Abstract
This study focuses on the application of combining membrane bioreactor (MBR) treatment with reverse osmosis (RO) or nanofiltration (NF) membrane treatment for removal of pharmaceuticals and personal care products (PPCPs) in municipal wastewater. Twenty-seven PPCPs were measured in real influent with lowest average concentration being trimethoprim (7.12 ng/L) and the highest being caffeine (18.4 ng/L). The results suggest that the MBR system effectively removes the PPCPs with an efficiency of between 41.08% and 95.41%, and that the integrated membrane systems, MBR-RO/NF, can achieve even higher removal rates of above 95% for most of them. The results also suggest that, due to the differences in removal mechanisms of NF/RO membrane, differences of removal rates exist. In this study, the combination of MBR-NF resulted in the removal of 13 compounds to below detection limits and MBR-RO achieved even better results with removal of 20 compounds to below detection limits.
Keywords: PPCPs, wastewater, membrane bioreactor, reverse osmosis, nanofiltration, removal mechanisms
1. Introduction
Pharmaceuticals and personal care products (PPCPs) are a class of emerging contaminants, which include commonly used medicinal, cosmetic and personal hygiene products. PPCPs have been widely detected in surface, ground, coastal and even drinking water [1,2,3,4]. Great concerns have been raised about PPCPs due to their potential adverse impacts on the ecological system and human health.
PPCPs contain a large and diverse group of organic compounds, including pharmaceutically active compounds, endocrine disrupting compounds and so on. This kind of compound has a potential physiological effect on the water environment, water ecology and human health. For example, it can cause fish’s nephridial tissue necrosis, influence the growth of alga and duckweed, enhance the microbial resistance to antibiotics, etc. [5,6,7]. Compared with common pollutants, this compound has strong polarity, low mass concentration and biological accumulation feature. In addition, it exists in bodies of water with very low concentration (<1 μg/L), which makes it quite difficult for urban sewage treatment plants to remove it [8]. Therefore, PPCPs have a huge potential ecological risk for both aquatic organisms and humans, and an efficient treatment process has become the focus of many scholars from home and abroad.
Many studies have explored different treatment methods to remove PPCPs from wastewater and receiving waters, including conventional activated-sludge, soil aquifer treatment, advanced oxidation process and biomembrane process [9,10,11,12,13,14,15]. Among them, biomembrane process is one of the most effective treatment processes. With the development of such technology, it has become more efficient, convenient and economically viable, and the membrane processing technique now plays an increasingly important role in sewage treatment. According to Jelena et al. [16] membrane bio-reactor (MBR) has a better effect (>80%) on most pharmaceuticals (naproxen 99.3%, ofloxacin 94.0%, bezafibrate 95.8%, and paroxetine 89.7%) than conventional activated-sludge. Katsuki et al. [17] have studied reverse osmosis (RO) membrane’s effect on 11 endocrine disrupting chemicals (EDCs) and pharmaceutically active compounds (PhACs), and it shows that the polyamide membrane has achieved a good removal effect on 2-naphthol, 4-phenylphenol, caffeine, bisphenol A, sulfamethoxazole and 17β-Estradiol with rate of 51% to 91%. Boleda et al. [18] and Sahar et al. [19] have proven that ultrafiltration (UF) - RO double membrane process has good removal efficiency (90% on average) on PhACs and EDCs. Dolar et al. [20] focus on the study of MBR-RO process’s efficiency on twenty multiple-class pharmaceuticals (including psychiatric drugs, macrolide antibiotics, β-blockers, vsulfonamide and fluoroquinolone antibiotics), and treatment has exhibited excellent overall removal of selected emerging contaminants with removal rates above 99%. Establishing MBR-NF(nanofiltration) treatment process, Alturki et al. [21] have studied different nanofiltration membranes’ efficiency to forty trace organics, including pharmaceutically active compounds, steroid hormones, industrial compounds and pesticides, and the removal rates are all above 95%.
Thus, the main goal of this work is to assess the removal efficiency (after each step and overall) of selected contaminants (27 PPCPs) from typical municipal wastewater by using an integrated membrane system (MBR-RO and MBR-NF) on a pilot scale.
2. Materials and Methods
2.1. MBR-RO/NF Pilot Plant
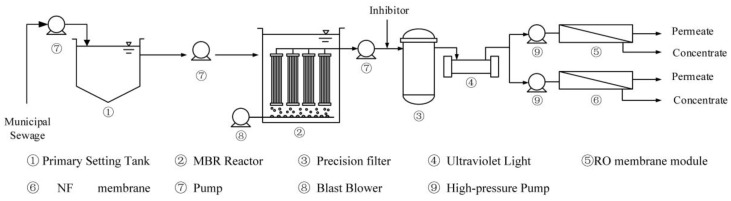
A pilot scale MBR-RO/NF system was employed in this study (Figure 1). The MBR system consisted of a stainless-steel reactor with an active volume of 9 L, one air pump, a pressure sensor, and influent and effluent pumps. Forty-eight membrane microfiltration (MF) membrane components supplied by PEIER (Jiangsu, China) were used in this apparatus (Table 1). This membrane had a nominal pore size of 0.08–0.3 μm. Each module had an effective membrane surface area of 0.034 m2. An electrical magnetic air pump (seko, Milano, Italy) with a maximum air flow rate of 630 L/min was used to aerate the MBR system and reduce fouling and formation of cake via a diffuser located at the bottom of the reactor. Transmembrane pressure was continuously monitored using a high-resolution pressure sensor which was connected to a personal computer for data recording purposes.
Figure 1.
Schematic representation of the MBR-RO/NF pilot plant showing different compartments, flow directions, main instruments and equipment.
Table 1.
Properties of three membrane modules.
| Membrane Component | Texture | Type | Rejection (%) a | Effective Area (m2) | General Operation Pressure (KPa) | General Operation Flux (LMH) | |
|---|---|---|---|---|---|---|---|
| Average | Minimum | ||||||
| RO | PA b | Duraslick RO 2540 | 98.6% (NaCl) | 97% (NaCl) | 2.5 | 1379 | 15–25 |
| NF | PA b | Duraslick NF-2540 | 98.6% (MgSO4) | 96% (MgSO4) | 2.2 | 690 | 15–25 |
| MBR | PVDF c + PET d | PEIER-B-80 | - | - | 0.8 | - | - |
a taking test after running 24 h, the average rejection of single membrane might be −15% ~ +25%; b PA: Polyamide; c PVDF: Polyvinylidene fluoride; d PET: Polyethylene terephthalate.
The temperature of the reactor was kept constant using a chiller/heater (Thermo NESLAB RTE-7, Bacchus Marsh, Australia), equipped with a stainless-steel heat exchanging coil. The temperature inside the reactor was 16.7 ± 0.5 °C during the entire sampling campaign. The flow rate of the influent pump was matched to that of the permeate pump to maintain a constant reactor volume. The MBR was operating with a hydraulic retention time (HRT) of 3.2 h, average pH value of 7.8. The solid retention time (SRT) was 40 days and the production flow was 0.8 m3/h during the sampling campaign.
The precision filter (Ф195 × 500, Tongzhou Zhiyuan, Beijing, China) and ultraviolet light (NLC-25/65, Tongzhou Zhiyuan, Beijing, China) were applied to prevent the NF/RO membrane from fouling by particles and bacteria, respectively. Research shows that ultraviolet (UV) alone (without NF or RO membrane) was not effective in reducing the PPCPs concentration [22,23,24].
The reverse osmosis and nanofiltration membrane (DuraslikTM , GE, California, USA) were used in this study, which had an effective membrane area of 2.5 m2 and 2.2 m2, respectively. The RO unit was equipped with a high-pressure pump (CDL2-11/1.1 KW, Nanfang Pump Industry, Suzhou, China) capable of providing pressures up to 1.32 MPa and a flow rate of 13 L/min. The NF unit was equipped with high-pressure pump (CDL2-5/0.55 KW, Nanfang Pump Industry, Suzhou, China) capable of providing pressures up to 0.7 MPa and flow rate of 13 L/min.
Permeate flow was measured by a digital flow meter (Sierra, Shanghai, China) connected to a PC, and the cross-flow rate was monitored using a rotameter.
2.2. Model Contaminants
The 27 kinds of PPCPs selected in the study are commonly found in wastewater and natural waters (see Table 2). The selected contaminants include organic compounds with molecular weights in the range between 194.19 g/mol (caffeine) and 837.05 g/mol (roxithromycin). The intrinsic hydrophobicities of these compounds vary significantly, as is also reflected by their octanol-water partitioning coefficient (LogKow) values.
Table 2.
Physicochemical properties of the selected compounds.
| Analytes | MW (g/mol) | Formula | CAS Number | LogKow | pKa * | Solubility * (mg/L) | Classification |
|---|---|---|---|---|---|---|---|
| Caffeine | 194.19 | C8H10N4O2 | 58-08-2 | −0.07 | 6.1; 0.73 | 2.16 × 104 | Stimulant |
| Atenolol | 266.34 | C14H22N2O3 | 29122-68-7 | 0.16 | 13.88; 9.16 | N/A | β--blocker |
| Metoprolol | 267.36 | C15H25NO3 | 51384-51-1 | 1.88 | 9.68 | N/A | β--blocker |
| Amoxicillin | 365.4 | C16H19N3O5S | 26787-78-0 | 0.91 | N/A | N/A | β-lactams Antibiotic |
| Trimethoprim | 290.32 | C14H18N4O3 | 738-70-5 | 0.87 | 6.3; 4.0; 7.2 | 12100 | Pyrimethamine antibiotic |
| Sulfadimidine | 278.33 | C12H14N4O2S | 57-68-1 | 0.89 | 7.4 | 1500 | Sulfonamides antibiotics |
| Sulfamethoxazole | 253.27 | C10H11N3O3S | 723-46-6 | 0.48 | 2.1; 5.81; 1.39 | 610 | Sulfonamides antibiotics |
| Norfloxacin | 319.33 | C16H18FN3O3 | 70458-96-7 | −1.03 | N/A | N/A | Fluoroquinolone antibiotics |
| Ofloxacin | 361.37 | C18H20FN3O4 | 82419-36-1 | −0.39 | N/A | N/A | Fluoroquinolone antibiotics |
| Ciprofloxacin | 331.34 | C17H18FN3O3 | 85721-33-1 | 0.28 | N/A | 3.0 × 104 | Fluoroquinolone antibiotics |
| Lomefloxacin | 351.35 | C17H19F2N3O3 | 98079-51-7 | −0.23 | N/A | N/A | Fluoroquinolone antibiotics |
| Enrofloxacin | 359.4 | C19H22FN3O3 | 93106-60-6 | −0.63 | N/A | N/A | Fluoroquinolone antibiotics |
| Oxytetracycline | 460.43 | C22H24N2O9 | 79-57-2 | −0.9 | 3.27 | N/A | Tetracycline antibiotics |
| Tetracycline | 444.44 | C22H24N2O8 | 60-54-8 | −1.37 | 3.30 | N/A | Tetracycline antibiotics |
| Chlortetracycline | 478.88 | C22H23ClN2O8 | 57-62-5 | −0.9 | 3.30 | N/A | Tetracycline antibiotics |
| Doxycycline | 444.44 | C22H24N2O8 | 564-25-0 | −0.02 | 3.30 | N/A | Tetracycline antibiotics |
| Clarithromycin | 747.95 | C38H69NO13 | 81103-11-9 | 3.16 | 8.9 | 0.33 | Macrolide antibiotics |
| Erythromycin-H2O | 715.916 | C37H65NO12 | 23893-13-2 | 3.06 | 8.9 | 1.44 | Macrolide antibiotics |
| Roxithromycin | 837.05 | C41H76N2O15 | 80214-83-1 | 1.7 | 8.8 | 0.019 | Macrolide antibiotics |
| Azithromycin | 748.98 | C38H72N2O12 | 83905-01-5 | 4.02 | 8.8 | N/A | Macrolide antibiotics |
| Carbamazpine | 236.27 | C15H12N2O | 298-46-4 | 2.25 | 13.90; −0.49 | 112 | Antiepilepti |
| Benzhabeite | 361.82 | C19H20ClNO4 | 41859-67-0 | N/A | N/A | N/A | Antihypercholesterolemic |
| Bisphenol A | 228.29 | C15H16O2 | 80-05-7 | 3.32 | 9.73 | 120 | Plasticizer |
| Nonylphenol | 220.35 | C15H24O | 25154-52-3 | 5.76 | 10.14 | N/A | Plasticizer |
| Estrone | 270.37 | C18H22O2 | 53-16-7 | 3.13 | 10.25; 10.5 | N/A | Hormone |
| 17β-Estradiol | 272.38 | C18H24O2 | 50-28-2 | 4.01 | 10.27; 10.4 | 3.6 | Hormone |
| Estriol | 288.38 | C18H24O3 | 50-27-1 | 2.45 | 10.25; >15 | 444 | Hormone |
2.3. Sample Collection and Analysis
Samples were taken during a one-week period and both influent and effluent sample were collected and analyzed for PPCP concentration. The sampling points were: (1) municipal wastewater-sewer (influent); (2) MBR effluent; and (3) permeate of RO/NF element. Water samples were collected in 1-L amber glass bottles. To ensure the stability of the target, the pH of the samples was adjusted to 3 with H2SO4 (40%). Samples were kept at 4 °C during preparation (1 h) and were vacuum filtered through 1.0 μm glass microfiber filter (Whatman, Maidstone, UK).
The analysis of the target compounds was based on a previously developed method [28]. Analytes were extracted using 500 mg/6 mL hydrophilic/lipophilic balance (HLB) cartridges (Waters, Millford, MA, USA). Cartridges were pre-conditioned with 5 mL of methanol alcohol, 5 mL of amprolium HCl and 3 mL of reagent water. The sample was loaded onto the cartridges at 4–5 mL/min, after which the cartridges were rinsed with 5 mL of methanol alcohol aqueous solution (5%), 5 mL of reagent water and dried with a stream of nitrogen for 40 min. Analytes were eluted with 10 mL of methanol followed by 5 mL of dichloromethane/acetone (7/3, v/v) methanol/MTBE into centrifuge tubes. The resulting extracts were concentrated using vacuum assisted evaporation to approximately 100 μL. The extracts were brought to a final volume of 1 mL with methanol. Analytes were separated using an Agilent (Waters, Milford, MA, USA) 1290 series ultra performance liquid chromatography (UPLC) system equipped with an OAsis HLB column (Waters, Milliford, MA, USA). Mass spectrometry was performed using an Agilent 6420 triple quadrupole mass spectrometer (Waters, Milford, MA, USA) with positive electro-spray modes (ESI+) [28].
Considering that PPCPs have rather different physicochemical characteristics, their removal during treatment is expected to be diverse. The literature shows that the removal efficiency is generally computed as the percentage of reduction between the dissolved aqueous phase concentration of the contaminant in the influent and that in the effluent. Except for a few studies, concentrations in sludge or suspended solid are generally not considered or measured, likely because of the difficulty to sample and analyze such complex matrices [29].
3. Results and Discussion
3.1. PPCPs in the Influent
In Table 3, the range of levels observed in influent wastewaters for each contaminant (with their mean values) are presented. Levels of target compounds were in the ng/L range but concentrations of some of them exceeded 1 μg/L range (caffeine, metoprolol and azithromycin). The process received domestic (100%) wastewater and thus high concentrations of selected compounds were found.
Table 3.
Concentration ranges and mean values (n = 6) of target contaminants in wastewater influent and limit of quantitation (LOQ) with standard deviation in parentheses (n = 6).
| Compounds | LOQ (ng/L) | Range (μg /L) | Mean (μg /L) |
|---|---|---|---|
| Caffeine | 10.6 (4.1) | 8.53–33.7 | 18.4 |
| Atenolol | 8.54 (3.32) | 0.012–0.409 | 0.166 |
| Metoprolol | 9.85 (4.56) | 0.437–3.21 | 1.73 |
| Amoxicillin | 6.11 (4.07) | 0.008–0.035 | 0.02 |
| Trimethoprim | 11.2 (7.35) | n.d. −0.023 | 0.007 |
| Sulfadimidine | 4.75 (2.32) | 0.005–0.131 | 0.059 |
| Sulfamethoxazole | 6.83 (3.22) | 0.012–0.092 | 0.037 |
| Norfloxacin | 10.63 (6.12) | 0.014–0.226 | 0.106 |
| Ofloxacin | 14.46 (10.32) | 0.1–0.912 | 0.560 |
| Ciprofloxacin | 3.65 (1.55) | n.d. −0.089 | 0.034 |
| Lomefloxacin | 2.12(1.46) | n.d. −0.0388 | 0.01 |
| Enrofloxacin | 3.56 (1.47) | n.d. −0.008 | 0.004 |
| Oxytetracycline | 2.66 (1.75) | 0.009–0.035 | 0.018 |
| Tetracycline | 2.32 (1.02) | 0.003–0.008 | 0.023 |
| Chlortetracycline | 4.11 (1.36) | n.d. −0.022 | 0.008 |
| Doxycycline | 2.33 (1.21) | n.d. −0.08 | 0.018 |
| Clarithromycin | 35.11 (15.88) | n.d. −1.26 | 0.368 |
| Erythromycin-H2O | 43.4 (45.9) | n.d. −0.082 | 0.020 |
| Roxithromycin | 35.3 (15.44) | n.d. −0.253 | 0.079 |
| Azithromycin | 23.12 (4.98) | 0.047–4.42 | 1.41 |
| Carbamazpine | 3.13 (1.78) | n.d. −0.032 | 0.014 |
| Benzhabeite | 20.3 (8.65) | 0.022–0.151 | 0.074 |
| Bisphenol A | 16.46 (6.21) | 0.3–1.52 | 0.833 |
| Nonylphenol | 15.2 (10.6) | 0.126–0.873 | 0.421 |
| Estrone | 7.65 (3.32) | 0.078–0.158 | 0.106 |
| 17β-Estradiol | 10.1 (4.40) | 0.011–0.054 | 0.030 |
| Estriol | 8.32 (4.67) | 0.042–0.162 | 0.092 |
n.d.: not detectable (below the LOQ).
Among the selected contaminates, the highest concentrations in influent were found for nervous stimulant caffeine (8.53–33.7 μg/L), β-blocker metoprolol (0.437–3.21 μg/L), macrolide antibiotics azithromycin (0.047–4.42 μg/L) and surfactant bisphenol A (up to 1.52 μg/L). Other authors [30,31,32] presented similar ranges (ng/L to μg/L) for the pharmaceuticals and personal care products found in the municipal wastewaters.
Caffeine was present in the highest concentration (33.7 μg/L) making it in agreement with concentrations presented by other authors [33,34]. The reason is that caffeine is widely distributed in coffee, tea and other beverages, and flow into sewage with residue [35]. Macrolide antibiotics are largely excreted into sewage in their unchanged forms at excretion rates greater than 60% and they are usually found in wastewater at high concentrations [30]. Relatively high concentrations of azithromycin (up to 4.42 μg/L) were observed. Despite its high consumption, other kinds of macrolide antibiotics (such as roxithromycin, clarithromycin, and erythromycin-H2O) were found at much lower levels (10 μg/L) than azithromycin. In addition, the same result has been found in other studies [36].
On the other hand, bisphenol-A was present in relatively high concentration (up to 1.5 μg/L), which is in agreement with concentrations presented by other authors [33]. Among many pollutants, estrone, 17β-Estradiol and estriol are the three estrogen compounds that gain the most attention in present studies. The concentration of 17β-Estradiol ranged 10.6–54.1 ng/L, while concentrations of the other two compounds are all at the 100 ng/L level, which is quite close to the concentration of estrogen compounds in Zhang’s [37] study.
3.2. Removal of PPCPs by MBR
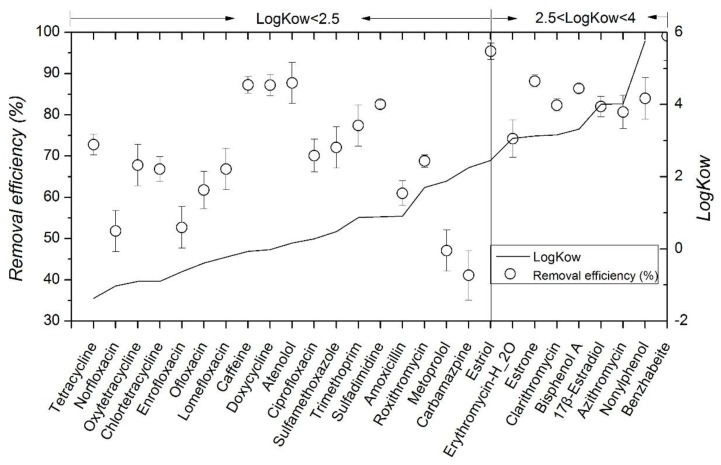
MBR’s average removal efficiency to the selected contaminants is presented in Figure 2. MBR’s main removal pathways to organic compounds are biodegradation (biotic)/absorption (abiotic) and biotransformation. Adsorption includes membrane surface adsorption and its settled layer adsorption. Biotic and abiotic process could not easily be distinguished in this study and therefore removal efficiency refers to overall removal result under two mechanisms.
Figure 2.
Removal efficiency of the model contaminants and their corresponding hydrophobicity (LogKow) by MBR treatment.
Given the diverse physicochemical properties of the 27 contaminants selected in this study, it is not surprising that their removal efficiency varied significantly. Lower removal is observed for carbamazpine (41%) and metoprolol (47%). In contrast, for several other compounds, including estriol (95%), benzhabeite (88%), caffeine (88%) and atenolol (87%), the removal efficiency is relatively high. This is in accordance with the research results of Alturki et al. [21].
For the macrolide antibiotics, MBR treatment shows mean removal efficiency from 74% to 82%. Under typical wastewater conditions, many macrolides can be adsorbed into biomass, mainly attributed to hydrophobic interactions due to their high LogKow partitioning coefficients (Table 1). Many macrolides are positively charged whereas sludge surface is predominantly negatively charged, which leads to adsorption of these compounds to biomass via cation exchange processes [38].
Sulfonamides is partly removed (61.4%) with MBR, likely due to a moderate sorption to sludge and to its limited biodegradability. Chemicals with LogKow < 2.5, as is the case with sulfamethoxazole and sulfadimidine, are considered to have low hydrophobic sorption potential. Göbel et al. [29] have described lower removal of sulfamethoxazole (37–38%) with a MBR system.
MBR’s removal efficiency rates on fluoroquinolones are 51.8% (norfloxacin), 70.1% (ciprofloxacin), 61.8% (ofloxacin), 66.9% (lomefloxacin) and 52.7% (enrofloxacin); its removal efficiency rates to tetracycline antibiotics are 67.80% (oxytetracycline), 72.79% (tetracycline), 66.8% (chlortetracycline) and 75.6% (doxycycline); and the LogKow values are all below 2.5. Therefore, this kind of compound has relatively low adsorption potential energy and weak hydrophobic property, which brings difficulty to MLSS’s (mixed liquor suspended solid) adsorption and further biodegradation process. As a result, MBR has a low removal efficiency to the three organisms.
Removal of carbamazepine is relatively poor (47.23%). Poor elimination of this neutral has been reported by many authors [16,39,40,41]. Carbamazpine possesses very weak biodegradability under low concentration, thus it is hard for activated sludge to conduct effective biodegradation. Although carbamazpine could adsorb on the surface of activated sludge, it fails to be degraded effectively. Besides, the carbamazpine adsorbed from activated sludge would flow out with MBR water.
MBR’s removal efficiency to retardant drugs are 87.7% (atenolol) and 47.1% (metoprolol). These removal rates are the same as the study by Radjenovic et al. [16], and much lower than those observed by Dalar et al. [20]. Maurer et al. [42] found that removal mechanism of β-blockers with MBR sludge is mainly biodegradation, whereas only in the case of propanolol, sorption was a possible removal method.
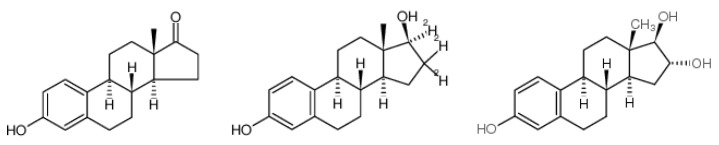
MBR’s removal efficiency to three estrogens is 88.2% (estrone), 82% (17β-Estradiol) and 95.4% (Estriol), and their LogKow values are 3.13, 4.01 and 2.45, so medium or high absorption potential energy is presented. According to the research, absorption would easily happen when OH hydroxy on benzene ring and C=O hydroxy on adsorbent form into hydrogen bond, and it is easier to remove [43]. Among the three estrogens, estriol has slightly lower LogKow value, but there are three hydroxyls in its molecular structure (Figure 3). Then, Estriol forms into hydrogen bond with C=O hydroxy on mixed liquid suspended solids (MLSS), which strengthens adsorption, and a higher removal efficiency is achieved.
Figure 3.
The chemical structure of Estrone/17β-Estradiol/Estriol.
3.3. Removal of PPCPs by MBR-RO/NF System
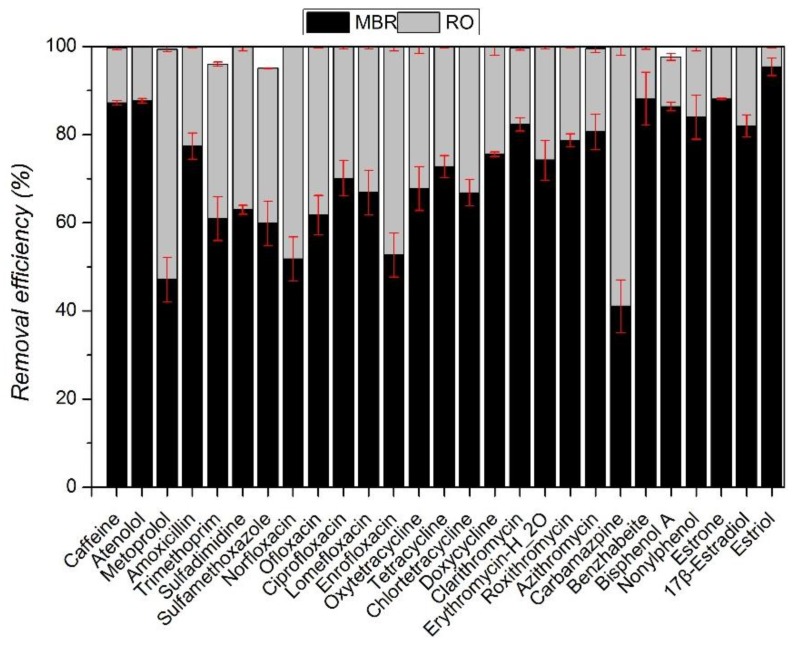
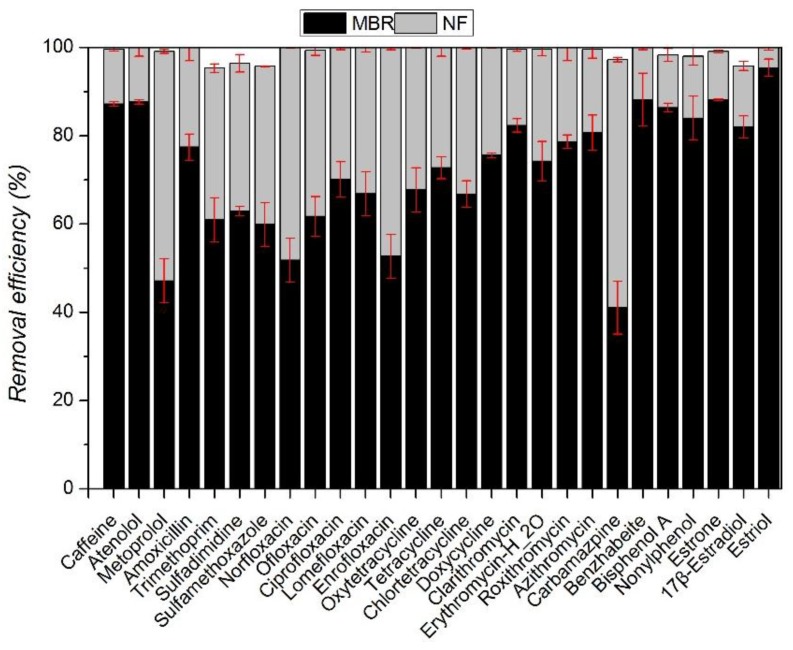
Results reported in Figure 2 reconfirm the limitation of MBRs with respect to the removal of some hydrophilic and biologically persistent trace organic compounds. However, since most of these problematic compounds are hydrophilic, there is a potential for use of NF/RO membranes to more effectively remove them. The overall removal of the 27 selected contaminants with MBR-RO/NF is presented in Figure 4. RO/NF membranes can both complement MBR treatment very well, since the majority of compounds studied in the influent were almost entirely removed or concentrations were below the detection limit of the analytical technique after RO/NF treatment. Overall removal rates are greater than 95%, which means that additional removal of selected contaminants with RO and NF membrane is higher than 95% and are in agreement with results obtained by other researchers [21,31,44,45]. Snyder et al. [46] obtained removal rates of various pharmaceuticals (antibiotics, psychiatric control, anti-inflammatory, etc.) higher than 90%. Wang et al. [47] found that most of the 40 trace organic compounds studied were effectively rejected by NF membrane.
Figure 4.
Overall removal efficiency of the selected contaminants by MBR-RO system.
Even though high rates of removal of most of the 27 compounds selected in this study were achieved with the combination of MBR-NF/RO treatment processes, there are also variations. The combination of MBR treatment and the NF membrane resulted in removal of 13 compounds to below detection limits (Figure 4). MBR and the RO membrane achieved even better results with removal of 20 compounds to below detection limits (Figure 5). Despite the significant variation in the concentrations of these compounds in the MBR effluent of up to 10 μg/L, their concentrations in the final RO permeate were only marginally above the analytical detection limits.
Figure 5.
Overall removal efficiency of the selected contaminants by MBR-NF system.
Removal of contaminants by RO is determined by complex interactions of electrostatic and other physical forces acting among the specific solute, the solution and the membrane itself. Main removal mechanisms in RO membranes are steric hindrance, electrostatic interaction and hydrophobic interaction between compounds and the membrane [17,40]. Considering the hydrophobicity, a compound that possesses strong hydrophobicity (LogKow > 2.5) can attach to the membrane’s polymer matrix, and the possible removal mechanism may be hydrophobicity interaction. In contrast, electrostatic attraction or repulsion forces can influence the rejection of some contaminants in RO membrane due to their charge. Generally, RO membranes can obtain a better removal performance, due to aperture of the reverse osmosis membrane is less than 1 nm, which can able to intercept most of soluble organic matters [48,49]. Among various parameters affecting removal of PPCPs by NF, namely physicochemical properties of the PPCPs (charge characteristics, hydrophobicity and molecular weight) and membranes (molecular weight cut off and surface charge), the molecular weight cut off (MWCO) effect was found to be the most critical aspect. The molecular cut off of DuraslikTM 2540 NF membrane is 300. For selected contaminants whose relative molecular weight is less than 300, such as sulfadimidine (278.33) and sulfamethoxazole (253.27), removal efficiency is lower, while, for pollutants whose molecular weight is more than 300, NF’s removal efficiency rate is above 99%.
4. Conclusions
The rejection performance of MBR-RO and NF process was tested with twenty-seven selected PPCPs from municipal wastewater in this study. The concentration variation of the selected contaminant in feed water was first monitored. Result showed that 27 PPCPs were in relatively high concentrations (caffeine, even up to 33.7 μg/L). Average concentrations of some PPCPs ranged from 7.1 ng/L (pyrimethamine antibiotic) and 75.9 ng/L (estrogen) to 468.5 ng/L (macrolide antibiotics). Removal efficiency of MBR varied significantly (41–95%) depending on compound, which was due to the diverse physicochemical properties of the 27 target compounds. High removal rates (to levels below LOQ) were achieved with the combination of MBR–RO/NF treatment for all compounds selected. Hydrophilic PPCPs compounds were effectively removed by NF/RO membranes selected in this study. Size exclusion and electrostatic attraction or repulsion were presumed to be the primary mechanisms involved in the removal of target compounds with RO membranes. Among various parameters affecting the removal of PPCPs by NF, the MWCO effect was found to be the most critical aspect.
Acknowledgments
This research project has been funded by the Major Science and Technology Program for Water Pollution Control and Treatment (No. 2012ZX07203-001-01), by the National Basic Research Program of China (No. 2012CB215303), and by Technology Fund of Beijing Municipal Research Institute of Environmental Protection (2017-B-02). The authors would like to thank Kasper Thomassen for the assistance.
Author Contributions
Yonggang Wang, Xu Wang, Changhong Sun and Guanyi Chen conceived and designed the experiments; Yonggang Wang and Xu Wang performed the experiments; Yonggang Wang, Xu Wang and Mingwei Li analysed the data; Jing Dong contributed reagents/materials/analysis tools. All authors contributed to writing the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vieno N., Tuhkanen T., Kronberg L. Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res. 2007;41:1001–1012. doi: 10.1016/j.watres.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Pessoa G.P., Souza N.C., Vidal C.B., Santos A.D. Occurrence and removal of estrogens in Brazilian wastewater treatment plants. Sci. Total Environ. 2014;490:288–295. doi: 10.1016/j.scitotenv.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Kim S.D., Cho J., Kim I.S., Vanderford B.J., Snyder S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007;41:1013–1021. doi: 10.1016/j.watres.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 4.Luo Y., Guo W., Ngo H.H., Nghiem L.D., Hai F.I., Zhang J., Liang S., Wang X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014;473–474:619–641. doi: 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- 5.Snyder S.A. Occurrence, Treatment, and Toxicological Relevance of EDCs and Pharmaceuticals in Water. Ozone Sci. Eng. 2008;30:65–69. doi: 10.1080/01919510701799278. [DOI] [Google Scholar]
- 6.Fent K., Weston A.A., Caminada D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006;76:122–159. doi: 10.1016/j.aquatox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Vethaak A.D., Lahr J., Schrap S.M., Voogt P.D. An integrated assessment of estrogenic contamination and biological effects in the aquatic environment of The Netherlands. Chemosphere. 2005;59:511–524. doi: 10.1016/j.chemosphere.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 8.Carmona E., Andreu V., Picó Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total Environ. 2014;484:53–63. doi: 10.1016/j.scitotenv.2014.02.085. [DOI] [PubMed] [Google Scholar]
- 9.Weber W.J., Smith E.H. Removing dissolved organic contaminants from water. Environ. Sci. Tehchnol. 1986;20:970–979. doi: 10.1021/es00152a002. [DOI] [PubMed] [Google Scholar]
- 10.Buser H.R., Poiger T., Müller M.D. Occurrence and Fate of the Pharmaceutical Drug Diclofenac in Surface Waters: Rapid Photodegradation in a Lake. Environ. Sci. Tehchnol. 1998;32:3449–3456. doi: 10.1021/es980301x. [DOI] [Google Scholar]
- 11.Lin A., Reinhard M. Photodegradation of common environmental pharmaceuticals and estrogens in river water. Environ. Toxicol. Chem. 2005;24:1303–1309. doi: 10.1897/04-236R.1. [DOI] [PubMed] [Google Scholar]
- 12.Kimura K., Hara H., Watanabe Y. Elimination of selected acidic pharmaceuticals from municipal wastewater by an activated sludge system and membrane bioreactors. Environ. Sci. Technol. 2007;41:3708–3714. doi: 10.1021/es061684z. [DOI] [PubMed] [Google Scholar]
- 13.Benitez F.J., Real F.J., Acero J.L. Removal of selected pharmaceuticals in waters by photochemical processes. J. Chem. Technol. Biotechnol. 2009;84:1186–1195. doi: 10.1002/jctb.2156. [DOI] [Google Scholar]
- 14.Rizzo L., Meric S., Kassinos D., Belgiorno V. Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res. 2009;43:979–988. doi: 10.1016/j.watres.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 15.Giri R.R., Ozaki H., Takayanagi Y., Takanami R. Efficacy of ultraviolet radiation and hydrogen peroxide oxidation to eliminate large number of pharmaceutical compounds in mixed solution. Int. J. Environ. Sci. Technol. 2011;8:19–30. doi: 10.1007/BF03326192. [DOI] [Google Scholar]
- 16.Radjenovic J., Petrovic M., Barceló D. Analysis of pharmaceuticals inwastewater and removal using amembrane bioreactor. Anal. Bioanal. Chem. 2007;387:1365–1377. doi: 10.1007/s00216-006-0883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura K., Toshima S., Amy G., Watanabe Y. Rejection of neutral endocrine disrupting compounds (EDCs) and pharmaceutical active compounds (PhACs) by RO membranes. J. Membr. Sci. 2004;245:71–78. doi: 10.1016/j.memsci.2004.07.018. [DOI] [Google Scholar]
- 18.Boleda R., Galceran T., Ventura F. Behavior of pharmaceuticals and drugs of abuse in a drinking water treatment plant (DWTP) using combined conventional and ultrafiltration and reverse osmosis (UF/RO) treatments. Environ. Pollut. 2011;159:1584–1591. doi: 10.1016/j.envpol.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 19.Sahar E., David I., Gelman Y., Chikurel H., Aharoni A., Messalem R., Brenner A. The use of RO to remove emerging micropollutants following CAS/UF or MBR treatment of municipal wastewater. Desalination. 2011;273:142–147. doi: 10.1016/j.desal.2010.11.004. [DOI] [Google Scholar]
- 20.Dolar D., Gros M., Rodriguez-Mozaz S., Barcelo D. Removal of emerging contaminants from municipal wastewater with an integrated membrane system, MBR-RO. J. Hazard. Mater. 2012;239–240:64–69. doi: 10.1016/j.jhazmat.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Alturki A.A., Tadkaew N., McDonaldb J.A., Khan S.J., Price W.E., Nghiem L.D. Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J. Membr. Sci. 2010;365:206–215. doi: 10.1016/j.memsci.2010.09.008. [DOI] [Google Scholar]
- 22.Kim I., Yamashita N., Tanaka H. Photodegradation of pharmaceuticals and personal care products during UV and UV/H2O2 treatments. Chemosphere. 2009;77:518–525. doi: 10.1016/j.chemosphere.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 23.Vagna D., Marotta R., Andreozzi R., Napolitana A. Kinetic and chemical seesssment of the UV/H2O2 treatment of antiepileptic drug carbamazepine. Chemosphere. 2004;54:497–505. doi: 10.1016/S0045-6535(03)00757-4. [DOI] [PubMed] [Google Scholar]
- 24.Pereira V.J., Weinberg H.S., Linden K.G., Singer P.C. UV degradation kinetics and modeling of pharmaceutical compounds in laboratory grade and surface water via direct and indirect photolysis at 254 nm. Envrion. Sci. Technol. 2007;41:1682–1688. doi: 10.1021/es061491b. [DOI] [PubMed] [Google Scholar]
- 25.Urase T., Kikuta T. Separate estimation of adsorption and degradation of pharmaceutical substances and estrogens in the activated sludge process. Water Res. 2005;39:1289–1300. doi: 10.1016/j.watres.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Suarez S., Carballa M., Omil F., LemaJ M. How are pharmaceutical and personal care products (PPCPs) removed from urban wastewaters? Rev. Environ. Sci. Bio-Technol. 2008;7:125–138. doi: 10.1007/s11157-008-9130-2. [DOI] [Google Scholar]
- 27.SRC (Syracuse Research Company) PhysProp Database. [(accessed on 2 July 2010)]; Available online: www.syrres.com/esc/physdemo.htm.
- 28.Vanderford B.J., Snyder S.A. Analysis of pharmaceuticals in water by isotope dilution liquid chromatography/tandem mass spectrometry. Environ. Sci. Technol. 2006;40:7312–7320. doi: 10.1021/es0613198. [DOI] [PubMed] [Google Scholar]
- 29.Miège C., Choubert J.M., Ribero L., Eusèbe M., Coquery M. Fate of pharmaceuticals and personal care products in wastewater treatment plants—Conception of a database and first results. Environ. Pollut. 2009;157:1721–1726. doi: 10.1016/j.envpol.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 30.Le-Minh N., Khan S.J., Drewes J.E., Stuetz R.M. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010;44:4295–4323. doi: 10.1016/j.watres.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Clara M., Strenn B., Gans O., Martinez E., Kreuzinger N., Kroiss H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005;39:4797–4807. doi: 10.1016/j.watres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Radjenovi J., Petrovi M., Barceló D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009;43:831–841. doi: 10.1016/j.watres.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 33.Kosma C.I., Lambropoulou D.A., Albanis T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014;466–467:421–438. doi: 10.1016/j.scitotenv.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 34.Cartagena P., Kaddouri M.E., Cases V., Trapote A., Prats D. Reduction of emerging micropollutants, organic matter, nutrients and salinity from real wastewater by combined MBR-NF/RO treatment. Sep. Purif. Technol. 2013;110:132–143. doi: 10.1016/j.seppur.2013.03.024. [DOI] [Google Scholar]
- 35.Gao J., O’Brien J., Peng D., Ortc C., Muellera J.F., Thaiad P.K. Measuring selected PPCPs in wastewater to estimate the population in different cities in China. Sci. Total Environ. 2016;568:164–170. doi: 10.1016/j.scitotenv.2016.05.216. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch R., Ternes T., Haberer K., Kratz K.L. Occurrence of antibiotics in the aquatic environment. Sci. Total Environ. 1999;225:109–118. doi: 10.1016/S0048-9697(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z., Feng Y., Gao P., Ren N. Occurrence and removal efficiencies of eight EDCs and estrogenicity in a STP. J. Environ. Monit. 2011;13:1366–1373. doi: 10.1039/c0em00597e. [DOI] [PubMed] [Google Scholar]
- 38.Göbel A., McArdell C.S., Joss A., Siegrist H., Giger W. Fate of sulfonamides macrolides, and trimethoprim in different wastewater treatment technologies. Sci. Total Environ. 2007;372:361–371. doi: 10.1016/j.scitotenv.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 39.Joss A., Keller E., Alder A.C., Göbel A., McArdell C.S., Ternes T., Siegrist H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005;39:3139–3152. doi: 10.1016/j.watres.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 40.Chon K., KyongShon H., Cho J. Membrane bioreactor and nanofiltration hybrid system for reclamation of municipal wastewater: Removal of nutrients, organic matter and micropollutants. Bioresour. Technol. 2012;122:181–188. doi: 10.1016/j.biortech.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 41.Clara M., Strenn B., Kreuzinger N. Carbamazepine as a possible anthropogenic marker in the aquatic environment: Investigations on the behaviour of Carbamazepine in wastewater treatment and during groundwater infiltration. Water Res. 2004;38:947–954. doi: 10.1016/j.watres.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 42.Maurer M., Escher B.I., Richle P., Schaffner C., Alder A.C. Elimination of β-blockers in sewage treatment plants. Water Res. 2007;41:1614–1622. doi: 10.1016/j.watres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Han J., Qiu W., Hu J., Gao W. Chemisorption of estrone in nylon microfiltration membranes: Adsorption mechanism and potential use for estrone removal from water. Water Res. 2012;46:873–881. doi: 10.1016/j.watres.2011.11.066. [DOI] [PubMed] [Google Scholar]
- 44.Joss A., Baenninger C., Foa P., Koepke S., Krauss M., McArdell C.S., Rottermann K., Wei Y., Zapata A., Siegrist H. Water reuse: >90% water yield in MBR/RO through concentration recycling and CO2 addition as scaling control. Water Res. 2011;45:6141–6151. doi: 10.1016/j.watres.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Gur-Reznik S., Koren-Menashe I., Heller-Grossman L., Rufel O., Dosoretz C.G. Influence of seasonal and operating conditions on the rejection of pharmaceutical active compounds by RO and NF membranes. Desalination. 2011;277:250–256. doi: 10.1016/j.desal.2011.04.029. [DOI] [Google Scholar]
- 46.Snyder S.A., Westerhoff P., Yoon Y., Sedlak D.L. Pharmaceuticals, Personal care products, and endocrine disruptors in water: Implication for the water industry. Environ. Eng. Sci. 2003;20:449–469. doi: 10.1089/109287503768335931. [DOI] [Google Scholar]
- 47.Wang X., Li B., Zhang T., Li X. Performance of nanofiltration membrane in rejecting trace organic compounds: Experiment and model prediction. Desalination. 2015;370:7–16. doi: 10.1016/j.desal.2015.05.010. [DOI] [Google Scholar]
- 48.Comerton A.M., Andrews R.C., Bagley D.M., Yang P. Membrane adsorption of endocrine disrupting compounds and pharmaceutically active compounds. J. Membr. Sci. 2007;303:267–277. doi: 10.1016/j.memsci.2007.07.025. [DOI] [Google Scholar]
- 49.Jacob M., Li C.C., Guigui C., Cabassud C., Lavison G., Moulin L. Performance of NF/RO process for indirect potable reuse: Interactions between micropollutants, micro-organisms and real MBR permeate. Desalination Water Treat. 2012;46:75–86. doi: 10.1080/19443994.2012.677507. [DOI] [Google Scholar]