Abstract
Background:
Curative plants have reportedly been used to make chewing sticks/toothbrushes intended for the treatment of oral diseases.
Objective:
The in vitro antibacterial activities of Azadirachta indica, Pongamia pinnata, Psidium guajava, and Mangifera indica were evaluated against Streptococcus mutans, along with the cytotoxicity and antioxidant and synergistic potentials. The effect of M. indica on the expression of crucial virulence genes spaP and gtfB of S. mutans was determined.
Materials and Methods:
The antibacterial activity was determined using a modified microdilution method. The antioxidant potential was evaluated using diphenyl picrylhydrazyl (DPPH), Griess reagent, and nitroblue tetrazolium calorimetric assays. The synergistic activity was investigated using a modified checkerboard method, while the cytotoxicity was determined according to a cell proliferation 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt assay. Reverse transcription was the chosen method for determining the difference in expression of the spaP and gtfB genes after treatment with the plant sample.
Results:
M. indica and A. indica had the highest antibacterial activity at concentrations of 0.3 mg/ml and 6.25 mg/ml, respectively. A. indica had the best free radical scavenging of DPPH, exhibiting 50% inhibition at 28.72 μg/ml; while M. indica showed better superoxide scavenging potential than the positive control quercetin. Both M. indica and A. indica had adequate activity against the nitric oxide-free radical (12.87 and 18.89 μg/ml, respectively). M. indica selectively reduced the expression of the gtfB gene, indicating a mechanism involving Glucotranferases, specifically targeting bacterial attachment.
SUMMARY
Mangifera indica and Azadirachta indica had very good antibacterial activity against Streptococcus mutans and moderate toxicity against Vero cells
M. indica had the best antioxidant capacity overall
M. indica reduced the expression of gtfB gene at 0.5 mg/ml.
Abbreviations used: AA: Ascorbic acid; BHI: Brain–heart infusion; CHX: Chlorhexidine; DPPH: Diphenyl picrylhydrazyl; DMSO: Dimethlysulfoxide; NBT: Nitroblue tetrazolium; NO: Nitric oxide;
Keywords: Antibacterial, antioxidant, gene expression, oral pathogens
INTRODUCTION
The mouth is often viewed as a separate entity to the rest of the body, as such, many people tend to neglect their oral health and often do not take the necessary steps required to prevent diseases such as dental caries.[1,2] These oral diseases have overwhelming consequences on the general quality of life, interfering with basic actions such as chewing and communicating.[2] The pain associated with toothaches has been linked to the loss in the working hours of many countries and was found to contribute to absenteeism in the workplace and schools.[3] This has led to many people turning to the roots and bark of curative plants in order to make chewing sticks/toothbrushes, primarily for maintaining good oral health and preventing the onset of oral diseases such as dental caries.[4,1,2]
Plants belonging to the families Meliaceae, Fabaceae, Myrtaceae, and Anacardiaceae have reported applications in the treatment of oral ailments such as toothache and oral thrush. The plants considered for the present study were Azadirachta indica, Pongamia pinnata, Psidium guajava, and Mangifera indica, commonly known as Neem, Karanj, Mango, and Guava, respectively. M. indica has been traditionally used as an ayurvedic component in the treatment of a variety of symptoms caused by oral diseases, such as healing wounds in the mouth that result from oral thrush as well as the treatment of toothache associated with dental caries. The reported traditional use of P. guajava in various parts of the world has been for the treatment for toothache and against caries, where the leaves, shoots, roots, and bark are used to make concoctions. The roots of P. pinnata have been traditionally used to clean gums and teeth, a preventative measure for the development of caries.[5] The stems, roots, and twigs of A. indica have been used to make chewing sticks and toothbrushes for the removal of plaque.[6]
With the development of high-throughput screening methods, it has become possible to conduct scientific validations of plants which are traditionally used for oral care.[7,8,9] Those plants that are able to specifically inhibit the activity of bacteria such as S. mutans, Gram-positive cocci, and the main causative agents of dental caries are of particular interest. In addition to their antibacterial activity, plants often possess natural antioxidants in the form of small secondary compounds, for instance, polyphenols, flavonoids, and other associated compounds.[10,11] These particular compounds are important in the prevention of disease development by reducing the oxidative damage that certain microorganisms such as S. mutans can cause, often as part of their own important cellular processes.[12,13,14,15] Medicinal plants can therefore be investigated for their radical scavenging potential.
Bacteria such as S. mutans possess important virulence factors that are important for the development and spread of disease in hosts.[16] S. mutans has three main virulence factors, namely, direct attachment, through unique enzymes on the surface of their membrane such as AntigenI/II and Glucotranferases (GTF); biofilm formation; and acid production.[17] Biofilm formation and acid production are directly dependent on successful attachment; therefore, a plant sample that can target attachment will effectively prevent disease development.[18] The spaP and gtfB genes encode for the enzymes AntigenI/II and GTF, respectively, and give S. mutans the unique ability to produce extracellular polysaccharides that allow it to bind onto the tooth surface.[19,20,21]
The antibacterial activities of A. indica, P. pinnata, P. guajava, and M. indica were tested against S. mutans (American Type Culture Centre [ATCC] 25175) using a calorimetric microdilution method. The radical scavenging potential of the samples was measured using the diphenyl picrylhydrazyl (DPPH), nitric oxide (NO), and nitroblue tetrazolium (NBT) scavenging assays. In addition to determining the antibacterial activity of the samples, it was assessed whether the inhibition of bacterial cells was due to a decrease in expression of the spaP and gftB genes in cells treated with plant extract. RNA was isolated from treated and untreated cells in order to compare the expression of the particular genes at mid-log phase. The main aim of this study was to determine the antibacterial and antioxidant activities as well as the possible mechanism of action of traditional medicinal plants used on a large scale from many decades ago.
MATERIALS AND METHODS
Materials
The leaves of the plants considered for the current study were gathered at the Hatia region of Ranchi district, Jharkhand, India. The plants were then identified at the Department of Botany, St. Xavier's College, Ranchi, Jharkhand, India, by Dr. Ajay Srivastava (HOD, Department of Botany, St. Xavierme College, Ranchi, Jharkhand, India). A herbarium specimen voucher for each plant was kept at the herbarium of the department of Botany at St. Xavier's College.
DPPH and PrestoBlue® were procured from Sigma Aldrich, South Africa. Brain–heart infusion (BHI) broth and agar, Anaerocult®, and McFarland standard 1 were purchased from Merck Chemicals (Pty) Ltd. (Wadeville, South Africa). Chlorhexidine (CHX) gluconate was bought from Dental Warehouse (Sandton, South Africa) and S. mutans were bought from the ATCC 25175.
Plant extract preparation
The leaves of each of the selected plants were dried out at room temperature and then subsequently ground to a fine powder. The powdered material was then placed on a shaker for 48 h, using ethanol as an extractant. After filtering the plant material, the ethanol was evaporated using a rotary evaporator.
Antibacterial assays
Oral pathogen
S. smutans (ATCC 25175) cells were proliferated on BHI agar (Merck Chemicals (Pty) Ltd., Wadeville, South Africa) placed in an anaerobic jar containing Anaerocult® A (Merck KGaA Darmstadt, Germany) which provided the anaerobic condition required for growth. This was done for 48 h at 37°C, with subculturing done weekly. The inocula were obtained by adding S. mutans colonies to BHI broth, until the concentration of the bacterial cells aligned with McFarland standard 1 (3 × 108 cfu/ml), determined spectrophotometrically.
The bacterial inoculum was prepared by suspending S. mutans colonies in BHI broth until turbidity was compatible with McFarland Standard 1 (3 × 108 cfu/ml) (Merck Chemicals [Pty] Ltd., Wadeville, South Africa).[22]
PrestoBlue® colorimetric assay to determine the minimum inhibitory concentration of each plant extract
The microplate method as described by Eloff[23] and Seukep et al.[24] was adapted and used to determine the inhibitory potential of the plant samples by calculating the concentration at which the plant samples inhibited the growth of the tested bacteria,; minimum inhibitory concentration (MIC). PrestoBlue® was used as the colorimetric indicator reagent in the assay. The plant extracts were dissolved in 100% acetone (Merck Chemicals [Pty] Ltd), which was shown to be nontoxic to the bacterial cells and diluted down in a 96-well plate enriched with BHI broth (Merck Chemicals [Pty] Ltd) to concentrations varying from 12.5 to 0.1 mg/ml. Five percent CHX gluconate was used as a positive control with concentrations ranging from 3.8 × 104 to 12.5 mg/ml, after which 3 × 108 cfu/ml of 24 h-old inocula were added to the plates and incubated at 37°C for a further 24 h before adding the color indicator PrestoBlue®.[25,23] The MIC was taken as the concentration at which there was no color change from blue to pink, indicating bacterial growth inhibition.
Synergistic antibacterial activity
The synergy between the different plants was determined by combining the checkerboard and ratio methods as described by Lall et al., Eloff, and Orhan et al.[25,23,26] In order to determine the combined antimicrobial interaction between M. indica and A. indica, P. pinnata, and P. guajava, the samples were prepared to concentrations ranging from 0.10 to 12.5 mg/ml. In a sterile 96-well plate, 100 μl of M. indica was then added in a ratio from 9:1 (90 μl M. indica: 10 μl A. indica) to 1:9 (10 μl M. indica: 90 μl A. indica); 9:1 (90 μl P. pinnata: 10 μl P. guajava) to 1:9 (10 μl P. pinnata: 90 μl P. guajava). The sample combinations in their ratios were then serially diluted down in 2-fold dilutions. A 100 μl solution of S. mutans inocula (3 × 108 cfu/ml) was added to the plates and incubated at 37°C, under anaerobic conditions for 24 h before adding the color indicator PrestoBlue®.[25,27]
Analysis
The fractional inhibitory concentration (FIC) was used to determine possible synergy, where the sum of FIC gave a Fractional Inhibitory Index (nFIC), determined using the following formula:
The Σ FIC was interpreted in several ways; if the Σ FIC was ≤0.50, the combination was synergistic; >0.5–1, the combination was additive; >1–≤4, the combination was noninteractive; or >4, the combination was antagonistic.
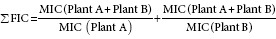
In vitro antioxidant activity
2,2-Diphenyl picrylhydrazyl assay
The degree of scavenging capacity for each plant sample was determined using the method published in the study by Henley-Smith et al.[28] with slight modifications. All reactions were carried out in 96-well plates. The plant samples and ascorbic acid (positive control) were prepared to stock solutions and then diluted down to concentrations varying from 500 to 3.9 μg/ml and 100 to 0.781 μg/ml, respectively. A 90-μl solution of 0.04 M DPPH was added to all the wells and the plates were incubated in a dark room for half an hour. In the presence of an antioxidant, the solution was reduced from purple to yellow/clear.
Nitric oxide radical inhibition activity
The method published in the study by Orhan et al.[26] with slight modifications was used to calculate the plant samplest ability to scavenge NO. The plant samples and ascorbic acid (positive control) were prepared to stock solutions of 10 mg/ml and serially diluted to final concentrations of 1000 μg/ml to 7.8 μg/m. After which 50 μl of sodium nitroprusside was added to each well and the plates were kept in light at 25°C for 1 h 30 min. Once the incubation period was complete, a 50 μl solution of Griess reagent was then added to each well barring the negative control, where dH2O was added in its place. For the NO scavenging assay, the presence of an antioxidant reduced the pink solution to a yellow color.
Nitroblue tetrazolium scavenging by alkaline dimethlysulfoxide method
The alkaline dimethlysulfoxide (DMSO) method was used to produce superoxide-free radicals (in this case, NBT), the reduction of which was determined in the presence and absence of a plant sample. All reactions were carried out in 96-well plates. The plant samples were prepared to stock solutions of 1000 to 7.8 μg/ml. The positive control, quercetin, was also prepared in the same way. A solution of 100 μl 100% DMSO was added to each well of the plate. After which, 10 μl of NBT was added. The presence of a reducing agent turned the solution from blue to blue-green, with the increase in the intensity of color change, indicating the increase in reducing power of the sample.
Statistical analysis
The ability of the plant samples to scavenge the free radicals in each antioxidant assay was determined using a BIO TEK Power-Wave XS multi-well reader (A.D.P., Weltevreden Park, South Africa) at a wavelength of 515 nm for DPPH, 546 nm for NO, and 560 for NBT, using KC Junior software (Highland Park, Winooski, Vermont, USA). The IC50 values of the samples were calculated using GraphPad Prism version 4 software (Graph Pad software version 4, San Diego, CA, USA) together with Windows Excel 2013.
Cytotoxicity
The potential toxicity of the plant samples on normal cells was determined using a cell proliferation kit II, where the effect that the samples had on Vero (monkey kidney) cells was measured using the 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)- 2H-tetrazolium-5-carboxanilide salt (XTT) method.[15] A solution of 100 μl of cells (1 × 105) was added to Mico plates, which were then incubated for 24 h allowing for the seeded cells to attach to the bottom of the plates. A stock solution of each sample was used to make dilutions ranging from 100 to 31 μg/ml, which were then added to the microplates and incubated for 48 h. The XTT reagent (0.3 mg/ml) was added and the plates were incubated for a further 1–2 h. Actinomycin D was used as a positive control. After incubation, the absorbance of the color was spectrophotometrically quantified using an ELISA plate reader, which measured the optical density at 490 nm with a reference wavelength of 690 nm.
RNA extraction, reverse transcription (cDNA synthesis), and reverse transcription-polymerase chain reactions
Having obtained good antibacterial and antioxidant activities by the M. indica plant extract, it was selected further for mechanistic studies. The change in the expression of virulence genes spaP and gtfB caused by treatment with this plant extract was determined using reverse transcription-polymerase chain reactions. Approximately 1 × 106 bacterial cells were treated with 500 μg/ml concentration of the M. indica sample in triplicate after which the treatments were incubated for 24 h at 37°C in an anaerobic environment. After incubation, intact RNA from untreated and cells treated with the plant extract was extracted using a Qiagen RNeasy® mini kit, with slight modifications.[29] A nanodrop spectrophotometer calculated the OD260/280 and OD260/230 ratios of each sample after which gel electrophoresis was carried out in order to scrutinize the quality of RNA. Five hundred nanograms of synthesized RNA was used to prepare a cDNA pool using the cDNA synthesis kit (Thermo scientific, SA). The cDNA was then used to conduct semi-quantitative Polymerase chain reaction (PCR), with Image J (NIH image, Biocompare, USA) and GraphPad Prism used to estimate the mRNA levels in treated and untreated cells.
RESULTS AND DISCUSSION
Potential antibacterial activity against Streptococcus mutans (in vitro)
Of the four samples tested, P. pinnata and P. guajava showed no significant antibacterial activity, with only occurring at the highest concentration tested, i.e. 12.5 mg/ml. M. indica had very good activity at 0.39 mg/ml, with A. indica showing moderate activity at 6.25 mg/ml [Table 1]. The positive control CHX had an MIC of 4.8 × 103 mg/ml, which corresponded to that reported in literature.[28]
Table 1.
Summary of the concentrations of the plant samples at which 50% of the free radical were scavenged (IC50) in in vitro antioxidant assays
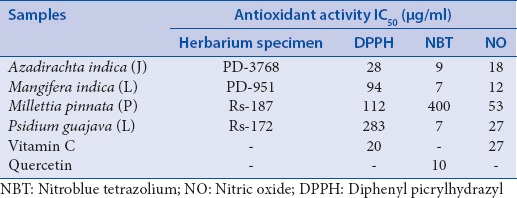
A plant extract is regarded as having antimicrobial activity if it has an MIC value of 8 mg/ml or lower.[30,23] A specific interpretation according to the study by Kuete[31] was that the activity of a plant sample was regarded noteworthy when its MIC fell below a concentration of 0.1 mg/ml and adequate when MIC values fell between 0.1 and 0.625 mg/ml, with samples having activity above 0.625 mg/ml indicating low activity. Of the four samples present, the extract of M. indica exhibited the best antibacterial activity, with its activity showing better activity than that reported for the leaf extracts of Eucalyptus spathulata (which has been known to be added in toothpastes and mouthwash), which had an MIC of 50 mg/ml when tested against S. mutans.[32]
Antioxidant capacity
The radical scavenging activities of the different plant samples using different antioxidant assays are summarized in Table 1.
The antioxidant activities of the samples were expressed by an IC50 value, which was used to define the concentration of the tested sample at which 50% of the free radical was scavenged. A low IC50 value indicated good activity of that sample against that particular free radical.[33]
A. indica had the best activity against DPPH (IC50 value of 28 μg/ml), with P. guajava exhibiting little-to-no DPPH scavenging activity with an IC50 value of 283 μg/ml. The positive control ascorbic acid had an IC50 value of 20 μg/ml. From the samples tested, M. indica had the best scavenging capacity against NO, with an IC50 value of 12 μg/ml, which was similar to that of the positive control ascorbic acid (IC50 value of 4 μg/ml). In general, most of the plant samples showed good activity against the NBT-free radical, with M. indica and P. guajava having the lowest IC50 value of 7 μg/ml, which was even better than that of quercetin, the positive control which had an IC50 value of 10 μg/ml. P. pinnata was the only sample that did not exhibit any NBT scavenging activity.
Many bacterial species use oxidation as a part of their pathogenesis, where many of them attack important molecules such as DNA and other molecules in order to use them as building blocks for bacterial cellular components.[10,34] The production of superoxide has been reported in diseases such as dental caries, where these free radicals are important for converting the environment in the oral cavity to one that is conducive to the establishment of other bacteria. Therefore, a sample with good antioxidant activity may directly interfere with the successful establishment of bacteria such as S. mutans in the oral cavity.[35]
All the plant samples in the present study generally exhibited some antioxidant activity across the board. The antioxidant activity that was of particular interest was that of M. indica, particularly against the NO radical. This antioxidant activity is of particular importance because the production of NO is an important part of the pathogenesis of certain oral pathogens, as it forms part of the inflammation process (acts as an activation agent). The good NO activity exhibited by M. indica also correlated with its good antibacterial activity. M. indica had the overall best antioxidant activity of all the plant samples tested. The reducing properties of the samples may be due to the presence of reductones (molecules that break free radical chains by donating hydrogen atoms). In particular, M. indica contains the pharmacologically and medicinally important chemical mangiferin, which is a polyphenolic antioxidant.[36]
Synergistic assay
The MIC value of the combination of M. indica and A. indica was 6.25 mg/ml and for P. pinnata and P. guajava was 6.25 mg/ml. These values were used to calculate the ΣFIC indices. The Σ hei for M. indica and A. indica was 16 and for P. guajava and P. pinnata was 1, indicating that a combination of M. indica and A. indica was antagonistic and that of P. guajava and P. pinnata was additive.
Reverse transcription-polymerase chain reaction
The degree of expression of the spaP and gtfB mRNAs in bacterial cells treated with the M. indica sample was evaluated by reverse transcription-polymerase chain reactions. The gene for glutamine (glnA) was used as the housekeeping gene. The levels of mRNA expressed for the gtfB gene were expressed at a lower rate in the presence of the plant extract (P< 0.01) while the levels of mRNAs expressed for spaP were found to be the same in treated and untreated cells (P > 0.01) [Figures 1 and 2]. This suggested that the plant sample did not inhibit the production of the Antigen I/II, expressed for by the spaP gene. It may have instead targeted the production of the GTF enzyme, encoded for by the gtfB gene at the transcription level.
Figure 1.
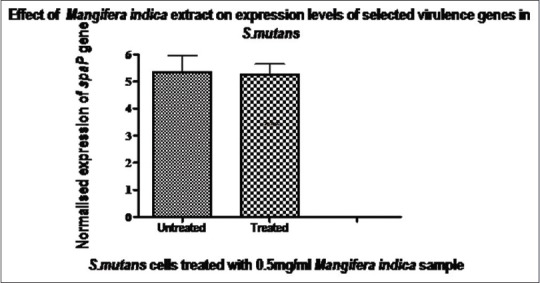
Expression of the spaP gene in treated and untreated cells. Expressions of the spaP gene were normalized to that of the glnA reference gene. Data are expressed as mean ± standard deviation, n = 6, t-test, P > 0.05, not statistically significant. This indicated that there was no difference in expression of the spaP gene in untreated and treated cells. This indicated that the sample had no effect on the synthesis of AntigenI/II at the transcription level
Figure 2.
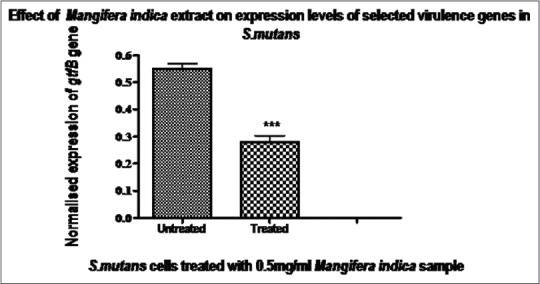
Expression of the mRNA levels of the gtfB gene in treated and untreated cells. Expressions of the gtfB gene were normalized to that of the glnA reference gene. Data are expressed as mean ± standard deviation, n = 6, t-test, P < 0.05, represents a statistical significance when compared to the untreated. The plant sample caused a reduction in mRNA levels for this gene, indicating that the sample may have an effect on the synthesis of the glucotransferase enzyme at transcription level
Cytotoxicity
The plant samples were tested for toxicity against Vero cells, and of all the samples, P. guajava showed acute toxicity to the cells, with an IC59 value of 23.63 μg/ml. M. indica, which was chosen for further investigation in the study, showed moderate toxicity (IC50 of 64.72 μg/ml). The other samples also showed moderate toxicity at concentrations ranging from 35.88 to 58.69 μg/ml. Actinomycin D had an MIC of 2 × 103 μg/ml, which was comparable to that reported in literature.
CONCLUSION
Of all the samples tested, M. indica (Mango) showed significant results for antibacterial activity. The results provided some scientific validation for the traditional use of M. indica leaf extract which inhibited some characteristics of S. mutans and thus may be considered beneficial for oral care.[37] Therefore, this study provides a significant contributor to antibacterial and antioxidant activities. Of interest would be M. indica, which had the best antibacterial activity as well as generally the best antioxidant activity. More importantly, this plant sample may possess a unique mechanism of action, which involves targeting the expression of the gftB gene, thereby targeting bacterial attachment.
Financial support and sponsorship
This study was financially supported by National Research Fund, University of Pretoria.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005;83:661–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Sheiham A. Oral health, general health and quality of life. Bull World Health Organ. 2005;83:644. [PMC free article] [PubMed] [Google Scholar]
- 3.Selwitz RH, Ismail AI, Pitts NB. Dental caries. The Lancet. 2007 Jan 12;369(9555):51–9. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 4.Homer KA, Manji F, Beighton D. Inhibition of protease activities of periodontopathic bacteria by extracts of plants used in Kenya as chewing sticks (mswaki) Arch Oral Biol. 1990;35:421–4. doi: 10.1016/0003-9969(90)90203-m. [DOI] [PubMed] [Google Scholar]
- 5.Arote SR, Yeole PG. Pongamia pinnata L: A comprehensive review. Int J Pharm Tech Res. 2010;2:2283–90. [Google Scholar]
- 6.Prashant GM, Chandu GN, Murulikrishna KS, Shafiulla MD. The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivarius, Streptococcus mitis, and Streptococcus sanguis: An in vitro study. Indian J Dent Res. 2007;18:148–51. doi: 10.4103/0970-9290.35822. [DOI] [PubMed] [Google Scholar]
- 7.Nigro SA, Makunga NP, Grace OM, Bornman CH. Medicinal plants at the ethnobotany–biotechnology interface in Africa. S Afr J Bot. 2004;70:89–96. [Google Scholar]
- 8.Patwardhan B. Ethnopharmacology and drug discovery. J Ethnopharmacol. 2005;100:50–2. doi: 10.1016/j.jep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Upadhyay B, Singh KP, Kumar A. Pharmacognostical and antibacterial studies of different extracts of Euphorbia hirta L. J Phytol. 2010;2:56–60. [Google Scholar]
- 10.Abdel-Hameed ES. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009;114:1271–7. [Google Scholar]
- 11.Alinezhad H, Azimi R, Zare M, Ebrahimzadeh MA, Eslami S, Nabavi SF, et al. Antioxidant and antihemolytic activities of ethanolic extract of flowers, leaves, and stems of Hyssopus officinalis L. Var. angustifolius. Int J Food Properties. 2013;16:1169–78. [Google Scholar]
- 12.Gutiérrez RM, Mitchell S, Solis RV. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2008;117:1–27. doi: 10.1016/j.jep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Rajkapoor B, Burkan ZE, Senthilkumar R. Oxidants and human diseases: Role of antioxidant medicinal plants – A review. Pharmacol Online. 2010;1:1117–31. [Google Scholar]
- 14.Rajkumar M, Ae N, Prasad MN, Freitas H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010;28:142–9. doi: 10.1016/j.tibtech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Zheng C, Huang D, Liu L, Björkholm M, Holm G, Yi Q, et al. Cytotoxic T-lymphocyte antigen-4 microsatellite polymorphism is associated with multiple myeloma. Br J Haematol. 2001;112:216–8. doi: 10.1046/j.1365-2141.2001.02552.x. [DOI] [PubMed] [Google Scholar]
- 16.Ajdić D, McShan WM, McLaughlin RE, Savić G, Chang J, Carson MB, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A. 2002;99:14434–9. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decker EM, Dietrich I, Klein C, von Ohle C. Dynamic production of soluble extracellular polysaccharides by Streptococcus mutans. Int J Dent. 2011;2011:435830. doi: 10.1155/2011/435830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ofek I, Hasty DL, Sharon N. Anti-adhesion therapy of bacterial diseases: Prospects and problems. FEMS Immunol Med Microbiol. 2003;38:181–91. doi: 10.1016/S0928-8244(03)00228-1. [DOI] [PubMed] [Google Scholar]
- 19.Ajdić D, Pham VT. Global transcriptional analysis of Streptococcus mutans sugar transporters using microarrays. J Bacteriol. 2007;189:5049–59. doi: 10.1128/JB.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limsong J, Benjavongkulchai E, Kuvatanasuchati J. Inhibitory effect of some herbal extracts on adherence of Streptococcus mutans. J Ethnopharmacol. 2004;92:281–9. doi: 10.1016/j.jep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Nakano K, Lapirattanakul J, Nomura R, Nemoto H, Alaluusua S, Grönroos L, et al. Streptococcus mutans clonal variation revealed by multilocus sequence typing. J Clin Microbiol. 2007;45:2616–25. doi: 10.1128/JCM.02343-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McFarland J. The nephelometer: An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J Am Med Assoc. 1907;49:1176–8. [Google Scholar]
- 23.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–3. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 24.Seukep JA, Sandjo LP, Ngadjui BT, Kuete V. Antibacterial activities of the methanol extracts and compounds from Uapaca togoensis against Gram-negative multi-drug resistant phenotypes. S Afr J Bot. 2016;103:1–5. [Google Scholar]
- 25.Lall N, Henley-Smith CJ, De Canha MN, Oosthuizen CB, Berrington D. Viability reagent, PrestoBlue®, in comparison with other available reagents, utilized in cytotoxicity and antimicrobial assays. Int J Microbiol. 2013;2013:420601. doi: 10.1155/2013/420601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orhan G, Bayram A, Zer Y, Balci I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J Clin Microbiol. 2005;43:140–3. doi: 10.1128/JCM.43.1.140-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Rapper S, Van Vuuren SF, Kamatou GP, Viljoen AM, Dagne E. The additive and synergistic antimicrobial effects of select frankincense and myrrh oils – A combination from the pharaonic pharmacopoeia. Lett Appl Microbiol. 2012;54:352–8. doi: 10.1111/j.1472-765X.2012.03216.x. [DOI] [PubMed] [Google Scholar]
- 28.Henley-Smith CJ, Steffens FE, Botha FS, Lall N. Predicting the influence of multiple components on microbial inhibition using a logistic response model – A novel approach. BMC Complement Altern Med. 2014;14:190. doi: 10.1186/1472-6882-14-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren Z, Chen L, Li J, Li Y. Inhibition of Streptococcus mutans polysaccharide synthesis by molecules targeting glycosyltransferase activity. J Oral Microbiol. 2016;8:31095. doi: 10.3402/jom.v8.31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akpata ES, Akinrimisi EO. Antibacterial activity of extracts from some African chewing sticks. Oral Surg Oral Med Oral Pathol. 1977;44:717–22. doi: 10.1016/0030-4220(77)90381-4. [DOI] [PubMed] [Google Scholar]
- 31.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010;76:1479–91. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 32.Mohammed NA. In vitro: Antimicrobial activity of leaves extracts of Eucalyptus spathulata against Streptococcus mutans and Candida albicans. J Al Rafidain Univ Coll. 2014;33:183–94. [Google Scholar]
- 33.du Toit R, Volsteedt Y, Apostolides Z. Comparison of the antioxidant content of fruits, vegetables and teas measured as vitamin C equivalents. Toxicology. 2001;166:63–9. doi: 10.1016/s0300-483x(01)00446-2. [DOI] [PubMed] [Google Scholar]
- 34.Mayur B, Sancheti S, Shruti S, Sung-Yum S. Antioxidant and glucosidase inhibitory properties of Carpesium abrotanoides L. J Med Plants Res. 2010;4:1547–53. [Google Scholar]
- 35.Rajkumar V, Guha G, Kumar RA, Lazar M. Evaluation of antioxidant activities of Bergenia ciliata rhizome. Rec Nat Prod. 2010;4:38. [Google Scholar]
- 36.Shah KA, Patel MB, Patel RJ, Parmar PK. Mangifera indica (mango) Pharmacogn Rev. 2010;4:42. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosa RT, Napimoga MH, Höfling JF, Gonçalves RB, Rosa EA. Clonal characterization of Streptococcus mutans strains by multilocus enzyme electrophoresis. Braz J Microbiol. 2006;37:17–9. [Google Scholar]


