Abstract
Background:
Tripterygium wilfordii (TW) is widely employed to treat rheumatoid arthritis and autoimmune disorders clinically, which, however, accompany with disturbing hepatotoxicity and nephrotoxicity. The previous research showed that Panax notoginseng (PN) compatibly and significantly reduces the TW-induced hepatotoxicity.
Objective:
To explore the underlying mechanism, the present study was designed to reveal the influence of PN on the intestinal absorption process of TW-derived active components in rat.
Materials and Methods:
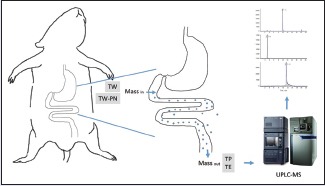
An in situ single-pass intestinal perfusion technique was established and preformed to obtain the perfusate samples of triptolide (TP), tripterine (TE), TW extract, and TW-PN extract. A rapid and sensitive ultra-performance liquid-chromatography tandem mass spectrometry method was subsequently developed and validated to determine the concentrations of TP and TE in the perfusate samples. Then, the absorption parameters, effective permeability, absorption rate constant, and percentage of 10 cm intestinal absorption were calculated strictly.
Results:
The final data indicated that both TP and TE have no special absorption site in the intestine and are primarily absorbed in a passive manner. Otherwise, the absorption of TP was decreased from compatibility of PN, but the absorption of TE was enhanced.
Conclusion:
The absorption reduction of TP and absorption elevation of TE from TW initiated by the combination of PN are contributed to attenuate the toxicity and reinforce the therapeutic efficacy of TW. It is practically reasonable of usage of TW compatibility with PN clinically.
SUMMARY
Panax notoginseng (PN) regulated the absorption process of Tripterygium wilfordii (TW) in intestine
Both triptolide (TP) and tripterine (TE), two typical components of TW, have no special absorption site in the intestine and are primarily absorbed in a passive manner
PN decreased the absorption of TP and enhanced the absorption of TE in the intestine.
Abbreviations used: 10 cm% ABS: percentage of 10 cm intestinal absorption, DMARDs: Disease-modifying antirheumatic drugs, GU: Glycyrrhiza uralensis, Ka: Absorption rate constant, NSAIDs: Nonsteroidal anti-inflammatory drugs, Peff: Effective permeability, PN: Panax notoginseng, QC: Quality control, RA: Rheumatoid arthritis, RG: Rehmannia glutinosa, SPIP: Single-pass intestinal perfusion, TE: Tripterine, TP: Triptolide, TW: Tripterygium wilfordii, UPLC-MS/MS: Ultra-performance liquid-chromatography tandem mass spectrometry.
Keywords: Rhizoma notoginseng, single-pass intestinal perfusion, tripterine, Tripterygium wilfordii, triptolide, ultra-performance liquid-chromatography tandem mass spectrometry
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease which commonly leads to cartilage and bone damage as well as disability. The causes of RA still remain unknown, but the combination of genetic, environmental, and host factors may be involved in.[1,2,3] Currently, clinic usually use nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, and traditional Chinese medicine as the regular and primary candidates for the treatment of RA. However, the overall therapeutic effect has been far from satisfaction due to the side effects of these chemicals and herbs. Because of the failure to ameliorate the long-term course of disease and adversely gastrointestinal and cardiac effects, NSAIDs are no longer the first-line drugs for the treatment of RA.[4,5,6] DMARDs are now the mainstay of therapeutic approach to RA, but the adverse effects of DMARDs cannot be ignored such as nausea, hepatotoxicity, blood dyscrasias, and interstitial lung disease.[7,8] Wilfordii glycosides tablets, one type of traditional Chinese medicine that used clinically for the treatment of RA, also is limited to its clinical application due to the narrow therapeutic window and serious toxicity on several organs such as liver and kidney.[9,10,11,12,13]
Qingluo Tongbi decoction is an effective multiherb formula for RA. In our previous studies, it has been demonstrated to be definitely positive and exhibited inconspicuous side effects clinically for several decades.[14,15,16,17] The basic constitute of Qingluo Tongbi decoction are Tripterygium wilfordii (TW), Panax notoginseng (PN), Caulis Sinomenii, Rehmannia glutinosa (RG), and Bombyx batryticatus in a proportion of 15:3:15:15:10. Based on the long-term clinic application, a novel-reduced formula was developed with TW, PN, and RG in a ratio of 20:6:25. Combining with PN and TW, the sovereign medicinal responsible for the curative actions of Qingluo Tongbi decoction on RA has showed obviously lowered hepatotoxicity. However, to the best of our knowledge, the mechanisms involved in the synergistic effect of herbs in Qingluo Tongbi were still vague. Meanwhile, triptolide (TP) together with other components have been certified to be the reason of the clinical efficacy of TW, and the degree of toxicity is closely depending on the pharmacokinetic characteristics of TP in vivo.[18,19,20,21,22,23,24,25] Under the guidance of traditional Chinese medicine remedy system, compatibility with various medicinal is an essential principal for clinicians according to the patient's conditions. The main aim of compatibility of Chinese medicine is to strengthen the principal curative action and counteract the toxicity or side effects. Therefore, it is very interesting to elucidate the medicinal interaction within Chinese medicine formula.
Nowadays, more and more attentions had been paid to promote the efficacy of TW on RA and diminish the followed toxicity. Ma et al.[26] analyzed the efficacy of TW combining with Glycyrrhiza uralensis (GU) through comparison of the pharmacokinetics and toxic properties between TW and TW-GU, which provided intriguing result that the combination of GU can significantly enhance the effect of anti-inflammatory and analgesic of TW. Another report from Liu et al.[27] indicated that GU can accelerate the metabolism and excretion of TP, and can reduce the tissue distribution concentration and contributes to decrease the toxicity of TP. Liu et al.[28,29] further studied the toxicity-reducing effect on TP combined with Pteris multifida Poir and found that Pteris multifida Poir reduces the toxicity of TP and maintains its immune suppression, antiinflammatory, and analgesic activities. The interesting investigation from Qin et al.[30] also demonstrated the curative effect of tripterygium glycosides on adjuvant-induced arthritis in rats and its adverse action on liver and kidney function compatible with astragalosides, which indicated that astragalosides enhance the efficacy and decrease the toxicity of tripterygium glycosides.
According to these studies on enhancing efficacy and decreasing toxicity of TW, it is important to explore the underlying rule of decreasing toxicity through the usage compatibility. Our previous study has showed that PN actually decreases the toxicity of TW.[16] Therefore, we need further study to clarify the potential mechanism of decreasing toxicity. TW contains several natural active components mainly including diterpenoid, triterpenoid, and alkaloid.[31,32,33,34,35] TP and tripterine (TE) considered to be the primarily effective components in the treatment of RA and attracted considerable attention. In the present investigation, both TP and TE were chosen to be the index components [Figure 1]. In our previous study, we determined the plasma concentration-time profiles and the distribution characteristics in liver, heart, spleen, and lung and kidney tissues of TP in rats after oral administration of the aqueous extract of TW and TW-PN and the result showed that PN could decrease the toxicity of TW. However, TE cannot be determined in the plasma of rats. To clarify the change of TP and TE after TW compatibility with PN, we use single-pass intestinal perfusion (SPIP) method to study the intestinal absorption characteristics of TP and TE. It is the first time to establish an effective method to monitor two index components derived from multiherb remedies simultaneously in rat, and it will be helpful to understand the interaction of TW and PN in vivo in the current study. This study was mainly concentrated on: (1) investigating the intestinal absorption of TP and TE by using SPIP method performed in rats; (2) establishing an ultra-performance liquid-chromatography tandem mass spectrometry (UPLC-MS/MS) method to determine the content of TP and TE in perfusate before and after SPIP operation and calculating the absorption parameters of TP and TE such as effective permeability (Peff), absorption rate constant (Ka), and percentage of 10 cm intestinal absorption (10 cm%ABS); and (3) comparing the absorption characteristics of TP and TE in intestine to clarify the toxicity-decreasing mechanism of compatibility of TW-PN in a ratio of 20:6. Furthermore, this study is also going to provide more biopharmaceutical basis of the design of clinic application of Qingluo Tongbi decoction and TW in inflammatory and autoimmune diseases.
Figure 1.
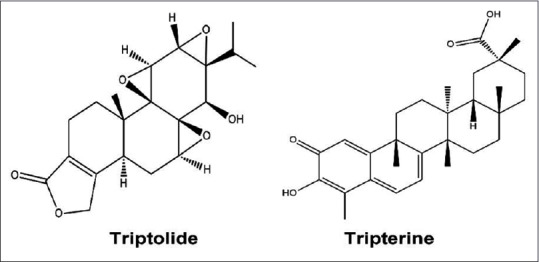
Chemical structure of triptolide and tripterine
MATERIALS AND METHODS
Chemicals and reagents
TP (purity: 98%, batch number: 111567-200505) and fenofibrate (purity: 98%, batch number: 100733-200401) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). TE (purity: 98%, batch number: ZL150520) was from Nanjing Zelang Medical Technology Co., Ltd.(Nanjing, China). TW was purchased from Xichang Materials Company of Sichuan (batch number: 120620), while PN was purchased from Bozhou Medicine Company of Anhui (batch number: 111208), and they were kindly authenticated by Dr. Qinan Wu, Professor of Pharmacognosy (College of Pharmacy, Nanjing University of Chinese Medicine, Nanjing, China). Normal saline was purchased from Nanjing Bianzheng Medical Technology Co., Ltd.(Nanjing, China). Acetonitrile and methanol used for UPLC were of chromatographic grade (Merck, Darmstadt, Germany). Ethyl acetate was of analytical grade (Nanjing Chemical Reagent Co., Ltd., Nanjing, China). Mill-Q water (Millipore, Bedford, MA, USA) was used throughout the study.
Apparatus
These primary apparatuses adopted in the present study were BT100-1 L constant flow peristalsis pump (Longer Pump, UK), AB SCIEX Triple Quad™ 4500 liquid chromatography tandem mass spectrometry (AB SCIEX, USA), electrospray ion source, Shimadzu LC-30A UPLC system (Shimadzu, Japan), MS105 electronic balance (Mettler-Toledo, Swiss), Ultra-pure water system (Millipore, USA), Multifuge X1R refrigerated centrifuge (Thermo, USA), Votex Genius 3 vortex mixing equipment (IKA, Germany), FE20 PH meter (Mettler-Toledo, Swiss), and CentriVap® centrifugal concentrator (LABCONCO, USA).
Preparation of perfusate
The stock solution of TP (150 μg/mL), TE (60 μg/mL), and internal standard fenofibrate (50 μg/mL) were prepared by dissolving an accurately weighed amount of the chemicals in methanol, respectively, and then stored at 4°C until use.
The procedure involving smashing the herb materials of TW and extracting with boiling water (1:11, w/v) for 1.5 h was repeated with another boiling water (1:7, w/v) for 1 h. The extract of TW was filtered with gauze and then merged. Evaporate the extract to about 300 mL by rotary evaporation under vacuum at 65°C. The concentrated solution of TW extract was further diluted in Hank's balanced salt solution (HBSS) solution (pH = 7.4) to produce three kinds of intestinal perfusate solution as TW-H, TW-M, and TW-L. The corresponding concentrations of TP in intestinal perfusate solution were 32, 16, and 8 μg/mL, and the concentrations of TE were 10, 5, and 2.5 μg/mL. A total of 1560 g mixed pieces of TW (1200 g) and PN (360 g) were extracted through the same process mentioned above. The obtained solution was further diluted in HBSS solution (pH = 7.4) to produce three kinds of intestinal perfusate solution as TW-PN-H, TW-PN-M, and TW-PN-L. The corresponding concentrations of TP in each intestinal perfusate solution were 24, 12, and 6 μg/mL, and the concentrations of TE were 2, 1, and 0.5 μg/mL. All the intestinal perfusate solution was stored at 4°C until use.
Animal experiment and samples collection
Male Sprague-Dawley rats (250 ± 15 g) supplied by Nanjing University of Chinese Medicine Animal Center were used. These animals were maintained with humidity of 55% ±5%, temperature of 24°C ± 2°C, and 12 h light/dark cycle for 1 week before the experiment. Animal welfare and experimental procedures were strictly in accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council, 1996). The study protocol and the total number of rat were approved by the Animal Care and Use Committee of Nanjing University of Chinese Medicine. Forty-eight rats were randomized into eight groups (TP, TE, TW-H, TW-M, TW-L, TW-PN-H, TW-PN-M, TW-PN-L, n = 6 for each group).
Rats fasted for 16 h with free access to water before the perfusion study were anesthetized with 10% chloral hydrate solution (3.4 mL/kg, i. p.). A laparotomy was made through a midline incision of about 4 cm to separate the duodenum, jejunum, ileum, and colon from the abdominal cavity, and approximately 10 cm of the four intestinal segments were exposed. Both ends of each measured intestinal segments were cannulated with silicone tubes. First, the intestinal lumen was cleaned by normal saline (37°C) perfusion through the inlet until the effluent from the outlet was judged to be free of feces and clear. The perfusion solution was pumped by peristaltic pump through the intestine at a flow rate of 1.0 mL/min. Following that, the perfusion solution to the intestinal lumen was changed into a constant flow rate of 0.2 mL/min. After 30 min, effluent solution was collected from the outlets of the four intestinal segments during each 15-min period (30–45, 45–60, 65–80, 80–95, 95–110 min) into preweighted vials. Then, the weight of each perfusion solution was analyzed. During the perfusion operation, these exposed intestines were covered with a gauze that had been moistened by frequent applications of warm (37°C) normal saline and kept warm by a small lamp placed over the area. At the end of the sampling, animals were euthanized with saturated potassium chloride solution by intracardiac injection, according to protocols of euthanasia in experimental animals. After death, the four intestinal segments were removed for measurements of length and radius (l and r, respectively). Duodenum segment was measured from 1 cm below the pylorus, jejunum segment was measured from 15 cm below the pylorus, ileum segment was measured from 20 cm above the cecum, and colon segment was measured from 1 cm below the cecum.
Sample preparation
The intestinal perfusion solution samples for UPLC-MS/MS analysis were prepared as follows. The internal standard fenofibrate (20 μL, 0.4 μg/mL) was added to intestinal perfusion solution samples (1.0 mL) in a 10.0 mL centrifuge tube. After vibrating with 4 mL ethyl acetate for 3 min, the intestinal perfusion solution samples were centrifuged at 3000 rpm for 10 min, and the supernatants were transferred into a new 10.0 mL tube. Then, another 4 ml ethyl acetate was added to the residue followed by vibrating for 3 min and centrifuged at 3000 rpm for 10 min. The two supernatants were merged and evaporated to dryness by the centrifugal concentrator at 40°C. The residue was dissolved by 1.0 mL acetonitrile followed by vibrating for 3 min and centrifuging at 12000 rpm for 10 min. At last, 100 μL of the supernatant was injected into the UPLC-MS/MS system for analysis.
Ultra-performance liquid-chromatography conditions and ultra-performance liquid-chromatography-mass spectrometry analysis
An Agilent Zorbax Eclipse Plus C18 column (2.1 mm × 150 mm, 3.5 μm, Agilent, USA) was employed, and the column temperature was kept at 30°C. Mobile phase A consisted of acetonitrile and mobile phase B was 0.1% formic acid (v/v) in water. The gradient conditions were as follows: 0–1 min, 50% A; 1–5 min, 50%–80% A; 5–7 min, 80% A; 7–15 min, 80%–98% A; 15–15.5 min, 98% A; 15.5–16 min, 98%–50% A; and 20.5 min, 50% A. The injection volume was 2 μL. The total run time was 20.5 min at a flow rate of 0.2 mL/min.
For the MS detection, an electrospray ionization source operating in the positive ion mode was used. The tune parameters used for data acquisition were the ion source temperature of 500°C; a spray voltage of 5000 V; curtain gas of 15 psi; nebulizer gas of 40 psi; heating gas of 55 psi; and collision activation dissociation gas value of 4 psi. Nitrogen (99.995% purity) was used as the desolvation and collision gas. The MRM acquisition method was run in unit resolution (0.7 amu) in both Q1 and Q3. The m/z of TP and TE was 361.1/145.1 and 451.2/201.1, respectively. The corresponding declustering potential was 120V and 80V, and the collision energy was 37eV and 34 eV.
Method validation
The methods of quantitative analysis of TP and TE in perfusate samples were validated according to the requirement of biopharmaceutical analysis, which was examined for specificity, linearity, precision, extraction recovery, and stability under the UPLC analytical conditions.
The specificity was evaluated by comparing blank perfusate, perfusate mixed by TP and TE, TW perfusate, and TW-PN perfusate. The precision was determined from interday and intraday using five sets of quality control (QC) samples and was expressed by the relative standard deviation (RSD %), which was estimated as follows: RSD (%) = (SD/the observed concentrations of replicate analyses of QC samples [Cobs]) ×100. The QC samples of TP were diluted by TP-containing HBSS stock solution (pH = 7.4) to produce three TP QC samples: TP-H (7.50 μg/mL), TP-M (1.86 μg/mL), and TP-L (0.47 μg/mL). Each concentration of QC samples of TP included five samples. The QC samples of TE were prepared by the same method as QC samples of TP. The QC samples of TE were TE-H (3.00 μg/mL), TP-M (0.75 μg/mL), and TP-L (0.19 μg/mL).
The extraction recoveries were determined by calculating the ratio of TP detected in QC samples of TP against those initially added in HBSS solution and by calculating the ratio of TE detected in QC samples of TE against those initially added in HBSS solution.
The stability of the method was evaluated by analyzing QC samples mixed with TP (7.50 μg/mL) and TE (3.00 μg/mL) at 37°C for 0 h as well as at 37°C for 2 h.
Calculation
The absorption parameters of TP and TE were calculated according to the methods as previously described.[36,37]
The Peff in the SPIP studies was calculated by gravimetric method, and the volume of perfusate was corrected and calculated by the following equation (equation (1)):

Where Q = constant perfusate flux of the peristaltic pump (0.236 mL/min), Cout = outlet drug concentration, Cin = inlet drug concentration, Qout = outlet perfusate volume of each intestinal segment during the 15-min period, Qin = inlet perfusate volume of each intestinal segment during the 15-min period, r = radius of every intestinal segment (duodenum, jejunum, ileum, colon), l = actual length of every intestinal segment (duodenum, jejunum, ileum, colon).
The Ka was calculated through the equation below (equation (2)):

Where Q = constant perfusate flux of the peristaltic pump (0.236 mL/min), Cout = outlet drug concentration, Cin = inlet drug concentration, r = radius of every intestinal segment (duodenum, jejunum, ileum, colon), l = actual length of every intestinal segment (duodenum, jejunum, ileum, colon).
The 10 cm%ABS was calculated through the equation below (equation (3)):
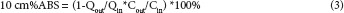
Where Cout = outlet drug concentration, Cin = inlet drug concentration, Qout = outlet perfusate volume of each intestinal segment during the 15-min period, Qin = inlet perfusate volume of each intestinal segment during the 15-min period.
Statistical analysis
The statistical analyses were performed by SPSS version 16.0 (IBM company, USA). Statistical comparison of Peff, Ka, and 10 cm%ABS was performed using one-way ANOVA. All values are expressed as mean ± SD. Means were assumed to be statistically significant when P < 0.05 and obviously significant when P < 0.01.
RESULTS
Method validation
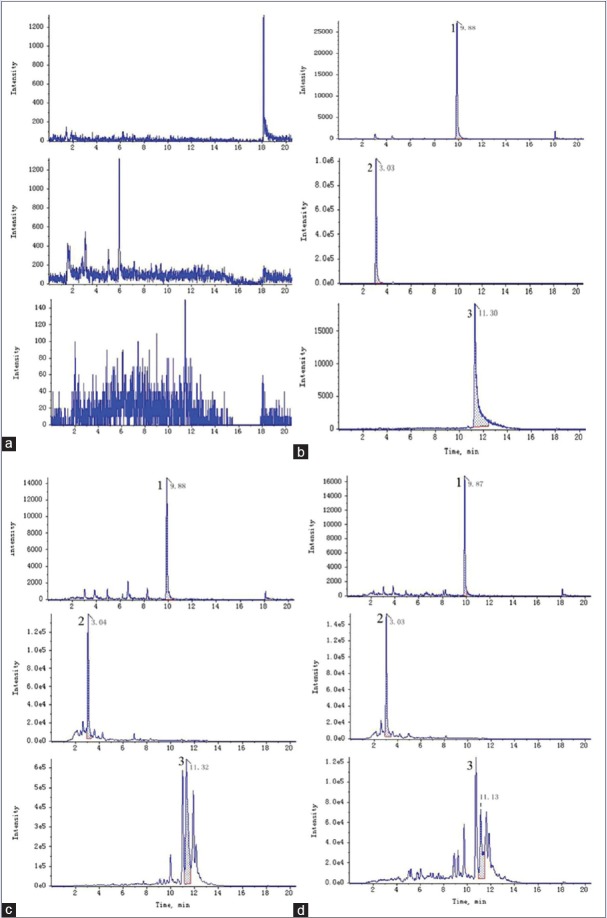
The retention time of fenofibrate, TP, and TE were 9.87, 3.02, and 11.32 min, respectively, and no significant endogenous peaks were observed within the time when TP, TE, and fenofibrate were detected. Figure 2 shows the representative UPLC chromatograms. The standard calibration curve of TP was linear over the range 0.12–30 μg/mL with good linearity (R2 = 0.9981), and the typical equation for the calibration curve was Y = 2.03X + 19.96. The standard calibration curve of TE was linear over the range 0.05–12 μg/mL with good linearity (R2 = 0.9984), and the typical equation for the calibration curve was Y = 8.23X-0.02. The method showed perfect precision within the intraday and interday. The analytical precision of TP and TE in perfusate samples are shown in Table 1. The extraction recoveries of TP were 85.91 ± 6.31%, 87.29 ± 3.67%, and 90.04 ± 5.16% at the concentrations of 0.47, 1.86, and 7.50 μg/mL, respectively. The extraction recoveries of TE were 91.47 ± 4.16%, 90.96 ± 4.62%, and 89.79 ± 5.74% at the corresponding concentrations of 0.19, 0.75, and 3.00 μg/mL. The range of stability of TP was 97.51%–97.69%, and the range of stability of TE was 93.10%–96.23%, and there is no significant difference between the initial and tested concentrations. The UPLC method was validated to be reproducible and reliable to determine TP and TE in perfusate samples.
Figure 2.
Representative chromatograms of different perfusion samples; (a) Blank perfusion solution; (b) Mixed standard solution; (c) Tripterygium wilfordii perfusion sample; (d) Tripterygium wilfordii-Panax notoginseng perfusion sample; 1: Fenofibrate; 2: Triptolide; 3: Tripterine
Table 1.
Analytical precision of triptolide and tripterine in perfusate samples (interday n=5; intraday n=5)
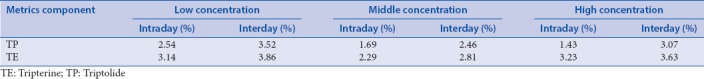
The absorption profiling of triptolide and tripterine in the intestine
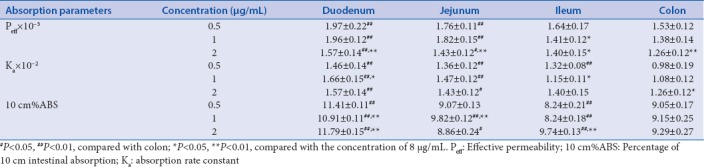
SPIP were performed at the different intestinal segments of rats using TP solution (8 μg/mL) and TE solution (2 μg/mL) to study the absorption characteristics of TP and TE in these four intestinal segments. Table 2 shows the absorption parameters of TP and TE in the four intestinal segments. There was no significant difference between the absorption parameters of TP in colon segment with other intestinal segments, which showed that the absorptive profiling in colon segment was similar to small intestinal segments. This data show that TP has no special absorption site. However, obvious difference of Ka of TE was observed between the colon segment and others. No significant variation of the Peff and 10 cm%ABS of TE was observed between the colon and other intestinal segments yet. Moreover, the values of 10 cm%ABS of TE were much less than that of 10 cm%ABS of TP. This result indicated the more poorly intestinal absorption of TE than TP.
Table 2.
Absorption parameters of triptolide and tripterine in the intestinal segments of rats (n=5, χ̄±s)

The absorption properties of triptolide and tripterine in Tripterygium wilfordii extract in the intestine
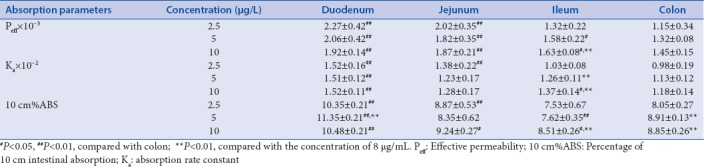
In this study, three different concentrations of TW extract were used to investigate the absorption characteristics of TP and TE in four intestinal segments. Tables 3 and 4 show the absorption parameters of two components in four intestinal segments with low, medium, or high concentrations of TW extracts. The Ka of TP and TE in the four intestinal segments did not change obviously with the increase of the TW extract concentration. Comparing with the absorbing parameters of the monomers TP and TE [Table 2], it was found that the Ka and Peff of TP and TE in TW extract are less than that of perfusate of TP and TE monomers, which may be related to the change in intestinal microenvironment of rat induced by other chemicals in the TW extract. Although the Peff of TP was significantly elevated with the increase of concentration of TW in jejunum and colon, the Ka of TP in these segments reached no statistical significance. Moreover, the Peff and Ka of TP in the small intestine were obviously larger than that of the colon segment, which is commonly accepted that absorption rate of TP in small intestine is greater than the colon. Naturally, compared with small intestine, the absorption extent of TP was significantly decreased in the colon due to the lower rate of absorption and the unavoidable decreased concentration of TP [Table 3]. Basically, similar tendencies were got in the absorption of TE in the four intestinal segments with TW perfusate. The descending order of absorption rate of TE in the four intestine segments was duodenum, jejunum, ileum, and colon. However, the order of absorption extent from high to low was duodenum, jejunum, colon, and ileum. The higher absorption rates in the small intestine, especially in the duodenum and jejunum instead of ileum, lead to the greater 10 cm%ABS than the colon [Table 4].
Table 3.
Absorption parameters of triptolide in the intestinal segments of rats with Tripterygium wilfordii (n=6, χ̄±s)
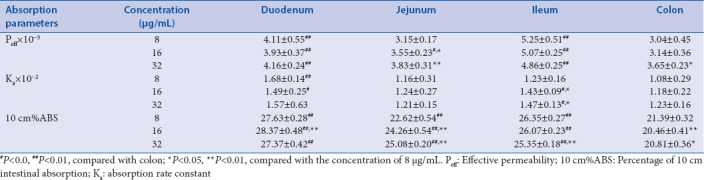
Table 4.
Absorption parameters of tripterine in the intestinal segments of rats with Tripterygium wilfordii (n=6, χ̄±s)
The absorption characteristics of triptolide and tripterine in Tripterygium wilfordii-Panax notoginseng extract in the intestine
In this study, three different concentrations of TW-PN extract were utilized to investigate the absorption characteristics of TP and TE in the four intestinal segments. Table 5 and Table 6 show the absorption parameters of these two components in four intestinal segments with low, medium, and high concentrations of TW-PN extracts. The Ka of TP and TE in the four intestinal segments did not significantly increase along with the concentration elevation of the TW-PN extracts. For the transport dynamics of TP, absorption rate of TP in small intestine segments is higher than that of the colon especially in duodenum and ileum, which, combined with the higher concentration of TW-PN extract, contribute to the larger absorption extent of TP in small intestine represented by 10 cm%ABS [Table 5]. Compared with the absorption parameters of TW [Table 3], both absorption rate and extent of TP were decreased in the TW-PN perfusate, which is responsible for the detoxification of PN on TW in some extent. For the diffusion of TE, although the absorption rates are higher in the small intestinal segments than the colon especially in duodenum and jejunum, the extent of absorption of TE was obviously greater than that of jejunum with concentration of 2 ug/mL and that of ileum with concentration of 0.5 and 1 ug/mL. Moreover, with the increase of concentrations, no obvious enhancement of Ka of TE in the intestinal segments was observed [Table 6]. Compared with the absorption process of TE in TW, the absorption rates and extent of TE had been increased in some extent under condition of TW-PN combination, which contributes to strength the therapeutic efficacy of TW for RA through TE. The converse modulation on TP and TW from compatibility of PN cannot realize simply through regulating the dose of TW alone.
Table 5.
Absorption parameters of triptolide in the intestinal segments of rats with Tripterygium wilfordii-Panax notoginseng (n=6, χ̄±s)
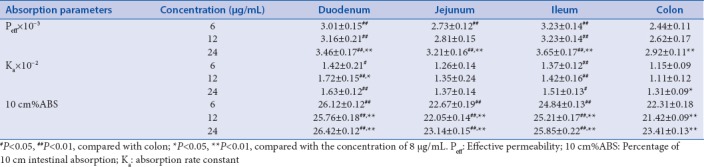
Table 6.
Absorption parameters of tripterine in the intestinal segments of rats with Tripterygium wilfordii-Panax notoginseng (n=6, χ̄±s)
DISCUSSION
The pivotal reference properties for designing a safe and effective drug are absorption, distribution, metabolism, and excretion. Data and information obtained from pharmacokinetics studies help to clarify the complex interactions of clinical drugs. The most common and convenient route for traditional Chinese medicine is oral drug administration. To predict the adsorption extent of oral drug, it is important to select lead candidate in the process of drug development. In the previous study, we found that in Qingluo Tongbi decoction, the liver toxicity of TW can be diminished by the subsidiary medicinal, and PN is the most pivotal candidate of the formula showing detoxification.[16] It is well known that TP is the main component of TW responsible for those adverse effects. Therefore, the regularity of TP dissolution in Qingluo Tongbi decoction was investigated to reveal the chemical basis related to TW compatibility in detoxification, and the TW-induced liver toxicity was found to be decreased by combination of PN.[15] Moreover, further study was performed to reveal the mechanisms underlying the phenomenon of hepatotoxicity-attenuated of TW-PN usage compatibility with metabolomics assay.[14] Based on these investigations, advanced study about the synergistic effect of the compatibility between TW and PN on the absorption mechanism in rat intestine was performed, which provided more definite evidence for the use of TW in clinical practice.
To investigate the absorption process, several intestinal perfusion methods have been developed as absorption models over the years, among which SPIP is the more popular model designed to estimate drug absorption properties with continuous fluid flow through the intestine. It is generally considered to provide better control of the hydrodynamics and increased surface area.[38] The advantages of SPIP model include an intact blood supply; multiple samples may be taken, thus enabling kinetic studies to be performed. The limitation of the SPIP model is the assumption that all drug passes into portal vein, which may not be valid in some circumstances. When the candidate drug under study is metabolized by the enterocytes, drug disappearance from the lumen will not completely reflect drug appearance into the blood. Due to the fact that traditional issues of drug metabolism has prefered to focus on hepatic biotransformation, the effect of intestinal bio-transformations has commonly been considered as an ignored factor.[39] However, apart from these shortcomings, the SPIP model has still proved a powerful research tool. In the model of SPIP, Peff is a useful index to estimate the absorption extent. Several factors implied to affect Peff including physiochemical properties of the drugs such as lipophilicity, molecular size, hydrogen bonding capacity, polar surface area, and also physiological factors in the membrane such as passive versus active transport and location in the intestine. As shown in the present study, the absorption extent, Peff, and absorption rate, Ka, of TP in TW were decreased after combining with PN. TP is the main toxic component of TW; the reduction of absorption extent and absorption rate indicate that the usage compatibility of TW-PN can contribute to decrease the toxicity of TW effectively. On the other hand, a little elevation in absorption rate of TE was observed. Furthermore, the 10 cm%ABS of TE in TW-PN extract in the four intestinal segments were increased following the combinational usage of TW-PN, which shows that the intestinal absorption of TE is improved and responsible for the amelioration of therapeutic effect under condition of usage compatibility of TW and PN. In summary, the usage compatibility of TW and PN may lead to enhance the efficacy of TW and decrease its toxicity. According to this study, TP could be absorbed in general intestinal tract without specific absorption sites. The 10 cm%ABS of TE is less than that of TP, which shows that the intestinal absorption of TE is poorer than TP. The similar result was found by other investigations.[40]
It is well known that TW is commonly used in the clinic for the treatment of inflammatory and autoimmune disorders, especially RA.[30,41] The application of TW, however, is limited due to its narrow therapeutic window and serious toxicity on several tissue or organs such as liver and kidney. The toxicity of TW is closely related to the components of TW, that is to say, some active ingredients also act as the toxic candidates such as TP. TP is the representative component of diterpenoids in TW. Reduction of TP exposure is closely associated with the decreasing toxic on liver and kidney. In the present investigation, PN reduced TP adsorption process from TW in the intestine. It is hard to objectively justify the benefit of compatibility of TW and PN since the less level entering the body indicated less efficiency. However, natural medicine and traditional Chinese medicine usually consist a serious of components with similar chemical core that share mimic targets or signaling pathway in a synergistic manner. Although TE is an important ingredient of triterpenes in TW and shown positive and definite pharmacology actions,[42,43] the absorption level is commonly too low to be detected in the plasma or tissue. One of the advantages of compatibility is to ameliorate the intracorporal content of active components. Obvious absorption of TE was demonstrated in the rat intestine with TW extract. Meanwhile, the tendency to enhance absorption of TE was maintained in the rat intestine with TW-PN extract. This showed that the absorption of triterpenes from TW is promoted by the usage compatibility of PN, which maintains the therapeutic effect of TW on autoimmune disorders under condition of a relative lower level of TP in the body.
CONCLUSION
In the current study, it is the first time to establish an effective method to demonstrate whether PN might contribute to reduce TW-induced acute hepatotoxicity in vivo. According to the absorption parameters of TP and TE in these four intestinal segments, it indicated that the absorption of TP in small intestine was reduced by PN, which led to lower liver and kidney toxicity. Meanwhile, the absorption of TE was increased after the usage compatibility of PN contributing to the more beneficial therapeutic. Consequently, the combination of TW and PN can effectively decrease the toxicity and reinforce the pharmacological actions of the formula Qingluo Tongbi decoction in the clinic.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–72. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 2.Radic M, Martinovic Kaliterna D, Radic J. Overview of vasculitis and vasculopathy in rheumatoid arthritis – Something to think about. Clin Rheumatol. 2013;32:937–42. doi: 10.1007/s10067-013-2273-8. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Dey M, Yang H, Poulev A, Pouleva R, Dorn R, et al. Anti-inflammatory and immunosuppressive compounds from Tripterygium wilfordii. Phytochemistry. 2007;68:1172–8. doi: 10.1016/j.phytochem.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Scott PA, Kingsley GH, Smith CM, Choy EH, Scott DL. Non-steroidal anti-inflammatory drugs and myocardial infarctions: Comparative systematic review of evidence from observational studies and randomised controlled trials. Ann Rheum Dis. 2007;66:1296–304. doi: 10.1136/ard.2006.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salphati L, Childers K, Pan L, Tsutsui K, Takahashi L. Evaluation of a single-pass intestinal-perfusion method in rat for the prediction of absorption in man. J Pharm Pharmacol. 2001;53:1007–13. doi: 10.1211/0022357011776252. [DOI] [PubMed] [Google Scholar]
- 6.Schaffer D, Florin T, Eagle C, Marschner I, Singh G, Grobler M, et al. Risk of serious NSAID-related gastrointestinal events during long-term exposure: A systematic review. Med J Aust. 2006;185:501–6. doi: 10.5694/j.1326-5377.2006.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 7.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: A systematic literature research. Ann Rheum Dis. 2009;68:1100–4. doi: 10.1136/ard.2008.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abasolo L, Leon L, Rodriguez-Rodriguez L, Tobias A, Rosales Z, Maria Leal J, et al. Safety of disease-modifying antirheumatic drugs and biologic agents for rheumatoid arthritis patients in real-life conditions. Semin Arthritis Rheum. 2015;44:506–13. doi: 10.1016/j.semarthrit.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Li XX, Du FY, Liu HX, Ji JB, Xing J. Investigation of the active components in Tripterygium wilfordii leading to its acute hepatotoxicty and nephrotoxicity. J Ethnopharmacol. 2015;162:238–43. doi: 10.1016/j.jep.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Drescher S, Glaeser H, Mürdter T, Hitzl M, Eichelbaum M, Fromm MF, et al. P-glycoprotein-mediated intestinal and biliary digoxin transport in humans. Clin Pharmacol Ther. 2003;73:223–31. doi: 10.1067/mcp.2003.27. [DOI] [PubMed] [Google Scholar]
- 11.Cibere J, Deng Z, Lin Y, Ou R, He Y, Wang Z, et al. A randomized double blind, placebo controlled trial of topical Tripterygium wilfordii in rheumatoid arthritis: Reanalysis using logistic regression analysis. J Rheumatol. 2003;30:465–7. [PubMed] [Google Scholar]
- 12.Yang JH, Luo SD, Wang YS, Zhao JF, Zhang HB, Li L, et al. Triterpenes from Tripterygium wilfordii hook. J Asian Nat Prod Res. 2006;8:425–9. doi: 10.1080/10286020500172665. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Jiang ZZ, Liu J, Huang X, Wang T, Liu J, et al. Sex differences in subacute toxicity and hepatic microsomal metabolism of triptolide in rats. Toxicology. 2010;271:57–63. doi: 10.1016/j.tox.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Zhang Q, Liu M, Zhang X, Shi D, Guo L, et al. Increased involvement of Panax notoginseng in the mechanism of decreased hepatotoxicity induced by Tripterygium wilfordii in rats. J Ethnopharmacol. 2016;185:243–54. doi: 10.1016/j.jep.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Liu MZ, Zhang XL, Pan LM, Cheng HB, Guo LW, Zhu HX, et al. Chemical basis of Tripterygium wilfordii compatibility for attenuation in Qing-Luo-Tong-Bi Decoction. Chin J Exp Tradit Med Form. 2014;20:107–12. [Google Scholar]
- 16.Zhou C, Zhou LL, Liu ZP, Feng Z, Zhou XP. Study on mechanism of decreased hepatotoxicity with the usage compatibility based on the hepatotoxicity. Pharmacol Clin Chin Mater Med. 2013;29:106–9. [Google Scholar]
- 17.Shi DL, Zhu HX, Pan LM, Guo LW. Research progress on attenuation of compatibility of Chinese medicine and Tripterygium. Chin Pharm Aff. 2009;23:1136–40. [Google Scholar]
- 18.Li XJ, Jiang ZZ, Zhang LY. Triptolide: Progress on research in pharmacodynamics and toxicology. J Ethnopharmacol. 2014;155:67–79. doi: 10.1016/j.jep.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Jin H, Li C, Hou Y, Mei Q, Fan D, et al. Heat shock protein 72 protects kidney proximal tubule cells from injury induced by triptolide by means of activation of the MEK/ERK pathway. Int J Toxicol. 2009;28:177–89. doi: 10.1177/1091581809337418. [DOI] [PubMed] [Google Scholar]
- 20.Xu LY, Chen HB, Xu HB, Yang XL. Anti-tumour and immuno-modulation effects of triptolide-loaded polymeric micelles. Eur J Pharm Biopharm. 2008;70:741–8. doi: 10.1016/j.ejpb.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Lu Y, Xiao C, Lu C, Niu X, He X, et al. Comparison of toxic reaction of Tripterygium wilfordii multiglycoside in normal and adjuvant arthritic rats. J Ethnopharmacol. 2011;135:270–7. doi: 10.1016/j.jep.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Zhou ZL, Yang YX, Ding J, Li YC, Miao ZH. Triptolide: Structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat Prod Rep. 2012;29:457–75. doi: 10.1039/c2np00088a. [DOI] [PubMed] [Google Scholar]
- 23.Ding H, Wu JY, Tong J, Yuan XF, Chen J, Shi GG. The study of triptolide acute toxicity and its mechanism. Chin Tradit Herbal Drugs. 2004;27:115–8. [Google Scholar]
- 24.Shao F, Wang GJ, Xie HT, Zhu XY, Sun JG, A JY. Pharmacokinetic study of triptolide, a constituent of immunosuppressive Chinese herb medicine, in rats. Biol Pharm Bull. 2007;30:702–7. doi: 10.1248/bpb.30.702. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Yang J, Gao YX, Wu CL. Triptolide poisonous side effect–liver hurt and mechanism preliminary study. Chin J Pract Chin Mod Med. 2006;19:2832–3. [Google Scholar]
- 26.Ma Z, Zhang Y, Liang MX. Toxicity reducing and efficacy enhancing research on rheumatoid arthritis effect of Tripterygium compatible with Licorice. Asia Pac Tradit Med. 2014;10:9–11. [Google Scholar]
- 27.Liu JQ, Li Q, Zhang R, Liu F, Zhang W, He ZH, et al. LC-MS/MS studies on effect of Glycyrrhiza uralensis on metabolism, distribution and excretion of triptolide in rat. Chin J Pharm Anal. 2010;30:1664–71. [Google Scholar]
- 28.Liu JQ, Hong Q, Zhang W, Shi M, Zhang R, Gu B. Studies on the toxicity-reducing and activity-maintaining effects of triptolide combined with Pteris multifida. Chin Hosp Pharm J. 2010;30:443–6. [Google Scholar]
- 29.Liu JQ, Zhang W, Gao SL, Zhang R, Xu L, Li Q, et al. Protective effect of Pteris multifida against Triptolide-induced hepatic injury in mice. J China Pharm. 2010;21:4033–5. [Google Scholar]
- 30.Qin S, Li Q, Yu Q, Wang ZH, Sun JH, Zhu CL, et al. Toxicity-reducing and Action-enhancing Effects of Tripterginum Wilfordii polyglycoside combined with astragalosides on rat adjuvant arthritis. J Math Med. 2016;29:540–2. [Google Scholar]
- 31.Xiao SJ, Liu ZP, Zhou M, Wei WX, Liu LH, Zhou JX, et al. Chemical constituents of Triptergyium wilfordii Hook. f. Natl Prod Res Dev. 2011;23:1–3. [Google Scholar]
- 32.Guo J, Liu L, Wang Z, Bi Z, Wang H, Ye W. Chemical constituents of Tripterygium glycosides. Res Pract Chin Med. 2011;25:41–4. [Google Scholar]
- 33.Ma Z, Liang M, Zhang Y. Research progress of chemical components and pharmacological activity of traditional Chinese medicine Tripterygium wilfordii. Asia Pac Tradit Med. 2011;7:157–60. [Google Scholar]
- 34.Xu J, Lu J, Sun F, Zhu H, Wang L, Zhang X, et al. Terpenoids from Tripterygium wilfordii. Phytochemistry. 2011;72:1482–7. doi: 10.1016/j.phytochem.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Tian Y, Hao S, Ma Y, Zhang Z, Wang J. Separation and identification of chemical composition in Tripterygium wilfordii root. J Shenyang Pharm Univ. 2010;27:715–8. [Google Scholar]
- 36.Nie SF, Pan WS, Yang XG, Liu HF, Liu ZD. Evaluation of gravimetry in the rat single-pass intestinal perfusion technique. Chin New Drugs J. 2005;14:1176–9. [Google Scholar]
- 37.Huang SH, Long XY, Yuan F, Chen L, Cai BL, Qiu HL. Transport of puerarin in rat intestine in situ by modified gravimetry and phenol red assay. J Guangdong Pharm Univ. 2012;28:603–7. [Google Scholar]
- 38.Yu LX, Lipka E, Crison JR, Amidon GL. Transport approaches to the biopharmaceutical design of oral drug delivery systems: Prediction of intestinal absorption. Adv Drug Deliv Rev. 1996;19:359–76. doi: 10.1016/0169-409x(96)00009-9. [DOI] [PubMed] [Google Scholar]
- 39.Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80:1029–36. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue J, Jia XB, Tan XB, Wang JJ, Chen Y, Zhang LY. Studies on rat intestinal absorption of triptolide in situ. Chin Tradit Herbal Drugs. 2010;41:86–9. [Google Scholar]
- 41.Marks WH. Tripterygium wilfordii hook F. Versus sulfasalazine in the treatment of rheumatoid arthritis: A well-designed clinical trial of a botanical demonstrating effectiveness. Fitoterapia. 2011;82:85–7. doi: 10.1016/j.fitote.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Zhang YY, Tan HW, Jia YF, Li D. Therapeutic effect of tripterine on adjuvant arthritis in rats. J Ethnopharmacol. 2008;118:479–84. doi: 10.1016/j.jep.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 43.Lu C, Yu X, Zuo K, Zhang X, Cao C, Xu J, et al. Tripterine treatment improves endothelial progenitor cell function via integrin-linked kinase. Cell Physiol Biochem. 2015;37:1089–103. doi: 10.1159/000430234. [DOI] [PubMed] [Google Scholar]