Abstract
Background:
Addiction to ketamine is becoming a serious public health issues, for which there exists no effective treatment. Rhynchophylline (Rhy) is an alkaloid extracted from certain Uncaria species that is well known for both its potent anti-addictive and neuroprotective properties. Increasing evidence supports the contributions of cAMP response element binding protein (CREB), nuclear receptor-related-1 (Nurr1), and brain-derived neurotrophic factor (BDNF) in modulating neural and behavioral plasticity which was induced by addictive drugs.
Objective:
To investigate the effects of Rhy on the behavior and the levels of phosphorylated CREB (p-CREB), Nurr1, and BDNF in the hippocampus of ketamine-induced conditioned place preference (CPP) rats.
Materials and Methods:
CPP paradigm was used to establish the model of ketamine-dependent rats and to evaluate the effect of Rhy on ketamine dependence. The expressions of p-CREB, Nurr1, and BDNF were tested by Western blotting and immunohistochemistry.
Results:
We observed that Rhy can reverse the behavior preference induced by ketamine CPP training. At the same time, expression of p-CREB, Nurr1, and BDNF, which was significantly increased by ketamine, was restored in the Rhy -treated group.
Conclusion:
This study indicates that Rhy can reverse the reward effect induced by ketamine in rats and the mechanism can probably be related to regulate the hippocampal protein expression of p-CREB, Nurr1, and BDNF.
SUMMARY
P-CREB, Nurr1 and BDNF play an important role in the formation of ketamine-induced place preference in rats
Rhynchophylline reversed the expression of p-CREB, Nurr1 and BDNF which was activated by ketamine in the hippocampus
Rhynchophylline demonstrates the potential effect of mediates ketamine induced rewarding effect.
Abbreviations used: Rhy: Rhynchophylline; CREB: cAMP response element binding protein; Nurr1: Nuclear receptor-related-1; BDNF: Brain-derived neurotrophic factor; CPP: Conditioned place preference; NMDA: N-methyl-D-aspartic acid; METH: Methamphetamine; CNS: Central nervous system; PFA: Paraformaldehyde; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; LTP: long-term potentiation.
Keywords: Brain-derived neurotrophic factor, conditioned place preference, ketamine, nuclear receptor-related-1, phosphorylated cAMP response element binding protein, rhynchophylline
INTRODUCTION
Ketamine is a noncompetitive antagonist of N-methyl-D-aspartic acid (NMDA) glutamate receptor that was clinically employed for an effective anesthetic for half a century. Ketamine is also a “club drug” that has been widely abused in various places of entertainment.[1] Although potent anti-addictive properties have been reported in both experimental and clinical trials,[2,3] a great deal of evidence has shown that ketamine does indeed have a high potential for abuse and addiction. Rats will appear compulsive ketamine-seeking behavioral,[4] reverse natural behavioral preference,[5] and increase locomotor activity, following repetitive ketamine administration.[6] Clinical observation has shown that long-lasting and unconstrained ketamine injections in the treatment of depression can lead to drug addiction.[7] Ketamine abuse, a chronic and complex neurological disorder as other drug addiction, can affect protein translation in multiple regions of the brain,[8] leading to degenerative changes in the nervous system.[9]
At present, opioid receptor agonists, opioid receptor antagonists, and nonopioid receptor agonists are the three types of medications mainly used for anti-addiction therapy. Opioid receptor agonists including methadone can detoxify the addiction rapidly but have high chance of misuse so they have restriction in use.[10] Opioid antagonists successfully shorten the withdrawal process but fail to relieve withdrawal symptoms.[11] In addition, clonidine, a nonopioid receptor agonist commonly utilized to relieve symptoms from the addiction substance in the clinic, has been associated with obvious adverse reactions, including sedation and hypotension.[12] Moreover, none of the above medications is suggested to be therapeutic for addiction to newer, synthetic drugs such as ketamine. As no effective drug therapy currently exists that can alleviate ketamine addiction, more and more research aims to identify potential anti-addiction substances in plants.
Several constituents within herbs exhibit notable anti-addiction effects, but the mechanisms of these effects are poorly understood.[13] Rhynchophylline (Rhy) [Figure 1] is a predominant tetracyclic oxindole alkaloid derived from Uncaria rhynchophylla that is routinely prescribed to treat symptoms related to drug addiction.[14] Studies have shown that Rhy has various beneficial effects, being anti-addictive, anti-arrhythmic, anticonvulsant, anti-anxiety, and anti-hypertensive, as well as exhibiting sedative and neuroprotective properties in various models.[15,16,17,18] Rhy can alleviate methamphetamine (METH)-induced neurotoxicity in rat cortical neurons[19] and inhibit Ca2 + influx to prevent glutamate-induced neuronal death in vitro.[20] Thus, Rhy is widely used to treat central nervous system (CNS) disorders, including convulsions, numbness, and lightheadedness, as well as cardiovascular disease, such as hypertension. Rhy is also a noncompetitive antagonist of NMDA glutamate receptor[21] and our previous studies have demonstrated that Rhy can remarkably eliminate the rewarding effect and decrease the NR2B level in the brain of amphetamine-induced conditioned place preference (CPP) rats,[22] mice,[23] and zebrafish.[24] Furthermore, recent research has shown that the decreased levels of phosphorylated cAMP response element binding protein (p-CREB) and p-Fos in the brain are responsible for the therapeutic effects of Rhy on METH-induced CPP rats.[25] As Rhy can relieve amphetamines-associated addiction, we hypothesized that Rhy may be anti-addictive for other drugs, such as ketamine. We therefore established a ketamine-dependent model of rats using CPP paradigm and examined behavior effect of Rhy on behavioral preference rats and then further elucidate the possible molecular mechanisms by measuring levels of p-CREB, nuclear receptor related-1, (Nurr1), and brain-derived neurotrophic factor (BDNF) in the hippocampus. Our findings indicate that the abnormally increased levels of p-CREB, Nurr1, and BDNF in the hippocampus are associated with ketamine addiction and the effect of Rhy on counteracting the ketamine CPP behavior may be involved with restoring the level of p-CREB, Nurr1, and BDNF.
Figure 1.
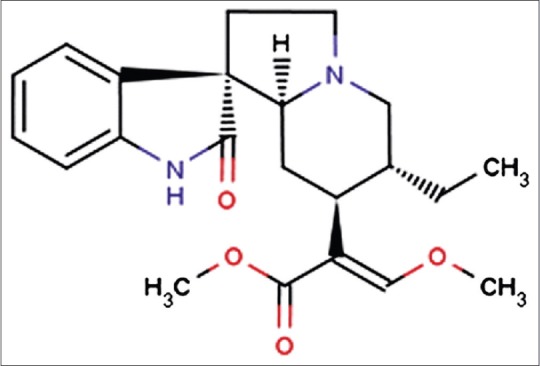
Molecular structure of rhynchophylline
MATERIALS AND METHODS
Animals
Sprague–Dawley rats, weighing 180–220 g, were bought from Southern Medical University Animals Center (Guangzhou, China) and adapted to the housing conditions with a 12-h light/dark cycle, stable temperature (22°C ± 1°C), controlled humidity (60% ± 5%), food and water ad libitum. Rats were handled 1 week before behavioral tests. All experiments were deferred to the Guidelines of National Institutes of Health and were approved by the Animal Care and Use Committee of the Southern Medical University.
Drug and treatment
Ketamine hydrochloride (Fujian Gutian Medicine Company, China) and Rhy (purity ≥98%; National Pharmaceutical Engineering Center, China) were dissolved in physiological saline, which will hereinafter be referred to saline.[25] All injections were administered intraperitoneally (i.p.) at a volume of 10 ml/kg.
Conditioned place preference
According to our published procedures,[26] we used CPP experiments as the biased procedure. Training took place in an apparatus consisting of two equal size chambers (30 cm × 30 cm × 30 cm) which were separated by a sliding door. One chamber is white and rough, and the other is black and smooth. On day 1, as an adaptive operation, rats were allowed to freely access the apparatus for 15 min. On day 2 and day 3, rats were pretested 15 min/day and recorded for the time spent in each compartment to determine an innate preference. Rats showing preference to the white compartment in the pretesting phase were excluded. From day 4 to day 7, rats assigned to ketamine CPP treatment were trained two daily conditioning sessions per day. In brief, rats were confined to the white compartment for 1 h after receiving ketamine (10 mg/kg) injection and confined to black compartment for 1 h after receiving equal volume of saline with an interval of 8 h. To evaluate the effect of Rhy on the expression of ketamine-induced CPP, rats assigned to ketamine CPP training were further divided into three groups randomly with eight rats per group. Each group was administered saline or Rhy (varied from 30 to 60 mg/kg) once a day, 12 h after the ketamine injection on days 5, 6, and 7. The dose and time points of drug administration were selected based on our previous work.[23,25] Rats assigned to saline CPP (as control) were treated the same as ketamine CPP, except that both sessions were paired with a saline injection. Twenty-four hours after the last conditioning session, rats were allowed to free access to the apparatus for 15 min in a drug-free state and measured the time spent in each compartment. The CPP score was determined by the change of activity time of rats in white compartment before and after the conditioning sessions.[27]
Immunohistochemistry and data analysis
After the CPP test, four rats per group were randomly assigned to immunohistochemistry test. Rats were deeply anesthetized using 3% sodium pentobarbital i.p (30 mg/kg) and perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4) intracardially. The brains were then removed and postfixed in 4% PFA immediately. After dehydration and paraffin embedding, the hippocampus was cut into 5-μm sections using a Leica RM2135 microtome. Immunohistochemistry was performed as described in product manual of streptavidin-biotin complex with peroxidase (Rabbit IgG) Kit (Boster, China). Antibodies for rabbit anti-p-CREB (1:150 dilution; Abcam, UK), rabbit anti-Nurr1 (1:50 dilution; Santa Cruz, USA), and rabbit anti-BDNF (1:150 dilution; Abcam, UK) were used to determine the levels of p-CREB, Nurr1, and BDNF. Five slices per rat containing hippocampus were selected, and then, three horizons of each slice were randomly observed in the light microscope (×40). Positive expression of p-CREB, Nurr1, and BDNF was defined as appearance of brown particles within the cell. We used Image-pro plus software (IPP 6.0, Media Cybernetics Inc., Silver Spring, Maryland, USA) to measure positive cells’ integrated optical density, and the relative content for p-CREB, Nurr1, and BDNF was represented by the mean value of each group.
Western blotting and data analysis
After the CPP test, four rats per group were randomly assigned to Western blot test. Rats were decapitated and the hippocampus was isolated by gross dissection. To detect CREB, p-CREB, Nurr1, and BDNF, extracts were prepared from individual rats using RIPA lysis buffer (Beyotime, China) with protease inhibitors and phosphatase inhibitors. Homogenized tissue was sonicated and centrifuged at 14,000 g for 15 min at 4°C. The supernatants were collected and employed for protein determination using the bicinchoninic acid method. The protein samples were mixed with 2× loading buffer and denatured at 95°C for 5 min. Samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis before electrophoretic transfer onto a polyvinylidene difluoride membrane (Millipore, USA). Primary antibodies for rabbit anti-CREB (1:1000; Abcam, UK), rabbit anti-p-CREB (1:1000; Abcam, UK), rabbit anti-Nurr1 (1:200; Santa Cruz), and rabbit anti-BDNF (1:1000; Abcam, UK) and horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:8000; Millipore, USA) were used to detect the expression of CREB, p-CREB, Nurr1, and BDNF and then visualized by enhanced chemiluminescent detection (Millipore, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (housekeeping gene) expression is shown as a loading control using rabbit anti-GAPDH antibody (1:3000; CWBIO, China). The results normalized for each membrane were expressed as percentages of saline-treated controls. Relative band intensities were quantified by Gel-Pro Analyzer (Media Cybernetics, USA).
Statistical analysis
Data are summarized as the mean ± standard error of mean. Statistical analysis was performed with SPSS software (version 20.0 for Windows, Chicago, IL, USA). All data were analyzed using one-way analysis of variance followed by a post hoc test (two-tailed) with Bonferroni correction when equal variances assumed or with Tamhane's T2 when not assumed. We considered differences significant at P < 0.05.
RESULTS
Rhynchophylline reversed the behavioral responses to ketamine
Given that Rhy is an NMDA receptor which can counteract to amphetamine- and METH-induced place preference,[22,25] here, we determined whether Rhy can reverse the behavioral preference induced by ketamine. As CPP is one of the most popular experiments to assess the reward effects of drugs,[28] we successfully established a ketamine addiction model of rats by four consecutive ketamine CPP training using a dose of 10 mg/kg. Compared with the control group, ketamine significantly increased the time difference in white compartments between post- and pre-ketamine CPP training (P < 0.01), as shown in Figure 2. Two different doses of Rhy were applied to testify the effect on ketamine addiction and find out which dose would be better. Compared with ketamine CPP group, low-dose Rhy (30 mg/kg) administration reduced the time difference induced by ketamine (P < 0.05), while the high dose of Rhy (60 mg/kg) reduced the time difference even more significantly (P < 0.01) [Figure 2].
Figure 2.
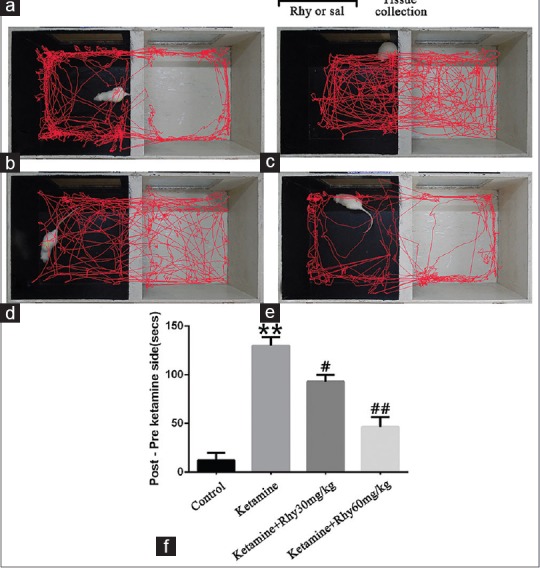
Rhynchophylline prevents ketamine-induced conditioned place preference. (a) The schematic of experimental design for conditioned place preference testing. (b-e) Representative running trajectory of rats in the conditioned place preference compartments recorded and analyzed with the Noldus Ethovision XT 8.5 software; b-e represent the control conditioned place preference group, ketamine conditioned place preference group, ketamine with 30 mg/kg rhynchophylline group and ketamine with 60 mg/kg rhynchophylline group, respectively. (f) Time difference between post ketamine training and pre-ketamine training. Data are expressed as mean values ± standard error of the mean for 8 rats per group. **P < 0.01 versus the control conditioned place preference group;#P < 0.05,##P < 0.01 versus the ketamine conditioned place preference group via Bonferroni post hoc analysis after one-way analysis of variance
Rhynchophylline regulated the levels of phosphorylated cAMP response element binding protein, nuclear receptor-related-1, and brain-derived neurotrophic factor to relieve the ketamine-dependent behavior
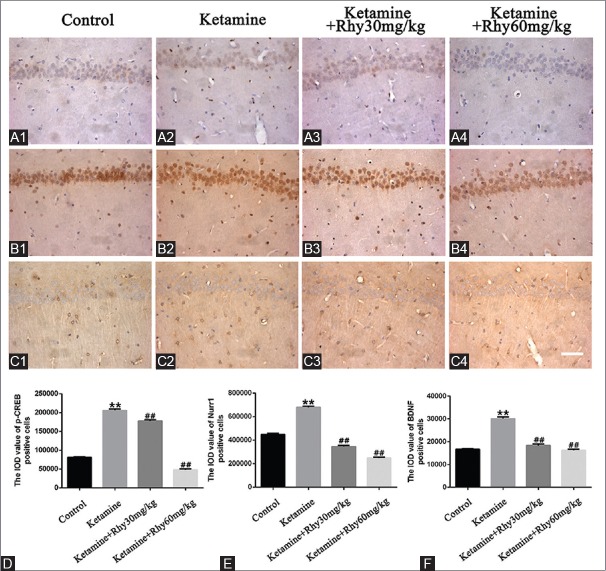
To find out the possible molecular mechanism involved the behavioral changes by ketamine and Rhy, first, we used immunohistochemistry to detect the levels of Nurr1 and BDNF and the active state of CREB (p-CREB) in the hippocampus of CPP rats. As shown in Figure 3, the levels of Nurr1, BDNF, and p-CREB, which were significantly higher in ketamine CPP group than that of the control group (P < 0.01), were significantly downregulated after Rhy administration (P < 0.01).
Figure 3.
Expression of p-CREB, Nurr1 and BDNF in the hippocampus of rats detected by immunohistochemistry. p-CREB (A1-A4), Nurr1 (B1-B4) and BDNF (C1-C4) were detected using immunohistochemistry. Photomicrographs are representative of results from multiple brain sections obtained from four animals in each treatment group. Quantitative analysis is shown in (D-F). Scale bar: 50 μm. Data are expressed as mean values ± standard error of mean. **P < 0.01 versus the control group;##P < 0.01 versus the ketamine conditioned place preference group via Bonferroni post hoc analysis after one-way analysis of variance. p-CREB: Phosphorylated cAMP response element binding protein, Nurr1: Nuclear receptor-related-1, BDNF: Brain-derived neurotrophic factor
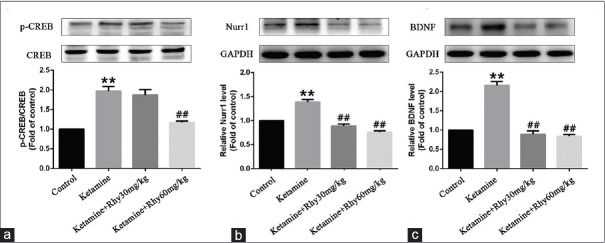
In addition to immunohistochemistry, the expression of BDNF, Nurr1, and p-CREB in the hippocampus rats was also assessed using Western blot analysis. In line with immunohistochemical results, ketamine significantly upregulated the expression of BDNF, Nurr1, and p-CREB (P < 0.01). Both 30 mg/kg Rhy and 60 mg/kg Rhy significantly reduced BDNF and Nurr1 levels (P < 0.01) in the hippocampus of ketamine-induced CPP rats, but only 60 mg/kg Rhy treatment reversed the level of p-CREB significantly (P < 0.01) [Figure 4].
Figure 4.
Expression of p-CREB, Nurr1 and BDNF in the hippocampus of rats detected by Western blotting (a-c). p-CREB was normalized to total CREB, Nurr1, and BDNF were normalized to glyceraldehyde-3-phosphate dehydrogenase. Data are shown as mean values ± standard error of mean for 4 rats per group. **P < 0.01 versusthe control group; ##P < 0.01 versus the ketamine group via Bonferroni posthoc analysis after one-way analysis of variance. p-CREB: Phosphorylated cAMP response element binding protein, Nurr1: Nuclear receptor-related-1, BDNF: Brain-derived neurotrophic factor
DISCUSSION
There are three neural circuits involved in motivation and reward: mesocortic pathway, mesolimbic pathway, and nigrostriatal pathway.[29] Drugs of abuse “hijack” these natural reward circuitries, leading to abnormal craving and compulsive drug-seeking behavior. The fact that drug-associated stimuli can trigger relapse even if abstinence is achieved suggests that it may be an aberrant form of learning and memory.[30] A common mechanism in drug addiction such as cocaine which is related to long-term potentiation (LTP). More and more proof indicates that the reason of addiction is the stubborn persistence of addiction memory.[31] Hence, we decided to focus our investigation on the hippocampus which is the main region to engage learning and plays a vital part in processing drug-related memory.[32] Rats after ketamine CPP training established connection between drug and the context of white compartment, spent more time in white compartment, indicating drug-associated memory triggered the rats’ craving behavior.
Drug addiction is a complicated brain disease that results in large-scale protein and/or gene expression changes, making addictive behavior extraordinarily steady and enduring.[33] As a cellular transcription factor, CREB is central to learning and memory storage.[34] p-CREB transcribes a host of genes and activates a cascade of events which cause tolerance and dependence adaptations.[35] One of the CREB's downstream targets is Nurr1, an immediate-early gene which might be associated with learning and memory processes.[36] It has been shown that Nurr1 is implicated in the pathogenesis of neuropsychiatric disorders, such as drug dependence, schizophrenia, and attention deficit hyperactivity disorder.[37,38,39,40] Another downstream target of CREB is BDNF which is distributed throughout the entire CNS and expressed strongest in the hippocampus.[41] It is considered to be a psychostimulant-regulated immediate early gene that involves in regulating synaptic plasticity and an important protein highly related to drug addiction.[42,43] As drug addiction is considered as a maladaptive learning and memory and the critical molecules include CREB, Nurr1, and BDNF, we focused on CREB, Nurr1, and BDNF as ideal candidates for studying the mechanisms of ketamine addiction and the role of Rhy in ketamine deaddiction. Our results showed that ketamine reversed the rats’ natural preference of black and upregulated p-CREB, Nurr1, and BDNF in the hippocampus. Studies have shown that the increased p-CREB may enhance BDNF transcription, leading to increased neuroplasticity and addiction.[44] Besides, BDNF can facilitate the autophosphorylation of tyrosine protein kinase B, leading to activation of CREB.[45] Therefore, the mutual regulation between CREB and BDNF may be involved in the mechanism of ketamine addiction. In this study, we observed that Rhy counteracts the CPP behavior and downregulates the levels of p-CREB, Nurr1, and BDNF, which were elevated by ketamine. The findings of this study indicate a critical role for Rhy in inhibiting ketamine dependence and the mechanism probably be related to regulate the hippocampal protein expression of p-CREB, Nurr1, and BDNF.
NMDA receptor, which can mediate synaptic plasticity processes such as LTP and long-term depression, is crucial in learning and memory.[46] Evidence supports that NMDA receptor inhibition prevents tolerance, sensitization, and seeking behavior to cocaine[47,48] and reduces locomotor activities induced by opiates such as cocaine and heroin.[49] It is quite intriguing that, on the one hand, ketamine can inhibit NMDA receptor to disrupt cocaine self-administration,[50] and, on the other hand, it is highly addictive. Studies indicate that CREB and BDNF are downstream targets of NMDA receptor[51,52] and Nurr1 is a key regulator of NMDA receptor to modulate neuronal survival.[28] The expression of p-CREB, BDNF, and Nurr1 elevated by ketamine indicates that there must be another pathway to strongly trigger these three molecules even if ketamine can counteract with NMDA receptor to downregulate them. As an NMDA receptor antagonist, Rhy may reverse the rewarding effect of ketamine by blocking NMDA receptor and the cascade signaling, downregulating the p-CREB, BDNF, and Nurr1.
CONCLUSION
The present results underscore the effect of Rhy in the inhibition of drug addiction induced by ketamine and link this action with reduced levels of p-CREB, Nurr1 and BDNF in the hippocampus. Although these results broaden our understanding of Rhy on ketamine addiction, it is still not intact. The exact mechanisms of ketamine addiction and Rhy anti-addiction are needed to illuminate in the future research.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (No. 81229003, 81673628); the Guangzhou Major Science and Technology Project (No. 20Foundation of Guangdong Province, China (No. 2014A030310251).1300000050); and the Natural Science.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Morgan CJ, Curran HV, Independent Scientific Committee on Drugs Ketamine use: A review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 2.Krupitsky EM, Grinenko AY. Ketamine Psychedelic Therapy (KPT): A review of the results of ten years of research. J Psychoactive Drugs. 1997;29:165–83. doi: 10.1080/02791072.1997.10400185. [DOI] [PubMed] [Google Scholar]
- 3.Inturrisi EC. Preclinical evidence for a role of glutamatergic systems in opioid tolerance and dependence. Sem Neurosci. 1997;9:110–9. [Google Scholar]
- 4.Winger G, Hursh SR, Casey KL, Woods JH. Relative reinforcing strength of three N-methyl-D-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther. 2002;301:690–7. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Kato H, Aoki T, Tsuda M, Narita M, Misawa M, et al. Effects of the non-competitive NMDA receptor antagonist ketamine on morphine-induced place preference in mice. Life Sci. 2000;67:383–9. doi: 10.1016/s0024-3205(00)00639-1. [DOI] [PubMed] [Google Scholar]
- 6.Trujillo KA, Zamora JJ, Warmoth KP. Increased response to ketamine following treatment at long intervals: Implications for intermittent use. Biol Psychiatry. 2008;63:178–83. doi: 10.1016/j.biopsych.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet U. Long-term ketamine self-injections in major depressive disorder: Focus on tolerance in ketamine's antidepressant response and the development of ketamine addiction. J Psychoactive Drugs. 2015;47:276–85. doi: 10.1080/02791072.2015.1072653. [DOI] [PubMed] [Google Scholar]
- 8.Tedesco V, Ravagnani C, Bertoglio D, Chiamulera C. Acute ketamine-induced neuroplasticity: Ribosomal protein S6 phosphorylation expression in drug addiction-related rat brain areas. Neuroreport. 2013;24:388–93. doi: 10.1097/WNR.0b013e32836131ad. [DOI] [PubMed] [Google Scholar]
- 9.Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, et al. Frontal white matter abnormalities following chronic ketamine use: A diffusion tensor imaging study. Brain. 2010;133:2115–22. doi: 10.1093/brain/awq131. [DOI] [PubMed] [Google Scholar]
- 10.Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. A review of opioid dependence treatment: Pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2010;30:155–66. doi: 10.1016/j.cpr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Gowing L, Ali R, White JM. Opioid antagonists under heavy sedation or anaesthesia for opioid withdrawal. Cochrane Database Syst Rev. 2010;(1):CD002022. doi: 10.1002/14651858.CD002022.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;(5):CD002024. doi: 10.1002/14651858.CD002024.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abenavoli L, Bardazzi G, Cracolici F, Quaranta C, Santini G, Graziosi S, et al. Complementary therapies for treating alcoholism First Annual Meeting by Complementary Medicine Research Group of the Italian Society for Alcohol Studies-May 5, 2006, Florence, Italy. Fitoterapia. 2008;79:142–7. doi: 10.1016/j.fitote.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Shi JS, Yu JX, Chen XP, Xu RX. Pharmacological actions of Uncaria alkaloids, rhynchophylline and isorhynchophylline. Acta Pharmacol Sin. 2003;24:97–101. [PubMed] [Google Scholar]
- 15.Amat M, Ramos C, Pérez M, Molins E, Florindo P, Santos MM, et al. Enantioselective formal synthesis of ent-rhynchophylline and ent-isorhynchophylline. Chem Commun (Camb) 2013;49:1954–6. doi: 10.1039/c2cc38540f. [DOI] [PubMed] [Google Scholar]
- 16.Cao W, Wang Y, Lv X, Yu X, Li X, Li H, et al. Rhynchophylline prevents cardiac dysfunction and improves survival in lipopolysaccharide-challenged mice via suppressing macrophage I-κBα phosphorylation. Int Immunopharmacol. 2012;14:243–51. doi: 10.1016/j.intimp.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhou JY, Chen J, Zhou SW, Mo ZX. Individual and combined effects of rhynchophylline and ketamine on proliferation, NMDAR1 and GluA2/3 protein expression in PC12 cells. Fitoterapia. 2013;85:125–9. doi: 10.1016/j.fitote.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Zhou JY, Zhou SW. Isorhynchophylline: A plant alkaloid with therapeutic potential for cardiovascular and central nervous system diseases. Fitoterapia. 2012;83:617–26. doi: 10.1016/j.fitote.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Mo ZX, Xu DD, Yung KK. Effects of rhynchophylline on rat cortical neurons stressed by methamphetamine. Pharmacologyonline. 2006;3:856–61. [Google Scholar]
- 20.Shimada Y, Goto H, Itoh T, Sakakibara I, Kubo M, Sasaki H, et al. Evaluation of the protective effects of alkaloids isolated from the hooks and stems of Uncaria sinensis on glutamate-induced neuronal death in cultured cerebellar granule cells from rats. J Pharm Pharmacol. 1999;51:715–22. doi: 10.1211/0022357991772853. [DOI] [PubMed] [Google Scholar]
- 21.Kang TH, Murakami Y, Matsumoto K, Takayama H, Kitajima M, Aimi N, et al. Rhynchophylline and isorhynchophylline inhibit NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 2002;455:27–34. doi: 10.1016/s0014-2999(02)02581-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhou JY, Mo ZX, Zhou SW. Rhynchophylline down-regulates NR2B expression in cortex and hippocampal CA1 area of amphetamine-induced conditioned place preference rat. Arch Pharm Res. 2010;33:557–65. doi: 10.1007/s12272-010-0410-3. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Liu W, Peng Q, Jiang M, Luo C, Guo Y, et al. Effect of rhynchophylline on conditioned place preference on expression of NR2B in methamphetamine-dependent mice. Biochem Biophys Res Commun. 2014;452:695–700. doi: 10.1016/j.bbrc.2014.08.127. [DOI] [PubMed] [Google Scholar]
- 24.Jiang M, Chen Y, Li C, Peng Q, Fang M, Liu W, et al. Inhibiting effects of rhynchophylline on zebrafish methamphetamine dependence are associated with amelioration of neurotransmitters content and down-regulation of TH and NR2B expression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;68:31–43. doi: 10.1016/j.pnpbp.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Peng QX, Lin XL, Luo CH, Jiang MJ, Mo ZX, et al. Effect of rhynchophylline on the expression of p-CREB and sc-Fos in triatum and hippocampal CA1 area of methamphetamine-induced conditioned place preference rats. Fitoterapia. 2014;92:16–22. doi: 10.1016/j.fitote.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhou JY, Mo ZX, Zhou SW. Effect of rhynchophylline on central neurotransmitter levels in amphetamine-induced conditioned place preference rat brain. Fitoterapia. 2010;81:844–8. doi: 10.1016/j.fitote.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–12. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barneda-Zahonero B, Servitja JM, Badiola N, Miñano-Molina AJ, Fadó R, Saura CA, et al. Nurr1 protein is required for N-methyl-D-aspartic acid (NMDA) receptor-mediated neuronal survival. J Biol Chem. 2012;287:11351–62. doi: 10.1074/jbc.M111.272427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arias-Carrión O, Stamelou M, Murillo-Rodríguez E, Menéndez-González M, Pöppel E. Dopaminergic reward system: A short integrative review. Int Arch Med. 2010;3:24. doi: 10.1186/1755-7682-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf ME. Addiction: Making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–57. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- 31.Nestler EJ. Neurobiology. Total recall-the memory of addiction. Science. 2001;292:2266–7. doi: 10.1126/science.1063024. [DOI] [PubMed] [Google Scholar]
- 32.Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7:e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyman SE. Addiction: A disease of learning and memory. Am J Psychiatry. 2005;162:1414–22. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 34.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–86. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Peña de Ortiz S, Maldonado-Vlaar CS, Carrasquillo Y. Hippocampal expression of the orphan nuclear receptor gene hzf-3/nurr1 during spatial discrimination learning. Neurobiol Learn Mem. 2000;74:161–78. doi: 10.1006/nlme.1999.3952. [DOI] [PubMed] [Google Scholar]
- 37.Smith KM, Bauer L, Fischer M, Barkley R, Navia BA. Identification and characterization of human NR4A2 polymorphisms in attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:57–63. doi: 10.1002/ajmg.b.30127. [DOI] [PubMed] [Google Scholar]
- 38.Cheng MC, Hsu SH, Chen CH. Chronic methamphetamine treatment reduces the expression of synaptic plasticity genes and changes their DNA methylation status in the mouse brain. Brain Res. 2015;1629:126–34. doi: 10.1016/j.brainres.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Mizuo K, Okazaki S. Acute ethanol administration increases Mir-124 expression via histone acetylation in the brain. J Alcohol Drug Depend. 2016;4:2. [Google Scholar]
- 40.Xing G, Zhang L, Russell S, Post R. Reduction of dopamine-related transcription factors Nurr1 and NGFI-B in the prefrontal cortex in schizophrenia and bipolar disorders. Schizophr Res. 2006;84:36–56. doi: 10.1016/j.schres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, et al. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–9. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 42.Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–58. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu K, Lipsky RH. Repeated ketamine administration alters N-methyl-D-aspartic acid receptor subunit gene expression: Implication of genetic vulnerability for ketamine abuse and ketamine psychosis in humans. Exp Biol Med (Maywood) 2015;240:145–55. doi: 10.1177/1535370214549531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Covington HE, 3rd, Maze I, Sun H, Bomze HM, DeMaio KD, Wu EY, et al. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron. 2011;71:656–70. doi: 10.1016/j.neuron.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–8. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bäckström P, Hyytiä P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–80. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- 48.De Montis MG, Devoto P, Meloni D, Gambarana C, Giorgi G, Tagliamonte A, et al. NMDA receptor inhibition prevents tolerance to cocaine. Pharmacol Biochem Behav. 1992;42:179–82. doi: 10.1016/0091-3057(92)90463-p. [DOI] [PubMed] [Google Scholar]
- 49.Pulvirenti L, Swerdlow NR, Koob GF. Nucleus accumbens NMDA antagonist decreases locomotor activity produced by cocaine, heroin or accumbens dopamine, but not caffeine. Pharmacol Biochem Behav. 1991;40:841–5. doi: 10.1016/0091-3057(91)90095-j. [DOI] [PubMed] [Google Scholar]
- 50.Dakwar E, Hart CL, Levin FR, Nunes EV, Foltin RW. Cocaine self-administration disrupted by the N-methyl-D-aspartate receptor antagonist ketamine: A randomized, crossover trial. Mol Psychiatry. 2017;22:76–81. doi: 10.1038/mp.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park H, Popescu A, Poo MM. Essential role of presynaptic NMDA receptors in activity-dependent BDNF secretion and corticostriatal LTP. Neuron. 2014;84:1009–22. doi: 10.1016/j.neuron.2014.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;20:3529–36. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]