cardiovascular disorders. Eur J Pharmacol. 2013;711:57–62. doi: 10.1016/j.ejphar.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Mordi I, Mordi N, Delles C, Tzemos N. Endothelial dysfunction in human essential hypertension. J Hypertens. 2016;34:1464–72. doi: 10.1097/HJH.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 14.Rochette L, Lorin J, Zeller M, Guilland JC, Lorgis L, Cottin Y, et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol Ther. 2013;140:239–57. doi: 10.1016/j.pharmthera.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Higashi Y, Sasaki S, Nakagawa K, Ueda T, Yoshimizu A, Kurisu S, et al. A comparison of angiotensin-converting enzyme inhibitors, calcium antagonists, beta-blockers and diuretic agents on reactive hyperemia in patients with essential hypertension: A multicenter study. J Am Coll Cardiol. 2000;35:284–91. doi: 10.1016/s0735-1097(99)00561-6. [DOI] [PubMed] [Google Scholar]
- 16.Dorenkamp M, Riad A, Stiehl S, Spillmann F, Westermann D, Du J, et al. Protection against oxidative stress in diabetic rats: Role of angiotensin AT (1) receptor and beta 1-adrenoceptor antagonism. Eur J Pharmacol. 2005;520:179–87. doi: 10.1016/j.ejphar.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Shang F, Zhang J, Li Z, Zhang J, Yin Y, Wang Y, et al. Cardiovascular protective effect of metformin and telmisartan: Reduction of PARP1 activity via the AMPK-PARP1 cascade. PLoS One. 2016;11:e0151845. doi: 10.1371/journal.pone.0151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He DY, Dai SM. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora pall. a traditional Chinese herbal medicine. Front Pharmacol. 2011;2:10. doi: 10.3389/fphar.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng ZX, Huang HD, Deng SG. Effects of paeoniflorin on isolated thoracic aorta rings of rats and its possible mechanism. Chin J Exp Tradit Med Formulae. 2011;17:190–3. [Google Scholar]
- 20.Qian GQ, Ding J, Zhang X, Yin X, Gao Y, Zhao GP, et al. Preconditioning with glycyrrhizic, ferulic, paeoniflorin, cinnamic prevents rat hearts from ischemia/reperfusion injury via endothelial nitric oxide pathway. Pharmacogn Mag. 2015;11:292–6. doi: 10.4103/0973-1296.153081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Suo ZW, Dong H, Zhang T, Yang RN, Mo X. Effect of paeoniflorin on nitric oxide level and expression of eNOS in pulmonary microvascular endothelial cells. Chin J Anim Sci. 2010;46:54–7. [Google Scholar]
- 22.Su-Hong C, Qi C, Bo L, Jian-Li G, Jie S, Gui-Yuan L, et al. Antihypertensive effect of Radix Paeoniae alba in spontaneously hypertensive rats and excessive alcohol intake and high fat diet induced hypertensive rats. Evid Based Complement Alternat Med. 2015;2015:731237. doi: 10.1155/2015/731237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao JL, Ji X, He TC, Zhang Q, He K, Zhao Y, et al. Tetrandrine suppresses cancer angiogenesis and metastasis in 4T1 tumor bearing mice. Evid Based Complement Alternat Med. 2013;2013:265061. doi: 10.1155/2013/265061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su J, Xu HT, Yu JJ, Gao JL, Lei J, Yin QS, et al. Luteolin ameliorates hypertensive vascular remodeling through inhibiting the proliferation and migration of vascular smooth muscle cells. Evid Based Complement Alternat Med. 2015;2015:364876. doi: 10.1155/2015/364876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: A new target for treatment? Circulation. 2001;104:735–40. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 26.Hu X, Li B, Lei SS, Su J, Lv GY, Chen SH. Effect of three unhealthy lifestyle factors on blood pressure, blood biochemistry and microcirculation of rats. J Zhejiang Chin Med Univ. 2014;38:608–12. [Google Scholar]
- 27.Lv GY, Li D, Chen SH, Su J, Xia CQ, Ji X. Effect of microcirculation blood flow measurement by laser doppler on pharmacodynamics of Chinese herbal medicine in three-high animal model. Chin J Microcirc. 2012;22:84–5. [Google Scholar]
- 28.Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: The janus face of prostacyclin. Br J Pharmacol. 2005;146:834–45. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lüscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–8. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- 30.Zhang JX, Yang JR, Chen GX, Tang LJ, Li WX, Yang H, et al. Sesamin ameliorates arterial dysfunction in spontaneously hypertensive rats via downregulation of NADPH oxidase subunits and upregulation of eNOS expression. Acta Pharmacol Sin. 2013;34:912–20. doi: 10.1038/aps.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing-implications in hypertension. J Mol Cell Cardiol. 2015;83:112–21. doi: 10.1016/j.yjmcc.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Y, Tang X, Xie L, Meng G, Ji Y. Aliskiren improves endothelium-dependent relaxation of thoracic aorta by activating PI3K/Akt/eNOS signal pathway in SHR. Clin Exp Pharmacol Physiol. 2016;43:450–8. doi: 10.1111/1440-1681.12550. [DOI] [PubMed] [Google Scholar]
- 33.de Nigris F, Balestrieri ML, Williams-Ignarro S, D'Armiento FP, Lerman LO, Byrns R, et al. Therapeutic effects of autologous bone marrow cells and metabolic intervention in the ischemic hindlimb of spontaneously hypertensive rats involve reduced cell senescence and CXCR4/Akt/eNOS pathways. J Cardiovasc Pharmacol. 2007;50:424–33. doi: 10.1097/FJC.0b013e31812564e4. [DOI] [PubMed] [Google Scholar]
- 34.Figueroa XF, González DR, Martínez AD, Durán WN, Boric MP. ACh-induced endothelial NO synthase translocation, NO release and vasodilatation in the hamster microcirculation in vivo. J Physiol. 2002;544:883–96. doi: 10.1113/jphysiol.2002.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgess G, Hoogkamer H, Collings L, Dingemanse J. Mutual pharmacokinetic interactions between steady-state bosentan and sildenafil. Eur J Clin Pharmacol. 2008;64:43–50. doi: 10.1007/s00228-007-0408-z. [DOI] [PubMed] [Google Scholar]
- 36.Liu ZQ, Jiang ZH, Liu L, Hu M. Mechanisms responsible for poor oral bioavailability of paeoniflorin: Role of intestinal disposition and interactions with sinomenine. Pharm Res. 2006;23:2768–80. doi: 10.1007/s11095-006-9100-8. [DOI] [PubMed] [Google Scholar]
- 37.Gao LN, Zhang Y, Cui YL, Akinyi OM. Comparison of paeoniflorin and albiflorin on human CYP3A4 and CYP2D6. Evid Based Complement Alternat Med. 2015;2015:470219. doi: 10.1155/2015/470219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder BD, Rowland A, Polasek TM, Miners JO, Doogue MP. Evaluation of felodipine as a potential perpetrator of pharmacokinetic drug-drug interactions. Eur J Clin Pharmacol. 2014;70:1115–22. doi: 10.1007/s00228-014-1716-8. [DOI] [PubMed] [Google Scholar]